Abstract
Depletion of β-catenin impairs regeneration of the rapid turn-over gut epithelial cells, but appears dispensable for that of the slow turn-over mature hepatocytes in mice until 1 y of age. As the life span of mature murine hepatocytes is about 400 d, we studied conditional β-catenin knockout mice (Alb-Cre;Ctnnb1flx/flx) until 20 mo of age to determine the function of β-catenin in the postnatal liver. β-catenin was absent from the hepatocytes of β-catenin knockout mice 4 wk after delivery. From 9 mo of age, hepatocytes were gradually replaced by newly formed β-catenin-positive hepatocytes, which constituted about 90% of hepatocytes at 18–20 mo of age. This process was accompanied by active proliferation of bile duct/ductule cells. β-catenin-positive hepatocytes exhibited elevated proliferation activity and expression of progenitor cell markers, but lower albumin and Cre. This might explain their intact β-catenin protein, and suggest their origins from hepatic progenitor cells. Liver tumors arose spontaneously from β-catenin-positive cells, and tumorigenesis was accelerated by hepatitis B X protein. These results indicate β-catenin critical for the regeneration of mature hepatocytes. Failure to regenerate mature hepatocytes results in proliferation of hepatic progenitor cells that are able to maintain liver function but are predisposed to form liver tumors.
Keywords: HBx protein, liver cancer, postnatal liver regeneration
The Wnt/β-catenin pathway has long been considered involved in embryonic liver development and hepatocarcinogenesis (1, 2). However, its role in the regeneration of mature hepatocytes has remained inconclusive.
In normal mature hepatocytes, the activity of this pathway is tightly controlled. Most cytosolic β-catenin is phosphorylated by glycogen synthase kinase 3β at specific serine/threonine residues, resulting in its degradation. Somatic Wnt-activation mutations of β-catenin and Axin-1 genes were identified in 20% to 30% of hepatocellular carcinoma (HCC) cases (2–4). In addition, down-regulation of two negative regulators of this pathway, APC and E-cadherin (2, 5), and overexpression of the Fzd type 7 receptor were frequently observed in HCC (6), all of which result in accumulation of β-catenin. Therefore, β-catenin accumulation in the cytoplasm and nucleus is present in 50% to 70% of HCCs (7). Mutations of β-catenin also occur in the majority of childhood hepatoblastomas (8) and in a subgroup of adult hepatic adenomas predisposing to HCC (9). Activation of the Wnt/β-catenin pathway is thus considered a key event in hepatocarcinogenesis.
Several transgenic (Tg) mouse models were established to investigate the role of an activated Wnt/β-catenin pathway in hepatocarcinogenesis. Tg mice expressing a Wnt-activating mutant of β-catenin in mature hepatocytes developed hepatomegaly with elevated proliferation activity but low compensatory apoptosis, but which was insufficient to cause HCC (10, 11). Additional events, such as H-ras activation or diethylnitrosamine treatment, are required for HCC formation (11, 12). Therefore, activation of β-catenin could accelerate liver regeneration, and could be critical for the development of HCC in the presence of other oncogenic events.
The role of β-catenin in liver regeneration and carcinogenesis has also been examined using conditional β-catenin–KO mouse models. Specific deletion of β-catenin in hepatoblasts was lethal for embryos at the embryonic day 17 stage, showing an overall deficient of parenchymal hepatocytes (13), These results indicate the critical role that β-catenin in the proliferation and differentiation of embryonic hepatoblasts. However, when β-catenin was conditionally knocked out in hepatocytes after birth, the mice showed only a 10% to 20% reduction in liver mass during the 12-mo follow-up period (14, 15). This suggest that β-catenin is less critical for regeneration in the postnatal liver than in the embryonic liver. Unexpectedly, the β-catenin–KO mice, similar to constitutively activated β-catenin Tg mice, are also more susceptible to diethylnitrosamine-induced HCC (16, 17). Thus, results from KO mouse models do not indicate a critical role for β-catenin in postnatal liver regeneration, and its role in tumorigenesis warrants clarification.
The liver is considered a slowly renewing organ, and the mean lifespan of mature hepatocytes is approximately 400 d in mice (18, 19). We propose that, if β-catenin is critical for regeneration of mature hepatocytes that have reached their normal lifespan, its deletion will not have a detectable effect if the period of observation does not exceed the normal lifespan of mature hepatocytes. This is because most mature hepatocytes do not undergo active replication within their lifespan. It is noteworthy that the follow-up period in most postnatal β-catenin–KO mouse studies was usually less than 12 mo (14, 15, 17), which may have been of insufficient duration and resulted in underestimation of the role of β-catenin in regeneration of mature hepatocytes. Therefore, we propose to study β-catenin–KO mice for 18 to 20 mo, a period that exceeds the normal lifespan of mature hepatocytes.
