Abstract
Elucidating the key signal transduction pathways essential for both antipsychotic efficacy and side-effect profiles is essential for developing safer and more effective therapies. Recent work has highlighted noncanonical modes of dopamine D2 receptor (D2R) signaling via β-arrestins as being important for the therapeutic actions of both antipsychotic and antimanic agents. We thus sought to create unique D2R agonists that display signaling bias via β-arrestin–ergic signaling. Through a robust diversity-oriented modification of the scaffold represented by aripiprazole (1), we discovered UNC9975 (2), UNC0006 (3), and UNC9994 (4) as unprecedented β-arrestin–biased D2R ligands. These compounds also represent unprecedented β-arrestin–biased ligands for a Gi-coupled G protein–coupled receptor (GPCR). Significantly, UNC9975, UNC0006, and UNC9994 are simultaneously antagonists of Gi-regulated cAMP production and partial agonists for D2R/β-arrestin-2 interactions. Importantly, UNC9975 displayed potent antipsychotic-like activity without inducing motoric side effects in inbred C57BL/6 mice in vivo. Genetic deletion of β-arrestin-2 simultaneously attenuated the antipsychotic actions of UNC9975 and transformed it into a typical antipsychotic drug with a high propensity to induce catalepsy. Similarly, the antipsychotic-like activity displayed by UNC9994, an extremely β-arrestin–biased D2R agonist, in wild-type mice was completely abolished in β-arrestin-2 knockout mice. Taken together, our results suggest that β-arrestin signaling and recruitment can be simultaneously a significant contributor to antipsychotic efficacy and protective against motoric side effects. These functionally selective, β-arrestin–biased D2R ligands represent valuable chemical probes for further investigations of D2R signaling in health and disease.
Keywords: functional selectivity, ligand bias
G protein–coupled receptors (GPCRs) signal not only via canonical pathways involving heterotrimeric large G proteins, but also via noncanonical G protein–independent interactions with other signaling proteins including, most prominently, β-arrestins (1–4). The process by which GPCR ligands differentially modulate canonical and noncanonical signal transduction pathways is a phenomenon known as “functional selectivity” (5, 6). Such functionally selective ligands preferentially engage either canonical or noncanonical GPCR pathways (7, 8). Clearly, the discovery of ligands with discrete functional selectivity profiles will be extremely useful for elucidating the key signal transduction pathways essential for both the therapeutic actions and the side effects of drugs (6). Understanding which signaling pathways contribute to antipsychotic efficacy and side effects, for instance, will in turn enable the design of better antipsychotic drug candidates and, ultimately, lead to safer and more effective therapies for patients. However, only a small number of functionally selective GPCR ligands have been reported to date (5–9). In addition to the paucity of such ligands, very little purposeful attention has been devoted to creating and annotating ligands with distinct patterns of functional selectivity. In particular, although β-arrestin–biased ligands of Gq and Gs-coupled GPCRs are known (9, 10), β-arrestin–biased GPCR ligands that selectively activate β-arrestin signaling pathways over Gi-coupled pathways have not been reported.
Aripiprazole (OPC-14597, 1), an FDA-approved atypical antipsychotic drug, was one of the first functionally selective D2 receptor (D2R or D2) ligands identified (7, 11, 12). Although aripiprazole was initially described as a partial D2R agonist, on the basis of assays performed in whole animals and isolated tissues (13–15), it was later demonstrated that aripiprazole could behave as a full agonist, a partial agonist, or an antagonist at D2R depending upon the signaling readout and cell type interrogated (7, 11, 16, 17). However, structure–functional–selectivity relationships (SFSR) of the aripiprazole scaffold have not been studied and only modest structure activity relationships (SAR) have been reported (15, 18). In this paper, we report our discovery that UNC9975 (2), UNC0006 (3), and UNC9994 (4), analogs of aripiprazole, represent unprecedented β-arrestin–biased D2R ligands; these compounds are also unique β-arrestin–biased ligands for Gi-coupled GPCRs. Here, we describe the design, synthesis, and in vitro and in vivo activities of these unique β-arrestin–biased chemical probes. We demonstrate, using wild-type and β-arrestin-2 knockout mice, that the atypical antipsychotic-like profile of these β-arrestin–biased D2R agonists requires β-arrestin-2. The development of these unique, functionally selective, β-arrestin–biased D2R ligands provides the biomedical community with valuable chemical tools for probing signaling pathways essential for antipsychotic efficacy and side effects.
Results
Discovery of β-Arrestin–Biased Dopamine D2 Ligands UNC9975, UNC0006, and UNC9994.
Because aripiprazole (1) has been shown to be a functionally selective D2R ligand (7, 11) and because the SFSRs of the aripiprazole scaffold have not been studied, we intensely explored four regions of the aripiprazole template to identify D2R ligands with distinct functional selectivity patterns (Fig. 1). We investigated various substituents (mono- and disubstitution) on the left-hand side phenyl ring, several cyclic amino groups, and various linkers in the middle region, along with a variety of bicyclic aromatic moieties at the right-hand side of the scaffold. Our intention was to modulate both the conformational preferences and substituent binding effects while retaining the core pharmacophore. In total, we prepared >150 unique compounds using the synthetic procedures described in SI Methods. These compounds were subsequently evaluated in dopamine D2 receptor binding, Gi-regulated cAMP production, and G protein–independent β-arrestin-2 translocation assays. Although most of these compounds (15 representative compounds are summarized in Table S1) strongly activated both Gi-regulated cAMP accumulation and G protein–independent β-arrestin-2 recruitment in a manner similar to aripiprazole, we discovered UNC9975 (2), UNC0006 (3), and UNC9994 (4) as unprecedented β-arrestin–biased D2R ligands, which were simultaneously partial agonists of β-arrestin-2 translocation and antagonists of Gi-regulated cAMP production (see below).
Fig. 1.
Discovery of unprecedented β-arrestin–biased D2R ligands UNC9975, UNC0006, and UNC9994 via exploring multiple regions of the aripiprazole scaffold.
