Abstract
Heat-shock factor 1 (HSF1) is the master transcriptional regulator of the cellular response to heat and a wide variety of other stressors. We previously reported that HSF1 promotes the survival and proliferation of malignant cells. At this time, however, the clinical and prognostic significance of HSF1 in cancer is unknown. To address this issue breast cancer samples from 1,841 participants in the Nurses’ Health Study were scored for levels of nuclear HSF1. Associations of HSF1 status with clinical parameters and survival outcomes were investigated by Kaplan–Meier analysis and Cox proportional hazard models. The associations were further delineated by Kaplan–Meier analysis using publicly available mRNA expression data. Our results show that nuclear HSF1 levels were elevated in ∼80% of in situ and invasive breast carcinomas. In invasive carcinomas, HSF1 expression was associated with high histologic grade, larger tumor size, and nodal involvement at diagnosis (P < 0.0001). By using multivariate analysis to account for the effects of covariates, high HSF1 levels were found to be independently associated with increased mortality (hazards ratio: 1.62; 95% confidence interval: 1.21–2.17; P < 0.0013). This association was seen in the estrogen receptor (ER)-positive population (hazards ratio: 2.10; 95% confidence interval: 1.45–3.03; P < 0.0001). In public expression profiling data, high HSF1 mRNA levels were also associated with an increase in ER-positive breast cancer-specific mortality. We conclude that increased HSF1 is associated with reduced breast cancer survival. The findings indicate that HSF1 should be evaluated prospectively as an independent prognostic indicator in ER-positive breast cancer. HSF1 may ultimately be a useful therapeutic target in cancer.
Keywords: heat-shock protein 90, signature, pathology, heat-shock response, immunohistochemistry
Heat-shock factor 1 (HSF1) is a multifaceted transcription factor that governs the cellular response to disruptions in protein homeostasis. To protect the proteome under various physiologic stresses, HSF1 drives the production of classic heat-shock proteins (HSPs), such as HSP27, HSP70, and HSP90, that act as protein chaperones. This heat-shock response is an ancient adaptive mechanism that is present in eukaryotes from yeast to humans (1–3). The functions of HSF1 are not limited to increasing the expression of chaperones, however. HSF1 also modulates the expression of hundreds of genes other than chaperones that are critical for survival under an array of potentially lethal stressors (4, 5). As a result, HSF1 influences fundamental cellular processes such as cell-cycle control, protein translation, and glucose metabolism (5, 6).
We have demonstrated that the multifaceted ability of HSF1 to promote survival in the face of lethal stressors is co-opted by cancer cells (6). In the malignant state, a litany of stressful conditions arises from the tumor microenvironment and from drastic internal changes in core cellular physiology that are hallmarks of cancer (7, 8). HSF1 permits cancer to cope with these diverse malignancy-associated stressors. In doing so, it allows tumors to reconfigure their metabolism, physiology, and protein homeostasis networks to enable oncogenesis. The ultimate result is the enhanced proliferation and increased fitness of malignant cells as they emerge (6, 9, 10).
HSPs have received considerable attention as prognostic biomarkers in cancer (11). This focus on HSPs in cancer has uncovered their potential as therapeutic targets, with HSP90 inhibitors being the first modulators of chaperone activity to show clinical utility (12, 13). Although it has been observed that HSF1 levels are elevated in cancer cell lines and tumor tissues, including breast cancer (14–16), HSF1 has not yet been evaluated as a prognostic marker in cancer.
HSF1 normally shuttles between the nucleus and the cytoplasm, but, when activated by stressors, it accumulates within the nucleus. Increased levels of HSF1 facilitate survival to stress (17) by coordinating a range of fundamental cellular processes, including glucose metabolism, cell-cycle control, protein translation, and ribosome biogenesis (6). In preliminary studies in human breast cancer samples, we observed a striking increase in the levels of HSF1 as well as a shift in its localization from the cytoplasm to the nucleus. Based on our previous work in mouse tumor models, we hypothesized that this increase in nuclear HSF1 might be associated with poor prognosis. To address this hypothesis, we examined the relationship between HSF1, clinicopathological characteristics, and survival outcomes among 1,841 women with invasive breast cancer who participated in the Nurses’ Health Study (NHS). Our findings show that HSF1 is an independent prognostic indicator of poor outcome in breast cancer. This work highlights the potential of HSF1 as both a prognostic marker and a promising therapeutic target.
Results
Characterization of HSF1 Antibody and HSF1 Expression in Breast Cancer.
We first verified the specificity of a commercially available Heat-shock factor 1 (HSF1) antibody cocktail on samples from Hsf1 wild-type and null mice. A strong immunoreactive band of the expected size for HSF1 was present in wild-type lysates but was absent in lysates from Hsf1 null mice (Fig. 1A). To provide a rigorous control for immunohistochemistry (IHC), we used the antibody at a very high concentration with longer development time and an ultrasensitive detection method. This approach allowed us to pick up a strong nuclear signal even in the control samples, which we could then compare with the knockout tissues under the same conditions. There was absolutely no signal in the knockout tissues, establishing the specificity of the antibody (Fig. 1B).
Fig. 1.
HSF1 protein is increased in breast cancer. (A) Characterization of HSF1 antibody. Immunoblot analysis of spleen lysates from HSF1 wild-type (+/+) and HSF1 null (−/−) mice. (B) IHC of mouse brain from HSF1 wild-type and HSF1 null mice, long development. (Scale bars: 20 μM.) (C Upper) HSF1 immunoblot analysis of matched pairs of invasive ductal carcinoma and adjacent normal breast from seven patients. (Lower) Protein staining for loading comparison.
