Abstract
Long-term memory (LTM) formation has been linked with functional strengthening of existing synapses and other processes including de novo synaptogenesis. However, it is unclear whether synaptogenesis can contribute to LTM formation. Here, using α-calcium/calmodulin kinase II autophosphorylation-deficient (T286A) mutants, we demonstrate that when functional strengthening is severely impaired, contextual LTM formation is linked with training-induced PSD95 up-regulation followed by persistent generation of multiinnervated spines, a type of synapse that is characterized by several presynaptic terminals contacting the same postsynaptic spine. Both PSD95 up-regulation and contextual LTM formation in T286A mutants required signaling by the mammalian target of rapamycin (mTOR). Furthermore, we show that contextual LTM resists destabilization in T286A mutants, indicating that LTM is less flexible when synaptic strengthening is impaired. Taken together, we suggest that activation of mTOR signaling, followed by overexpression of PSD95 protein and synaptogenesis, contributes to formation of invariant LTM when functional strengthening is impaired.
Keywords: synaptic plasticity, hippocampus, immediate-early gene, reconsolidation
A fundamental question in neuroscience concerns the cellular mechanisms that lead to long-term memory (LTM) formation (1–3). It has been shown that LTM formation is associated with and demands strengthening of existing synapses, so-called functional plasticity (3–5), but it may also be linked with a more overt form of structural plasticity, an increase in synapse density (6–9). It is, however, unclear whether de novo synaptogenesis is critical for LTM formation.
The participation of synaptogenesis for LTM formation can be investigated when synaptic strengthening is fully blocked. It would be ideal to also block training-induced changes in neuronal excitability that have been implicated in memory formation (10). However, technically this block may not be achievable as, for example, there are distinct types of synaptic strengthening. As a proxy, here we have studied T286A missense mutant mice lacking autophosphorylation at threonine 286 of the α-isoform of calcium/calmodulin-dependent kinase II (αCaMKII), a major protein of glutamatergic synapses in the forebrain (11). The T286A mutants have fully blocked NMDA receptor-dependent synaptic strengthening at hippocampal CA1 synapses (11–14), and they have impaired regulation of the postburst afterhyperpolarization (AHP) in CA1 pyramidal neurons (15). Accordingly, T286A mutants are deficient in contextual fear conditioning after a single training trial (16). However, after five massed training trials, T286A mutants can form contextual LTM (16) and this type of LTM is hippocampus-dependent (14).
Here, we have used T286A mutants as tools to investigate the mechanism of LTM formation when NMDA receptor-dependent synaptic strengthening is blocked. Our study provides evidence that activation of mTOR signaling in the hippocampus, followed by overexpression of PSD95 protein and persistent generation of multiinnervated spines (MIS), a particular type of synapse that is characterized by several presynaptic terminals contacting the same postsynaptic spine, can contribute to LTM formation when synaptic strengthening is severely impaired.
Results
Impaired Induction of Immediate-Early Gene (IEG) Expression in the T286A Mutants Despite Contextual LTM Formation.
T286A mutants have substantial deficits in synaptic strengthening induced by electrical stimulation (10–14). The expression of the IEGs Zif268 and c-Fos has been shown to be a prerequisite for late LTP (17, 18). Thus, analysis of Zif268 and c-Fos expression allowed us to test whether training-induced synaptic strengthening is impaired in the absence of αCaMKII autophosphorylation. We used a foreground conditioning protocol that induced equally contextual LTM for both genotypes (WT: 73 ± 3%; T286A: 69 ± 6%, freezing 24 h after conditioning; n = 6; P > 0.05).
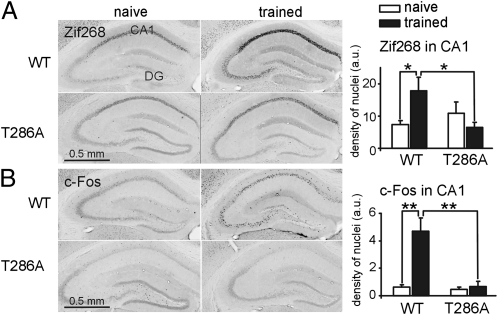
Zif268 and c-Fos expression was analyzed by immunohistochemistry in the hippocampal area CA1 and dentate gyrus (Fig. 1), and in the amygdala, neocortex, and striatum (Tables S1 and S2) 75 min after conditioning for both genotypes. Analysis of Zif268 and c-Fos in the hippocampal area CA1 revealed training-induced expression of both proteins in WT, but not in T286A, mice (training × genotype interaction for Zif268: P < 0.05; for c-Fos: P < 0.001). In dentate gyrus, Zif268 expression was not significantly regulated, but c-Fos expression was increased by training only in WT mice (P < 0.001). Furthermore, Zif268 expression was up-regulated after training in the infralimbic cortex (Table S1) and c-Fos in the amygdala and striatum in WT, but not mutant, mice (Table S2).
