Abstract
Somatic cells can be reprogrammed to induced pluripotent stem cells (iPSCs) by expressing four transcription factors: Oct4, Sox2, Klf4, and c-Myc. Here we report that enhancing RA signaling by expressing RA receptors (RARs) or by RA agonists profoundly promoted reprogramming, but inhibiting it using a RAR-α dominant-negative form completely blocked it. Coexpressing Rarg (RAR-γ) and Lrh-1 (liver receptor homologue 1; Nr5a2) with the four factors greatly accelerated reprogramming so that reprogramming of mouse embryonic fibroblast cells to ground-state iPSCs requires only 4 d induction of these six factors. The six-factor combination readily reprogrammed primary human neonatal and adult fibroblast cells to exogenous factor-independent iPSCs, which resembled ground-state mouse ES cells in growth properties, gene expression, and signaling dependency. Our findings demonstrate that signaling through RARs has critical roles in molecular reprogramming and that the synergistic interaction between Rarg and Lrh1 directs reprogramming toward ground-state pluripotency. The human iPSCs described here should facilitate functional analysis of the human genome.
Keywords: embryonic stem cell, RAREoct, piggyBac transposition, SH-iPSC
Four transcription factors—Oct4, Sox2, c-Myc, and Klf4 —or their variants can reprogram cells of somatic lineages in the mouse and human to iPSCs (1–6). However, the reprogramming mechanism is still obscure, which hinders wider application. In the mouse, even after prolonged expression of the four factors (4F), only a small percentage of cells can be fully reprogrammed (7, 8). In contrast, reprogramming by nuclear transfer and cell fusion is quicker and more efficient (9), indicating that additional key reprogramming factors may yet to be identified. Reprogramming human somatic cells to iPSCs is usually performed in human ES cell culture medium containing FGF. Consequently, human iPSCs, like human ES cells, are more similar to mouse epiblast stem cells (EpiSCs) than to blastocyst-derived mouse ES cells (10, 11).
Here, we report that overexpressing RA receptors (RARs) profoundly promoted reprogramming. Specifically, addition of Rarg and Lrh-1 to the reprogramming factor mixture rapidly activated the endogenous Oct4 locus expression, which permitted fast and efficient reprogramming comparable to nuclear transfer or cell fusion. Expressing the same set of six transcription factors readily produced human iPSCs that closely resembled mouse ESCs in many aspects. Importantly, these human iPSCs are independent of ectopic expression of any of the exogenous factors, and should therefore facilitate functional dissection of human genome and modeling human diseases.
Results
Signaling Through RARs Is Required in Reprogramming.
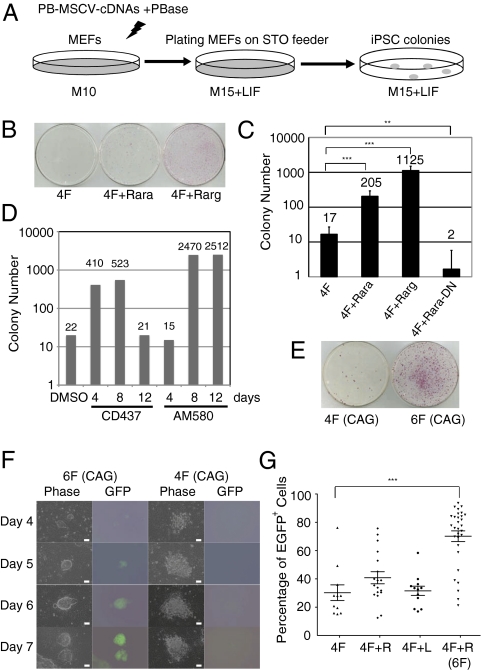
In an effort to identify new reprogramming factors, we analyzed several developmentally important signaling pathways. Among them, RA signaling has complex functions during vertebrate development and normally induces ES cell differentiation at high concentration (12). We thus asked whether RA signaling would play any roles in reprogramming, with the presumption that RA signaling would hinder reprogramming. To test this hypothesis, we cloned cDNAs of Oct4, Sox2, Klf4, cMyc, Rara (RA receptor-α), Rarg, and Rara-DN (dominant-negative form of Rara) (13) into a piggyBac (PB)-murine stem cell virus (MSCV) vector in which expression of the cDNAs was controlled by the LTR promoter/enhancer from MSCV (Fig. S1A). PB transposition facilitates efficient integration of the PB transposon into the genome (14) and can be used as a nonviral approach to deliver reprogramming factors (15, 16). Although exogenous c-Myc is not essential for reprogramming, we still included it in this study because omission of it reduces reprogramming efficiency and delays the process (8). The reported tumorigenic problem associated with using c-Myc was not observed in our study partly because the PB transposon vector, unlike retroviruses or lentiviruses, does not have a strong enhancer/promoter (14). The individual PB-MSCV-cDNA vectors were transfected into mouse embryonic fibroblasts (MEFs) together with a plasmid transiently expressing PB transposase (PBase; Fig. 1A). Transfection of the 4F were used as control (Fig. 1B). In the other three transfections, 4F were coelectroporated with Rarg (4F+Rarg), Rara (4F+Rara), or Rara-DN (4F+Rara-DN).
Fig. 1.
