Abstract
Psychological stress causes adaptive changes in the nervous system directed toward maintaining homoeostasis. These biochemical and structural mechanisms regulate animal behavior, and their malfunction may result in various forms of affective disorders. Here we found that the lipocalin-2 (Lcn2) gene, encoding a secreted protein of unknown neuronal function, was up-regulated in mouse hippocampus following psychological stress. Addition of lipocalin-2 to cultured hippocampal neurons reduced dendritic spine actin's mobility, caused retraction of mushroom spines, and inhibited spine maturation. These effects were further enhanced by inactivating iron-binding residues of Lcn-2, suggesting that they were facilitated by the iron-free form of Lcn-2. Concurrently, disruption of the Lcn2 gene in mice promoted stress-induced increase in spine density and caused an increase in the proportion of mushroom spines. The above changes correlated with higher excitability of CA1 principal neurons and with elevated stress-induced anxiety in Lcn-2−/− mice. Our study demonstrates that lipocalin-2 promotes stress-induced changes in spine morphology and function to regulate neuronal excitability and anxiety.
Keywords: restraint stress, limbic system, remodeling
Stress triggers a variety of adaptive cellular processes that help to maintain nervous system homeostasis and to shape the adequate behavioral response of an animal (1). The nature and extent of these region-specific alterations depends on the duration and severity of the traumatic experience (1–4). Failure to adjust biochemical and structural properties of neurons in stress-sensitive brain regions often results in affective disorders that can be as diverse as their underlying causes.
Despite considerable effort (1–5), the mechanisms of structural and functional neuronal plasticity underlying the stress response are not well understood. It is known that multiple stress-related pathways affect dendritic structure and spine motility, density, shape, and receptor composition within the spine (6, 7). These changes in dendritic spine morphology and function affecting synaptic and local circuit organization may compose a cellular substrate for altered emotional responses (1–3).
Identifying the roles of different pathways involved in stress-induced plasticity is fundamental for our understanding of how diverse mechanisms may result in similar neuronal phenotypes to culminate in a common behavioral outcome—the development of anxiety disorders. In this respect, glucocorticoids and glucocorticoid-regulated genes are instrumental in modulating stress-related neuronal machinery, central nervous system physiology, and animal behavior (8). However, neuronal functions of numerous glucocorticoid-regulated genes are still unclear. Lipocalin-2, a member of the family of over 20 small secreted proteins serving diverse cellular roles (9), caught our attention because it is up-regulated by glucocorticoids (10) and has been implicated in tissue restructuring, organ involution, and cellular invasiveness in other systems (11, 12). These findings suggest that lipocalin-2 might play a role in glucocorticoid-regulated processes such as stress-induced structural remodeling of neuronal connections (2, 8, 13, 14). However, the involvement of lipocalin-2 in psychological stress has not been studied, and its neuronal function is unknown.
To examine the role of lipocalin-2 in the stress response, we investigated morphological, biochemical, and behavioral signatures of stress in wild-type and lipocalin-2–deficient mice. We show here that in the mouse hippocampus lipocalin-2 is up-regulated by restraint stress to control dendritic spine density, dynamics, maturation, neuronal excitability, and finally, anxiety. Our findings establish lipocalin-2 as a critical mediator of the stress response at the subcellular, cellular, and behavioral levels.
Results
Lipocalin-2 Is Expressed in Neurons and Up-Regulated in the Hippocampus by Stress.
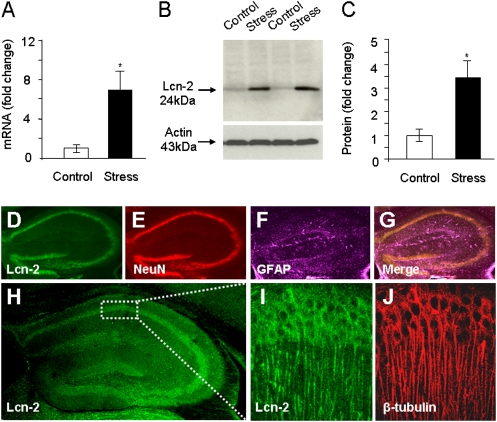
The hippocampus is critically involved in numerous aspects of the stress response (2, 3, 8, 13, 15). To examine whether the lipocalin-2 gene (Lcn2) is regulated in this brain region by psychological stress, we performed quantitative RT-PCR (qRT-PCR) using RNA extracted from the hippocampi of mice subjected to a single 6-h restraint. We found that under these conditions Lcn2 was up-regulated sevenfold (Fig. 1A; n = 5/group; P < 0.05). Similarly, Western blotting demonstrated an up-regulation of lipocalin-2 levels in the hippocampus after stress (Fig. 1 B and C; n = 3/group; P < 0.05), suggesting that the transcriptional regulation of the Lcn2 gene is accompanied by a parallel change in its translation.
Fig. 1.