UNC9975, UNC0006, and UNC9994 Do Not Activate D2-Mediated Gi-Regulated Inhibition of cAMP Production.
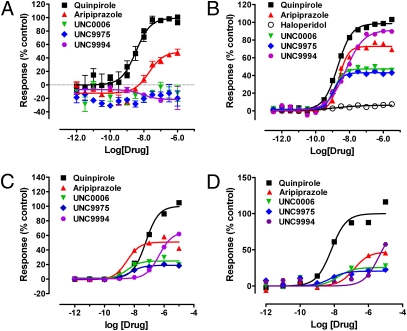
UNC9975, UNC0006, and UNC9994 were initially evaluated in the D2-mediated cAMP accumulation assay, which measures inhibition of isoproterenol-stimulated cAMP production via the Gi-coupled signaling pathway (19). UNC9975, UNC0006, and UNC9994 did not activate this Gi-mediated signaling pathway, in stark contrast to aripiprazole, which was a potent partial agonist (EC50 = 38 nM, Emax = 51%) (Fig. 2A). As expected, quinpirole (20) was a potent full agonist (Fig. 2A) whereas common typical and atypical antipsychotic drugs were antagonists in this assay. When tested for agonist activity at D2 receptors to decrease basal cAMP in the absence of isoproterenol, none of the compounds exhibited activity. In addition, HEK293T cells devoid of D2 receptors were assayed in parallel and displayed no inhibition of isoproterenol-stimulated cAMP, either by aripiprazole or by UNC9975 (Fig. S1), demonstrating that the effect observed in D2 receptor-expressing cells is solely due to signaling through the D2 receptors.
Fig. 2.
UNC9975, UNC0006, and UNC9994 are functionally selective, β-arrestin–biased dopamine D2 partial agonists. (A) Activity of UNC9975, UNC0006, UNC9994, aripiprazole, and quinpirole in the D2-mediated Gi-coupled isoproterenol-stimulated cAMP production assay using HEK293T cells expressing the dopamine D2 receptor and GloSensor-22F. UNC9975, UNC0006, and UNC9994 did not activate this Gi-mediated signaling pathway whereas aripiprazole (EC50 = 38 nM, pEC50 = 7.4 ± 0.1, Emax = 51 ± 5%) was a partial agonist and quinpirole (EC50 = 3.2 nM, pEC50 = 8.49 ± 0.07, Emax = 100 ± 3%) was a full agonist. Data are representative of at least two independent experiments. (B) Activity of UNC9975, UNC0006, UNC9994, aripiprazole, quinpirole, and haloperidol in the D2-mediated β-arrestin-2 translocation Tango assay using HTLA cells transfected with a D2V2-TCS-tTA construct. UNC9975 (EC50 = 1.1 nM, pEC50 = 8.95 ± 0.03, Emax = 43 ± 0.5%), UNC0006 (EC50 = 1.2 nM, pEC50 = 8.91 ± 0.03, Emax = 47 ± 1%), UNC9994 (EC50 = 6.1 nM, pEC50 = 8.22 ± 0.09, Emax = 91 ± 3%), and aripiprazole (EC50 = 2.4 nM, pEC50 = 8.62 ± 0.03, Emax = 73 ± 1%) were partial agonists whereas haloperidol (antagonist control) had no agonist activity. Quinpirole (EC50 = 2.0 nM, pEC50 = 8.70 ± 0.05, Emax = 100 ± 2%) was used as a positive control. Data are representative of at least two independent experiments. (C) Activity of UNC9975, UNC0006, UNC9994, aripiprazole, and quinpirole in the D2-mediated β-arrestin-2 translocation DiscoveRx assay with 20 h stimulation. UNC9975 (EC50 = 5.7 nM, pEC50 = 8.24 ± 0.20, Emax = 19 ± 1%), UNC0006 (EC50 = 3.2 nM, pEC50 = 8.49 ± 0.15, Emax = 25 ± 1%), UNC9994 (EC50 = 448 nM, pEC50 = 6.35 ± 0.07, Emax = 64 ± 2%), and aripiprazole (EC50 = 3.4 nM, pEC50 = 8.47 ± 0.08, Emax = 51 ± 1%) were partial agonists. Quinpirole (EC50 = 56 nM, pEC50 = 7.25 ± 0.04, Emax = 100 ± 2%) was used as a positive control. Data are representative of at least two independent experiments. (D) Activity of UNC9975, UNC0006, UNC9994, aripiprazole, and quinpirole in the D2-mediated BRET-based β-arrestin-2 recruitment assay using HEK293 cells expressing GRK2. UNC9975 (EC50 = 6.0 nM, pEC50 = 8.22 ± 0.49, Emax = 20 ± 3%), UNC0006 (EC50 = 17 nM, pEC50 = 7.77 ± 0.38, Emax = 25 ± 4%), UNC9994 (EC50 > 1,000 nM, Emax > 50%), and aripiprazole (EC50 = 145 nM, pEC50 = 6.84 ± 0.18, Emax = 47 ± 4%) were all partial agonists that promote β-arrestin recruitment to D2 receptors. Quinpirole (EC50 = 6.7 nM, pEC50 = 8.17 ± 0.15, Emax = 100 ± 5%) was used as a positive control. Data are representative of at least three independent experiments.
UNC9975, UNC0006, and UNC9994 Induce D2-Mediated β-Arrestin-2 Translocation as Partial Agonists.