Critical roles of RA signaling in reprogramming. (A) Schematic of the reprogramming strategy. M10 and M15 are media for MEFs and ES cells, respectively. (B and C) Expressing Rara or Rarg, together with 4F, drastically promoted reprogramming, whereas expressing Rara-DN blocked reprogramming. Reprogrammed colonies were visualized by AP staining (**P < 0.005 and ***P < 0.0005). Rara-DN, dominant-negative form of Rara. (D) Temporal requirement of RA signaling in reprogramming. After transfection of 4F, Rarg agonist CD437 (0.1 μM) or Rara agonist AM580 (0.01 μM) was immediately added to the medium for several time lengths. Control, DMSO, the solvent for CD437 and AM580. (E) Six factors increased reprogramming efficiency. AP+ colonies were scored on day 10 after transfection. (F) Rapid reprogramming by 6F. Activation of endogenous Oct4 was detected by GFP expression at several time points after transfection. (Scale bars: 10.0 μm.) (G) Six factors improved iPSC quality based on GFP expression from the Oct4 locus. Individual colonies were picked on day 10 after transfection and expanded in 96-well plates for flow cytometry analysis (***P < 0.0005).
Three weeks after transfection, ES-like colonies started to appear. By day 30, there were one or even two orders of magnitude more alkaline phosphatase (AP)-positive ES cell-like colonies in 4F+Rara and 4F+Rarg, respectively, compared with the 4F control (Fig. 1 B and C). In contrast, expressing the Rara-DN almost completely blocked reprogramming (Fig. 1C). Therefore, RA signaling through RARs has critical roles in reprogramming MEFs. We subsequently examined the specificity and the temporal requirement of RA signaling in reprogramming by using a synthetic RA agonist specific to Rarg, CD437 (17). After transfecting MEFs with 4F, addition of CD437 in the medium for up to 8 d substantially increased the number of AP+ iPSC colonies, but longer treatment reduced the colony number, indicating a temporal requirement for RA signaling in reprogramming (Fig. 1D). The similar phenotype was observed for a synthetic RA agonist specific to Rara, AM580 (Fig. 1D).
These surprising results prompted us to further investigate RA signaling in reprogramming. Previous biochemical studies suggested that heterodimers of RARs and retinoid X receptors (RXRs) promote expression of the key pluripotency factor Oct4 in a ligand-dependent manner (18). To monitor activation of the endogenous Oct4, we used MEFs from the Oct4-GFP transgenic reporter mouse line in which Oct4 reactivation could be visualized under fluorescence microscope (7). To make activation of the endogenous Oct4 locus also selectable, we made another Oct4 reporter mouse line in which the IRES-Puro-GFP cassette was targeted to the 3′UTR of the Oct4 locus (Fig. S1 B–D). The knock-in ES cells survived 2.0 μg/mL or higher concentrations of puromycin.
Consistent with previous reports that the majority of colonies obtained by 4F are partially reprogrammed (7), most AP+ colonies in the aforementioned transfection experiments, whether using 4F alone or 4F+Rarg (Fig. 1 B and C), were neither resistant to 2.0 μg/mL puromycin nor GFP+, indicating that they were in a pre-iPSC state with incomplete Oct4 activation (7). Compared with WT ES cells, these pre-iPSC clones expressed lower levels of key pluripotency genes, and remained highly methylated in the promoters of Oct4 and Nanog genes (Fig. S1 E and F). The temporal requirement for RA signaling in reprogramming at relatively early stages of reprogramming (Fig. 1D) was supported by the fact that treating the pre-iPSC clones did not increase GFP+ cells (Fig. S1G). Therefore, although enhancing RA signaling by overexpressing Rarg increased the AP+ colony number as much as 100 fold, it did not substantially improve the overall iPSC quality, nor did it alter the reprogramming kinetics.
Rapid and Efficient Reprogramming of MEFs by Coexpression of Rarg and Lrh-1.
We next investigated additional genetic factors that could cooperate with Rarg in reprogramming. Biochemical studies showed that in differentiated mouse ES cells and embryonal carcinoma cells, COUP-TFs and other repressors silence the Oct4 locus by binding to RAREoct, a composite RA responsive element in the Oct4 promoter (19, 20). Besides RAR:RXR heterodimers, RARs and members of Nr5a steroid hormone receptor family (Sf-1 or Lrh-1) also form heterodimers which compete against COUP-TFs for RAREoct and maintain Oct4 expression in embryonal carcinoma cells in a likely ligand-independent manner (21). We thus examined Rarg and Lrh-1 for their possible synergistic interaction in reprogramming.
We constructed two unique PB vectors, PB-CAG-OCKS and PB-CAG-RL, in which cDNAs of Oct4, c-Myc, Klf4 and Sox2 (OCKS), Rarg and Lrh-1 (RL) were linked by T2A, respectively, and were under control of the CAG promoter (Fig. S1A). PB-CAG-OCKS (4F) or PB-CAG-OCKS plus PB-CAG-RL (6F) was transfected into the Oct4-GFP reporter MEFs. On day 10, there were as many as 20 times more AP+ colonies in the 6F plates compared with the 4F control (Fig. 1E). Remarkably, most 6F iPSC colonies on day 10 (>80%) were GFP+, indicating that these cells expressed high levels of the endogenous Oct4. There were many more 6F-reprogrammed colonies on day 30 compared with expressing 4F. We did not score them because, similar to subcloning mouse ES cells, by then these 6F colonies were overgrown and became differentiated.