Our initial results showed that β-catenin was absent from hepatocytes at the age of 1 mo. From 9 mo of age, hepatocytes were gradually replaced by newly formed β-catenin(+) hepatocytes, which constituted approximately 90% of hepatocytes at 18 to 20 mo of age. We then analyzed changes in liver histology to determine the ontogeny of the newly formed β-catenin(+) cells and evaluated their susceptibility for liver tumorigenesis by crossing with HBx Tg mice. The uncharted role of β-catenin in regeneration of postnatal hepatocytes and in HBx-induced HCC was investigated.
Results
Replacement of β-Catenin(−) Hepatocytes by β-Catenin(+) Hepatocytes in Liver of β-Catenin–KO Mice During Long-Term Follow-Up.
To generate KO mice with β-catenin–free mature hepatocytes, we crossed Ctnnb1flx/flx mice with Alb-Cre mice. The extent of β-catenin deletion in mature hepatocytes in β-catenin–KO mice was evaluated by Western blotting with the proteins extracted from the liver/purified hepatocytes of 2-mo-old mice. The results supported the efficient depletion of β-catenin from mature hepatocytes in this mouse model, with the details as described in SI Results and Fig. S1.
To test our hypothesis that a long follow-up that exceeds the lifespan of mature hepatocytes is required to evaluate the effect of β-catenin deletion from the postnatal liver, we followed KO mice until 18 to 20 mo of age. First, we examined changes in the expression pattern of β-catenin in liver tissues collected from WT and KO mice from 10 d to 18 mo of age by using Western blotting and immunohistochemical (IHC) staining. Intriguingly, a gradual recovery of β-catenin was observed in the liver of β-catenin–KO mice after 7 mo of age (Fig. 1A), which almost completely recover to the level close to that of WT mice at 18 mo of age (Fig. 1B).
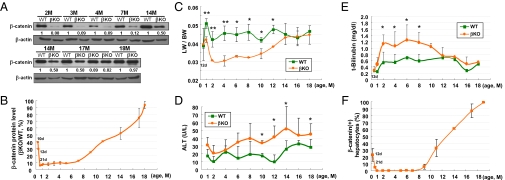
Fig. 1.
β-Catenin(−) hepatocytes in β-catenin–KO mouse liver are gradually replaced by β-catenin(+) hepatocytes in mice older than 7 mo. (A) Liver tissues were collected from 2- to 18-mo-old β-catenin–KO and WT mice for Western blotting. The relative ratios of the amount of β-catenin in the liver of β-catenin–KO mice to that in the WT mice at various ages are indicated at the bottom of each pair of samples, and illustrated in a quantitative manner (B). Several parameters were compared between the β-catenin–KO and WT mice, including LW/BW (C), ALT (D), and total bilirubin (E). (F) The percentages of β-catenin(+) hepatocytes in the livers of KO mice at different ages are illustrated schematically according to IHC staining results. All graphic results represent the mean values of each group of samples with SE bars; data were analyzed by using a t test (*P < 0.05 and **P < 0.01).
The ratios of liver weight (LW) to body weight (BW) and levels of serum alanine aminotransferase (ALT) and total bilirubin were determined at different ages. The LW/BW ratios of WT and KO mice were similar at 12 d of age. However, the ratio for KO mice was approximately 30% lower than that of WT mice from the age of 1 mo, and remained so until 10 mo of age (Fig. 1C). After that, the ratio for β-catenin–KO mice increased gradually and reached a level close to that of the WT mice at 14 mo of age (Fig. 1C). Levels of ALT and total bilirubin were higher in β-catenin–KO mice than in WT mice during the follow-up, but remained within the normal range (Fig. 1 D and E).
The recovery of β-catenin in the liver of KO mice was confirmed by IHC staining, with the representative results shown in Fig. S2 and the quantitative data summarized in Fig. 1F. Most hepatocytes were β-catenin(−) at the age of 2 to 7 mo; the only positive signals were detected in nonparenchymal cells (Fig. S2, arrowheads, 2M). However, 3% to 5% of hepatocytes in the liver of KO mice at the age of 9 mo expressed β-catenin (Fig. S2, arrows, 9M and 11M), which gradually formed β-catenin(+) cell clusters (Fig. S2, 9M and 11M). The proportions of these cells increase to approximately 30%, 60%, and 90% at 11, 14, and 18 mo of age, respectively (Fig. 1F and Fig. S2).
To examine if the replacement event also occur in the liver of β-catenin–KO mice underwent partial hepatectomy (PHx), we compared the regeneration and protein expression patterns in serial livers collected after PHx of 2-mo-old WT and β-catenin–KO mice. The results indicated that the replacement also occurs in the liver of β-catenin–KO mice, with the details as described in SI Results and Fig. S3.
β-Catenin(+) Cells May Be Derived from Hepatic Progenitor Cells.