Lipocalin-2 is expressed by neurons and up-regulated by stress in the hippocampus. qRT-PCR (A) and Western blotting (B and C) revealed an up-regulation of the lipocalin-2 (Lcn2) gene and protein after restraint stress in the hippocampus. Lipocalin-2 was expressed in the pyramidal cell layers of CA1–CA3 and in the granule cell layers of the dentate gyrus and highly colocalized with the neuronal marker NeuN (D–G). High expression of lipocalin-2 was also observed along β-tubulin–positive apical dendritic processes (H–J). *P < 0.05. Data are expressed as mean ± SEM.
We next investigated the pattern of up-regulation of lipocalin-2 expression in the hippocampus by immunohistochemistry and found high levels of lipocalin-2 in the pyramidal and granule cell layers of the hippocampal formation following stress (Fig. 1 D and H). Neuronal adaptation to external stimuli can be modulated by a variety of cell types, including astrocytes (16), and thus we investigated the phenotype of the lipocalin-2–positive cells by triple immunohistochemistry using cell type-specific markers (Fig. 1 D–G). Lipocalin-2 colocalized well with the neuronal marker NeuN and, to a lesser extent, with the astrocyte marker glial fibrillary acidic protein (GFAP), indicating that it was predominantly expressed by neurons. Further immunostaining revealed high amounts of lipocalin-2 present along β-tubulin–positive apical dendrites of the CA1–CA3 pyramidal neurons, suggesting that it may play a role in regulating dendrite or spine morphology (Fig. 1 H–J). We also detected considerable amounts of lipocalin-2 in the brain parenchyma, consistent with previous reports of its extracellular release (17).
Lipocalin-2 Regulates Actin Mobility, Promotes Spine Elimination, and Regulates Stress-Induced Changes in Spine Density and Morphology.
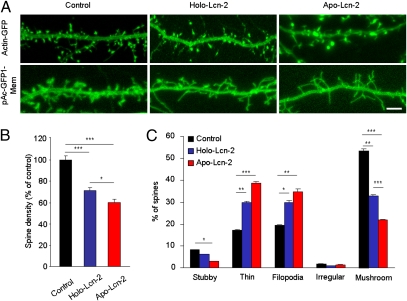
Lipocalin-2 was previously shown to promote deramification of microglial cells (18). Moreover, high levels of extracellular and dendrite-associated lipocalin-2 (Fig. 1 D and H–J) suggested a putative role in modulating neuronal morphology. Therefore, we examined its role in regulating the density and shape of dendritic spines. To this end, we coexpressed either unlinked green fluorescent protein (GFP), β-actin–GFP (enriched in spines), PSD-95-GFP (postsynaptic density specific), or a membrane-targeted GFP (consisting of N-terminal 20 amino acids of neuromodulin fused with AcGFP1) in cultured hippocampal neurons to visualize spines and cellular morphology (see SI Materials and Methods). Irrespective of the construct used, confocal microscopy revealed a ∼30% decrease in spine density after application of the wild-type lipocalin-2 (Holo-Lcn-2) for 3 d, suggesting its role in spine destabilization and elimination (Fig. 2 A and B; n = 22 and 21 neurons in the control and Holo-Lcn-2 groups, respectively; P < 0.001). In particular, analysis of dendritic spine morphology upon treatment with Holo-Lcn-2 revealed a ∼40% decrease in the proportion of mushroom spines (Fig. 2C; n = 22 and 21 neurons in the control and Holo-Lcn-2 groups, respectively; P < 0.01) accompanied by an increase in the percentage of thin spines (P < 0.01) and filopodia (P < 0.05).
Fig. 2.
Lipocalin-2 promotes dendritic spine retraction and affects spine morphology in an iron-dependent fashion. Cultured hippocampal neurons were transfected with β-actin–GFP or with pAcGFP1-Mem, treated with either wild-type (Holo-Lcn-2) or iron-free (Apo-Lcn-2) lipocalin-2 (examples in A), and dendritic spine density was calculated (quantified in B). Holo-Lcn-2 treatment caused a decrease in spine density, and this effect was potentiated following Apo-Lcn-2 application. Incubation of neurons with Holo-Lcn-2 resulted in an increase in the proportion of thin spines and filopodia accompanied by a decrease in the density of mushroom spines (C). This effect was augmented in the Apo-Lcn-2–treated group. *P < 0.05; **P < 0.01; ***P < 0.001. (Scale bar in A, 5 μm.) Data are expressed as mean ± SEM.
Because dendritic plasticity has been linked to iron metabolism (19), we next asked whether the effect of lipocalin-2 could be associated with its ability to bind and transport iron. To this end, we mutated the lipocalin-2 iron-binding residues and treated hippocampal neurons in culture with the modified protein (Apo-Lcn-2). We found that, upon Apo-Lcn-2 treatment, the decrease in the spine density and the increase in the proportion of immature spines were enhanced further (Fig. 2C; n = 15 neurons; P < 0.05 and P < 0.001 vs. Holo-Lcn-2 for the spine density and percentage of mushroom spines, respectively).