Besides producing more GFP+ iPSCs, 6F also dramatically accelerated reprogramming. As early as 4 d after transfection of 6F into the Oct4-GFP reporter MEFs, microscopic ES cell-like GFP+ colonies appeared (Fig. 1F). Even when 2.0 μg/mL puromycin selection was applied 1 day after transfection of 6F, a few Puror cells survived and grew to GFP+ iPSC colonies. Puromycin at 2.0 μg/mL kills MEFs and partially reprogrammed iPSCs within 24 h. Therefore, following 6F expression, the Oct4 locus in MEFs was rapidly activated. Although expressing 4F (OCKS) also led to formation of a few loose colonies after 4 d, GFP+ cells were not found in these colonies until day 7 after transfection (Fig. 1F), consistent with previous reports (22). The full activation of the endogenous Oct4 locus was further characterized in flow cytometry. The majority of primary 6F iPSC colonies expanded in 96-well plates were GFP+ (Fig. 1G). Similar to 4F plus Rarg, 4F plus Lrh-1 did not obviously improve iPSC quality nor accelerate reprogramming (Fig. 1G).
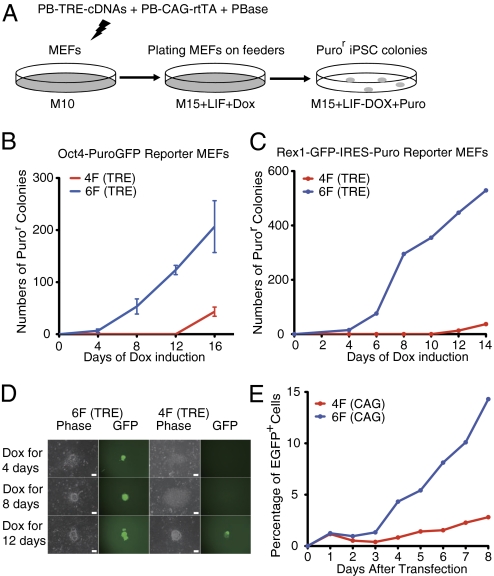
Previous studies suggested that exogenous factor-induced reprogramming is a stochastic process (23–25). Approximately 10 to 16 d of continuous expression of the 4F in MEFs is needed before some reprogrammed cells become independent of exogenous factor expression (24). To test how fast the 6F reprogramming achieves this state, we expressed the six factors under the control of doxycycline (Dox)-inducible tetracycline response element (TRE) promoter in PB-TRE-OCKS and PB-TRE-RL (Fig. S1A). These two PB transposons, the PBase plasmid, as well as PB-CAG-rtTA that expressed the reverse tetracycline transactivator (Fig. S1A), were cotransfected into the Oct4-GFP reporter MEFs. Dox was added to the medium immediately after transfection and was subsequently removed at several time points. Puromycin was added after Dox removal to select for cells expressing proper levels of Oct4 (Fig. 2A). GFP+ and Puror cells were detected as early as 4 d after 6F expression (Fig. 2B). Remarkably, some of these day-4 colonies already expressed SSEA-1 and Nanog (Fig. S2A), and later formed large iPSC colonies in the absence of Dox. This is consistent with a recent report that activation of Nanog coincides with irreversible commitment to reprogramming, or “point of no return” (23).
Fig. 2.
Rarg and Lrh-1 synergistically promote reprogramming. (A) Schematic of the Tet-On reprogramming strategy. (B and C) Rapid activation of endogenous Oct4 (B) or Rex1 (C) by 6F. Expression of 6F for 4 d was sufficient to fully activate endogenous Oct4 (B) or Rex1 (C) to obtain Dox-independent iPSCs. (D) Morphology and GFP expression of Dox-independent colonies from Rex1-GFP reporter MEFs. (Scale bars: 10.0 μm.) (E) Kinetics of the Oct4 locus activation in Oct4-GFP reporter MEFs. The blue or red dots represent percentages of GFP+ cells in 6F or 4F transfections, respectively.
Rex1 is expressed in mouse ES cells but not in EpiSCs (10, 11) and represents a better marker for ground state pluripotency than Oct4. We made the Rex1-GFP mouse line where the GFP-IRES-Puro cassette was inserted into the Rex1 locus (26), and used MEFs from this line for reprogramming. Dox-independent GFP+ and Puror colonies again appeared only after 4 d Dox treatment if 6F were used (Fig. 2 C and D), and prolonged expression of 6F further increased iPSC colony number (Fig. 2 B and C). In contrast, if only 4F were expressed, it took at least 12 d to obtain Dox-independent Puror or GFP+ colonies (Fig. 2 B–D). Therefore, synergistic interaction between Rarg and Lrh-1 dramatically accelerated reprogramming by rapidly activating key endogenous pluripotency genes including Oct4, Rex1, and Nanog.
To investigate dynamic expression of the endogenous Oct4 locus, we transfected either 4F alone (PB-CAG-OCKS) or plus PB-CAG-RL (6F) in Oct4-GFP reporter MEFs, and performed flow cytometric analysis (Fig. 2E and Fig. S2 B and C). Twenty-four hours after transfection of 6F, approximately 1% of MEFs became GFP+. Some of these GFP+ were of similar sizes to MEFs, but others were smaller (Fig. S2B). From day 3, the small GFP+ cells increased rapidly and steadily, and by day 8, they accounted for 11% of total cells (Fig. S2B). In contrast, expressing 4F did not substantially increase GFP+ cell numbers (Fig. S2B) in this period.
To test whether expressing Rarg and Lrh-1 was able to directly activate Oct4 in MEFs, we performed a reporter assay using a luciferase expression cassette linked to the Oct4 promoter (Fig. S2D). Expression of Rarg or Lrh-1 alone, or 4F, did not have a substantial effect on the luciferase reporter expression (Fig. S2E). However, coexpressing Rarg and Lrh-1 increased the reporter expression by four to five fold (Fig. S2E), similar to the synergistic interaction of Rarg and Sf-1 (21).