To assess the effects of these compounds on recruiting β-arrestin-2 to D2 receptors, we used a D2-mediated β-arrestin-2 translocation Tango assay that is highly sensitive to β-arrestin-2 recruitment (21). In this assay, UNC9975, UNC0006, and UNC9994 were potent (EC50 < 10 nM) partial agonists for β-arrestin-2 recruitment to D2 receptors, similar to aripiprazole (Fig. 2B). Whereas UNC9975 (Emax = 43%) and UNC0006 (Emax = 47%) were less efficacious than aripiprazole (Emax = 73%), UNC9994 (Emax = 91%) was more efficacious than aripiprazole and approached the activity of the full agonist quinpirole. Haloperidol (22), a typical antipsychotic (along with all other typical and atypical antipsychotic drugs tested) did not activate D2-mediated β-arrestin-2 translocation. Because HTLA cells (a HEK293-derived cell line stably expressing a tTA-dependent luciferase reporter gene and a β-arrestin-2-TEV fusion protein) transfected with a D2V2-TCS-tTA construct (21) were used in this assay, we tested these compounds in parallel in HTLA cells transfected with a V2-TCS-tTA construct (21) to investigate the possibility that the observed effect was due to compound acting via the V2 tail or some other nonreceptor-mediated arrestin pathway. As expected, UNC9975, UNC0006, aripiprazole, and quinpirole (a positive control for D2) were inactive in V2 receptor-expressing cells whereas arginine vasopressin (a positive control for V2) was a full agonist (Fig. S2A). These results demonstrate that the effect of UNC9975, UNC0006, UNC9994, and aripiprazole observed in D2V2 receptor-expressing cells is due to the compounds acting via the D2 receptor, not at the V2 tail.
To confirm this finding from the Tango assay, which has downstream amplification of signaling, we tested these compounds in an orthologous assay: the D2-mediated β-arrestin-2 recruitment DiscoveRx approach, which uses a β-galactosidase fragment complementation-based technology to monitor the interaction of β-arrestin with GPCRs (23). As shown in Fig. 2C and Fig. S2B, UNC9975 (EC50 = 5.7 nM), UNC0006 (EC50 = 3.2 nM), and aripiprazole (EC50 = 3.4 nM) potently activated D2-mediated β-arrestin-2 translocation. Although UNC9994 (EC50 = 448 nM) was less potent, this compound (Emax = 64%) had a higher efficacy than aripiprazole (Emax = 51%), UNC9975 (Emax = 19%), and UNC0006 (Emax = 25%) (Fig. 2C). Quinpirole (EC50 = 56 nM, Emax = 100%) was used as a positive control in this assay. These findings are consistent with the results obtained in the D2 β-arrestin-2 Tango assay.
Aripiprazole has been previously reported to be either an antagonist (17) or a partial agonist (24) for β-arrestin-2 recruitment using the bioluminescence resonance energy transfer (BRET)-based assay. Therefore, we also assessed the effects of UNC9975, UNC0006, and UNC9994 on D2-mediated β-arrestin-2 recruitment using this BRET-based approach (17). In HEK293T cells wherein G protein–coupled receptor kinase 2 (GRK2) along with the D2-dopamine receptor was expressed, aripiprazole, UNC9975, UNC0006, UNC9994, and aripiprazole all displayed partial agonist activity for D2-mediated β-arrestin-2 recruitment (Fig. 2D). Although UNC9994 was less potent than aripiprazole, UNC9975, and UNC0006, UNC9994 had a higher efficacy than aripiprazole, UNC9975, and UNC0006 (Fig. 2D). Notably, compound activity to recruit β-arrestin-2 was sensitive to the expression of GRK2 in this BRET-based assay, as all compounds required GRK2 coexpression to induce detectable BRET signals in response to β-arrestin-2 recruitment (Fig. 2D and Fig. S2C). Therefore, using three orthogonal assay platforms, we confirmed that UNC9975 and UNC0006 were potent partial agonists for β-arrestin-2 recruitment with slightly lower efficacy than aripiprazole whereas UNC9994 was a higher efficacy and lower potency partial agonist for β-arrestin-2 translocation compared with aripiprazole.
We next examined the effects of UNC9975 and UNC0006 on inducing D2 receptor endocytosis (internalization) by assessing the cell surface fluorescence of D2 receptors using flow cytometry (25) in HEK293 cells coexpressing GRK2 and β-arrestin-2. D2 receptor internalization in response to treatment with UNC9975, UNC0006, and aripiprazole (1 μM for 1 h) did not reach statistical significance (Fig. S3). No internalization was observed in cells treated with haloperidol (as a negative control). As expected, quinpirole (a positive control) significantly increased receptor internalization.
To assess the effects of UNC9975 and UNC0006 on β-arrestin–mediated signaling, we evaluated these two compounds in the extracellular signal-regulated kinase (ERK) phosphorylation (p-ERK) reporter assay, which is capable of detecting extended β-arrestin–mediated ERK phosphorylation [following a relatively long incubation (4 h)] (10). In the HEK293T cells transfected with D2 receptors, UNC9975 (EC50 = 2.2 nM, Emax = 32%) and UNC0006 (EC50 = 3.2 nM, Emax = 33%) were potent partial agonists (Fig. S2A). The potencies and efficacies of UNC9975 and UNC0006 are similar to those of aripiprazole (EC50 = 1.8 nM, Emax = 39%) in this assay. Importantly, coexpression of β-arrestin-2 and GRK2 significantly enhanced the efficacies of UNC9975, UNC0006, and aripiprazole in this signaling assay (Fig. S4B). In addition, UNC9975 (Emax = 13%) and UNC0006 (Emax = 11%) were inactive whereas aripiprazole was a potent partial agonist (EC50 = 6.3 nM, Emax = 49%) in the D2 dopamine receptor-mediated p-ERK immunofluorescence assay (Fig. S4C), which measures the rapid phase of G protein–mediated ERK phosphorylation (5 min incubation) (26). These results are consistent with the cAMP biosensor assay results (see above) and further indicate that UNC9975 and UNC0006 lack appreciable agonism via canonical Gi-dependent pathways.
Taken together, these multiple functional activity profiling studies demonstrate that UNC9975, UNC0006, and UNC9994 are β-arrestin–biased D2R ligands that selectively activate β-arrestin recruitment and signaling and are simultaneously inactive at Gi-mediated signal transduction pathways.
Selectivity of UNC9975, UNC0006, and UNC9994 and PK Parameters of UNC9975.