Fig. 1.
Impaired Zif268 and c-Fos protein expression in the dorsal hippocampus in T286A mutants despite contextual LTM formation. Zif268 (A) and c-Fos (B) immunostaining (density of stained nuclei) was analyzed in the area CA1 of the dorsal hippocampus 75 min after conditioning. All graphs in the paper show mean ± SEM. Statistical significance coding used on all figures: *P < 0.05, **P < 0.01, ***P < 0.001. n = 6 or as indicated.
Moreover, we analyzed the immediate-early transcription factor Nur77 (Fig. S1 and Table S3), a gene shown to be specifically induced by context–shock association (19). Nur77 expression was up-regulated after conditioning in the analyzed brain areas only in WT mice, but not in T286A mutants.
In addition, we confirm that a very strong electrical stimulation cannot induce late CA1-LTP in the T286A mutants (Fig. S2).
LTM Formation in T286A Mutants Is Linked to Synaptogenesis.
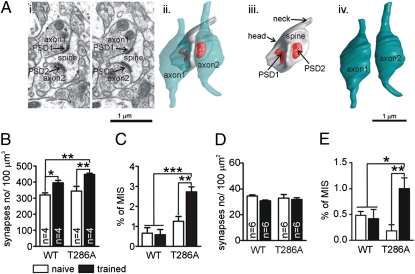
Our IEG mapping studies indicate that contextual LTM formation in T286A mutants occurs despite severe impairments in synaptic strengthening, and previous lesion studies established that it is hippocampus-dependent (14). Thus, it appeared plausible that contextual LTM formation was explained by training-induced synaptogenesis in the hippocampus. We used electron microscopy to investigate this idea, analyzing synaptogenesis in the stratum radiatum of CA1 in the dorsal hippocampus. We analyzed 1,000–1,200 μm3 of tissue per animal 2 h after training and 1,200 μm2 of tissue per animal 24 h after training (Fig. 2). There was a significant effect of training on synapse density in WT and mutant mice 2 h (post hoc analysis: P < 0.05 for naïve vs. trained WT mice; P < 0.01 for naïve vs. trained T286A mutants; P > 0.05 for naïve mutants vs. naïve and trained WT mice: P < 0.01 for trained mutants vs. naïve and trained WT mice) (Fig. 2B), but not 24 h after training (Fig. 2D). For both genotypes, there was no difference in percentage of increase in total synapse density 2 h after training and, at 24 h, there was no increase (Fig. S3).
Fig. 2.
Training-induced generation of MIS in T286A mutants but not in WT mice. (Ai) Two serial electron micrographs with a single spine innervated by two axonal boutons (axon1 and axon2), each with single postsynaptic density (PSD1 and PSD2). (Aii) 3D reconstructions of this MIS. The red color indicates the PSDs contacting the spine. (Aiii) The spine without the axonal boutons. (Aiv) Two axonal boutons. Synapse density and MIS were analyzed 2 h after conditioning in stratum radiatum of hippocampal area CA1 by using 3D electron microscopy (B and C) and 24 h after training by using 2D electron microscopy (D and E).
Our analysis also included a rare species of synaptic connections: multiinnervated spines (MIS) that are characterized by several presynaptic terminals contacting the same postsynaptic spine (Fig. 2A). The analyses of MIS (Fig. 2 C and E) shows, that conditioning significantly increased the number of MIS in mutants 2 and 24 h after training, but not in WT mice (2 h: training × genotype interaction: P < 0.05, P < 0.001 for trained mutants vs. naïve and trained WT mice, P < 0.01 for trained vs. naïve T286A mutants, P > 0.05 for naïve mutants vs. naïve and trained WT mice; 24 h: training × genotype interaction: P < 0.01, P < 0.05 for trained mutants vs. naïve and trained WT mice, and P < 0.01 for trained vs. naïve T286A mutants). Thus, LTM formation in T286A mutants was accompanied by synaptogenesis that included persistent generation of MIS.
Training-Induced PSD95 Up-Regulation in T286A Mutants.