Finally, we examined the effect of expressing the two additional factors on cell cycle. In MEFs, coexpression of Rarg and Lrh-1 did not appear to substantially alter cell cycle in the cell proliferation assay (Fig. S2F). Therefore, efficient reprogramming by synergistic interaction of Rarg and Lrh-1 is likely a result of activation or stabilization of expression of Oct4 and other key pluripotency genes.
Mouse iPSCs Produced by Expressing the Six Factors Are Germline-Competent.
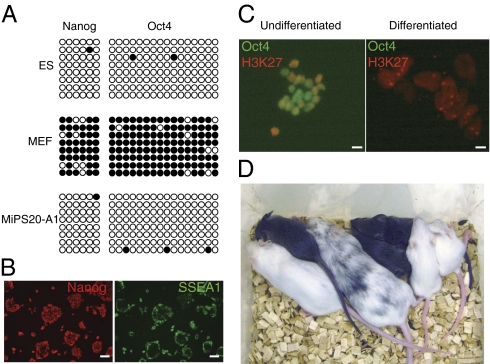
To further confirm pluripotency of the iPSCs produced by expressing 6F, iPSCs of more than 20 Dox-independent lines were characterized in vitro and in vivo. Both immunostaining and quantitative RT-PCR (qRT-PCR) analyses demonstrated that iPSCs expressed proper levels of pluripotency genes (Fig. S3 A–C). Furthermore, the promoter regions of Oct4 and Nanog showed nearly complete DNA demethylation (Fig. 3A).
Fig. 3.
Characterization of Dox-independent mouse iPSCs produced by 6F. (A) Nearly complete demethylation in the promoters of Oct4 and Nanog in the iPSCs. (B) Pluripotency of iPSCs in N2B27/2i medium as demonstrated by Nanog and SSEA-1 immunostaining. (Scale bars: 10.0 μm.) (C) Both X chromosomes were active in undifferentiated female iPSCs produced with 6F. The differentiated female iPSCs lost Oct4 expression and had one inactivated X chromosome, which was decorated by H3K27me3 staining. (Scale bars: 40.0 μm.) (D) Contribution of iPSCs to the germline in chimeras. The chimeras were crossed to WT C57BL6 females (albino). Germline transmission pups were the agouti ones.
N2B27/leukemia inhibitory factor (LIF) medium supplemented with two inhibitors, PD0325901 (MAPK inhibitor) and CHIR99021 (glycogen synthase kinase 3 inhibitor), supports the growth of ground state mouse ES cells (27). The 6F iPSCs proliferated well and kept pluripotent in the N2B27/LIF/2i medium without feeders (Fig. 3B). Both X chromosomes in mouse female ES cells are in a preinactivation state while mouse EpiSCs have already undergone X inactivation (28). As shown in Fig. 3C, the female iPSCs had two active X chromosomes.
The iPSCs were able to differentiate to somatic cell types representing all three germ layers in vitro and in vivo (Fig. S3 D and E). Moreover, iPSCs efficiently contributed to the germline in chimeras (Fig. 3D). Importantly, because most iPSC lines did not express detectable levels of the exogenous factors (Fig. S3F), the chimeras did not develop tumors (18 mo; n = 40).
6F Combination Readily Produces Human iPSCs That Resemble Mouse ES Cells.
The MEF reprogramming results prompted us to explore whether these factors would also play similar roles in reprogramming human somatic cells. Computational analysis showed that the RAREoct at the Oct4 locus is highly conserved in several mammalian genomes (Fig. S4A). In both the mouse and human genomes, approximately 30 to 40 loci contain RAREoct-like elements (Datasets S1 and S2). However, among these loci, the Oct4 locus was the only one shared between the two species, suggesting functional significance of this element. Indeed, recent experimental evidence also supports a possible role for RAREoct in regulating Oct4 expression in human cells (29).
We first attempted to produce human iPSCs by expressing 6F by using the CAG promoter. The PB-CAG transposons carrying the six human cDNAs were transposed into human neonatal foreskin dermal fibroblast (HDFn) cells. As early as 7 d after transfection, colonies formed in both human ES cell medium [KnockOut serum replacement (KSR)/FGF] and mouse ES cell medium (M15/LIF). We did not further characterize the FGF colonies because expressing 4F could also produce them (4, 6). Instead, we focused on the colonies in M15/LIF medium that were morphologically similar to mouse ES cell colonies (e.g., compact raised colonies or 3D, high nucleus-to-cytoplasm ratios, and prominent nucleoli; Fig. S4B). These colonies were picked on day 12 to 14 and were subsequently cultured by using standard mouse ES cell protocols on STO feeders (Fig. S4B). The primary 4F colonies in M15/LIF medium were morphologically distinct from the 6F ones (Fig. S4B), and no stable lines could be established from them, consistent with a previous report (4). Besides their ability to proliferate in regular mouse ES cell medium, the 6F human iPSCs expressed key pluripotency genes and human ES cell markers (Fig. S4 C and D), and could also be maintained in N2B27/2i/LIF medium (Fig. S4B). Despite expressing exogenous factors from the CAG promoter, these cells formed teratomas in immune-compromised NSG mice (30) with cell types representing all three germ layers (Fig. S4E). Furthermore, even after extensive in vitro culture, these iPSCs retained the normal karyotype, demonstrating that these cells were genetically stable (Fig. S4F). By using Y chromosome polymorphism markers, we also confirmed the iPSCs’ HDFn origin (Table S1).