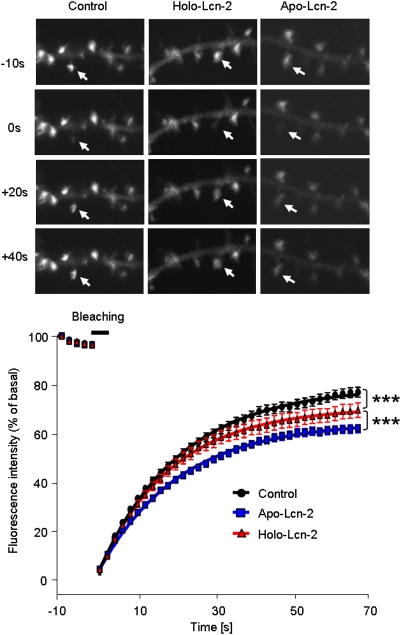
Recent reports suggest that actin, enriched and undergoing rapid turnover in spines, constitutes a converging point for multiple pathways regulating dendritic structural plasticity and may serve as the major driving force behind spinogenesis and/or spine retraction (20). It has been demonstrated that the mobility of the filamentous form of actin (F-actin)—an essential component of the spine cytoskeleton—regulates spine shape (21). In addition, spine morphology and plasticity is modulated by dynamic changes in F-actin/G-actin equilibrium (22). To investigate if lipocalin-2 affects actin dynamics and mobility in spines, we expressed GFP-tagged actin in cultured neurons to examine its fluorescent recovery after photobleaching (FRAP) (23). We focused on mushroom spines because this type was most significantly affected by lipocalin-2. After comparing recovery curves, we observed a decrease in actin's mobile fraction following the application of Holo-Lcn-2 (Fig. 3; 78.2 ± 0.9% in control vs. 69.5 ± 1.1% in Holo-Lcn-2; P < 0.001). Treatment with Apo-Lcn-2 resulted in a further decrease in this parameter (Fig. 3; 63.2 ± 0.7%; P < 0.001 vs. Holo-Lcn-2; n = 50, 45, and 51 spines analyzed in the control, Holo-, and Apo-Lcn-2 groups, respectively), which coincided with a higher rate of spine elimination after Apo-Lcn-2 treatment (Fig. 2 A and B).
Fig. 3.
Lipocalin-2 reduces dendritic spine actin turnover in an iron-dependent manner. Neurons were transfected with β-actin–GFP and treated with either wild-type lipocalin-2 (Holo-Lcn-2) or its iron-free form (Apo-Lcn-2). Actin fluorescence in single spines was bleached with a short laser pulse, and fluorescent recovery was measured over time (Upper) Representative examples. (Lower) Quantification. Holo-Lcn-2 treatment resulted in a decrease in actin's mobile fraction in spines, and the effect was potentiated when Apo-Lcn-2 was applied instead. ***P < 0.001. Data are expressed as mean ± SEM.
We then examined whether lipocalin-2 affected hippocampal dendritic spine density in vivo. To this end, we labeled the CA1 and CA3 neurons with DiI in hippocampal slices collected from stress-naive Lcn-2+/+ and Lcn-2−/− mice and examined spine density and morphology according to the previously published criteria (24). Stress-naive Lcn-2−/− animals showed higher spine density in both CA1 and CA3 (Fig. 4A; n = 29 and 20 neurons from six animals per group for CA1 and CA3; P < 0.05 and P < 0.01, respectively) compared with wild-type controls (n = 33 and 20 neurons from six mice per group for CA1 and CA3, respectively), consistent with the role of lipocalin-2 in spine elimination. Baseline spine morphology did not differ between the genotypes (Fig. 4 B and C).
Fig. 4.
Lipocalin-2 regulates basal and stress-induced changes in dendritic spine density and morphology. Dendritic spine density in DiI-labeled neurons was analyzed in hippocampal CA1–CA3 subfields of Lcn-2+/+ and Lcn-2−/− mice before and after restraint stress (A). Stress caused an increase in spine density in the CA1–CA3. Lcn-2 deficiency facilitated spinogenesis and promoted spine maturation in the CA1–CA3 as evidenced by an increase in the percentage of mushroom spines accompanied by a decrease in the proportion of thin and stubby spines (B and C). *P < 0.05; **P < 0.01; ***P < 0.001. Data are expressed as mean ± SEM.
To investigate if lipocalin-2 affected experience-driven spine plasticity, we performed DiI labeling in Lcn-2+/+ and Lcn-2−/− neurons after stress and compared spine density with the neurons from stress-naive mice [F(3,99) = 14.14 and F(3,100) = 18.78 for CA1 and CA3, respectively]. We found an ∼15–20% increase in spine density after stress in CA1 (n = 20 from six mice) and in CA3 (n = 20 neurons from six mice) of wild-type animals (Fig. 4A; P < 0.01 and P < 0.001 vs. stress-naive for CA1 and CA3, respectively). This effect was enhanced in CA1 by the deletion of the Lcn2 gene (Fig. 4A; P < 0.05 vs. stressed Lcn-2+/+ animals; n = 22 neurons from six mice).