We next tried to address the origin of the β-catenin(+) hepatocytes that replaced the β-catenin(−) hepatocytes in β-catenin–KO mice. Intriguingly, we observed a significant increase in the number of bile duct/ductule cells and ductular reactions in liver tissues collected from the KO mice (Fig. S2), which was confirmed by their positive signals for cytokeratin 19 (CK19) staining (Fig. 2A). This pattern began at the age of 11 mo, gradually increased until the age of 18 mo, and was strongly correlated with the increase in the number of β-catenin(+) cells (Fig. 2A and Fig. S2). This correlation was also observed in liver that was regenerating after PHx (Fig. S4). Some of the proliferating bile duct cells were close to the clusters of β-catenin(+) hepatocytes (Fig. S2, 11–19M, and Fig. S4, 2–6M). It thus raises the possibility that the β-catenin(+) hepatocytes were derived from hepatic progenitor cells, such as oval cells or bipotential cells in the ductules, which gave rise to hepatocytic and biliary lineages of cells.
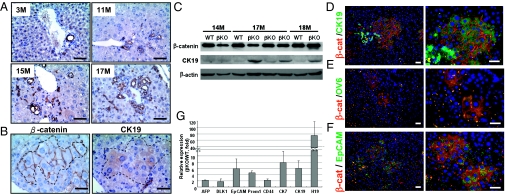
Fig. 2.
β-Catenin(+) hepatocytes in the β-catenin–KO mice could be derived from hepatic progenitor cells. (A) Liver tissues collected from KO mice at various ages were stained with CK19 antibody, with the ductule cells showing strong positive CK19 signals. (B) Continuous liver sections collected from a 14-mo-old KO mouse were stained with CK19 and β-catenin antibodies, with areas showing positive signals indicated by the dotted lines. (C) Proteins extracted from the livers of WT and KO mice at the ages of 14, 16, 17, and 18 mo were processed for Western blotting by probing with antibodies as indicated. (D) Double staining of the liver tissues collected from a 15-mo-old β-catenin–KO mouse, with β-catenin antibody in combination with antibodies against CK19 (D), OV6 (E), and EpCAM (F) (Scale bar: 50 μM.) (G) The mRNA levels of several hepatic progenitor markers were compared between liver tissues collected from WT and β-catenin–KO mice (18 mo old; n = 4). Results are expressed as the ratio of the amount of mRNA in liver from KO mice to that from the WT mice.
CK19 staining also revealed that some of the β-catenin(+) cell clusters were weakly positive for CK19 (Fig. 2B and Fig. S4), suggesting their early exit from the population of bipotential progenitor cells. Western blotting further supported an increase in CK19 in the liver of KO mice, in accordance with the increase of β-catenin(+) hepatocytes (Fig. 2C). Double staining was conducted to validate that β-catenin(+) clusters of cells in close proximity to the proliferative ductule cells are CK19-positive (Fig. 2D). Within the same areas, we noted several distinctly ovoid cells with scant cytoplasm, a characteristic of oval cells, that showed strong positive signals for β-catenin and CK19 (Fig. S5). These results supported that the β-catenin(+) hepatocytes are immature hepatocytes, which could be derived from oval cells or bipotential progenitor cells.
To clarify this, we conducted double-staining by using β-catenin antibody in combination with antibodies against two progenitor cell markers, OV6 and epithelial cell adhesion molecule (EpCAM). Some β-catenin(+) cluster of cells were positive for OV6 and EpCAM (Fig. 2 E and F), supporting their identity as hepatic progenitor cells but not mature hepatocytes. This was further validated by the elevated mRNA expression of a panel of progenitor cell markers, including AFP, DLK1, EpCAM, Prom1, CD44, CK7, CK19, and H19, in the liver collected from β-catenin–KO mice than that from WT control mice (19-mo-old; Fig. 2G).
Failure of β-Catenin Deletion in β-Catenin(+) Progenitor Cells Is Attributed to Low Albumin Promoter Activity.
To determine why β-catenin is not knocked out in β-catenin(+) immature hepatocytes, DNA from the liver of 17-mo-old KO mice was processed for genotyping. The Cre-loxP recombination was not successful in this liver compared with that of 2-mo-old mice in which β-catenin deletion occurred (Fig. 3A, lane 4 vs. lanes 2 and 3), suggesting the β-catenin(+) hepatocytes were derived from cells not undergoing recombination. The sequencing analysis excluded the possibility of mutations in the Cre and loxP sites in β-catenin(+) hepatocytes. Therefore, we investigated whether this was a result of insufficient Cre expression caused by low albumin promoter activity in immature hepatocytes.
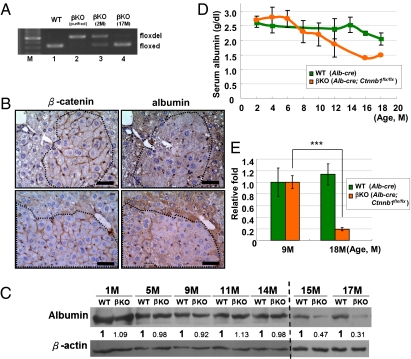
Fig. 3.