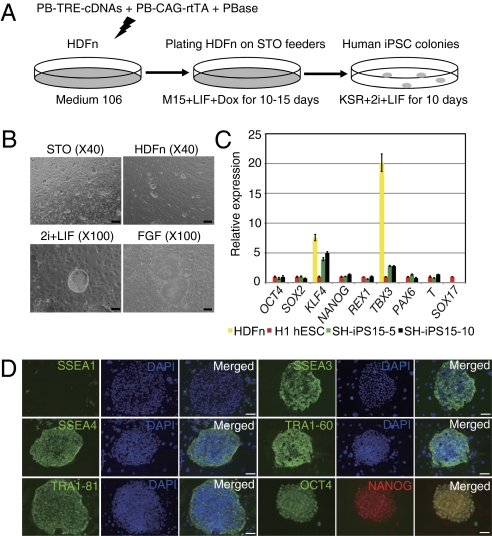
To establish human iPSC lines that were independent of expression of the exogenous reprogramming factors, we used the Tet-On system to express 6F. The PB-TRE-cDNA transposons were cotransfected with the PB-CAG-rtTA plasmid into HDFn cells (Fig. 4A). Colonies consisting of ES cell-like cells appeared after 8 to 10 d of Dox induction, which expressed the endogenous NANOG (Fig. S5A). Dox could be removed from the medium from as early as day 8 after transfection (Table S2), and the cells were subsequently cultured in DMEM/F12 medium supplemented with 20% KSR, the two inhibitors, and human LIF (KSR/2i/LIF). From 1 million human neonatal dermal fibroblast cells, we typically obtained 800 to 1,000 colonies and most were Dox-independent (Table S2). The primary colonies on day 25 were dissociated and plated on STO feeders in the KSR/2i/LIF medium (Fig. 4B).
Fig. 4.
Production and characterization of human iPSCs that are free of exogenous factor expression. (A) Reprogramming HDFn cells using the Tet-On 6F. (B) Typical human iPSC colony morphology on the STO or HDFn feeders in the KSR/2i/LIF medium (passage 10, top two panels). (Scale bars: 4.0 μm.) The bottom two panels are representative human iPSC colonies growing in KSR/2i/LIF medium (compact and 3D) or human ES medium KSR/FGF (monolayer and flattened). (Scale bars: 10.0 μm.) (C) qRT-PCR analysis of key genes in parental HDFn, human iPSCs, and H1 hESC cells. HiPS15-5 and -10 were two independent iPSC lines derived from a male neonatal dermal fibroblast line (passage 20). Expression was relative to Gapdh and normalized against gene expression in H1 hESCs growing in KSR/FGF medium. (D) Immunostaining of human iPSCs for hESC pluripotency markers: SSEA1, SSEA3, SSEA4, TRA-1–60, and TRA-1–81 (cell surface markers) and OCT4 and NANOG. (Scale bars: 20.0 μm.).
The Dox-independent human iPSCs expressed endogenous OCT4, SOX2, and NANOG at levels generally comparable to that in human ES cells (Fig. 4C and Fig. S5B), and were positive for human ES cell surface markers (Fig. 4D and Fig. S5C). Critically, expression of the exogenous factors in these cells was undetectable (Fig. S5D), which confirmed that pluripotency was self-sustainable in the KSR/2i/LIF medium. Even after extensive in vitro culture, these iPSCs retained the normal karyotype demonstrating that these cells were genetically stable (Fig. S5E). Human iPSCs could differentiate to cells of three germ layers in vitro (Fig. S5F), and form mature teratomas when injected into the NSG mice (Fig. S5G). By using the same strategy, we were also able to produce iPSCs in the KSR/2i/LIF medium from primary human adult dermal fibroblast (HDFa) cells (Fig. S6A and Table S2). In qRT-PCR analysis, these adult cell-derived iPSCs expressed high levels of endogenous pluripotency genes but low levels of differentiation markers (Fig. S6B). Furthermore, global gene expression analysis of these iPSC lines and those from neonatal fibroblast cells showed that they had similar expression patterns, which were different from the parental cells (Fig. S6C and Dataset S3). Importantly, expression of key endogenous pluripotency genes was stable during establishment of human iPSC lines and in the maintenance of these cells (Fig. S6D). Similar to iPSCs from neonatal fibroblast cells, the adult fibroblast cell-derived iPSCs also had a normal karyotype after 20 passages (Fig. S6E).
The ground-state mouse female ES cells or iPSCs have two active X chromosomes, whereas human female ES cells show signs of X chromosome inactivation detected by XIST expression or punctuate immunostaining pattern for H3K27me3 (31). qRT-PCR detected little XIST in our female iPSCs compared with the parental female dermal fibroblast cells (Fig. S6 F and G), suggesting a lack of X-inactivation in these iPSCs. To confirm this observation, we measured expression of a subset of X-linked genes, namely CXORF15, PLS3, RBBP7, and UTX. In all cases, these genes were expressed at higher levels than those of the fibroblasts and human male ES cells (Fig. S6H). To distinguish from the human iPSCs produced in conventional human ES cell medium containing FGF, we named these FGF-independent human iPSCs Sanger human iPSCs (SH-iPSCs).
Similar to mouse ES cells, SH-iPSCs were easily expanded, and were usually passaged every 3 d after dissociating into single cells in a 1:3 split ratio with a cell cycle time of approximately 22 to 24 h. Cell proliferation analysis proved that many more SH-iPSCs (approximately seven times) were undergoing cell division than human ES cells cultured in KSR/FGF medium (Fig. S7A). Furthermore, the promoter regions of OCT4 and NANOG were almost completely demethylated (Fig. S7B).