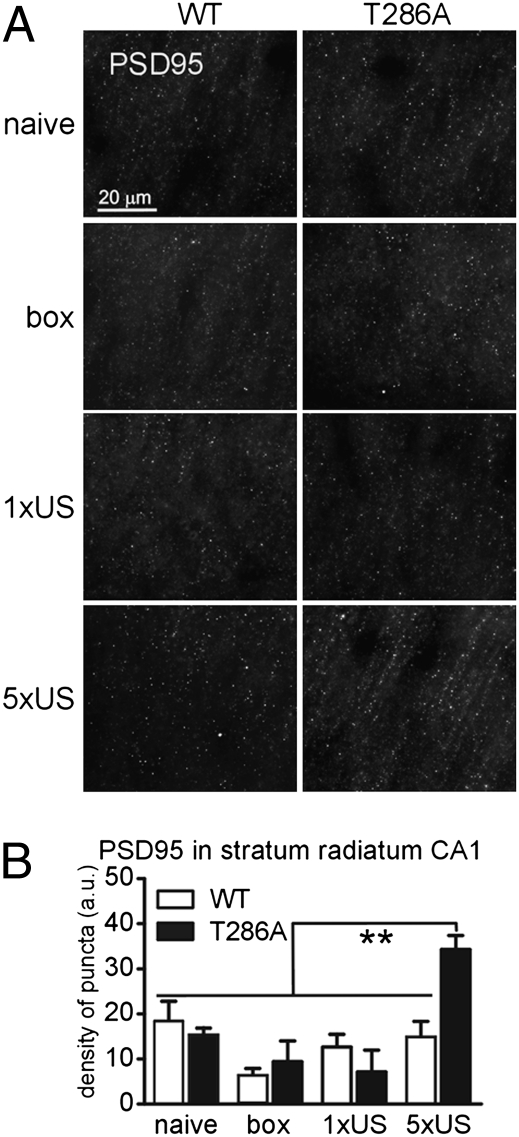
The postsynaptic scaffolding protein PSD95 has been linked with synaptogenesis (20). Furthermore, in hippocampal slice cultures overexpression of PSD95 leads to MIS formation, in particular (21). This fact, together with our finding that contextual LTM in T286A mutants is linked with synaptogenesis including persistent MIS generation, suggested that PSD95 overexpression is involved in memory formation in T286A mutants. We tested this idea by studying PSD95 expression in the stratum radiatum of area CA1 of the dorsal hippocampus 2 h after conditioning (Fig. 3). The analysis revealed that there was a significant increase in density of PSD95+ puncta after training only in T286A mutants, but not in WT mice (genotype × training interaction: P < 0.05) (Fig. 3B). Importantly, one-trial training, which does not lead to contextual LTM formation in T286A mutants (16), as well as exposure to the context alone, were not sufficient to induce PSD95 up-regulation (Fig. 3B). Additionally, we found that conditioning increased the level of polymerized actin only in T286A mutants, but not in WT mice (Fig. S4).
Fig. 3.
Training-induced PSD95 protein overexpression in T286A mutants. PSD95 expression was analyzed on the brain sections 2 h after conditioning (5xUS). The control animals were taken from the home cage (naïve), exposed to the experimental context without shock presentation (box) or to single-trial (1xUS), the paradigm which does not induce LTM in the mutants (16). (A) Representative microphotographs. (B) The density of PSD95+ puncta was analyzed in the stratum radiatum of the CA1 area of the dorsal hippocampus.
Contextual LTM Formation in T286A Mutants, but Not in WT Mice, Requires mTOR Signaling in the Dorsal Hippocampus.
PSD95 protein expression is regulated by mammalian target of rapamycin (mTOR) and is sensitive to inhibitors of protein synthesis, but not block of transcription (22, 23). Thus, if training-induced PSD95 up-regulation were important for LTM formation in T286A mutants, then blockers of protein synthesis and mTOR signaling should impair LTM formation, whereas blockers of mRNA synthesis would have no effect.
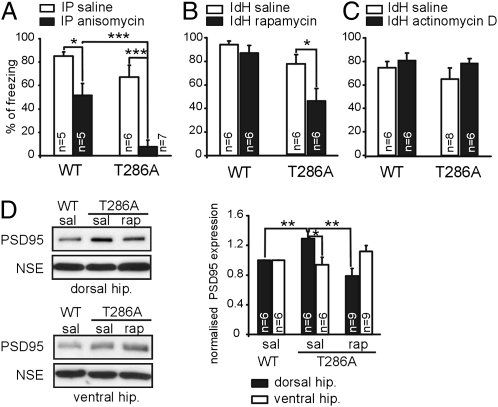
First, we tested this idea with systemic administration of anisomycin, a general protein synthesis blocker, after conditioning (Fig. 4A). The anisomycin treatment impaired contextual LTM formation both in T286A and WT mice (WT: P < 0.05; T286A: P < 0.001).