We next examined the expression of HSF1 in invasive carcinoma and matched normal adjacent breast tissue from seven patients by immunoblot analysis (Fig. 1C). More HSF1 was present in the tumors than in the matched controls in all cases. Interestingly, there was a strong HSF1 band in three of seven tumor samples and moderate-to-weak bands in the remaining tumors. The variation observed in this pilot study indicated that human breast tumors express HSF1 in different amounts and encouraged us to examine whether the amount of HSF1 protein expression correlates with prognosis.
As a transcription factor, HSF1 is active only in the nucleus. We first examined the localization and expression levels of HSF1 in tumor cells versus normal cells by IHC in a small panel of breast carcinoma tissue sections. The staining conditions that we established for the human study were different from those used for the mouse control. More dilute antibody and shorter development times were used to produce staining that was within the linear, dynamic range so that differences could be appreciated.
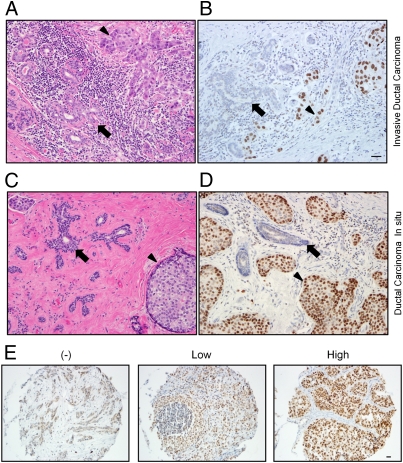
A striking difference between malignant cells and the adjacent normal breast epithelium was apparent (Fig. 2 A and B and Fig. S1). Under the IHC conditions optimized to capture differences in protein levels, no nuclear HSF1 was detectable in normal breast epithelium (n = 40) in nearly all cases, whereas there was nuclear staining in the majority of breast tumors. In samples of normal breast and in the tumors lacking nuclear HSF1, there was often a weak cytoplasmic signal. At higher antibody concentrations, we noted infrequent HSF1-expressing cells in occasional lobules (Fig. S2). The increase in HSF1 levels in invasive tumors supported the concept that HSF1 is activated in the malignant state.
Fig. 2.
HSF1 is increased and localized to the nucleus in invasive and in situ breast carcinoma. Photomicrographs of H&E-stained sections and HSF1 IHC of invasive ductal carcinoma (A and B) and the preinvasive lesion, DCIS (C and D). Nonneoplastic breast epithelium is indicated by arrows, and neoplastic cells are indicated by the arrowheads. (E) Representative photomicrographs of tumors from the NHS TMAs that were stained by HSF1 IHC and that were scored as having either no (−), low, or high nuclear HSF1 expression. This example with no nuclear HSF1 expression (−) demonstrates weak immunoreactivity in the cytoplasm. (Scale bar: 20 μM.)
Interestingly, in 20 HSF1-positive tumors, there was widespread uniform expression of HSF1 throughout the tumor cell nuclei but not in normal breast epithelial cells that were infiltrated by the tumor (Fig. 2 A and B). The uniform intensity of HSF1 expression is important to contrast with the variable patterns seen with most prognostic markers that are surveyed in human tumor sections with IHC. HSF1 staining was not stronger in tumor cells at the center of the tumor versus those at the stromal interface (Fig. S3 A and B) or regions of necrosis where microenvironmental stress was likely to be severe (Fig. S3C). Staining intensity also did not depend on the distance from stromal desmoplasia, inflammation, or microvasculature (Fig. S3 C and D). These observations suggest that increases in HSF1 in tumor cells are not principally attributable to external microenvironmental stress but more commonly result from internal, cell-autonomous factors.
The findings in 10 in situ carcinomas were similar to those in invasive cancer. In the majority of ductal carcinoma in situ (DCIS) cases, there was increased nuclear HSF1 compared with neighboring normal breast epithelium (Fig. 2 C and D). The levels of HSF1 were also uniform in the DCIS cells. These findings suggest that HSF1 expression is elevated during the in situ stage of malignant transformation and before invasion. Altogether, these immunoblot and pilot IHC findings justified an in-depth analysis of HSF1 protein expression in a large breast cancer cohort.
Nuclear HSF1 Is Highest in High-Grade Breast Cancer and Is Associated with Advanced Clinical Stage at Diagnosis.
We evaluated 1,841 invasive breast cancer cases from the NHS for HSF1 localization and expression. The cases were scored by a semiquantitative assessment of nuclear staining intensity. Nuclear staining was classified as either negative or positive. The positive cases had uniform expression of HSF1 throughout nearly all of the tumor cells. These positive cases were further subdivided by the intensity of nuclear HSF1 staining into either low or high groups (Fig. 2E).
There were 404 (21.9%) cases negative for nuclear HSF1 and 1,437 that had detectable nuclear HSF1 (78.1%), with 882 (47.9%) demonstrating low-level and 555 (30.2%) demonstrating high-level HSF1. Levels of HSF1 expression differed by histological grade (P < 0.0001): 40.5% of well-differentiated low-grade carcinomas were HSF1-negative and only 14.4% showed high nuclear HSF1 (Table 1); conversely, in poorly differentiated high-grade cancers, only 13.0% were HSF1-negative and 48.1% showed high HSF1 expression. Levels of HSF1 also differed by clinical parameters. Compared with HSF1-negative tumors, those with nuclear HSF1 expression were more likely to be diagnosed at a more advanced clinical stage (P < 0.0001) (Table 1). HER2-positive and triple-negative tumors are generally more aggressive than estrogen receptor (ER)-positive tumors. Compared with HSF1-negative tumors, high HSF1 tumors were more likely to be ER-negative (P < 0.0001), HER2-positive (P = 0.0003), and triple-negative (P = 0.0084), supporting an association between HSF1 expression and a more malignant phenotype.