Consistent with their potencies in D2 functional assays, UNC9975 and UNC0006 displayed high affinities (Ki < 10 nM) similar to aripiprazole in the D2 antagonist radioligand competition binding assay whereas UNC9994 displayed a lower binding affinity (Ki = 79 nM) to D2R than UNC9975, UNC0006, and aripiprazole (Table 1). Although UNC9975, UNC0006, and UNC9994 also had high affinity for the D3-dopamine receptor, they displayed low affinities for other dopamine receptors (i.e., D1, D4, and D5; Table 1). At serotonin [as known as 5-hydroxytryptamine (5-HT or 5HT)] receptors, UNC9975, UNC0006, and UNC9994 displayed moderate to high binding affinities (Ki = 0.6–500 nM) for 5HT2A, 5HT2B, 5HT2C, and 5HT1A, but were significantly less potent in functional assays [Ca2+ mobilization fluorometric imaging plate reader (FLIPR) or cAMP biosensor]; UNC9975, UNC0006, and UNC9994 were antagonists at 5HT2A and 5HT2B and agonists at 5HT2C and 5HT1A. In addition, UNC9975, UNC0006, and UNC9994 had relatively high affinities to H1-histamine receptor (Ki < 10 nM) but were less potent antagonists in H1 functional assays. In general, UNC9975 and UNC0006 displayed a similar GPCR selectivity profile to aripiprazole. UNC9994 was less potent against most of these GPCRs compared with UNC9975, UNC0006, and aripiprazole.
Table 1.
Radioligand binding and functional activities of UNC9975, UNC0006, and UNC9994 at select GPCRs
| Binding affinity or potency, nM* | ||||
| Receptor/assay | Aripiprazole | UNC9975 | UNC0006 | UNC9994 |
| D2/binding (Ki) | 8.0 | 2.6 | 5.0 | 79 |
| D1/binding (Ki) | 895 | 1,040 | 825 | 4,000 |
| D3/binding (Ki) | 19 | 11 | 16 | 17 |
| D4/binding (Ki) | 251 | 178 | 200 | 138 |
| D5/binding (Ki) | 1,051 | 513 | 615 | >10,000 |
| 5HT2A/binding (Ki) | 40 | 7.4 | 16 | 140 |
| 5HT2A/FLIPR (pA2) | 417 | 102 | 331 | 6,600 |
| 5HT2B/binding (Ki) | 1.4 | 1.1 | 0.6 | 25 |
| 5HT2B/FLIPR (IC50) | 98 | 76 | 115 | 501 |
| 5HT2C/binding (Ki) | 250 | 99 | 115 | 512 |
| 5HT2C/FLIPR (EC50) | 1,900 | 324 | 363 | 1,550 |
| 5HT1A/binding (Ki) | 18 | 29 | 60 | 26 |
| 5HT1A/cAMP (EC50) | 450 | 1,800 | 2,500 | 933 |
| H1/binding (Ki) | 6.0 | 6.1 | 4.5 | 2.4 |
| H1/FLIPR (pA2) | 52 | 35 | 22 | 79 |
*Ki, IC50, pA2, or EC50 values are the average of at least two duplicate experiments with SD values that are threefold less than the average.
In mouse pharmacokinetic (PK) studies, both UNC9975 and aripiprazole displayed high exposure levels in brain and excellent CNS penetration (Table S2). Although the brain exposure level of UNC9975 was about threefold lower, UNC9975 had a longer half-life in brain and a higher brain/plasma ratio over 24 h compared with aripiprazole. The excellent in vivo PK parameters of UNC9975 make it a suitable tool for in vivo pharmacodynamic studies.
UNC9975 and UNC9994 Exhibit Antipsychotic Activity in Vivo That Is Attenuated in β-Arrestin-2 Knockout Mice.
To correlate the in vitro functional selectivity profiles with potential in vivo therapeutic effects, we evaluated the inhibition of psychostimulant or psychotomimetic-induced hyperlocomotion in mice by UNC9975, both of which are well-established pharmacological mouse models for assessing potential antipsychotic activity (27, 28). We first evaluated the ability of UNC9975 to inhibit d-amphetamine–induced hyperlocomotion as detailed previously (27) in inbred C57BL/6 mice. As shown in Fig. 3A, i.p. administration of UNC9975 dose dependently inhibited d-amphetamine–induced hyperlocomotion. UNC9975 exhibited an efficacy and potency similar to aripiprazole (ED50 = 0.38 mg/kg vs. 0.36 mg/kg) in this in vivo model (Fig. 3B).
Fig. 3.
UNC9975 exhibits potent antipsychotic-like activity in mouse hyperlocomotion studies that is attenuated in β-arrestin-2 knockout mice. (A) Locomotor responses in inbred C57BL/6 mice are shown as 5-min binned intervals to vehicle or different doses (i.p.) of UNC9975 followed 30 min later by 3 mg/kg d-amphetamine (AMPH, i.p.). (B) Bar graph of distance traveled after AMPH administration (30- to 70-min time interval). C57BL/6 mice were given vehicle or different doses of UNC9975 or aripiprazole 30 min before AMPH treatment. n = 8 animals/group. *P < 0.05, vs. vehicle + 3 mg/kg AMPH group. (C and D) Locomotor activities shown as 5-min binned intervals of wild-type (WT) or β-arrestin-2 knockout (β-ARR2 KO) littermate mice given vehicle or different doses of UNC9975 followed 30 min later with 6 mg/kg phencyclidine (PCP, i.p.). (E) Bar graph of distance traveled by WT and β-ARR2 KO mice after PCP administration (30- to 70-min time interval) as shown in C and D. n = 14 WT and β-ARR2 KO pairs/group. *P < 0.05, vs. vehicle + 6 mg/kg PCP group. (F) Bar graph of distance traveled by WT and β-ARR2 KO mice after aripiprazole injection followed by PCP treatment (30- to 70-min time interval). n = 8 littermate WT and β-ARR2 KO pairs/group. *P < 0.05, vs. vehicle + 6 mg/kg PCP group.