Failure of β-catenin deletion in β-catenin(+) hepatic progenitor cells is attributed to low albumin promoter activity. (A) Genotyping analysis of DNA collected from livers of KO mice (lane 2, 3 mo old; lane 4, 17 mo old) and control WT mice (lane 1, 2 mo old), and of control hepatocytes purified by collagenase perfusion from the KO mouse (lane 2, 2 mo old). (B) Continuous liver tissue sections collected from 15-mo-old β-catenin–KO mice were processed for IHC staining with albumin and β-catenin antibodies. (C) Proteins extracted from the livers of WT and KO mice were processed for Western blotting probing with albumin and β-actin antibodies. (D) Serum albumin levels of WT and KO mice of various ages were determined. (E) The mRNA levels of Cre in livers from Alb-Cre (nondeleted β-catenin) and Alb-Cre;Ctnnb1flx/flx (β-catenin–KO) mice at the ages of 9 and 18 mo were examined by quantitative PCR (n = 3). The mean for 9-mo-old mice was standardized to a value of 1.0, and data were analyzed by using a t test (***P < 0.001). (Scale bar: 50 μM.)
To address this, liver tissues collected from KO mice were processed for albumin staining. The clusters of β-catenin(+) hepatocytes showed a weaker signal than the β-catenin(−) hepatocytes (Fig. 3B). The decrease in albumin expression in the liver of KO mice was supported by Western blotting (Fig. 3C), and also by a decrease in serum albumin level (Fig. 3D). Quantitative RT-PCR revealed the mRNA level of Cre in liver from 18-mo-old KO mice was less than that from 9-mo-old KO mice, consistent with the decrease of albumin protein level (Fig. 3E). Therefore, failure of β-catenin deletion in β-catenin(+) immature hepatocytes could occur because of weak albumin promoter activity in these less-differentiated progenitor cells, leading to insufficient Cre expression for successful recombination.
Hepatic Tumors Develop from Clusters of β-Catenin(+) Hepatic Progenitor Cells Spontaneously or When Induced by HBx Oncoprotein.
The replication activity between β-catenin(+) and β-catenin(−) hepatocytes was first compared by Ki-67 staining in liver tissues collected from a 14-mo-old KO mouse and from a KO mouse 6 mo after PHx. Representative results are shown in Fig. S6 A and B, with the quantification results summarized in Fig. S6 C and D, respectively. Ten to 20% of hepatocytes in the β-catenin(+) clusters of hepatocytes were Ki-67–positive, and very few of the β-catenin(−) hepatocytes were Ki-67–positive, indicating that β-catenin(+) hepatocytes have higher proliferative activity than β-catenin(−) hepatocytes.
During follow-up of the β-catenin KO mice, we noted that liver tumors arose spontaneously in some of these β-catenin–KO mice, but not in the control group of Ctnnb1flx/flx mice (n > 30). Six adenomas and one HCC were identified among 12 KO mice at the end of the 20-mo follow-up period (H&E staining; Fig. S7). IHC staining showed that these liver tumors and the adjacent nontumorous tissues all consisted of β-catenin(+) hepatocytes (Fig. S7B). However, the CK19 signal was stronger in the tumor tissues than in adjacent nontumorous tissues (Fig. S7C). The results suggest that β-catenin(+) progenitor cells in the liver of KO mice have a high tumorigenic potential.
We then crossed Alb-Cre;Ctnnb1flx/flx mice with HBx Tg mice (HBx;Ctnnb1flx/flx) to determine whether the tumorigenic potential of β-catenin(+) hepatic progenitor cells is enhanced by the carcinogenic HBx protein. As expected, the replacement event also occurred in the liver of HBx;Alb-Cre;Ctnnb1flx/flx mice, but at a faster rate than in Alb-Cre;Ctnnb1flx/flx mice. Replacement started at 3 to 4 mo of age, and more than 90% of hepatocytes were β-catenin(+) by 8 mo of age (Fig. 4 A and B). The rate of liver tumor development was also much faster in HBx;Alb-Cre;Ctnnb1flx/flx mice than in HBx;Ctnnb1flx/flx and Alb-Cre;Ctnnb1flx/flx mice (representative images of the livers are shown in Fig. 4C, and the incidence of tumors is summarized in Fig. 4D). The tumors not only developed earlier (8–9 mo of age), but were so numerous that they covered the entire liver, which were preceded by multiple nodules apparent from 6 to 7 mo of age (Fig. 4C). In contrast, HBx;Ctnnb1flx/flx and Alb-Cre;Ctnnb1flx/flx mice had fewer than three tumors per liver.
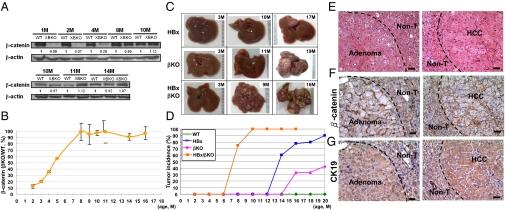
Fig. 4.
Crossing of β-catenin–KO mice with HBx Tg mice resulted in the formation of multiple liver tumors arising from β-catenin(+) hepatic progenitor cells. (A) Protein expression of β-catenin and HBx in the livers of WT and KO mice at various ages was analyzed by Western blotting. The amount of β-catenin in the KO mice relative to that of the control WT mice at the same age is indicated at the bottom of each pair of samples, and quantitative results are shown (B). (C) Gross views of whole livers harvested from HBx Tg mice, β-catenin–KO mice, and HBx;Alb-Cre;Ctnnb1flx/flx mice at the indicated ages. (D) Incidence of tumors in the various strains of mice at various ages. (E) Representative liver tumors from HBx;Alb-Cre;Ctnnb1flx/flx mice were processed for H&E staining and IHC staining with antibodies against β-catenin (F) and CK19 (G). (Scale bar: 50 μM.)