Table 1.
Means and frequencies of participants’ characteristics by HSF1 status from NHS (1976–1996)
| Characteristic | No HSF1 | Low HSF1 | High HSF1 |
| n (%) | 404 (21.9) | 882 (47.9) | 555 (30.2) |
| Age at diagnosis, mean (n), y | 57.8 (404) | 56.8 (882) | 57.6 (555) |
| Menopausal status at diagnosis, n* (%) | |||
| Premenopausal | 74 (18.6) | 219 (25.3) | 109 (20.2) |
| Postmenopausal | 325 (81.5) | 648 (74.7) | 432 (79.9) |
| ER status, n* (%) | |||
| Positive | 334 (82.7) | 702 (79.4) | 412 (71.2) |
| Negative | 70 (17.3) | 182 (20.6) | 167 (28.8) |
| HER2 status, n* (%) | |||
| Positive | 23 (5.8) | 95 (10.7) | 81 (14.1) |
| Negative | 375 (94.2) | 794 (89.3) | 494 (85.9) |
| Triple-negative tumors, n* (%) | |||
| Yes | 49 (12.2) | 122 (13.7) | 108 (18.7) |
| No | 353 (87.8) | 766 (86.3) | 471 (81.4) |
| Nodal involvement, n (%) | |||
| None | 290 (71.8) | 590 (66.9) | 324 (58.4) |
| 1–3 | 72 (17.8) | 166 (18.8) | 134 (24.1) |
| 4–9 | 26 (6.4) | 78 (8.8) | 55 (9.9) |
| ≥10 | 16 (4.0) | 48 (5.4) | 42 (7.6) |
| Tumor size, n (%) | |||
| ≤2 cm | 301 (74.5) | 589 (66.8) | 295 (53.2) |
| >2 cm | 103 (25.5) | 293 (33.2) | 260 (46.9) |
| Histological grade, n* (%) | |||
| I (low) | 143 (35.8) | 159 (18.2) | 51 (9.3) |
| II (intermediate) | 199 (49.8) | 543 (62.1) | 284 (51.7) |
| III (high) | 58 (14.5) | 173 (19.8) | 214 (39.0) |
| Stage,†n (%) | |||
| I | 239 (59.2) | 452 (51.3) | 217 (39.1) |
| II | 114 (28.2) | 283 (32.1) | 225 (40.5) |
| III | 51 (12.6) | 147 (16.7) | 113 (20.4) |
| Chemotherapy, n* (%) | |||
| Yes | 101 (33.2) | 263 (41.9) | 217 (50.6) |
| No | 203 (66.8) | 365 (58.1) | 212 (49.4) |
| Hormone treatment, n* (%) | |||
| Yes | 207 (68.8) | 415 (66.3) | 280 (66.0) |
| No | 94 (31.2) | 211 (33.7) | 144 (34.0) |
| Radiation treatment, n* (%) | |||
| Yes | 136 (44.4) | 275 (43.7) | 185 (43.3) |
| No | 170 (55.6) | 354 (56.3) | 242 (56.7) |
*n for these characteristics does not add up to total studied (n = 1,841) because of missing information.
†Stage I: tumor size ≤2 cm and no nodal involvement; stage II: tumor size ≤2 cm and 1–3 nodes, 2–4 cm and 0–3 nodes, or 4+ cm and 0 nodes; stage III: tumor size ≤2 cm and 4+ nodes, 2–4 cm and 4+ nodes, or >4 cm and 1+ nodes.
HSF1 Accumulates in the Nuclei of in Situ Carcinomas.
Nuclear HSF1 was detected in 84.5% of the DCIS cases. The frequency and levels of HSF1 expression were similar between DCIS and invasive cancer, confirming our earlier observations on a small number of tumor sections. No statistically significant association was found between HSF1 expression and DCIS nuclear grade, however (Table S1). Our limited sample size of DCIS cases (n = 200) may have limited the power to detect such an association. Nonetheless, these observations highlight that HSF1 is activated before malignant cells gain the ability to invade across the basement membrane.
HSF1 Expression Is Associated with Reduced Survival in Breast Cancer.
We next investigated the relationship between HSF1 expression and breast cancer survival. A total of 1,841 women met inclusion criteria such as the absence of metastases at the time of diagnosis (see SI Materials and Methods for the full list of inclusion criteria). Median follow-up time was 14.9 y. Kaplan–Meier curves show that women with HSF1-positive tumors had worse survival rates relative to women with HSF1-negative tumors (P < 0.0001) (Fig. 3A). Although a suggestive association was observed in the HER2-positive population (P = 0.14) (Fig. 3B), no significant association was seen in triple-negative cases (P = 0.63) (Fig. 3C). Because of the relatively small number of cases in the ER-negative groups, the study is likely underpowered to observe an effect in those populations. However, in women with ER-positive tumors, a strong association was observed between HSF1-positive tumors and worse outcome (P < 0.0001) (Fig. 3D).
Fig. 3.
HSF1-positive tumors are associated with decreased survival in ER-positive breast cancer. (A) Kaplan–Meier analysis of all individuals with breast cancer that were scored in this study. (B–D) Kaplan–Meier analysis of participants with HER2-positive (HER2+) breast cancer (B), triple-negative breast cancer (C), and ER-positive (ER+) breast cancer (D) that had HSF1 in the nucleus (HSF1 +) or that had no detectable nuclear HSF1 (HSF1 −). In these analyses, low and high nuclear HSF1 expressors were included in the HSF1 + group. (E and F) Kaplan–Meier analysis of individuals with ER+, HER2+, and triple-negative breast cancer (E) or with only ER+ breast cancer (F) expressing no nuclear HSF1, low nuclear HSF1, or high nuclear HSF1. Data are from the NHS (1976–1997). Log-rank P values are shown.