Fig. 4.
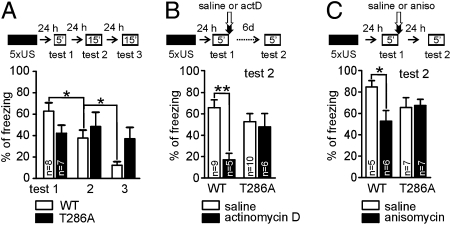
Contextual LTM formation requires mTOR signaling in the dorsal hippocampus in the T286A mutants. WT mice and T286A mutants were trained with massed foreground conditioning. (A) Anisomycin (225 mg/kg) was intraperitoneally (IP) injected immediately after training. Contextual LTM was tested 24 h later. Rapamycin (2.5 μg per side) (B) or actinomycin D (10 ng per side) (C) were bilaterally injected into dorsal hippocampus (IdH) immediately after training and 24 h after conditioning the animals were tested for contextual LTM. (D) Training-induced PSD95 overexpression requires mTOR signaling in the IdH in the T286A mutants. Rapamycin (2.5 μg per side) was bilaterally injected into IdH immediately after conditioning. The ventral and dorsal hippocampi were collected 2 h after training, and lysates were assayed by immunoblotting. *P < 0.05; **P < 0.01; ***P < 0.001.
Second, we used rapamycin as a drug that specifically inhibits mTOR signaling. We blocked mTOR signaling in the dorsal CA1 area of the hippocampus by using bilateral injections of rapamycin immediately after conditioning, using a dose previously shown to block fear LTM formation when injected into rat lateral amygdala (24) (Fig. 4B). Rapamycin treatment impaired contextual LTM only in T286A mutants, but not in WT littermates (T286A: P < 0.05; WT: P > 0.05).
Next, we used actinomycin D, a drug that blocks transcription. Actinomycin D was bilaterally applied into dorsal CA1 area of the hippocampus immediately after conditioning in a dose previously shown to block contextual LTM formation when injected into rat amygdala (25). Actinomycin D did not block LTM formation in both genotypes (Fig. 4C). The lack of an actinomycin D treatment effect was not due to inactivity of the drug used, because injection of actinomycin D into the dorsal hippocampus after memory reactivation had a specific effect (Fig. S5 and Fig. 5B). Hence, our findings suggested that mTOR-dependent processes in the dorsal CA1 area of the hippocampus are involved in contextual LTM formation in T286A mutants.
Fig. 5.
Contextual LTM in T286A mutants is less flexible than in WT mice. WT mice and T286A mutants were conditioned and then underwent extinction training (A) or were tested for memory destabilization after memory reactivation, using injection of actinomycin D (actD) (bilateral, intrahippocampal injection, 10 ng per side) (B) or anisomycin (i.p., 225 mg/kg) (C) to block memory reconsolidation. *P < 0.05; **P < 0.01.
Rapamycin Blocks the Training-Induced PSD95 Up-Regulation in T286A Mutants.
To further verify the role of PSD95 in LTM formation in T286A mutants, we asked whether the rapamycin treatment blocks not only LTM formation but also the training-induced PSD95 expression (Fig. 4D). Consequently, WT and mutant mice were trained and rapamycin was bilaterally injected immediately after conditioning. The tissue from dorsal and ventral hippocampi was collected 2 h after conditioning and immunoblots were performed.
In agreement with our previous finding, PSD95 protein expression after training in the dorsal hippocampus was higher in T286A mutants than in WT mice (WT saline vs. T286A saline: P < 0.01). Furthermore, rapamycin blocked the increased PSD95 expression in the trained T286A mutants (T286A saline vs. T286A rapamycin: P < 0.01). The increased PSD95 expression was found only in dorsal hippocampus, but not in the ventral hippocampus. Further, the rapamycin treatment showed only an effect on PSD95 expression in the dorsal hippocampus, where the drug was injected. Thus, we showed that rapamycin injected into dorsal hippocampus of T286A mutants blocked both contextual LTM formation (Fig. 4B) and impaired the training-induced increase in PSD95 protein expression (Fig. 4D).
LTM in T286A Mutants Is Resistant to Extinction and Destabilization.