In contrast to dependence on Lif/Jak/Stat pathway for pluripotency maintenance in mouse ES cells, human ES cells derived from blastocysts are usually cultured in FGF-containing medium, and LIF alone is insufficient to maintain pluripotency (32). SH-iPSCs did not require FGF in the medium. Instead, they grew well and kept pluripotency gene expression in KSR/2i/LIF medium even in the presence of the FGF receptor inhibitor SU54021 (Fig. S7C). Human ES cells are resistant to JAK inhibitor (JAKi) that blocks STAT3 phosphorylation. If a JAKi was added to the medium, SH-iPSCs lost expression of OCT4, NANOG, and REX1 and were differentiated (Fig. S7C). Mouse EpiSCs and human ES cells are differentiated in the presence of BMP4 (10, 33), and SH-iPSCs proliferated well in the presence of BMP4. Finally, compared with human ES cells, SH-iPSCs expressed lower levels of lineage-specific genes such as PAX6, GATA6, and SOX17 (Fig. S7C).
To investigate whether SH-iPSCs had the potential to become human ES cell-like cells, we cultured them in KSR/FGF medium. After three passages, the FGF-cultured human iPSCs became morphologically similar to human ES cells (i.e., flattened colonies and difficult to passage; Fig. 4B). These cells still expressed comparable levels of pluripotency genes such as OCT4, NANOG, and REX1, but KLF4 expression decreased four to five fold. They also displayed a down-regulation of STELLA and NODAL expression (Fig. S7D), but retained human ES cell markers such as SSEA3 (Fig. S7E). Furthermore, the FGF-cultured female SH-iPSCs has increased XIST expression and decreased X-linked gene expression, indicating X-inactivation in these cells (Fig. S6 G and H). When the culture medium had been switched back to KSR/2i/LIF, these FGF-adapted iPSCs appeared differentiated and expressed high levels of SOX1 with concomitant loss of OCT4 and NANOG expression (Fig. S7D), resembling the behavior of human ES cells (32). To further compare SH-iPSCs cultured in 2i/LIF medium, the FGF-cultured SH-iPSCs and human ES cells, we performed global gene expression analysis. Expression profiles of FGF-cultured SH-iPSCs and human ES cells were similar to each other, but were distinct from that of the 2i/LIF-cultured iPSCs (Fig. S7F and Dataset S4). Stem cells express telomerase and human ES cells have high telomerase activity (32). Human iPSCs growing in the 2i/LIF medium had approximately 10-fold higher telomerase activity than that in human ES cells or the FGF-cultured iPSCs (Fig. S7G). These results thus demonstrated that SH-iPSCs growing in KSR/2i/LIF medium had the potential to covert to cells similar to human ES cells, but not vice versa. The ability of SH-iPSCs to adapt to the FGF medium condition is reminiscent of the observation that different stem cells could be cultured out of inner cell mass of the mouse blastocyst (34), and is also similar to mouse ES cells differentiated into EpiSCs by withdrawal of LIF and cultured in FGF medium (28). Therefore, the human iPSCs produced in this study were more immature than human ES cells, and resembled the ground state mouse ES cells in aspects of growth properties, signaling dependence, X-inactivation status, and gene expression (27).
Discussion
RARs activates Oct4 expression via binding of RAR:RXR heterodimers to RAREoct or through synergistic binding of RARs and SF-1 (Lrh-1) to RAREoct (18, 21). These heterodimers subsequently recruit coactivators, such as CBP/p300, P/CAF, and SRC1/TIF2 (12), to the Oct4 locus to stabilize or maintain its activation status and to facilitate further chromatin remodelling. Consequently, one major limiting step in somatic cell reprogramming, namely activation of the endogenous Oct4 locus (35, 36), is overcome almost immediately after exogenous factor expression. In Oct4-GFP reporter MEFs, GFP+ cells were detected as early as day 3 to 4 after expressing 6F, which is comparable to somatic cell nuclear transfer (37) or cell fusion (38). Therefore, technically, 6F should facilitate further development of nongenetic modifying reprogramming such as using episome and mRNA (39, 40).
Besides its classical roles, recent studies show that RARa has noncanonical modes of action by interacting with estrogen receptor (ER)-binding sites and cooccupying regulatory regions together with ER-α in an ER-dependent manner (41, 42). Besides the heterodimers between RARs and RXRs, or between RARG and LRH1, in binding RAREoct to promote OCT4 expression, RARs may bind additional genomic loci with other partners in a ligand-independent manner to promote reprogramming, which would represent additional examples of their noncanonical function. Therefore, both canonical (RAR:RXR) and noncanonical (RAR:LRH1 or other partners) modes of action of RARs are likely to be needed for efficient reprogramming. Further investigation will provide insights into the molecular mechanism of RARs in reprogramming.
While this study was being finalized, another report showed that overexpressing Lrh-1 or Sf1 by retrovirus could moderately enhance reprogramming and even replace exogenous Oct4 in reprogramming at a low efficiency (43). In the same study, however, the investigators did not find any obvious effect of RARs on reprogramming. This discrepancy is likely because RA signaling in reprogramming is dose- and time-dependent, which could be overshadowed by the retrovirus delivery approach. Recent evidence also suggests that Lrh-1 acts downstream of canonical Wnt signaling and regulates the Nanog and Tbx3 pluripotency signaling axis (44), which may explain the ability of expressing Lrh-1 to reliably reset EpiSCs to ground state pluripotency (45). Therefore, Lrh-1 could promote activation of other key pluripotency genes at late stages of reprogramming besides the synergic interaction with Rarg in early Oct4 activation and stabilization.