The types of liver tumors in HBx;Alb-Cre;Ctnnb1flx/flx mice were confirmed histopathologically (Fig. 4E). IHC analysis revealed that most tumors and adjacent nontumorous tissues were β-catenin(+), with a stronger CK19 signal in the tumor than in the adjacent nontumorous tissue (Fig. 4G). These results support the high potential of β-catenin(+) hepatic progenitor cells for HCC formation, which is potentiated by the presence of carcinogenic agents such as HBx.
Discussion
The present study demonstrated that a 18- to 20-mo follow-up of β-catenin–KO mice is necessary to elucidate the role of β-catenin in postnatal liver regeneration. After a period equivalent to the normal lifespan of mature hepatocytes, the liver of KO mice was gradually replaced by β-catenin(+) hepatic progenitor cells, which constituted more than 90% of hepatic cells at the end of the follow-up period.
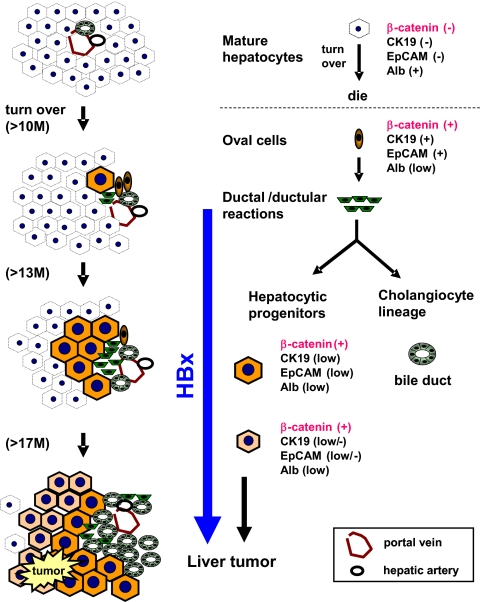
Apparently, β-catenin(−) mature hepatocytes in KO mice are healthy and functional throughout their normal lifespan, as revealed by the liver function indicators of ALT and bilirubin, and by histology (Fig. 1). However, the proliferation capability of such cells seems to be compromised (Fig. S6 A and C), which was also evident after PHx (Fig. S6 B and D). In such an environment, hepatic progenitor cells proliferate to maintain normal liver mass and function. This was implied by an increase in the number of β-catenin(+) cells that had a high expression level of the hepatic progenitor markers, CK19, EpCAM, AFP, DLK1, Prom1, CD44, CK7, and H19 (Fig. 2G). This also explains the increase in the number of bile ductule cells that accompanied the increase of immature hepatocytes in the KO liver after 12 mo of age (Fig. 2 and Fig. S2). Both groups of cells could be derived from the bipotential hepatic oval cells, which is consistent with the presence of numerous oval cell-like cells in close proximity to the amplifying β-catenin(+) hepatocytes and ductule cells (Fig. S5). Such oval cells undergo differentiation to the β-catenin(+) immature hepatocytes before final maturation, which eventually became the major population in the aged liver. The liver functions executed by β-catenin(+) immature hepatocytes in 18-mo-old mice remain normal, as reflected by normal bilirubin and ALT levels (Fig. 1 D and E). However, the cells showed a higher carcinogenic potential for subsequent tumor formation, either spontaneously or as greatly accelerated by the HBx protein. The details of this proposed scheme for the replacement and carcinogenic events in the liver of β-catenin KO mice is shown in Fig. 5.
Fig. 5.
Schematic illustration of the replacement and carcinogenic events in the liver of β-catenin–KO mice, showing changes in cell populations at different ages. The liver consisted of approximately 99% β-catenin(−) mature hepatocytes from day 15 after birth for the normal hepatocyte lifespan (>10–12 mo), after which the hepatocytes underwent regeneration. Because hepatocytes lacking β-catenin lose the ability to regenerate, hepatic oval cells proliferate and differentiate into hepatocytes and cholangiocytes. These two cell lineages gradually replaced the entire liver mass. During active proliferation, most hepatic progenitor cells underwent maturation arrest and became dedifferentiated via an unknown mechanism. These progenitor-derived immature hepatocytes showed a high potential to develop into liver tumors, including adenoma and HCC, and this process was accelerated by HBx.
It is noteworthy that a similar replacement event in the epithelium of small intestine of β-catenin–KO mice has been reported (20). This occurred 5 d after induction of Cre expression, a period equivalent to the lifespan of small intestine cells. The results suggest that β-catenin is critical for maintenance of normal regeneration of intestinal epithelial cells and also of mature hepatocytes in the gastrointestinal tract. The mechanism(s) underlying β-catenin regulation of the proliferation of mature hepatocytes are currently under investigation with the use of this model.