We analyzed the properties of contextual LTM formed in the absence of αCaMKII autophosphorylation. We studied extinction of contextual LTM by consecutively reexposing to the training context without foot shock (Fig. 5A, Top). We found a significant difference in freezing between both genotypes during extinction training (genotype × day of training interaction: P < 0.01). Although in WT mice extinction of contextual freezing was found (first vs. second session: P < 0.05; second vs. third: P < 0.05), there was no such extinction of contextual LTM in T286A mutants (first vs. second: P > 0.05; second vs. third session: P > 0.05). Next, we tested memory destabilization, which is induced by memory reactivation during brief reexposure to the experimental context without electric shock presentation (26) (Fig. 5 B and C). Immediately after reactivation of LTM by reexposure to the conditioning context, actinomycin D (Fig. 5B) was bilaterally applied into dorsal hippocampus, to block memory reconsolidation, and then contextual LTM was tested 6 d later (Fig. 5B, Top). We found that injection of actinomycin D significantly impaired contextual LTM in WT mice, but not in T286A mutants (drug × genotype interaction: P < 0.05). The same effect was observed when anisomycin was injected i.p. (Fig. 5C). Injection of anisomycin significantly impaired contextual LTM in WT mice, but not in T286A mutants (drug × genotype interaction: P < 0.05, P < 0.05 for saline vs. anisomycin-treated WT mice). Thus, our experiments show that T286A mutants have impaired memory destabilization.
Discussion
Hippocampus-dependent LTM formation has been associated with de novo synaptogenesis, a form of structural plasticity (7, 8, 27). Those studies, however, did not establish whether synaptogenesis contributes to memory as functional plasticity and modulation of neuronal excitability could be sufficient. Using αCaMKII autophosphorylation-deficient (T286A) mutants, which have impaired NMDA receptor-dependent synaptic strengthening (10–14) and deficits in the regulation of the AHP in CA1 pyramidal neurons (15), we demonstrate that contextual LTM formation is linked with PSD95 up-regulation and persistent MIS generation. Both, the PSD95 up-regulation and contextual LTM formation in T286A mutants require the mTOR kinase in the hippocampus. Furthermore, we show that modification of contextual LTM is impaired in T286A mutants, suggesting that LTM formed with impaired NMDA receptor-dependent synaptic plasticity is less flexible than normal LTM.
Function of Synaptogenesis and MIS in LTM.
MIS develop by the attraction of presynaptic terminals onto existing spines (21, 28). Thus, MIS formation is a specific form of synaptogenesis that seems to serve the purpose of strengthening and/or modifying existing inputs onto neurons. Here, we show that persistent MIS generation coincides with contextual LTM formation in T286A mutants, and not in WT mice. In T286A mutants training-induced MIS generation occurred in the dorsal hippocampal area CA1, which is critical for contextual LTM formation (29).
MIS generation is not detectable when total synapse numbers are analyzed, because MIS is a rare synapse type (≈1% of total synapses). We detected a transient increase in total synaptogenesis after contextual fear conditioning in T286A mutants and WT mice. It remains unclear whether this transient synaptogenesis contributes to contextual LTM formation in both genotypes.
The T286A mutants can form contextual LTM although they have fully blocked NMDA receptor-dependent synaptic strengthening in hippocampal area CA1, as indicated by previous electrophysiological experiments (10–14). We confirmed that late CA1 LTP is blocked after strong electrical stimulation, and we show that training-induced synaptic strengthening is deficient in hippocampal area CA1, in the amygdala, neocortex, and striatum, and in the T286A mutants, using imaging of the expression of three IEGs (Zif268, c-Fos, and Nur77) that are critical transcription factors for long-lasting synaptic strengthening (17, 18). Therefore, we suggest that MIS generation contributes to contextual LTM formation in T286A mutants, when NMDA receptor-dependent synaptic strengthening is impaired. Additionally, our findings demonstrate that training-induced synaptogenesis including MIS generation does not require αCaMKII autophosphorylation. αCaMKII autophosphorylation constrains training-induced MIS generation.
Function of mTOR-PSD95 Pathway in LTM.
Psd95 mRNA is dendritically localized and locally translated via activation of the mTOR pathway (22, 23, 30, 31). In hippocampal slice cultures, overexpression of PSD95 induces MIS formation (21). We analyzed PSD95 expression in hippocampal area CA1 after contextual fear conditioning and show that only under a condition that leads to LTM formation is there a pronounced training-induced PSD95 overexpression in the T286A mutants. Thus, the persistent increase in MIS in T286A mutants accompanies training-induced PSD95 overexpression in agreement with the hippocampal slice culture experiments (21). We show also that the training-induced PSD95 overexpression in T286A mutants is blocked by the mTOR inhibitor rapamycin. Furthermore, posttraining injection of rapamycin into the dorsal hippocampus blocked contextual LTM formation in T286A mutants. These findings indicate that local translation of PSD95 regulated by mTOR contributes to contextual LTM formation when NMDA receptor-dependent synaptic strengthening is impaired.
Properties of LTM When Synaptic Strengthening Is Impaired.