We also examined survival considering HSF1 status in three categories: HSF1-negative, HSF1-low, and HSF1-high groups. Survival decreased as HSF1 levels increased from none to low and then further to high (P < 0.0001), suggesting a dose-dependent association between HSF1 and survival outcomes (Fig. 3E). Dose dependence was not seen for HER2-positive (P = 0.22) and triple-negative (P = 0.74) populations but was present in patients with ER-positive tumors (P < 0.0001) (Fig. 3F).
In Multivariate Models, HSF1 Is a Significant Independent Predictor of Worse Outcome.
To account for the effects of all variables on the relationship between HSF1 levels and survival, we assessed this relationship with several multivariate models. Across all cases, adjusting for age (Table 2, model 1), HSF1-positive tumors were associated with a 74% increase in breast cancer mortality [Table 2; hazards ratio (HR): 1.74; 95% confidence interval (CI): 1.35–2.25; P < 0.0001] relative to HSF1-negative tumors. After adjusting for age, ER status, date of diagnosis, stage, grade, and treatment variables (radiotherapy, chemotherapy, and endocrine therapy) (Table 2, model 2), HSF1-positive tumors were associated with a 50% increase in breast cancer mortality (Table 2; HR: 1.50; 95% CI: 1.15–1.95; P = 0.0026). HSF1-low and HSF1-high tumors were associated with 45% (P = 0.008) and 62% (P = 0.001) increases in mortality, respectively (Table 3). Similar results were seen in the ER-positive population, with HSF1-positive tumors associated with 86% increased mortality (Table 2; HR: 1.86; 95% CI: 1.34–2.59; P = 0.0002). Among the ER-positive tumors, HSF1-low and HSF1-high tumors were associated with 75% and 110% increases in mortality, respectively (Table 3) relative to HSF1-negative, ER-positive tumors.
Table 2.
Multivariate analysis of breast cancer-specific mortality by HSF1 status (positive or negative)
|
n |
HR (95% CI) |
|||
| Models | Cases | Endpoints | HSF1-negative | HSF1-positive |
| All cases | ||||
| Model 1 | 1,841 | 463 | 1.00 | 1.74 (1.35–2.25) |
| Model 2 | 1,841 | 463 | 1.00 | 1.50 (1.15–1.95) |
| ER-positive cases | ||||
| Model 1 | 1,416 | 327 | 1.00 | 2.21 (1.60–3.06) |
| Model 3 | 1,416 | 327 | 1.00 | 1.86 (1.34–2.59) |
| ER-negative cases | ||||
| Model 1 | 403 | 135 | 1.00 | 0.86 (0.56–1.32) |
| Model 3 | 403 | 135 | 1.00 | 0.88 (0.570–1.39) |
| HER2-positive cases | ||||
| Model 1 | 194 | 71 | 1.00 | 2.06 (0.83–5.12) |
| Model 2 | 194 | 71 | 1.00 | 2.87 (1.12–7.39) |
| HER2-negative cases | ||||
| Model 1 | 1,621 | 386 | 1.00 | 1.61 (1.23–2.11) |
| Model 2 | 1,621 | 386 | 1.00 | 1.37 (1.04–1.80) |
| Triple-negative cases | ||||
| Model 1 | 268 | 86 | 1.00 | 0.88 (0.52–1.50) |
| Model 3 | 268 | 86 | 1.00 | 0.88 (0.50–1.53) |
| ER-positive with hormone therapy cases | ||||
| Model 1 | 700 | 122 | 1.00 | 2.77 (1.52–5.02) |
| Model 4 | 700 | 122 | 1.00 | 2.20 (1.19–4.05) |
| ER-positive without hormone therapy cases | ||||
| Model 1 | 247 | 38 | 1.00 | 3.22 (1.14–9.10) |
| Model 4 | 247 | 38 | 1.00 | 2.01 (0.69–5.88) |
Model 1: adjust for age at diagnosis (years); model 2: adjust for age at diagnosis (years), ER status (positive, negative), date of diagnosis (months), disease stage (I, II, III), grade (I, II, III), radiation treatment (yes, no, missing), and chemotherapy and hormonal treatment (no/no, yes/no, no/yes, yes/yes, missing); model 3: adjust for age at diagnosis (years), date of diagnosis (months), disease stage (I, II, III), grade (I, II, III), radiation treatment (yes, no, missing), and chemotherapy and hormonal treatment (no/no, yes/no, no/yes, yes/yes, missing); model 4: adjust for age at diagnosis (years), date of diagnosis (months), disease stage (I, II, III), grade (I, II, III), radiation treatment (yes, no, missing), and chemotherapy (yes, no, missing).
Table 3.
Multivariate analysis of breast cancer-specific mortality by HSF1 status (no, low, or high HSF1)
|
n |
HR (95% CI) |
||||
| Models | Cases | Endpoints | No HSF1 | Low HSF1 | High HSF1 |
| All cases | |||||
| Model 1 | 1,841 | 463 | 1.00 | 1.61 (1.23–2.11) | 1.97 (1.49–2.62) |
| Model 2 | 1,841 | 463 | 1.00 | 1.45 (1.10–1.91) | 1.62 (1.21–2.17) |
| ER-positive cases | |||||
| Model 1 | 1,416 | 327 | 1.00 | 1.98 (1.41–2.78) | 2.66 (1.87–3.79) |
| Model 3 | 1,416 | 327 | 1.00 | 1.75 (1.25–2.47) | 2.10 (1.45–3.03) |
Model 1: Adjust for age at diagnosis (years); model 2: adjust for age at diagnosis (years), ER status (positive, negative), date of diagnosis (months), disease stage (I, II, III), grade (I, II, III), radiation treatment (yes, no, missing), and chemotherapy and hormonal treatment (no/no, yes/no, no/yes, yes/yes, missing); model 3: adjust for age at diagnosis (years), date of diagnosis (months), disease stage (I, II, III), grade (I, II, III), radiation treatment (yes, no, missing), and chemotherapy and hormonal treatment (no/no, yes/no, no/yes, yes/yes, missing).