Replacement events were not reported in several previous studies in which the same conditional β-catenin–KO mice were used to investigate the role of β-catenin in postnatal liver regeneration (14, 15). The reason for the lack of a replacement event may reside with the follow-up periods used (usually <12 mo), which were shorter than the lifespan of normal hepatocytes. Until recently, three reports also mentioned the reappearance of β-catenin(+) cells in the β-catenin–KO liver, but none of them have identified the progenitor origin of these cells. Braeuning et al. reported the occurrence of small clusters of β-catenin(+) cells from 2 mo of age (21), which, however, did not show higher tumorigenic potential by treatment with the tumor promoting agent of phenobarbital (16). Sekine et al. reported replacement event in the liver of KO mice from 8 mo of age, and the β-catenin(+) cells were derived from cells that escaped Cre-mediated recombination as a result of the presence of mutations at loxP sites (22). However, loxP-site mutations were not detected in the β-catenin(+) hepatocytes in the present study. Instead, the expression of β-catenin was caused by low Cre expression secondary to weak albumin promoter activity in immature hepatocytes (Fig. 3). Factors responsible for such discrepancies warrant clarification. More recently, Thompson et al. reported a repopulation of β-catenin(+) hepatocytes in the β-catenin–KO mouse liver treated with 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC), a reagent for inducing chronic liver injury (23). The β-catenin(+) hepatocytes were enriched by DDC treatment but rarely detected in the baseline liver. These cells did not show the progenitor cell markers, which instead were characterized as the mature hepatocytes. Although such β-catenin(+) hepatocytes also showed higher proliferation activity, but no liver tumors were identified in this model during long-term follow-up. Therefore, the nature or the differentiation program of the DDC-induced β-catenin(+) hepatocytes could be different from the ones spontaneously repopulating in liver without any stimulus, as shown in our model. The details for the difference await further investigation.
It is well documented that, in normal liver, few hepatic progenitor cells are present, and mature hepatocytes are the main source of cells during normal liver regeneration. However, the regeneration capability of mature hepatocytes is significantly impaired in human liver diseases such as chronic hepatitis, alcoholic liver disease, and chronic cholestatic diseases. In that case, the number of hepatic progenitor cells increases and contributes to hepatic regeneration to maintain liver mass (24). It raises a possibility that human HCC may originate from enriched progenitor cells in the liver under conditions in which the proliferation of hepatocytes is inhibited. By tracing the mitochondrial DNA mutations, Lin et al. recently showed that some monoclonal human cirrhotic regenerative nodules could be derived from hepatic progenitor cells (25). However, the details still need to be studied by using appropriate animal models.
Our β-catenin–KO mice could be an appropriate animal model in which to address this issue. Because of the failure of active replication of β-catenin(−) mature hepatocytes, the livers of older KO mice are rich in β-catenin(+) hepatic progenitor cells. The potential of these progenitor cells for subsequent tumorigenesis can be addressed in this model. In our study, the older KO mice spontaneously developed liver tumors from the progenitor cells, according to their positive status for β-catenin and CK19 staining. It suggested that hepatic progenitor cells are more susceptible to tumor development than mature hepatocytes. This hypothesis was further supported by our trial to cross β-catenin–KO mice with HBx Tg mice. In the liver of HBx;Alb-Cre;Ctnnb1flx/flx mice, replacement with β-catenin(+) cells also occurred, starting from 3 mo of age and reaching 90% at 8 mo of age. Moreover, multiple liver tumors arose from numerous nodules that consisted of β-catenin(+) immature hepatocytes and covered the entire liver (Fig. 4C). This pattern differed from that in control HBx;Ctnnb1flx/flx and Alb-Cre;Ctnnb1flx/flx mice, in which tumors developed at the age of 17 to 20 mo and usually numbered fewer than three per liver. These results demonstrate the high carcinogenic potential of hepatic progenitor cells in HBx-induced carcinogenesis (17).
This unique pattern is noted very similar to that of human HCC arising from HBV-associated cirrhosis. The β-catenin(+) nodules in the HBx;Alb-Cre;Ctnnb1flx/flx mice are similar to the regenerative nodules of patients with cirrhosis, which are usually surrounded by many CK19- and EpCAM-positive cells (26). We thus propose a model in which the cirrhotic livers of patients with HBV are repopulated by hepatic progenitor cells, which predispose them to HCC when they are infected with HBV and exposed to the HBx carcinogenic protein. This hypothesis will be subjected to further testing using our animal model.