We next examined whether lipocalin-2 regulated activity-driven changes in spine morphology in the hippocampus. In keeping with our neuronal culture data (Fig. 2C), ANOVA revealed an increase in the proportion of mushroom spines in CA1 and CA3 of Lcn-2−/− but not Lcn-2+/+ mice after stress [Fig. 4 B and C; F(3,61) = 43.41 and F(3,49) = 43.33 for CA1 and CA3, respectively; n = 11–20 neurons from six mice per group; P < 0.001 vs. both stress-naive Lcn-2−/− mice and stressed Lcn-2+/+ animals], accompanied by a decrease in the percentage of thin spines in the CA1–CA3 regions of Lcn-2−/−mice [F(3,61) = 19.87 and F(3,49) = 45.02 for CA1 and CA3, respectively; P < 0.001 vs. nonstressed control]. The proportion of stubby spines was also reduced in Lcn-2−/− mice after stress [F(3,61) = 8.16 and F(3,49) = 7.99, P < 0.05 and P < 0.001 vs. nonstressed control for CA1 and CA3, respectively].
Lipocalin-2−/− Regulates Neuronal Excitability and Anxiety.
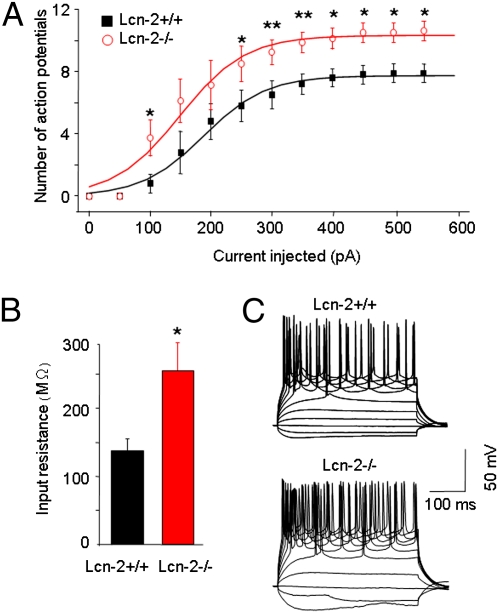
Changes in dendritic spine density or morphology can modify electrophysiological properties of neurons (25). Thus, using current clamp recordings, we examined whether lipocalin-2–induced changes in the dendritic spine phenotype could alter neuronal excitability. We found that pyramidal neurons of the CA1 in Lcn-2−/− mice were more excitable and fired more action potentials than neurons from Lcn-2+/+ mice under the same conditions, which is consistent with a higher number of dendritic spines in Lcn-2–deficient animals (Fig. 5 A–C).
Fig. 5.
Lipocalin-2–deficient hippocampal neurons show increased excitability. Current-clamp recordings revealed a higher neuronal firing rate in the pyramidal neurons of the CA1 region of Lcn-2−/− mice compared with Lcn-2+/+ animals (A). Disruption of the Lcn2 gene significantly increased the number of action potentials (A). A significant increase in the mean input resistance in Lcn-2−/− mice compared with Lcn-2+/+ animals was found (B). Voltage responses (representative traces in C) were recorded by current steps from −100 to +600 pA in 50 pA (starting membrane potential, −80 mV). *P < 0.05; **P < 0.01. Data are expressed as mean ± SEM.
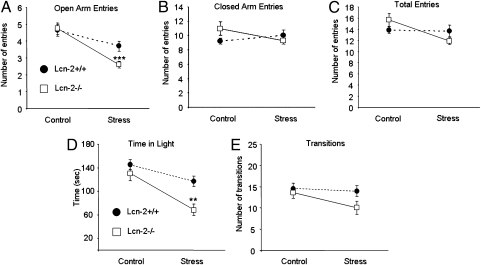
Adjustments in spine density, morphology, and neuronal excitability may result in alterations of the behavioral phenotype of an animal. One well-documented consequence of the restraint stress in mice is elevated anxiety (5). Thus, to assess the behavioral effects of lipocalin-2, we measured anxiety in Lcn-2+/+ and Lcn-2−/− mice in the elevated-plus maze. Although basal locomotor activity and anxiety were similar between the genotypes, we found that after stress Lcn-2−/− mice performed fewer entries into the open arms of the maze than their Lcn-2+/+ counterparts, indicating that genetic disruption of lipocalin-2 facilitated the development of anxiety (Fig. 6 A–C; n ≥ 20 animals/group, P < 0.001).
Fig. 6.
Lcn-2−/− mice show enhancement of stress-induced anxiety. Mice were subjected to restraint stress and behavioral responses in the elevated-plus maze, and the light–dark box was measured. Lcn-2−/− animals developed higher levels of anxiety compared with Lcn-2+/+ mice, as evidenced by a lower number of entries into the open arms of the elevated-plus maze after stress (A). The anxiogenic effect of the deletion of the Lcn2 gene was independent of general locomotor activity because the number of entries into closed arms (B) and the total number of arm entries (C) were similar in both genotypes. After stress Lcn-2−/− mice spent less time in the light compartment compared with Lcn-2−/− mice (D) whereas the total number of light–dark transitions (indicative of locomotor activity) was similar between the genotypes (E). **P < 0.01; ***P < 0.001. Data are expressed as mean ± SEM.