Our studies show that αCaMKII autophosphorylation-deficient mutants have impaired extinction and destabilization of LTM after retrieval. These observations indicate that not only formation of new memories but also modification of old memories by new experience during extinction requires the αCaMKII autophosphorylation, which is in agreement with a previous analysis of heterozygous T286A mutants (32). Furthermore, destabilization of LTM after retrieval requires αCaMKII autophosphorylation. Accordingly, our data indicates that αCaMKII autophosphorylation is not only involved in LTM formation, but also remodeling, because contextual LTM in the αCaMKII autophosphorylation-deficient mutants cannot be modified by new learning or destabilized after retrieval.
Conclusions
Our study suggests that activation of mTOR signaling, followed by overexpression of PSD95 protein and the generation of MIS, can contribute to LTM formation. MIS are persistently generated after training when functional strengthening of existing synapses is impaired. This finding suggests that MIS formation and functional strengthening might compete for LTM maintenance. Thus, the generation of MIS may underlie LTM formation in cognitive diseases with impaired functional strengthening.
Materials and Methods
Animals.
αCaMKII-T286A mice were generated and genotyped as described (10). All experiments were undertaken in accordance with the UK Animals (Scientific Procedures) Act 1986.
Intrahippocampal Drug Injection.
Cannulation of the hippocampus is described in SI Materials and Methods. Animals received bilateral drug injections (0.5 μL per side for 1 min) immediately after training or memory test (Fig. 4D) via intrahippocampal cannulae. After the infusion, the injector was left in place for an additional 30 s. The cannula placement was verified by histology, and only data from animals with correct cannula implants were included in statistical analyses.
Foreground Fear Conditioning, Extinction, and Reconsolidation.
Immunohistochemistry.
The protocol is described in detail in SI Materials and Methods. Immunostaining was analyzed with AxioImager Z1 microscope. Photomicrographs of stained brain sections were taken with a digital camera (AxioCam MRm, Zeiss). The TIFF format micrographs were analyzed by using ImageJ software. The threshold tool was used, which identifies objects distinct from the background based on coloring and intensity. Every sixth section from the dorsal hippocampus, prefrontal cortex, striatum, and amygdala was analyzed (33). Fluorescent immunostaining were analyzed with AxioImager Z1 microscope, with Plan-Apochromat 63× objective and Apotom (Zeiss). Four Z-stack micrographs (10 pictures per stack, every 0.5 μm) were taken with AxioCamMT3 M27 per brain section, and every sixth section through the dorsal hippocampus (stratum radiatum of CA1 field; Bregma −1.58 to −2.18 mm) was analyzed (on average five sections per animal). Z stacks were reconstructed with ImageJ software and processed with “find edges” and “max projection” functions. PSD95 immunostaining appeared as dots. Density of PSD95-positive dots was calculated with ImageJ software according to formula: dots number/area (density of puncta).
Electron Microscopy.
Mice were anesthetized and transcardially perfused with filtered 10 mM Pipes at pH 7.2, 139 mM NaCl, 2.7 mM KCl, 7.5 μM povidone-iodine (PVP) (40,000 Da), 19.4 mM glucose, 5 mM sodium nitrite, 2 U/mL heparin (Sigma) and fixed for 30 min in filtered 0.1 M Pipes at pH 7.2, 3% glutaraldehyde, 0.5% formaldehyde, 2.5% PVP (40,000 Da). Brains were dissected and postfixed overnight. Tissue blocks with a volume of 1 mm3 were selected from the CA1 regions of dorsal hippocampus. Specimens were postfixed for 90 min in 1% osmium tetroxide in 0.1 M phosphate buffer. They were then dehydrated in alcohol and embedded in epoxy resin (Premix Medium Resin Kit, TAAB Laboratories Equipment). For two-dimensional (2D) analysis (of the 24 h time point), ultrathin sections (70–85 nm) were cut from the face of each block and stained in uranyl acetate and lead citrate. Eighty-five to ninety photos were taken per animal from the neuropil of CA1, covering ≈1,200 μm2 per animal (microscope: Tecnai 2 BioTWIN). To prepare serial sections for 3D analysis for electron microscopy (for the analysis of the 2-h time point), a trapezoid area on the surface of the blocks containing CA1 region of the hippocampus were prepared with a glass knife (34, 35). Serial sections of gray/white color (60–70 nm) were cut with a Diatome diamond knife and allowed to form a ribbon on the surface of a water/ethanol solution (2–5% ethanol) and collected by using Pioloform-coated slot copper grids (up to 100 serial sections). Sections were counterstained with saturated ethanolic uranyl acetate, followed by lead citrate. Finally, sections were imaged by using an AMT XR60 12-megapixel camera in a JEOL 1400 electron microscope.