The first attempt to obtain mouse ES cell-like human iPSCs used a combination of genetic factors and chemical mixture of ALK5, GSK3, and MEK inhibitors (46). A recent study reported that human iPSCs derived from secondary human fibroblast cells (iPSC-derived) or from human ES cells could be maintained in N2B27/2i/LIF medium plus forskolin (47). These cells appeared to be similar to ground-state mouse ES cells, and thus provided independent evidence that ground-state pluripotency exists in humans. However, these cells could only be maintained for limited passages. Another study also produced mouse ES cell-like human iPCSs (called hLR5), which still relied on continuous expression of all the reprogramming factors (48). These cells, to some extent, are similar to the human iPSCs obtained by using PB-CAG-cDNAs in this study (Fig. S4B). However, hLR5 cells did not express SSEA-3 and SSEA-4 and other human ES cell surface markers. Instead, some hLR5 lines expressed high levels of SSEA-1, which is present in mouse ES cells but absent in human ES cells. Attempts were also made to use HDAC inhibitors such as butyrate, which appears to pull hESCs backward toward an earlier developmental stage while, at the same time, could push mouse ES cells forward to a developmental intermediate between mouse ESCs and EpiSCs (49). Compared with iPSCs produced in these reports, SH-iPSCs were not only directly reprogrammed from primary human fibroblast cells, but did not have detectable expression of any of the exogenous factors, and could be maintained for more than 50 passages in KSR/2i/LIF medium. Moreover, they were FGF-independent but relied on LIF–JAK–STAT pathway and could be handled by using the standard mouse ES cell protocols. SH-iPSCs therefore are similar in many aspects to the envisioned ground-state human pluripotent stem cells and should facilitate functional dissection of the human genome and for modeling human diseases.
Methods
Mouse ES and iPSC Culture.
Mouse ES cells and iPSCs were normally cultured in M15 medium: knockout DMEM, 15% FBS (HyClone), 1× glutamine-penicillin-streptomycin (Invitrogen), 1× nonessential amino acids (NEAA; Invitrogen), 0.1 mM β-mercaptoethanol (β-ME; Sigma), and 106 U/mL LIF (Millipore).
Human ES and iPSC Culture.
Human iPSCs reprogrammed by PB-CAG vectors were cultured in M15 medium, with 106 U/mL human LIF. Human iPSCs reprogrammed by PB-TRE vectors were maintained in DMEM/F12 with GlutaMAX (Invitrogen), 20% KnockOut serum replacement (Invitrogen), 106 U/mL human LIF, 1× NEAA, 0.1 mM β-ME (Sigma), and the inhibitors CHIR99021 (5 μM) and PD0325901 (1 μM).
The human ES cell lines BG01V/hOG (Invitrogen) and H1 (WiCell) were cultured in hESC medium: DMEM/F12 with GlutaMAX (Invitrogen), 20% KnockOut serum replacement (Invitrogen), 1× NEAA, 0.1 mM β-ME (Sigma), and 4.0 ng/mL bFGF (Invitrogen).
SI Methods and Tables S3 and S4 include further details of the study materials and methods.
Supplementary Material
Acknowledgments
We thank Prof. Austin Smith for the Oct4-Puro-IRES-EGFP cassette and for the Oct4-GFP MEFs, Dr. Fangtang Yang and Beiyun Fu for spectral karyotyping human iPSCs, Dr. Yali Xue for helping genotype human Y chromosome polymorphisms, and the RSF staff of the Sanger Institute for their assistance on the mouse work. We thank Dr. Peter W. Andrews for the human ES cell antibodies. P.L. thanks Prof. Mike Stratton, Prof. Janet Rossant, and Drs. Bill Skarnes, Derek Stemple, Andy Futreal, and Carol Smee for comments and suggestions on this manuscript. This work was supported by the Wellcome Trust (077186/Z/05/Z) (to P.L.) and by the China Scholarship Council (H.L.).
Footnotes
Conflict of interest statement: The authors declare a conflict of interest (such as defined by PNAS policy). The Genome Research Ltd has one patent application related to the subject of this paper.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1100893108/-/DCSupplemental.
References
- 1.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 3.Wernig M, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Park IH, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 6.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 7.Silva J, et al. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol. 2008;6:e253. doi: 10.1371/journal.pbio.0060253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakagawa M, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 9.Pasque V, Miyamoto K, Gurdon JB. Efficiencies and mechanisms of nuclear reprogramming. Cold Spring Harb Symp Quant Biol. 2010;75:189–200. doi: 10.1101/sqb.2010.75.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tesar PJ, et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- 11.Brons IG, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- 12.Niederreither K, Dollé P. Retinoic acid in development: Towards an integrated view. Nat Rev Genet. 2008;9:541–553. doi: 10.1038/nrg2340. [DOI] [PubMed] [Google Scholar]
- 13.Tsai S, et al. A mutated retinoic acid receptor-alpha exhibiting dominant-negative activity alters the lineage development of a multipotent hematopoietic cell line. Genes Dev. 1992;6(12A):2258–2269. doi: 10.1101/gad.6.12a.2258. [DOI] [PubMed] [Google Scholar]
- 14.Wang W, et al. Chromosomal transposition of PiggyBac in mouse embryonic stem cells. Proc Natl Acad Sci USA. 2008;105:9290–9295. doi: 10.1073/pnas.0801017105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woltjen K, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yusa K, Rad R, Takeda J, Bradley A. Generation of transgene-free induced pluripotent mouse stem cells by the piggyBac transposon. Nat Methods. 2009;6:363–369. doi: 10.1038/nmeth.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gianni M, Zanotta S, Terao M, Garattini S, Garattini E. Effects of synthetic retinoids and retinoic acid isomers on the expression of alkaline phosphatase in F9 teratocarcinoma cells. Biochem Biophys Res Commun. 1993;196:252–259. doi: 10.1006/bbrc.1993.2242. [DOI] [PubMed] [Google Scholar]