To examine whether the effect of lipocalin-2 on anxiety was test-specific, we subjected Lcn-2+/+ and Lcn-2−/− mice to restraint stress and measured their anxiety in the light–dark box (Fig. 6 D and E). We found that in Lcn-2+/+ animals stress resulted in the reduction of time spent in the illuminated part of the apparatus (n = 9–10; P < 0.05) and that this effect was exacerbated further by the deletion of the Lcn2 gene (n = 10–11; P < 0.01 between the genotypes after stress). Together with the results obtained using the elevated-plus maze, these data confirmed a general role of lipocalin-2 in stress-induced anxiety.
Discussion
Lipocalin-2 (also known as 24p3 or NGAL), a member of the lipocalin family, serves diverse cellular functions by binding a variety of hydrophobic molecules, interacting with specific cell-surface receptors, and regulating cellular iron concentration (9, 26). Although basal levels of lipocalin-2 are low, lipocalin-2 is rapidly inducible (10) and regulates innate immune responses (27) and cellular viability (28), migration, or morphology in a variety of tissues (11, 12). Here we found that the Lcn2 gene and protein are up-regulated in the hippocampus after psychological stress and contribute to stress-induced neuronal plasticity at the cellular and behavioral levels.
Lipocalin-2 is induced by glucocorticoids (10) and up-regulated in response to numerous adverse stimuli such as inflammation (29) and β-amyloid (30) or cerebral ischemia (31). We found that lipocalin-2 is up-regulated in the hippocampus by restraint stress. Thus, the induction of Lcn-2 in the nervous system likely represents a universal mechanism of cellular adaptation to unfavorable, potentially harmful situations. Such an idea is corroborated by the previously reported role of lipocalin-2 in regulating cellular vulnerability to injury and apoptosis (28, 32), a phenomenon observed in the central nervous system after severe or prolonged stress (33). Thus, lipocalin-2 likely constitutes an important element regulating brain allostasis in response to challenging external stimuli.
In addition to neurodegeneration, psychological stress triggers subtle alterations in neuronal morphology indicative of the early stages of injury. For example, long-term stress causes retraction of apical dendrites in the hippocampus (4), in some cases counterbalanced by an increase in density of spines (34, 35). It is also well accepted that noxious stimuli may result in changes in spine morphology (36). Although our stress paradigm (6 h daily restraint for 3 d) was unlikely to alter the complexity of dendritic arborisation, our data indicate that lipocalin-2 is an important regulator of stress-induced changes in dendritic spine density and morphology in the hippocampus. The effect of lipocalin-2 in regulating spine morphology and promoting spine elimination complements its previously reported effect on deramification of microglial cells and astrocytes (17, 18).
Dendritic spines are morphologically diverse, and their phenotype correlates with motility and synaptic strength. Spine size and shape are dynamic and modulated by input-specific neuronal activity (e.g., long-term potentiation or depression) that may transform thin spines into mushroom spines and vice versa (37). Aversive experience often leads to changes in synaptic physiology resulting from alterations in spine morphology (38). Stress modulates various components of the immune system such as glucocorticoids (8), proinflammatory cytokines (39), or lipocalin-2 (this study), which may significantly contribute to changes in spine morphology. We found that treatment of neurons with lipocalin-2 (mimicking its stress-induced up-regulation) caused a decrease in the proportion of mushroom spines with a concomitant increase in the percentage of thin ones. Concurrently, the disruption of the Lcn2 gene facilitated the formation of mushroom spines while decreasing the percentage of thin spines after stress. The mechanism underlying such a change in spine morphology is not clear but may involve differences in sensitivity of distinct spine subclasses toward the effects of lipocalin-2.
Higher spine density observed in Lcn-2−/− mice accompanied by a larger proportion of mushroom spines may have important physiological consequences. It is generally accepted that highly plastic, thin spines are involved in learning (“learning spines”) whereas mature, less motile mushroom spines, may remain unchanged over long periods and are implicated in the maintenance of the established neuronal networks and long-term memory (“memory spines”) (37, 40). Consequently, the increase in the proportion of mushroom spines observed in Lcn-2−/− mice after stress may result in more stable neuronal connections and promote anxiety-like behavior in this strain.
The increase in the proportion of mushroom spines observed in Lcn-2−/− mice after stress suggests the existence of stress-related molecular mechanisms facilitating spine maturation that are inhibited by lipocalin-2. Consequently, the morphological phenotype of dendritic spines upon stress would reflect a relative balance between opposite forces facilitating or attenuating their maturation. Our in vitro and ex vivo data suggest that Lcn-2 might serve as a “molecular brake,” imposing control over stress-associated spine maturation and therefore limiting the negative impact of stress on animal behavior. Binding and internalization of lipocalin-2 by its receptor 24p3R (41) could provide an efficient way of fine-tuning lipocalin-2 levels in discrete tissue compartments to regulate its impact on neuronal physiology.