We next evaluated the effects of UNC9975 on psychotomimetic-induced hyperlocomotion in response to the NMDA receptor antagonist phencyclidine (PCP) (28). Because β-arrestin-2 knockout mice have a reduced locomotor response to amphetamine (3), we used PCP to induce locomotion in these animals. To assess the potential involvement of β-arrestin in the effects of UNC9975, β-arrestin-2 knockout mice and wild-type littermate controls (29) were administered increasing doses of UNC9975 followed by PCP administration. UNC9975 potently and dose dependently inhibited PCP-induced hyperlocomotion in wild-type mice (Fig. 3C); however, this antipsychotic-like activity of UNC9975 was significantly attenuated in β-arrestin-2 knockout mice (Fig. 3D). Significantly, the in vivo potency of UNC9975 was greatly decreased in β-arrestin-2 knockout mice (β-arrestin-2 knockout ED50 = 0.75 mg/kg vs. wild-type ED50 = 0.26 mg/kg) (Fig. 3E). In striking contrast, aripiprazole displayed no significant differences in potency or efficacy in wild-type (ED50 = 0.13 mg/kg) and β-arrestin-2 knockout mice (ED50 = 0.13 mg/kg) (Fig. 3F).
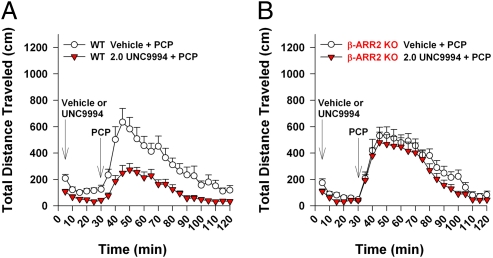
To confirm these findings, we next evaluated the effects of UNC9994 on PCP-induced hyperlocomotion in wild-type and β-arrestin-2 knockout mice. UNC9994 (2 mg/kg, i.p.) markedly inhibited PCP-induced hyperlocomotion in wild-type mice (Fig. 4A). Importantly, this significant antipsychotic-like activity of UNC9994 was completely abolished in β-arrestin-2 knockout mice (Fig. 4B).
Fig. 4.
UNC9994 exhibits potent antipsychotic-like activity in mouse hyperlocomotion studies that is completely abolished in β-arrestin-2 knockout mice. (A and B) Locomotor activities shown as 5-min binned intervals for wild-type (WT) or β-arrestin-2 knockout (β-ARR2 KO) littermate mice given vehicle or 2.0 mg/kg UNC9994 (i.p.) followed 30 min later with 6 mg/kg phencyclidine (PCP, i.p.). n = 10–13 WT and β-ARR2 KO pairs/group.
Because UNC9975 has 5HT2A antagonist activity (Table 1) and 5HT2A antagonism can be a significant contributor to the efficacy of antipsychotic drugs (30, 31), we evaluated SR46349B (a potent and selective 5HT2A antagonist) (32) and clozapine (a classic atypical antipsychotic with potent antagonist activity at 5HT2A and other aminergic GPCRs) (33) in the PCP-induced hyperlocomotion model in wild-type and β-arrestin-2 KO mice. We found that SR46349B and clozapine were equally effective at suppressing PCP-induced locomotion in β-arrestin-2 KO mice vs. wild-type mice (Fig. S5), indicating that antagonism of 5HT2A receptors is similar in both genotypes. These observations suggest that the attenuation in antipsychotic drug-like activity of UNC9975 in β-arrestin-2 KO mice is likely not due to activity at 5-HT2A receptors.
Taken together, our results strongly suggest that the antipsychotic drug-like activity of UNC9975 and UNC9994 requires β-arrestin and this is likely mediated through the dopamine D2 receptors in mice.
UNC9975 and UNC0006 Induce Catalepsy in β-Arrestin-2 Knockout Mice but Not in Wild-Type Mice.
To compare potential extrapyramidal side effects of UNC9975, UNC0006, and aripiprazole, we evaluated these compounds along with haloperidol (as a positive control) in a standard drug-induced catalepsy model (34), using wild-type and β-arrestin-2 knockout mice. As shown in Fig. 5 A and B, UNC9975, UNC0006, or aripiprazole (5.0 mg/kg) failed to significantly induce catalepsy in wild-type mice at either 30 or 60 min after treatment, whereas haloperidol (2.0 mg/kg) induced significant catalepsy at both time points. In notable contrast to the results in wild-type animals, the β-arrestin–biased dopamine D2 ligands UNC9975 and UNC0006 significantly induced catalepsy in β-arrestin-2 knockout mice 60 min after treatment (Fig. 5B). By comparison, aripiprazole, which activates both Gi-coupled and β-arrestin–mediated pathways, did not induce catalepsy in β-arrestin-2 knockout mice or in wild-type mice (Fig. 5B). Collectively, these results suggest that β-arrestin recruitment and signaling are protective against motoric side effects.
Fig. 5.
UNC9975 and UNC0006 induce catalepsy in β-arrestin-2 knockout mice but not in wild-type littermates. (A and B) Wild-type (WT) and β-arrestin-2 knockout (β-ARR2 KO) littermate mice were administered (i.p.) vehicle, 5.0 mg/kg UNC9975, 5.0 mg/kg UNC0006, 5.0 mg/kg aripiprazole, or 2.0 mg/kg haloperidol. Catalepsy was assessed 30 and 60 min after drug injection using the inclined screen test where latency to move was scored. n = 8 WT and β-ARR2 KO pairs/group. *P < 0.05, vs. vehicle controls.
Discussion
Through a combined medicinal chemistry and comprehensive in vitro and in vivo pharmacological profiling approach, we designed, synthesized, and characterized unique D2 β-arrestin–biased agonists and demonstrated their atypical antipsychotic drug-like activities in vivo. These chemical probes represent unprecedented functionally selective β-arrestin–biased dopamine D2R ligands that exhibit antipsychotic activity in vivo. This study thus represents a successful proof-of-concept for how functionally selective GPCR ligands can be discovered and validated. Additionally, our findings show that on the basis of the β-arrestin bias of these compounds, β-arrestin emerges as an important contributor to both antipsychotic drug efficacy and antipsychotic side effects.