Stereological Analysis.
Serial sections were aligned as JPEG images (software from http://synapses.clm.utexas.edu). Alignments of sections were made with full-eld images. Stereological analysis was performed as described (34, 35), with total tissue volumes of ≈1,000–1,200 μm3 (≈3,000 spines and synapses were analyzed). Synapses were defined as contacts between neurons containing at least one PSD with a spine being the part of the synapse that contains PSDs, but no vesicles and no microtubules, and a presynapse being the part of the synapse containing at least two to three vesicles. The number of synapses per unit area of photomicrograph were assessed. An additional category of spines was classified as multiinnervated (multisynaptic) where two or more presynaptic boutons originated from different axons have formed synapses with only one dendritic spine (Fig. 2A).
Spines with their PSDs and presynaptic boutons were reconstructed by using Trace 1.6b software (http://synapses.clm.utexas.edu). 3D reconstructions of selected multiinnervated spines and multisynaptic axonal boutons were imported to 3D Studio Max 8 software for rendering and subsequent rotation to display the optimal views of the reconstructed structures.
Western Blots.
Statistics.
For statistical analysis Student's t test, one-way analysis of variance (ANOVA), two-way ANOVA with repeated measures, and Student-Newman-Keuls multiple comparison post hoc tests were used when appropriate.
Supplementary Material
Acknowledgments
We thank Drs. Gerald Finnerty, Paul W. Frankland, Frank Hirth, Jacek Jaworski, Keiko Mizuno, and Jeffrey Vernon for helpful comments and Cristian Bodo for technical assistance. This work was supported by a Marie Curie postdoctoral fellowship (to K.R.) and funding from CAPES/BRAZIL (to G.S.P. and M.F.D.M.), Polish-Norwegian Grant PNRF-96 (to L.K.), and the Medical Research Council (to K.P.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109680108/-/DCSupplemental.
References
- 1.Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- 2.De Roo M, Klauser P, Garcia PM, Poglia L, Muller D. Spine dynamics and synapse remodeling during LTP and memory processes. Prog Brain Res. 2008;169:199–207. doi: 10.1016/S0079-6123(07)00011-8. [DOI] [PubMed] [Google Scholar]
- 3.Shema R, Sacktor TC, Dudai Y. Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKM zeta. Science. 2007;317:951–953. doi: 10.1126/science.1144334. [DOI] [PubMed] [Google Scholar]
- 4.Matsuo N, Reijmers L, Mayford M. Spine-type-specific recruitment of newly synthesized AMPA receptors with learning. Science. 2008;319:1104–1107. doi: 10.1126/science.1149967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- 6.Xu T, et al. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462:915–919. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leuner B, Falduto J, Shors TJ. Associative memory formation increases the observation of dendritic spines in the hippocampus. J Neurosci. 2003;23:659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Restivo L, Vetere G, Bontempi B, Ammassari-Teule M. The formation of recent and remote memory is associated with time-dependent formation of dendritic spines in the hippocampus and anterior cingulate cortex. J Neurosci. 2009;29:8206–8214. doi: 10.1523/JNEUROSCI.0966-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giese KP, Peters M, Vernon J. Modulation of excitability as a learning and memory mechanism: A molecular genetic perspective. Physiol Behav. 2001;73:803–810. doi: 10.1016/s0031-9384(01)00517-0. [DOI] [PubMed] [Google Scholar]
- 11.Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- 12.Yasuda H, Barth AL, Stellwagen D, Malenka RC. A developmental switch in the signaling cascades for LTP induction. Nat Neurosci. 2003;6:15–16. doi: 10.1038/nn985. [DOI] [PubMed] [Google Scholar]
- 13.Cooke SF, et al. Autophosphorylation of alphaCaMKII is not a general requirement for NMDA receptor-dependent LTP in the adult mouse. J Physiol. 2006;574:805–818. doi: 10.1113/jphysiol.2006.111559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irvine EE, et al. Properties of contextual memory formed in the absence of αCaMKII autophosphorylation. Mol Brain. 2011;4:8. doi: 10.1186/1756-6606-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohno M, Sametsky EA, Silva AJ, Disterhoft JF. Differential effects of alphaCaMKII mutation on hippocampal learning and changes in intrinsic neuronal excitability. Eur J Neurosci. 2006;23:2235–2240. doi: 10.1111/j.1460-9568.2006.04746.x. [DOI] [PubMed] [Google Scholar]