Another intriguing characteristic of the progenitor cells in this animal model was noted: in old β-catenin–KO mice, most β-catenin(+) cells derived from hepatic oval cells remained immature before the end stage of differentiation, with respect to their weak positivity for CK19 and low albumin expression. This suggests a terminal differentiation signal required for the hepatocyte maturation is compromised in β-catenin–KO mice. The critical role of β-catenin in mature hepatocytes for the terminal differentiation of progenitor cells was thus implicated. Aided by cell culture based assay, Mancone et al. demonstrated that the differentiation of hepatocytes induced by cell–cell contact could be mediated through the E-cadherin/β-catenin pathway, which was diminished by the active β-catenin (27). It raised a possibility that the membrane translocalization of β-catenin could contribute to hepatocyte differentiation, which was also supported by the matrigel-induced hepatocyte differentiation (28). We expect that this possibility can be firmly addressed in our animal model. For example, transplantation of the progenitor cells into the WT or the β-catenin–KO liver can help evaluate their subsequent differentiation status. Moreover, the “stemness” genes and underlying mechanisms which maintain the progenitor status of β-catenin(+) cells in this model could be further addressed prospectively.
Finally, this animal model provides a potential application for experiments which need large numbers of progenitor hepatocytes. For example, purification of the enriched progenitor cells from the livers of aged KO mice can help evaluate the feasibility and safety of novel stem cell therapy for a wide variety of medical and surgical patients with liver injury.
Materials and Methods
Hepatocyte-Specific β-Catenin–KO and HBx Tg Mice.
All of the animal experiments were approved by the institutional animal care and use committee at the National Taiwan University College of Medicine, and carried out according to the committee's guidelines. All mice were kept in the specific pathogen-free room.
The hepatocyte-specific albumin promoter-driven Cre recombinase Tg mice (Alb-Cre mice; C57BL/6) and the homozygous floxed β-catenin (exon 2–6) mice (Ctnnb1flx/flx mice; C57BL/6) were obtained from Jackson Laboratory (stock no. 003574 and 004152). These mice were used for the generation of β-catenin–KO mice, with genotype determined by PCR with the primer sets as previously described (Table S1) (29).
The HBx Tg mice were developed as described previously (30). The HBx gene is cloned in pAlb-In-pA-HS4 vector, with its expression driven by albumin promoter. HCC occurs in more than 90% of male Tg mice at age greater than 17 mo (3, 30).
Mouse Hepatocyte Isolation.
Mouse hepatocyte isolation was modified by two-step perfusion and collagenase digestion as described previously (31). Briefly, the mice were anesthetized with tribromoethanol (Sigma-Aldrich). Surgery was performed to insert a catheter into the hepatic vein. The livers were then perfused with calcium-free and magnesium-free Hanks buffer (Gibco) containing 0.025% collagenase type IV (Sigma-Aldrich) in Hanks buffer containing 5 mM calcium chloride. After perfusion, the livers were removed, and the cells were resuspended in hepatocyte wash medium (Gibco) and then filtered through a 100-μm cell strainer (BD Bioscience), counted, and tested for viability by using trypan blue exclusion assay.
Supplementary Material
Acknowledgments
This study was supported by National Science Council (Taiwan) Grant NSC100-2811-B-002-106; National Research Program of Genomic Medicine, National Science Council (Taiwan), Grant NSC99-3112-B-002-015; and National Health Research Institutes Grant NHRI-EX100-9832BI.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1116386108/-/DCSupplemental.
References
- 1.Nejak-Bowen KN, Monga SP. Beta-catenin signaling, liver regeneration and hepatocellular cancer: Sorting the good from the bad. Semin Cancer Biol. 2011;21:44–58. doi: 10.1016/j.semcancer.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cavard C, et al. Wnt/beta-catenin pathway in hepatocellular carcinoma pathogenesis and liver physiology. Future Oncol. 2008;4:647–660. doi: 10.2217/14796694.4.5.647. [DOI] [PubMed] [Google Scholar]
- 3.de La Coste A, et al. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci USA. 1998;95:8847–8851. doi: 10.1073/pnas.95.15.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Satoh S, et al. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet. 2000;24:245–250. doi: 10.1038/73448. [DOI] [PubMed] [Google Scholar]
- 5.Slagle BL, Zhou YZ, Birchmeier W, Scorsone KA. Deletion of the E-cadherin gene in hepatitis B virus-positive Chinese hepatocellular carcinomas. Hepatology. 1993;18:757–762. doi: 10.1002/hep.1840180402. [DOI] [PubMed] [Google Scholar]