Of the ER-positive patients, 74% (n = 700) received hormonal therapy. In this group, there was a significant association between HSF1-positive tumors and increased mortality (Table 2; HR: 2.20; 95% CI: 1.19–4.05; P = 0.0115). In women without hormonal therapy (26%, n = 247), the magnitude of the association was similar (Table 2; HR: 2.01; 95% CI: 0.69–5.88; P = 0.2002), but our analysis may have been underpowered to detect a significant association. Although the data may suggest that HSF1 can contribute to tamoxifen resistance, this effect will need to be evaluated in follow-up studies prospectively in a uniformly treated population.
Interestingly, in Kaplan–Meier analysis, a suggestive association between HSF1 status and survival in patients with HER2-positive tumors was observed (Fig. 3B). In multivariate model 2, accounting for additional covariates, the strength of association increased and was statistically significant (Table 2; HR: 2.87; 95% CI: 1.12–7.39; P = 0.0288). No association was observed between HSF1 status and survival among triple-negative patients (P = 0.64) in multivariate models.
HSF1 mRNA Expression Is Associated with Reduced Survival in Breast Cancer.
Finally, we examined whether the associations between HSF1 protein level and outcome in breast cancer could also be detected by using HSF1 mRNA levels. Because mRNA expression profiling data are not available from tumors in the NHS, we used data from the publicly available van de Vijver cohort (18) for this analysis. Consistent with our IHC analysis in the NHS, HSF1 mRNA levels were higher in ER-negative than in ER-positive (P < 0.0001) cancers. We analyzed survival with two HSF1 categories: HSF1-high and HSF1-low groups. Kaplan–Meier curves show that women with HSF1-high tumors in the van de Vijver cohort had worse survival relative to women with HSF1-low tumors (Fig. S4A; HR: 3.04; 95% CI: 1.95–4.75; P < 0.0001). The difference in survival between women with HSF1-high tumors and HSF1-low tumors was seen in the ER-positive (Fig. S4B; HR: 2.93; 95% CI: 1.63–5.26; P = 0.0003) but not in the ER-negative (Fig. S4C; HR: 0.74; 95% CI: 0.37–1.45; P = 0.3736) population.
Discussion
We previously reported a fundamental role for HSF1, a master regulator of protein homeostasis and cell survival, in tumor biology and cancer cell proliferation, for both mouse models and diverse human cell lines (6). Using the comprehensive resources of the NHS, we now demonstrate that, in a large number of clinical breast cancer samples, HSF1 is translocated to the nucleus, a change in localization that is required for its functions as a transcription factor. Moreover, nuclear localization of HSF1 and increased levels of HSF1 are associated with advanced clinical stage at the time of diagnosis and increased mortality, particularly in the ER-positive population.
Although a number of studies have examined individual HSPs, including HSP27, HSP70, and HSP90 (11), as prognostic indicators in cancer, most of these studies used relatively small patient cohorts followed for short periods of time, typically less than 10 y. In our study, we examined the relationship of HSF1 with outcome by using data from more than 1,800 patients, with some followed for longer than 25 y. We demonstrate that the prognostic value of HSF1 protein is retained after adjusting for age, stage, grade, and adjuvant therapy. Our study suggests that HSF1 status might identify patients with ER-positive tumors who may benefit from more aggressive therapeutic management and others for whom less intervention may be warranted. In addition, HSF1 levels identify a large patient population in which targeting protein homeostasis and HSF1 function may be of therapeutic benefit.
How might activation of HSF1 enable more aggressive breast cancer and lead to worse clinical outcomes? A part of the answer can be found in the elevated levels of HSPs that are characteristic of cancer. HSP elevation is driven by HSF1 responding to the complex protein-folding conditions that are common in malignancies, including increased protein load from dysregulation of the translation machinery (19), accumulation of mutated or fusion proteins (13), and imbalances in the stoichiometry of protein complexes because of aneuploidy (20). Although the malignant phenotype requires the support of the HSF1-driven chaperone machinery, HSF1's role is clearly much broader.
Malignant transformation alters cellular physiology and imposes significant metabolic and genetic stresses in addition to proteomic stresses. HSF1's impact on cell-cycle control, survival signaling, and energy metabolism during tumor initiation and progression may allow tumor cells to cope with these malignancy-associated stressors (6). In addition, HSF1 was recently reported to facilitate invasion in a melanoma model (21). Another possibility for fostering more aggressive cancer phenotypes is raised by work in fungal organisms. In yeast, HSP90, an important regulatory target of HSF1, potentiates the emergence of drug resistance by enabling the generation of greater phenotypic diversity (22). In human cancer, the general role of diversity in progression is less well-understood, but early data are intriguing (23). For example, in Barrett's esophagus, greater diversity in genetic and epigenetic parameters is associated with progression to invasive cancer (24). If HSF1 potentiates diversity, it may contribute to de novo and acquired drug resistance.
In our study, the association between HSF1 status and outcome was strongest in the ER-positive population. HSF1 was not associated with outcome in patients with triple-negative breast cancer. Of interest, shRNA knockdown of HSF1 in established cell lines shows that diverse molecular subtypes of breast cancer depend on HSF1 for growth in vitro (6). The present study may simply have been underpowered to observe an effect of HSF1 in triple-negative patients because of the much smaller number of such cases in the NHS cohort and their molecular heterogeneity. Alternatively, triple-negative tumors may have a decreased dependence on HSF1 because the underlying lesions do not require protection against diverse stresses or because other robust survival mechanisms are recruited. Additional work will be required to more definitively investigate HSF1 as a prognostic factor in ER-negative tumors. Other master regulators of survival responses should be investigated as well.