- 16.Irvine EE, Vernon J, Giese KP. AlphaCaMKII autophosphorylation contributes to rapid learning but is not necessary for memory. Nat Neurosci. 2005;8:411–412. doi: 10.1038/nn1431. [DOI] [PubMed] [Google Scholar]
- 17.Jones MW, et al. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat Neurosci. 2001;4:289–296. doi: 10.1038/85138. [DOI] [PubMed] [Google Scholar]
- 18.Fleischmann A, et al. Impaired long-term memory and NR2A-type NMDA receptor-dependent synaptic plasticity in mice lacking c-Fos in the CNS. J Neurosci. 2003;23:9116–9122. doi: 10.1523/JNEUROSCI.23-27-09116.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Hertzen LS, Giese KP. Memory reconsolidation engages only a subset of immediate-early genes induced during consolidation. J Neurosci. 2005;25:1935–1942. doi: 10.1523/JNEUROSCI.4707-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livneh Y, Feinstein N, Klein M, Mizrahi A. Sensory input enhances synaptogenesis of adult-born neurons. J Neurosci. 2009;29:86–97. doi: 10.1523/JNEUROSCI.4105-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikonenko I, et al. PSD-95 promotes synaptogenesis and multiinnervated spine formation through nitric oxide signaling. J Cell Biol. 2008;183:1115–1127. doi: 10.1083/jcb.200805132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belelovsky K, Kaphzan H, Elkobi A, Rosenblum K. Biphasic activation of the mTOR pathway in the gustatory cortex is correlated with and necessary for taste learning. J Neurosci. 2009;29:7424–7431. doi: 10.1523/JNEUROSCI.3809-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee CC, Huang CC, Wu MY, Hsu KS. Insulin stimulates postsynaptic density-95 protein translation via the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway. J Biol Chem. 2005;280:18543–18550. doi: 10.1074/jbc.M414112200. [DOI] [PubMed] [Google Scholar]
- 24.Parsons RG, Gafford GM, Helmstetter FJ. Translational control via the mammalian target of rapamycin pathway is critical for the formation and stability of long-term fear memory in amygdala neurons. J Neurosci. 2006;26:12977–12983. doi: 10.1523/JNEUROSCI.4209-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parsons RG, Riedner BA, Gafford GM, Helmstetter FJ. The formation of auditory fear memory requires the synthesis of protein and mRNA in the auditory thalamus. Neuroscience. 2006;141:1163–1170. doi: 10.1016/j.neuroscience.2006.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nader K, Hardt O. A single standard for memory: The case for reconsolidation. Nat Rev Neurosci. 2009;10:224–234. doi: 10.1038/nrn2590. [DOI] [PubMed] [Google Scholar]
- 27.Geinisman Y, Berry RW, Disterhoft JF, Power JM, Van der Zee EA. Associative learning elicits the formation of multiple-synapse boutons. J Neurosci. 2001;21:5568–5573. doi: 10.1523/JNEUROSCI.21-15-05568.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrak LJ, Harris KM, Kirov SA. Synaptogenesis on mature hippocampal dendrites occurs via filopodia and immature spines during blocked synaptic transmission. J Comp Neurol. 2005;484:183–190. doi: 10.1002/cne.20468. [DOI] [PubMed] [Google Scholar]
- 29.Rampon C, et al. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat Neurosci. 2000;3:238–244. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- 30.Swiech L, Perycz M, Malik A, Jaworski J. Role of mTOR in physiology and pathology of the nervous system. Biochim Biophys Acta. 2008;1784:116–132. doi: 10.1016/j.bbapap.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 31.Kelleher RJ, 3rd, Govindarajan A, Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron. 2004;44:59–73. doi: 10.1016/j.neuron.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 32.Kimura R, Silva AJ, Ohno M. Autophosphorylation of alphaCaMKII is differentially involved in new learning and unlearning mechanisms of memory extinction. Learn Mem. 2008;15:837–843. doi: 10.1101/lm.1049608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paxinos G, Franklin KJ. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 2001. [Google Scholar]
- 34.Popov V, Medvedev NI, Davies HA, Stewart MG. Mitochondria form a filamentous reticular network in hippocampal dendrites but are present as discrete bodies in axons: A three-dimensional ultrastructural study. J Comp Neurol. 2005;492:50–65. doi: 10.1002/cne.20682. [DOI] [PubMed] [Google Scholar]
- 35.Popov VI, et al. Remodelling of synaptic morphology but unchanged synaptic density during late phase long-term potentiation (LTP): A serial section electron micrograph study in the dentate gyrus in the anaesthetised rat. Neuroscience. 2004;128:251–262. doi: 10.1016/j.neuroscience.2004.06.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.