What is the mechanism of the effect of lipocalin-2 on dendritic spine density and morphology during stress? The actin cytoskeleton, highly enriched in spines, is critical for both spine formation and dynamics. It serves as a converging point of numerous cellular pathways controlling cellular shape and motility with a well-documented role in spine generation, elimination, and regulation of their size and shape (20). It is also known that actin dynamics underlie stimulus-driven morphological changes in spines (21, 42). These changes are often accompanied by dynamic shifts in F-actin/G-actin equilibrium (22). We found that treatment of neurons with wild-type lipocalin-2 (containing both iron-free and iron-bound molecules) significantly affects spine number and shape. This effect is accompanied by a decrease in actin's mobile fraction in mushroom spines. Thus, spine collapse, retraction, or morphological changes could result from modified actin mobility and, as a consequence, alter scaffolding support due to a shift in the mobile/immobile actin ratio.
Our results indicate that the iron-binding capabilities of lipocalin-2 are key to this process. When we disrupted the lipocalin-2 iron-binding properties (thus increasing the proportion of its iron-free form), actin's mobile fraction decreased further, the spine loss was enhanced, and the percentage of immature spines increased. Although under physiological conditions iron-free lipocalin-2 acts as an important regulator of the observed morphological changes, excess iron-free lipocalin-2 could be deleterious to neurons by sequestrating intracellular iron and shutting down iron-responsive genes. This is consistent with its previously reported detrimental effects in other systems (41) and with recent findings of deregulation of iron metabolism correlating with neurodegeneration in numerous pathologies including Parkinson, Alzheimer's, and Huntington disease (43). In concordance with our hypothesis, iron deficiency was shown to reduce the complexity of hippocampal dendritic arborisation (19, 43). Although lipocalin-2 is certainly not the only modulator of dendritic spine shape, its iron-free and iron-bound forms could play important roles in regulating actin mobility, structural plasticity, and, finally, stress-induced anxiety.
It is important to note that the effect of stress on spine density and morphology often depends on stress duration, the paradigm used, and the brain area examined (14, 44). Further studies are necessary to clarify the effect of lipocalin-2 during chronic vs. acute stress in different stress-related brain regions.
The hippocampus, amygdala, and medial prefrontal cortex collectively form the innate fear circuit; the role of the hippocampus in anxiety is well-documented (15). Although on the basis of our experiments we cannot exclude the involvement of other brain regions in the behavioral phenotype observed in Lcn-2−/− mice, it is likely that Lcn-2–mediated subcellular and cellular changes in the hippocampus significantly contribute to the development and behavioral manifestation of anxiety.
In summary, our findings establish lipocalin-2 as an important modulator of stress-induced changes in spine morphology, neuronal excitability, and anxiety. Lcn-2 or its molecular partners could become targets to combat stress-related disorders, including affective disorders.
Materials and Methods
Animals and Restraint Stress.
Experiments were performed on male wild-type (C57BL6) or Lcn-2−/− mice (27) backcrossed to C57BL6. Restraint stress was performed during the light period of the circadian cycle as described (5). Control animals were left undisturbed, and stressed animals were subjected to 6-h restraint stress sessions in a separate room for 3 d. The mice were placed in their home cages in wire-mesh restrainers secured at the head and tail ends with clips.
qRT-PCR.
Hippocampi were dissected from a coronal slice −0.58 to −2.3 mm relative to Bregma, homogenized (QIAzol lysis reagent, Qiagen), total-RNA-isolated (RNeasy lipid tissue mini kit, Qiagen), and converted to cDNA [Superscript III, Invitrogen, and oligo(dT) primers]. qRT-PCR for Lcn2 was performed using a Chromo4/PTC-200 thermal cycler (MJ Research) as follows: 95 °C for 15 min, 94 °C for 15 s, 55 °C for 30 s and 72 °C for 30 s; steps 2–4 were repeated 40 times with the primers CCCCATCTCTGCTCACTGTC and TTTTTCTGGACCGCATTG. The results were normalized to actin.
Western Blotting.
The hippocampi were dissected as above, homogenized in 0.1 M Tris, 0.1% Triton X-100, pH 7.4. Reduced (DTT) and denatured (100 °C for 5 min) samples were subjected to SDS/PAGE electrophoresis and transferred onto nitrocellulose membrane. After blocking [5% skim milk for 1 h at room temperature (RT)], the membranes were probed with goat anti–Lcn-2 antibody (1:500, 4 °C overnight; R&D) followed by a HRP-conjugated secondary antibody (1:1,000, 1 h at RT; Vector Labs) and the signal developed (Western blot luminol reagent; Santa Cruz). The results were normalized to actin (anti–β-actin antibody; Sigma).
Immunohistochemistry.
The brains were fixed in 4% paraformaldehyde in PBS overnight at 4 °C. Coronal sections (70-μm thick) were incubated in PBS-T (PBS containing 0.5% BSA, 0.02% Triton X-100, and normal serum 1:500) for 5 h at RT. Sections were then incubated with goat anti–Lcn-2 antibody (1:500; R&D) along with the mouse anti-NeuN (1:200; Chemicon) and chicken anti-GFAP (1:1,000; Dako) or with mouse anti–β-III tubulin (1/1000; Abcam) overnight at 4 °C in PBS-T. After washing, the sections were incubated overnight with appropriate AlexaFluor secondary antibodies (1:500; Molecular Probes) in the same buffer, washed, mounted on glass slides using Vectamount medium (Vector Laboratories), and photographed.