Our robust diversity-oriented synthetic approach capitalized on the aripiprazole scaffold to generate, initially, hundreds of analogs. Among them, UNC9975, UNC0006, and UNC9994 were discovered as unique, β-arrestin–biased functionally selective D2 ligands. These compounds are partial agonists that induce D2 receptor-mediated β-arrestin recruitment and signaling and simultaneously are inactive at Gi-dependent signaling. Further, it should be acknowledged that whereas UNC9975, UNC0006, and UNC9994 promoted the recruitment of β-arrestin to D2 receptors, these studies were performed in heterologous cells expressing β-arrestin-2 and GRK2. Whether these compounds stimulate β-arrestin recruitment and signaling in native D2 receptor-expressing neurons is the subject of ongoing investigations. Interestingly, although aripiprazole, UNC0006, and UNC9975 are all agonists for β-arrestin recruitment and signaling, in contrast to quinpirole, none of these ligands promoted robust internalization of D2 receptors, a trafficking phenomenon commonly involving β-arrestins (35). This inability of aripiprazole to induce significant receptor internalization is consistent with previous findings (7), suggesting that a common property of aripiprazole-based ligands may be the inability to promote robust D2 receptor internalization. This lack of internalization may bode well for the eventual translation of such β-arrestin–biased ligands to the clinic as drugs that induce internalization would ultimately induce tachyphylaxis and receptor down-regulation (36).
More significantly, the in vivo activity of UNC9975 indicated a strict requirement for β-arrestin-2 for both full antipsychotic activity and protection against motoric side effects. Similar to aripiprazole, this β-arrestin–biased ligand shows a potent ability to suppress both d-amphetamine and phencyclidine-induced hyperlocomotion in mice, indicating that the compound possesses antipsychotic drug-like activities in vivo. Significantly, the antipsychotic drug-like activities of UNC9975 were attenuated in β-arrestin-2 knockout mice, indicating that β-arrestin-2 is required in vivo for full activity. Interestingly, previous studies have determined that the locomotor responses induced by the nonselective direct (apomorphine) and indirect (amphetamine) agonists are attenuated in β-arrestin-2 knockout mice (3), Importantly, the mechanisms by which β-arrestin influences these locomotor responses in vivo and to what extent this β-arrestin-2 knockout effect is specific to D2 receptors are currently unknown. Here we observe that in β-arrestin-2 knockout mice the unique β-arrestin–biased ligands UNC9975 and UNC9994, but not the other atypical drugs including aripiprazole, clozapine, and SR46349B, show an attenuated ability to inhibit PCP-induced locomotion. These findings demonstrate that β-arrestin-2 is required for the full antipsychotic drug-like activity of UNC9975 and UNC9994. However, further mechanistic studies will be required to determine how D2-mediated β-arrestin recruitment and signaling by UNC9975 and UNC9994 are essential for these compounds’ antipsychotic activity in vivo.
With the exception of aripiprazole, all Food and Drug Administration-approved typical and atypical antipsychotic medications (e.g., haloperidol, chlorpromazine, clozapine, and risperidone) share the common property of antagonizing D2-mediated G protein–dependent and –independent signaling (17). Indeed, typical and atypical antipsychotic drugs are antagonists at Gi-mediated signaling and arrestin-ergic pathways. It is currently unknown what, if any activity, any of these compounds have at potential Go-mediated signaling. This antagonism at both signaling pathways is thought to underlie the therapeutic benefit to prevent psychotic symptoms but can also cause serious extrapyramidal side effects including catalepsy and other motor dyskenesias (37). Although aripiprazole, UNC0006, and UNC9975 do not induce catalepsy in wild-type mice at doses at which antipsychotic drug-like therapeutic activities are measured, UNC0006 and UNC9975 resemble haloperidol in inducing catalepsy in β-arrestin-2 knockout mice. This propensity to cause catalepsy in the absence of β-arrestin-2 suggests that UNC0006 and UNC9975 signal through β-arrestin-2 in vivo and that this signaling may protect against motoric side effects due to antagonism at D2R-mediated Gi pathways.
These findings have obvious implications for the development of therapeutic approaches for treating schizophrenia and related disorders. Atypical antipsychotic drugs, which differ from older medications (e.g., haloperidol and chlorpromazine) by virtue of their reduced propensity to induce motoric side effects, are among the most widely prescribed medications. Many pharmacologic strategies that aim to target a multiplicity of non-D2 receptor molecular targets (e.g., 5-HT2A inverse agonists, 5-HT2C agonists, mGluR2/3 agonists, NK-3 antagonists, sigma antagonists, D1- or D4-selective antagonists, and so forth) have led to a growing number of unsuccessful attempts to create safer and more effective atypical antipsychotic drugs (37–39). Our results suggest that atypical antipsychotic drugs with a unique mechanism of action may arise from β-arrestin–biased D2 ligands. Although we do not know whether such compounds will have therapeutic benefits over existing medications, it is tempting to speculate that they may demonstrate special efficacies in psychotic disorders with a mood dysfunction given the prominent role of arrestin-ergic signaling in the action of lithium and related compounds (40, 41).
Methods
A complete description of chemical synthesis for all compounds and protocols for all biological experiments is detailed in SI Methods. 1H and 13C NMR spectra of UNC9975 (Fig. S6), UNC0006 (Fig. S7), and UNC9994 (Fig. S8) are provided in SI Methods.
D2 β-Arrestin Recruitment Assay.