We also found that HSF1 retained its prognostic significance even when the analysis was restricted to ER-positive patients treated with tamoxifen, suggesting that HSF1 may contribute to tamoxifen resistance. In the majority of tamoxifen-resistant breast cancers, ER expression is maintained, suggesting that a complex series of events leads to tamoxifen unresponsiveness (25). Could the antagonistic effects of high HSF1 levels render cells functionally ER-negative despite the presence of ER protein? Calderwood and colleagues (15) have demonstrated that HSF1 can repress ER-dependent transcription. HSF1 exists in a complex with corepressors such as metastasis-associated protein 1 (MTA1) and components of the Mi-2/nucleosome remodeling and deacetylase (NuRD) complex. This complex can interact with promoters containing estrogen-response elements to inhibit estrogen-driven transactivation. Unlike tamoxifen, which selectively modulates ER function and slows breast cancer growth, repression of ER-responsive promoters by HSF1 might contribute to progression in the manner suggested for the Lim domain only 4 (LMO4) protein (26) and the MTA1s (“short”) variant of MTA1 (27, 28). Repression of ER-driven transactivation by these proteins may contribute to the development of an ER-negative phenotype similar to that seen in aggressive ER-negative tumors. The poor outcomes that are associated with increased HSF1 levels in ER-positive tumors are consistent with a similar role of HSF1 in malignant progression.
HSF1 was also associated with worse clinical outcomes in patients with HER2-positive breast cancer. Evidence for mechanistic interplay between HSF1 and HER2 has recently begun to emerge. HER2 and HSF1 work together to influence cancer metabolism by driving lactate dehydrogenase A (LDH-A) expression and shifting tumor cells into a glycolytic state (29). In addition, as we showed previously for RAS- and PDGF receptor-driven transformation (6), HSF1 is also important for HER2-driven transformation (9). HER2 or its ligand heregulin β1 also enhance the efficiency of transformation by increasing levels of HSF1 itself (16, 29). Consistent with HER2 increasing HSF1 levels, we observed that 88.4% of HER2-positive invasive tumors were HSF1-positive and 40.7% had high levels of HSF1, the greatest percentage of any molecular subtype.
Whether HSF1 status in breast cancer provides additional information beyond that of other available molecular prognostic assays, such as MammaPrint and Oncotype DX, is of great interest. An interesting next step will be to derive an mRNA signature of HSF1 activity in the malignant state from genome-wide promoter-occupancy analysis of HSF1 by using engineered human cell lines with different malignant potentials (30). Further work will be required to determine whether HSF1 protein status or a signature of its activity are predictive of therapeutic response. Because patients in the NHS received a variety of therapies, including cytotoxic chemotherapy, we were not able to determine whether HSF1 is a specific predictor for tamoxifen benefit or generalized drug resistance. This question can be evaluated in follow-up studies prospectively in a uniformly treated population.
The influence of HSF1 on the clinical outcome of breast cancer suggests that targeting protein homeostasis pathways may be of therapeutic value (31–33). Unfortunately, drugs that inhibit HSF1 selectively and with high efficacy have not been developed. Clinical efforts at targeting protein homeostasis pathways downstream of HSF1, however, have shown initial promise. For example, several potent, structurally diverse HSP90 inhibitors are currently in clinical trials. In breast cancer, the focus to date has been on HER2-positive disease, where addition of the first-generation HSP90 inhibitor tanespimycin (17-AAG) to trastuzumab therapy has demonstrated efficacy in patients who had previously progressed on trastuzumab alone (34–36).
An ongoing challenge in the development of HSP90 inhibitors, however, has been the identification of which patients are most likely to benefit from treatment with these drugs (37–40). The basal level of HSP90 per se has not proven to be predictive. The frustrating lack of predictive markers arises to a great extent because HSP90 does not act alone but rather as a part of a complex network of additional HSPs, cochaperones, and accessory molecules (13). This network's architecture makes the potential for cross-talk and compensation enormous. It may also explain the rather disappointing lack of power seen for elevation of single HSPs as independent predictors of response in cancers including breast (11).
In light of previous disappointing results with various individual HSPs as prognostic markers, the efficacy of HSF1, even as a single marker, in predicting the outcome of breast cancers is quite remarkable. It is likely that HSF1, as a dominant regulator of the entire heat-shock network, serves as a better indicator of the overall stress levels within a tumor and consequently the “load” on the HSP-based chaperone machinery. This load could determine which patients might benefit from a HSP90 inhibitor, either alone or in combination with other agents.
In summary, we demonstrate that HSF1 is associated with reduced survival in breast cancer patients, particularly among those with ER-positive disease. Future efforts to evaluate HSF1 as a prognostic factor for routine clinical management of ER-positive patients are warranted. The findings support efforts to identify drugs that specifically target HSF1 function and ongoing work to develop inhibitors of HSP90 and other downstream pathways regulated by HSF1.
Materials and Methods
Study Design and Population.
The NHS is a prospective cohort study initiated in 1976: 121,700 female US-registered nurses between the ages of 30 and 55 y completed a questionnaire on factors relevant to women's health with follow-up biennial questionnaires used to update exposure information and ascertain nonfatal incident diseases (41). See SI Materials and Methods for additional information about the cohort, selection criteria for outcome analysis, the covariates evaluated, and the statistical analysis.
Tissue Microarray (TMA) Construction.