Neuronal Cultures.
Hippocampi from P1 C57BL6 wild-type mice were chopped and placed in a dish containing 9.1 mM glucose, 25 mM Hepes, 5 mM KCl, and 120 mM NaCl. Tissue was digested (5 mg of pronase E and 5 mg of thermolysin; Sigma) in 10 mL of the buffer for 30 min at RT, triturated, and plated on poly–d-lysine (Sigma)-coated coverslips. After 24 h, 5 μM cytosine β–d-arabinofuranoside (Ara-C; Sigma) was added for 48 h. Neurons were maintained for 17–21 days in vitro (DIV) in Neurobasal medium with supplements at 37 °C in a humidified atmosphere of 5% CO2/95% air. Neurons were lipofectamine-transfected and treated with 100 ng/mL of wild-type (R&D) or iron-free lipocalin-2 for 3 d.
Site-Directed Mutagenesis, Protein Synthesis, and Purification.
Arg81, Lys125, and Lys134 forming the lipocalin-2 iron-binding center (26) were substituted with alanines by PCR-based site-directed mutagenesis. Both wild-type and mutated coding sequences were then expressed in BL21 DE3 pLysS (Invitrogen) and purified using a B-PER 6xHis Spin Purification Kit (Thermo Scientific).
Electrophysiology.
Recordings made from somata of CA1 pyramidal neurons (coronal 250-μm slices from 10- to 20-d-old mice). The cell membrane was clamped at −80 mV. Input resistance and instantaneous frequency were derived from traces in which cells were injected with 200-ms current pulses (−100 to +600 pA: 50 pA increments). The recording electrodes were borosilicate glass pipettes (2–4 MΩ) filled with (in mM): K-gluconate (130), KCl (4), EGTA (0.5), Hepes (10), and glucose (5). All of the experiments were performed at 25 °C.
Fluorescence Recovery After Photobleaching.
Neurons were transfected at DIV7 to express β-actin–GFP. FRAP experiments (23) were performed on mushroom spines of DIV 16–17 in a temperature-controlled chamber. Spines that bleached (488-nm laser line) more than 80% from the original intensity were used for data analysis. Fluorescent intensity before photobleaching was considered 100%, and the intensity on the first image after bleach was considered 0. Recovery was analyzed in areas including the bleached spine with background subtracted and was expressed as a proportion of the baseline fluorescence intensity.
DiI Labeling.
DiI labeling was performed as previously described (45). The brains were fixed (4% paraformaldehyde) and 170-μm coronal slices containing the hippocampus were cut on vibrating microtome. Small DiI crystals (Molecular Probes) were applied to the CA1 and CA3 pyramidal cell layers for 24 h. After further fixation, the sections were mounted and the dendritic tree (the middle portion of stratum radiatum of the CA1 and apical dendrites of the CA3) was visualized using a Zeiss LSM5 Exciter and analyzed in a blind fashion. Spine morphology was assessed according to previously published criteria (24): mushroom spines—<2 μm in length, >0.5 μm in width, and connected to the dendritic shaft by a narrower portion (neck); stubby spines—<2 μm in length, >0.5 μm in width, and lack of a defined neck; thin spines—<2 μm in length, <0.5 μm in , and with a neck; filopodia—>2 μm in length, <0.5 μm in width, without a distinct spine head, and irregular spines with more than one neck and/or head.
Behavioral Studies.
The elevated-plus maze apparatus (5) was made of two enclosed arms (50 × 10 × 30 cm) that formed a cross shape with the two open arms (50 × 10 cm). The maze was 55 cm above the floor and dimly illuminated. Mice were placed individually on the central platform and allowed to explore the apparatus for 5 min. The number of entries of the animal to closed or open arms was counted. The light–dark box apparatus consisted of the light and dark compartments connected by an open door. Each compartment was rectangular in shape (20 × 25 cm). The dark compartment was entirely enclosed. The apparatus was dimly illuminated. Mice were placed in the dark chamber, and the number of transitions between the two chambers and the time spent in each was measured for 5 min. Behavioral analyses were performed blind.
Statistics.
Student's t test or analysis of variance followed by Newman–Keuls posttest were used as appropriate. P values of less than 0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank Dr. Kenneth W. Young, Dr. Alan Aderem, Dr. Jacek Jaworski, and Dr. Yukiko Goda for comments, reagents, and mice. This work was supported by a Marie Curie Excellence Grant (MEXT-CT-2006-042265 from the European Commission) to R.P.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
See Commentary on page 18197.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107936108/-/DCSupplemental.