Recruitment of β-arrestin to agonist-stimulated D2L receptors was performed using a previously described “Tango”-type assay (21). Briefly, HTLA cells stably expressing β-arrestin-TEV protease and a tetracycline transactivator-driven luciferase were plated into 15-cm dishes in DMEM containing 10% FBS. Cells were transfected (calcium phosphate) with 20 μg of a D2V2-TCS-tTA construct (21). The next day, cells were plated in white, clear-bottom, 384-well plates (Greiner; 10,000 cells/well, 50 μL/well) in DMEM containing 1% dialyzed FBS. The following day, the cells were challenged with 10 μL/well of reference agonist (6 μM) or D2 test ligand (6 μM) ± reference agonist prepared in HBSS, 20 mM Hepes (pH 7.4), and 18% DMSO (final ligand concentrations are 1 μM, final DMSO concentration is 3%). After 18 h, the medium was removed and replaced with 1× BriteGlo reagent (Promega), and luminescence per well was read using a TriLux plate reader (1 s/well). Data were normalized to vehicle (0%) and quinpirole (100%) controls and regressed using the sigmoidal dose-response function built into GraphPad Prism 4.0.
In Vivo Studies in Mice.
All experiments were approved by the Institutional Animal Care and Use Committees at the University of North Carolina, Chapel Hill and Duke University. C57BL/6J wild-type and β-arrestin-2 knockout mice were housed under standard conditions: 12-h light/dark cycle with food and water provided ad libitum. Adult, age-matched male and female wild-type and β-arrestin-2 knockout drug-naive mice were used for all behavioral testing.
Locomotor activity was assessed under standardized environmental conditions in 21 × 21-cm Plexiglas chambers with photobeams spaced at 2.5 cm (AccuScan Instruments) as previously described (42). Mice were injected (i.p.) with vehicle (0.9% saline/0.2% acetic acid), aripiprazole (0.1, 0.25, 0.50, or 2.0 mg/kg), or UNC9975 (0.25, 0.50, or 2.0 mg/kg) and placed into the open field. For the studies with SR46349B and clozapine, mice were injected (i.p.) with vehicle (0.9% saline/50 mM tartaric acid), SR46349B (1.0 mg/kg), or clozapine (1.0 mg/kg) and placed into the open field. Thirty minutes later d-amphetamine (3 mg/kg) or phencyclidine (6.0 mg/kg) was administered and mice were immediately returned to the open field. Activity was monitored throughout this entire period. Horizontal activity was measured as the total distance traveled in centimeters. The means ± SEMs of the locomotor responses were analyzed using Graphpad Prism 5.0. To estimate the half-maximal inhibitory concentration (ED50), dose responses of total locomotor activity during the 90-min period after d-amphetamine or phencyclidine administration were plotted and best-fit decay curves were determined using a nonlinear regression one-phase decay equation. Cumulative locomotor responses were analyzed using a one-way ANOVA followed by Newman–Keuls multiple comparison tests with Graphpad Prism 5.0. Statistical significance was set at P < 0.05.
Supplementary Material
Acknowledgments
We thank Dr. J. Martin Herold for critical reading of synthetic procedures and characterization data and Dr. Gilad Barnea (Brown University) for supplying cells and cDNA for the Tango assay. We thank the National Institutes of Health (Grants U19MH082441S1, U19MH082441, R01MH61887, R01MH73853, R01DA022413, and R01MH054137) and the Lieber Center for Schizophrenia Research and Treatment for financial support and the National Institute of Mental Health Psychoactive Drug Screen Program for selectivity assay support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1104807108/-/DCSupplemental.
References
- 1.Luttrell LM, et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 2.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 3.Beaulieu JM, et al. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Abbas A, Roth BL. Arresting serotonin. Proc Natl Acad Sci USA. 2008;105:831–832. doi: 10.1073/pnas.0711335105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urban JD, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 6.Mailman RB. GPCR functional selectivity has therapeutic impact. Trends Pharmacol Sci. 2007;28:390–396. doi: 10.1016/j.tips.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urban JD, Vargas GA, von Zastrow M, Mailman RB. Aripiprazole has functionally selective actions at dopamine D2 receptor-mediated signaling pathways. Neuropsychopharmacology. 2007;32:67–77. doi: 10.1038/sj.npp.1301071. [DOI] [PubMed] [Google Scholar]
- 8.Kilts JD, et al. Functional selectivity of dopamine receptor agonists. II. Actions of dihydrexidine in D2L receptor-transfected MN9D cells and pituitary lactotrophs. J Pharmacol Exp Ther. 2002;301:1179–1189. doi: 10.1124/jpet.301.3.1179. [DOI] [PubMed] [Google Scholar]
- 9.Violin JD, Lefkowitz RJ. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28:416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Gesty-Palmer D, et al. A beta-arrestin-biased agonist of the parathyroid hormone receptor (PTH1R) promotes bone formation independent of G protein activation. Sci Transl Med. 2009;1:1ra1. doi: 10.1126/scitranslmed.3000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawler CP, et al. Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes. Neuropsychopharmacology. 1999;20:612–627. doi: 10.1016/S0893-133X(98)00099-2. [DOI] [PubMed] [Google Scholar]
- 12.Mailman RB, Murthy V. Third generation antipsychotic drugs: Partial agonism or receptor functional selectivity? Curr Pharm Des. 2010;16:488–501. doi: 10.2174/138161210790361461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kikuchi T, et al. 7-(4-[4-(2,3-Dichlorophenyl)-1-piperazinyl]butyloxy)-3,4-dihydro-2(1H)-quinolinone (OPC-14597), a new putative antipsychotic drug with both presynaptic dopamine autoreceptor agonistic activity and postsynaptic D2 receptor antagonistic activity. J Pharmacol Exp Ther. 1995;274:329–336. [PubMed] [Google Scholar]