The NHS breast cancer tissue-block collection and TMA assembly have been described previously (41, 42). Paraffin blocks were also obtained from the archives of Brigham and Women's Hospital to generate a TMA of normal breast epithelial lobules. See SI Materials and Methods for additional information.
IHC of Tissues.
Paraffin sections of human and mouse tissues and TMAs were stained with a rat monoclonal antibody mixture to HSF1 (RT-629-PABX; Thermo Scientific). In immunoblots, this reagent recognizes HSF1 both in its basal state and in the hyperphosphorylated form present in heat-shocked cells. Immunostained sections were reviewed by light microscopy and scored visually with a value assigned to each individual core. Scoring was based on a semiquantitative review of staining intensity with 0 indicating no nuclear staining, 1 indicating low-level nuclear staining, and 2 indicating strong nuclear staining for HSF1. See SI Materials and Methods for detailed IHC protocol and further description of scoring.
Immunoblotting.
Tissue blot IMB-130a from Imgenex was stained with a rat monoclonal antibody mixture to HSF1 (RT-629-PABX; Thermo Scientific). See SI Materials and Methods for detailed protocol.
Supplementary Material
Acknowledgments
We thank the participants and staff of the NHS cohort for their valuable contributions. We thank the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, and WY. We thank Terri Woo for expert assistance with IHC. We acknowledge grants from the Breast Cancer Research Foundation (New York, NY), National Cancer Institute Grant R01-CA146445-01, and Department of Defense Congressionally Directed Medical Research Program Breast Cancer Research Program Grant W81XWH-08-1-0282 BC-07456 (to T.A.I.); Public Health Service Grant CA087969 (to S.E.H.); National Cancer Institute Specialized Program of Research Excellence (SPORE) in Breast Cancer Grant CA089393 (to Dirk Iglehart, Dana-Farber Cancer Institute); funds from the Department of Health and Human Services and GlaxoSmithKline Grant WE234, EPI40307 (to R.M.T.); grants from the Susan G. Komen Breast Cancer Foundation (to L.W. and N.U.L.); funding from the Howard Hughes Medical Institute (to S.L.); American Cancer Society New England Division–SpinOdyssey Postdoctoral Fellowship PF-09-253-01-DMC (to M.L.M.); and National Institutes of Health Grant K08NS064168 and funds from the Marble Foundation and the V Foundation for Cancer Research (to S.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1115031108/-/DCSupplemental.
References
- 1.Rabindran SK, Giorgi G, Clos J, Wu C. Molecular cloning and expression of a human heat shock factor, HSF1. Proc Natl Acad Sci USA. 1991;88:6906–6910. doi: 10.1073/pnas.88.16.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiederrecht G, Seto D, Parker CS. Isolation of the gene encoding the S. cerevisiae heat shock transcription factor. Cell. 1988;54:841–853. doi: 10.1016/s0092-8674(88)91197-x. [DOI] [PubMed] [Google Scholar]
- 3.Xiao X, et al. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J. 1999;18:5943–5952. doi: 10.1093/emboj/18.21.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guertin MJ, Lis JT. Chromatin landscape dictates HSF binding to target DNA elements. PLoS Genet. 2010;6:e1001114. doi: 10.1371/journal.pgen.1001114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Page TJ, et al. Genome-wide analysis of human HSF1 signaling reveals a transcriptional program linked to cellular adaptation and survival. Mol Biosyst. 2006;2:627–639. doi: 10.1039/b606129j. [DOI] [PubMed] [Google Scholar]
- 6.Dai C, Whitesell L, Rogers AB, Lindquist S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell. 2007;130:1005–1018. doi: 10.1016/j.cell.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: Oncogene and non-oncogene addiction. Cell. 2009;136:823–837. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solimini NL, Luo J, Elledge SJ. Non-oncogene addiction and the stress phenotype of cancer cells. Cell. 2007;130:986–988. doi: 10.1016/j.cell.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Meng L, Gabai VL, Sherman MY. Heat-shock transcription factor HSF1 has a critical role in human epidermal growth factor receptor-2-induced cellular transformation and tumorigenesis. Oncogene. 2010;29:5204–5213. doi: 10.1038/onc.2010.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Min JN, Huang L, Zimonjic DB, Moskophidis D, Mivechi NF. Selective suppression of lymphomas by functional loss of Hsf1 in a p53-deficient mouse model for spontaneous tumors. Oncogene. 2007;26:5086–5097. doi: 10.1038/sj.onc.1210317. [DOI] [PubMed] [Google Scholar]
- 11.Ciocca DR, Calderwood SK. Heat shock proteins in cancer: Diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barginear MF, et al. The heat shock protein 90 chaperone complex: An evolving therapeutic target. Curr Cancer Drug Targets. 2008;8:522–532. doi: 10.2174/156800908785699379. [DOI] [PubMed] [Google Scholar]
- 13.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 14.Hoang AT, et al. A novel association between the human heat shock transcription factor 1 (HSF1) and prostate adenocarcinoma. Am J Pathol. 2000;156:857–864. doi: 10.1016/S0002-9440(10)64954-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khaleque MA, et al. Heat shock factor 1 represses estrogen-dependent transcription through association with MTA1. Oncogene. 2008;27:1886–1893. doi: 10.1038/sj.onc.1210834. [DOI] [PubMed] [Google Scholar]
- 16.Khaleque MA, et al. Induction of heat shock proteins by heregulin β1 leads to protection from apoptosis and anchorage-independent growth. Oncogene. 2005;24:6564–6573. doi: 10.1038/sj.onc.1208798. [DOI] [PubMed] [Google Scholar]