- 18.Ben-Shushan E, Sharir H, Pikarsky E, Bergman Y. A dynamic balance between ARP-1/COUP-TFII, EAR-3/COUP-TFI, and retinoic acid receptor:retinoid X receptor heterodimers regulates Oct-3/4 expression in embryonal carcinoma cells. Mol Cell Biol. 1995;15:1034–1048. doi: 10.1128/mcb.15.2.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pikarsky E, Sharir H, Ben-Shushan E, Bergman Y. Retinoic acid represses Oct-3/4 gene expression through several retinoic acid-responsive elements located in the promoter-enhancer region. Mol Cell Biol. 1994;14:1026–1038. doi: 10.1128/mcb.14.2.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sylvester I, Schöler HR. Regulation of the Oct-4 gene by nuclear receptors. Nucleic Acids Res. 1994;22:901–911. doi: 10.1093/nar/22.6.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnea E, Bergman Y. Synergy of SF1 and RAR in activation of Oct-3/4 promoter. J Biol Chem. 2000;275:6608–6619. doi: 10.1074/jbc.275.9.6608. [DOI] [PubMed] [Google Scholar]
- 22.Jaenisch R, Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132:567–582. doi: 10.1016/j.cell.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samavarchi-Tehrani P, et al. Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell. 2010;7:64–77. doi: 10.1016/j.stem.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 24.Stadtfeld M, Maherali N, Breault DT, Hochedlinger K. Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell. 2008;2:230–240. doi: 10.1016/j.stem.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li R, et al. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell. 2010;7:51–63. doi: 10.1016/j.stem.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 26.Guo G, Huang Y, Humphreys P, Wang X, Smith A. A PiggyBac-based recessive screening method to identify pluripotency regulators. PLoS ONE. 2011;6:e18189. doi: 10.1371/journal.pone.0018189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ying QL, et al. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo G, et al. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development. 2009;136:1063–1069. doi: 10.1242/dev.030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang HM, et al. Transcriptional regulation of human Oct4 by steroidogenic factor-1. J Cell Biochem. 2007;101:1198–1209. doi: 10.1002/jcb.21244. [DOI] [PubMed] [Google Scholar]
- 30.Ishikawa F, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor gamma chain(null) mice. Blood. 2005;106:1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen Y, et al. X-inactivation in female human embryonic stem cells is in a nonrandom pattern and prone to epigenetic alterations. Proc Natl Acad Sci USA. 2008;105:4709–4714. doi: 10.1073/pnas.0712018105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 33.Xu RH, et al. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol. 2002;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- 34.Chou YF, et al. The growth factor environment defines distinct pluripotent ground states in novel blastocyst-derived stem cells. Cell. 2008;135:449–461. doi: 10.1016/j.cell.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hochedlinger K, Plath K. Epigenetic reprogramming and induced pluripotency. Development. 2009;136:509–523. doi: 10.1242/dev.020867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boiani M, Eckardt S, Schöler HR, McLaughlin KJ. Oct4 distribution and level in mouse clones: Consequences for pluripotency. Genes Dev. 2002;16:1209–1219. doi: 10.1101/gad.966002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boiani M, et al. Variable reprogramming of the pluripotent stem cell marker Oct4 in mouse clones: Distinct developmental potentials in different culture environments. Stem Cells. 2005;23:1089–1104. doi: 10.1634/stemcells.2004-0352. [DOI] [PubMed] [Google Scholar]
- 38.Do JT, et al. Erasure of cellular memory by fusion with pluripotent cells. Stem Cells. 2007;25:1013–1020. doi: 10.1634/stemcells.2006-0691. [DOI] [PubMed] [Google Scholar]
- 39.Yu J, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warren L, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hua S, Kittler R, White KP. Genomic antagonism between retinoic acid and estrogen signaling in breast cancer. Cell. 2009;137:1259–1271. doi: 10.1016/j.cell.2009.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ross-Innes CS, et al. Cooperative interaction between retinoic acid receptor-alpha and estrogen receptor in breast cancer. Genes Dev. 2010;24:171–182. doi: 10.1101/gad.552910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heng JC, et al. The nuclear receptor Nr5a2 can replace Oct4 in the reprogramming of murine somatic cells to pluripotent cells. Cell Stem Cell. 2010;6:167–174. doi: 10.1016/j.stem.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 44.Wagner RT, Xu X, Yi F, Merrill BJ, Cooney AJ. Canonical Wnt/beta-catenin regulation of liver receptor homolog-1 (lrh-1) mediates pluripotency gene expression. Stem Cells. 2010;28:1794–1804. doi: 10.1002/stem.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo G, Smith A. A genome-wide screen in EpiSCs identifies Nr5a nuclear receptors as potent inducers of ground state pluripotency. Development. 2010;137:3185–3192. doi: 10.1242/dev.052753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li W, et al. Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell. 2009;4:16–19. doi: 10.1016/j.stem.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 47.Hanna J, et al. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc Natl Acad Sci USA. 2010;107:9222–9227. doi: 10.1073/pnas.1004584107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buecker C, et al. A murine ESC-like state facilitates transgenesis and homologous recombination in human pluripotent stem cells. Cell Stem Cell. 2010;6:535–546. doi: 10.1016/j.stem.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ware CB, et al. Histone deacetylase inhibition elicits an evolutionarily conserved self-renewal program in embryonic stem cells. Cell Stem Cell. 2009;4:359–369. doi: 10.1016/j.stem.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.