- 6.Merle P, et al. Functional consequences of frizzled-7 receptor overexpression in human hepatocellular carcinoma. Gastroenterology. 2004;127:1110–1122. doi: 10.1053/j.gastro.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Wong CM, Fan ST, Ng IO. beta-Catenin mutation and overexpression in hepatocellular carcinoma: Clinicopathologic and prognostic significance. Cancer. 2001;92:136–145. doi: 10.1002/1097-0142(20010701)92:1<136::aid-cncr1301>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 8.Cairo S, et al. Hepatic stem-like phenotype and interplay of Wnt/beta-catenin and Myc signaling in aggressive childhood liver cancer. Cancer Cell. 2008;14:471–484. doi: 10.1016/j.ccr.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Bioulac-Sage P, et al. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology. 2007;46:740–748. doi: 10.1002/hep.21743. [DOI] [PubMed] [Google Scholar]
- 10.Cadoret A, et al. Hepatomegaly in transgenic mice expressing an oncogenic form of beta-catenin. Cancer Res. 2001;61:3245–3249. [PubMed] [Google Scholar]
- 11.Nejak-Bowen KN, et al. Accelerated liver regeneration and hepatocarcinogenesis in mice overexpressing serine-45 mutant beta-catenin. Hepatology. 2010;51:1603–1613. doi: 10.1002/hep.23538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harada N, et al. Hepatocarcinogenesis in mice with beta-catenin and Ha-ras gene mutations. Cancer Res. 2004;64:48–54. doi: 10.1158/0008-5472.can-03-2123. [DOI] [PubMed] [Google Scholar]
- 13.Tan X, et al. Beta-catenin deletion in hepatoblasts disrupts hepatic morphogenesis and survival during mouse development. Hepatology. 2008;47:1667–1679. doi: 10.1002/hep.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan X, Behari J, Cieply B, Michalopoulos GK, Monga SP. Conditional deletion of beta-catenin reveals its role in liver growth and regeneration. Gastroenterology. 2006;131:1561–1572. doi: 10.1053/j.gastro.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 15.Sekine S, Lan BY, Bedolli M, Feng S, Hebrok M. Liver-specific loss of beta-catenin blocks glutamine synthesis pathway activity and cytochrome p450 expression in mice. Hepatology. 2006;43:817–825. doi: 10.1002/hep.21131. [DOI] [PubMed] [Google Scholar]
- 16.Rignall B, Braeuning A, Buchmann A, Schwarz M. Tumor formation in liver of conditional β-catenin-deficient mice exposed to a diethylnitrosamine/phenobarbital tumor promotion regimen. Carcinogenesis. 2011;32:52–57. doi: 10.1093/carcin/bgq226. [DOI] [PubMed] [Google Scholar]
- 17.Zhang XF, et al. Conditional beta-catenin loss in mice promotes chemical hepatocarcinogenesis: role of oxidative stress and platelet-derived growth factor receptor alpha/phosphoinositide 3-kinase signaling. Hepatology. 2010;52:954–965. doi: 10.1002/hep.23747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacDonald RA. “Lifespan” of liver cells. Autoradio-graphic study using tritiated thymidine in normal, cirrhotic, and partially hepatectomized rats. Arch Intern Med. 1961;107:335–343. doi: 10.1001/archinte.1961.03620030023003. [DOI] [PubMed] [Google Scholar]
- 19.Furuyama K, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 20.Ireland H, et al. Inducible Cre-mediated control of gene expression in the murine gastrointestinal tract: effect of loss of beta-catenin. Gastroenterology. 2004;126:1236–1246. doi: 10.1053/j.gastro.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 21.Braeuning A, et al. Phenotype and growth behavior of residual β-catenin-positive hepatocytes in livers of β-catenin-deficient mice. Histochem Cell Biol. 2010;134:469–481. doi: 10.1007/s00418-010-0747-1. [DOI] [PubMed] [Google Scholar]
- 22.Sekine S, Ogawa R, Kanai Y. Hepatomas with activating Ctnnb1 mutations in ‘Ctnnb1-deficient’ livers: a tricky aspect of a conditional knockout mouse model. Carcinogenesis. 2011;32:622–628. doi: 10.1093/carcin/bgr002. [DOI] [PubMed] [Google Scholar]
- 23.Thompson MD, et al. Spontaneous repopulation of beta-catenin null livers with beta-catenin-positive hepatocytes after chronic murine liver injury. Hepatology. 2011;54:1333–1343. doi: 10.1002/hep.24506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duncan AW, Dorrell C, Grompe M. Stem cells and liver regeneration. Gastroenterology. 2009;137:466–481. doi: 10.1053/j.gastro.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin WR, et al. The histogenesis of regenerative nodules in human liver cirrhosis. Hepatology. 2010;51:1017–1026. doi: 10.1002/hep.23483. [DOI] [PubMed] [Google Scholar]
- 26.Yamashita T, et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012–1024. doi: 10.1053/j.gastro.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mancone C, et al. Proteomic analysis reveals a major role for contact inhibition in the terminal differentiation of hepatocytes. J Hepatol. 2010;52:234–243. doi: 10.1016/j.jhep.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 28.Monga SP, Micsenyi A, Germinaro M, Apte U, Bell A. beta-Catenin regulation during matrigel-induced rat hepatocyte differentiation. Cell Tissue Res. 2006;323:71–79. doi: 10.1007/s00441-005-0045-8. [DOI] [PubMed] [Google Scholar]
- 29.Brault V, et al. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 30.Wu BK, et al. Blocking of G1/S transition and cell death in the regenerating liver of Hepatitis B virus X protein transgenic mice. Biochem Biophys Res Commun. 2006;340:916–928. doi: 10.1016/j.bbrc.2005.12.089. [DOI] [PubMed] [Google Scholar]
- 31.Yang WJ, et al. Hepatitis B virus X protein enhances the transcriptional activity of the androgen receptor through c-Src and glycogen synthase kinase-3beta kinase pathways. Hepatology. 2009;49:1515–1524. doi: 10.1002/hep.22833. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.