- 14.Inoue T, Domae M, Yamada K, Furukawa T. Effects of the novel antipsychotic agent 7-(4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butyloxy)-3,4-dihydro -2(1H)-quinolinone (OPC-14597) on prolactin release from the rat anterior pituitary gland. J Pharmacol Exp Ther. 1996;277:137–143. [PubMed] [Google Scholar]
- 15.Oshiro Y, et al. Novel antipsychotic agents with dopamine autoreceptor agonist properties: Synthesis and pharmacology of 7-[4-(4-phenyl-1-piperazinyl)butoxy]-3,4-dihydro-2(1H)-quinolinone derivatives. J Med Chem. 1998;41:658–667. doi: 10.1021/jm940608g. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro DA, et al. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 2003;28:1400–1411. doi: 10.1038/sj.npp.1300203. [DOI] [PubMed] [Google Scholar]
- 17.Masri B, et al. Antagonism of dopamine D2 receptor/beta-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc Natl Acad Sci USA. 2008;105:13656–13661. doi: 10.1073/pnas.0803522105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson DS, et al. Discovery of PF-00217830: Aryl piperazine napthyridinones as D2 partial agonists for schizophrenia and bipolar disorder. Bioorg Med Chem Lett. 2011;21:2621–2625. doi: 10.1016/j.bmcl.2011.01.059. [DOI] [PubMed] [Google Scholar]
- 19.Kimple AJ, et al. Structural determinants of G-protein alpha subunit selectivity by regulator of G-protein signaling 2 (RGS2) J Biol Chem. 2009;284:19402–19411. doi: 10.1074/jbc.M109.024711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsuruta K, et al. Evidence that LY-141865 specifically stimulates the D-2 dopamine receptor. Nature. 1981;292:463–465. doi: 10.1038/292463a0. [DOI] [PubMed] [Google Scholar]
- 21.Barnea G, et al. The genetic design of signaling cascades to record receptor activation. Proc Natl Acad Sci USA. 2008;105:64–69. doi: 10.1073/pnas.0710487105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Granger B, Albu S. The haloperidol story. Ann Clin Psychiatry. 2005;17:137–140. doi: 10.1080/10401230591002048. [DOI] [PubMed] [Google Scholar]
- 23.McGuinness D, et al. Characterizing cannabinoid CB2 receptor ligands using DiscoveRx PathHunter beta-arrestin assay. J Biomol Screen. 2009;14:49–58. doi: 10.1177/1087057108327329. [DOI] [PubMed] [Google Scholar]
- 24.Klewe IV, et al. Recruitment of beta-arrestin2 to the dopamine D2 receptor: Insights into anti-psychotic and anti-parkinsonian drug receptor signaling. Neuropharmacology. 2008;54:1215–1222. doi: 10.1016/j.neuropharm.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo N, et al. Impact of D2 receptor internalization on binding affinity of neuroimaging radiotracers. Neuropsychopharmacology. 2010;35:806–817. doi: 10.1038/npp.2009.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strachan RT, Sciaky N, Cronan MR, Kroeze WK, Roth BL. Genetic deletion of p90 ribosomal S6 kinase 2 alters patterns of 5-hydroxytryptamine 2A serotonin receptor functional selectivity. Mol Pharmacol. 2010;77:327–338. doi: 10.1124/mol.109.061440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlumberger C, et al. Comparison of the mGlu(5) receptor positive allosteric modulator ADX47273 and the mGlu(2/3) receptor agonist LY354740 in tests for antipsychotic-like activity. Eur J Pharmacol. 2009;623:73–83. doi: 10.1016/j.ejphar.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Nordquist RE, et al. Effects of aripiprazole/OPC-14597 on motor activity, pharmacological models of psychosis, and brain activity in rats. Neuropharmacology. 2008;54:405–416. doi: 10.1016/j.neuropharm.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Bohn LM, et al. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 30.Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21(2, Suppl):106S–115S. doi: 10.1016/S0893-133X(99)00046-9. [DOI] [PubMed] [Google Scholar]
- 31.Yadav PN, et al. The presynaptic component of the serotonergic system is required for clozapine's efficacy. Neuropsychopharmacology. 2011;36:638–651. doi: 10.1038/npp.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rinaldi-Carmona M, et al. Biochemical and pharmacological properties of SR 46349B, a new potent and selective 5-hydroxytryptamine2 receptor antagonist. J Pharmacol Exp Ther. 1992;262:759–768. [PubMed] [Google Scholar]
- 33.Coward DM. General pharmacology of clozapine. Br J Psychiatry Suppl. 1992:5–11. [PubMed] [Google Scholar]
- 34.Chipkin RE, et al. Pharmacological profile of SCH39166: A dopamine D1 selective benzonaphthazepine with potential antipsychotic activity. J Pharmacol Exp Ther. 1988;247:1093–1102. [PubMed] [Google Scholar]
- 35.Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: The role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- 36.Allen JA, Roth BL. Strategies to discover unexpected targets for drugs active at G protein-coupled receptors. Annu Rev Pharmacol Toxicol. 2011;51:117–144. doi: 10.1146/annurev-pharmtox-010510-100553. [DOI] [PubMed] [Google Scholar]
- 37.Roth BL, Sheffler DJ, Kroeze WK. Magic shotguns versus magic bullets: Selectively non-selective drugs for mood disorders and schizophrenia. Nat Rev Drug Discov. 2004;3:353–359. doi: 10.1038/nrd1346. [DOI] [PubMed] [Google Scholar]
- 38.Conn PJ, Roth BL. Opportunities and challenges of psychiatric drug discovery: Roles for scientists in academic, industry, and government settings. Neuropsychopharmacology. 2008;33:2048–2060. doi: 10.1038/sj.npp.1301638. [DOI] [PubMed] [Google Scholar]
- 39.Gray JA, Roth BL. The pipeline and future of drug development in schizophrenia. Mol Psychiatry. 2007;12:904–922. doi: 10.1038/sj.mp.4002062. [DOI] [PubMed] [Google Scholar]
- 40.Beaulieu JM, Gainetdinov RR, Caron MG. Akt/GSK3 signaling in the action of psychotropic drugs. Annu Rev Pharmacol Toxicol. 2009;49:327–347. doi: 10.1146/annurev.pharmtox.011008.145634. [DOI] [PubMed] [Google Scholar]
- 41.Beaulieu JM, et al. A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell. 2008;132:125–136. doi: 10.1016/j.cell.2007.11.041. [DOI] [PubMed] [Google Scholar]
- 42.Abbas AI, et al. PSD-95 is essential for hallucinogen and atypical antipsychotic drug actions at serotonin receptors. J Neurosci. 2009;29:7124–7136. doi: 10.1523/JNEUROSCI.1090-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.