- 17.Yang J, Bridges K, Chen KY, Liu AY. Riluzole increases the amount of latent HSF1 for an amplified heat shock response and cytoprotection. PLoS ONE. 2008;3:e2864. doi: 10.1371/journal.pone.0002864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van de Vijver MJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 19.Robert F, Pelletier J. Translation initiation: A critical signalling node in cancer. Expert Opin Ther Targets. 2009;13:1279–1293. doi: 10.1517/14728220903241625. [DOI] [PubMed] [Google Scholar]
- 20.Williams BR, et al. Aneuploidy affects proliferation and spontaneous immortalization in mammalian cells. Science. 2008;322:703–709. doi: 10.1126/science.1160058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott KL, et al. Proinvasion metastasis drivers in early-stage melanoma are oncogenes. Cancer Cell. 2011;20:92–103. doi: 10.1016/j.ccr.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cowen LE, Lindquist S. Hsp90 potentiates the rapid evolution of new traits: Drug resistance in diverse fungi. Science. 2005;309:2185–2189. doi: 10.1126/science.1118370. [DOI] [PubMed] [Google Scholar]
- 23.Michor F, Polyak K. The origins and implications of intratumor heterogeneity. Cancer Prev Res (Phila) 2010;3:1361–1364. doi: 10.1158/1940-6207.CAPR-10-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merlo LM, et al. A comprehensive survey of clonal diversity measures in Barrett's esophagus as biomarkers of progression to esophageal adenocarcinoma. Cancer Prev Res (Phila) 2010;3:1388–1397. doi: 10.1158/1940-6207.CAPR-10-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins MJ, Stearns V. Understanding resistance to tamoxifen in hormone receptor-positive breast cancer. Clin Chem. 2009;55:1453–1455. doi: 10.1373/clinchem.2009.125377. [DOI] [PubMed] [Google Scholar]
- 26.Singh RR, Barnes CJ, Talukder AH, Fuqua SA, Kumar R. Negative regulation of estrogen receptor α transactivation functions by LIM domain only 4 protein. Cancer Res. 2005;65:10594–10601. doi: 10.1158/0008-5472.CAN-05-2268. [DOI] [PubMed] [Google Scholar]
- 27.Manavathi B, Singh K, Kumar R. MTA family of coregulators in nuclear receptor biology and pathology. Nucl Recept Signal. 2007;5:e010. doi: 10.1621/nrs.05010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar R, et al. A naturally occurring MTA1 variant sequesters oestrogen receptor-α in the cytoplasm. Nature. 2002;418:654–657. doi: 10.1038/nature00889. [DOI] [PubMed] [Google Scholar]
- 29.Zhao YH, et al. Upregulation of lactate dehydrogenase A by ErbB2 through heat shock factor 1 promotes breast cancer cell glycolysis and growth. Oncogene. 2009;28:3689–3701. doi: 10.1038/onc.2009.229. [DOI] [PubMed] [Google Scholar]
- 30.Ince TA, et al. Transformation of different human breast epithelial cell types leads to distinct tumor phenotypes. Cancer Cell. 2007;12:160–170. doi: 10.1016/j.ccr.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Calderwood SK. Heat shock proteins in breast cancer progression—a suitable case for treatment? Int J Hyperthermia. 2010;26:681–685. doi: 10.3109/02656736.2010.490254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Billy E, Powers MV, Smith JR, Workman P. Drugging the heat shock factor 1 pathway: Exploitation of the critical cancer cell dependence on the guardian of the proteome. Cell Cycle. 2009;8:3806–3808. doi: 10.4161/cc.8.23.10423. [DOI] [PubMed] [Google Scholar]
- 33.Whitesell L, Lindquist S. Inhibiting the transcription factor HSF1 as an anticancer strategy. Expert Opin Ther Targets. 2009;13:469–478. doi: 10.1517/14728220902832697. [DOI] [PubMed] [Google Scholar]
- 34.Mayer IA. Treatment of HER2-positive metastatic breast cancer following initial progression. Clin Breast Cancer. 2009;9(Suppl 2):S50–S57. doi: 10.3816/CBC.2009.s.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Modi S, et al. Combination of trastuzumab and tanespimycin (17-AAG, KOS-953) is safe and active in trastuzumab-refractory HER-2 overexpressing breast cancer: A phase I dose-escalation study. J Clin Oncol. 2007;25:5410–5417. doi: 10.1200/JCO.2007.11.7960. [DOI] [PubMed] [Google Scholar]
- 36.Modi S, et al. HSP90 inhibition is effective in breast cancer: A phase II trial of tanespimycin (17-AAG) plus trastuzumab in patients with HER2-positive metastatic breast cancer progressing on trastuzumab. Clin Cancer Res. 2011;17:5132–5139. doi: 10.1158/1078-0432.CCR-11-0072. [DOI] [PubMed] [Google Scholar]
- 37.Kamal A, et al. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425:407–410. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- 38.Ramanathan RK, et al. Phase I pharmacokinetic and pharmacodynamic study of 17-dimethylaminoethylamino-17-demethoxygeldanamycin, an inhibitor of heat-shock protein 90, in patients with advanced solid tumors. J Clin Oncol. 2010;28:1520–1526. doi: 10.1200/JCO.2009.25.0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10:537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitesell L, Bagatell R, Falsey R. The stress response: Implications for the clinical development of hsp90 inhibitors. Curr Cancer Drug Targets. 2003;3:349–358. doi: 10.2174/1568009033481787. [DOI] [PubMed] [Google Scholar]
- 41.Hu R, et al. Androgen receptor expression and breast cancer survival in postmenopausal women. Clin Cancer Res. 2011;17:1867–1874. doi: 10.1158/1078-0432.CCR-10-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamimi RM, et al. Comparison of molecular phenotypes of ductal carcinoma in situ and invasive breast cancer. Breast Cancer Res. 2008;10:R67. doi: 10.1186/bcr2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.