References
- 1.Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- 2.Magariños AM, McEwen BS, Flügge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- 5.Pawlak R, Magarinos AM, Melchor J, McEwen B, Strickland S. Tissue plasminogen activator in the amygdala is critical for stress-induced anxiety-like behavior. Nat Neurosci. 2003;6:168–174. doi: 10.1038/nn998. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy MB, Beale HC, Carlisle HJ, Washburn LR. Integration of biochemical signalling in spines. Nat Rev Neurosci. 2005;6:423–434. doi: 10.1038/nrn1685. [DOI] [PubMed] [Google Scholar]
- 8.McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 9.Flower DR, North AC, Sansom CE. The lipocalin protein family: Structural and sequence overview. Biochim Biophys Acta. 2000;1482:9–24. doi: 10.1016/s0167-4838(00)00148-5. [DOI] [PubMed] [Google Scholar]
- 10.Liu Q, Nilsen-Hamilton M. Identification of a new acute phase protein. J Biol Chem. 1995;270:22565–22570. doi: 10.1074/jbc.270.38.22565. [DOI] [PubMed] [Google Scholar]
- 11.Ryon J, Bendickson L, Nilsen-Hamilton M. High expression in involuting reproductive tissues of uterocalin/24p3, a lipocalin and acute phase protein. Biochem J. 2002;367:271–277. doi: 10.1042/BJ20020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J, et al. Lipocalin 2 promotes breast cancer progression. Proc Natl Acad Sci USA. 2009;106:3913–3918. doi: 10.1073/pnas.0810617106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magarinos AM, Li CJ, Toth JG, Bath KG, Jing D, et al. Effect of brain-derived neurotrophic factor haploinsufficiency on stress-induced remodeling of hippocampal neurons. Hippocampus. 2011;21:253–264. doi: 10.1002/hipo.20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci USA. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adhikari A, Topiwala MA, Gordon JA. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron. 2010;65:257–269. doi: 10.1016/j.neuron.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirase H. A multi-photon window onto neuronal-glial-vascular communication. Trends Neurosci. 2005;28:217–219. doi: 10.1016/j.tins.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Lee S, et al. Lipocalin-2 is an autocrine mediator of reactive astrocytosis. J Neurosci. 2009;29:234–249. doi: 10.1523/JNEUROSCI.5273-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee S, et al. A dual role of lipocalin 2 in the apoptosis and deramification of activated microglia. J Immunol. 2007;179:3231–3241. doi: 10.4049/jimmunol.179.5.3231. [DOI] [PubMed] [Google Scholar]
- 19.Jorgenson LA, Wobken JD, Georgieff MK. Perinatal iron deficiency alters apical dendritic growth in hippocampal CA1 pyramidal neurons. Dev Neurosci. 2003;25:412–420. doi: 10.1159/000075667. [DOI] [PubMed] [Google Scholar]
- 20.Hotulainen P, Hoogenraad CC. Actin in dendritic spines: Connecting dynamics to function. J Cell Biol. 2010;189:619–629. doi: 10.1083/jcb.201003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matus A. Actin-based plasticity in dendritic spines. Science. 2000;290:754–758. doi: 10.1126/science.290.5492.754. [DOI] [PubMed] [Google Scholar]
- 22.Okamoto K-I, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat Neurosci. 2004;7:1104–1112. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- 23.Star EN, Kwiatkowski DJ, Murthy VN. Rapid turnover of actin in dendritic spines and its regulation by activity. Nat Neurosci. 2002;5:239–246. doi: 10.1038/nn811. [DOI] [PubMed] [Google Scholar]
- 24.Bourgin C, Murai KK, Richter M, Pasquale EB. The EphA4 receptor regulates dendritic spine remodeling by affecting beta1-integrin signaling pathways. J Cell Biol. 2007;178:1295–1307. doi: 10.1083/jcb.200610139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noguchi J, Matsuzaki M, Ellis-Davies GC, Kasai H. Spine-neck geometry determines NMDA receptor-dependent Ca2+ signaling in dendrites. Neuron. 2005;46:609–622. doi: 10.1016/j.neuron.2005.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goetz DH, et al. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10:1033–1043. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- 27.Flo TH, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 28.Kehrer JP. Lipocalin-2: Pro- or anti-apoptotic? Cell Biol Toxicol. 2010;26:83–89. doi: 10.1007/s10565-009-9119-9. [DOI] [PubMed] [Google Scholar]
- 29.Marques F, et al. Lipocalin 2 is a choroid plexus acute-phase protein. J Cereb Blood Flow Metab. 2008;28:450–455. doi: 10.1038/sj.jcbfm.9600557. [DOI] [PubMed] [Google Scholar]
- 30.Paratore S, et al. Genomic profiling of cortical neurons following exposure to beta-amyloid. Genomics. 2006;88:468–479. doi: 10.1016/j.ygeno.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 31.MacManus JP, et al. Translation-state analysis of gene expression in mouse brain after focal ischemia. J Cereb Blood Flow Metab. 2004;24:657–667. doi: 10.1097/01.WCB.0000123141.67811.91. [DOI] [PubMed] [Google Scholar]
- 32.Tong Z, et al. Neutrophil gelatinase-associated lipocalin as a survival factor. Biochem J. 2005;391:441–448. doi: 10.1042/BJ20051020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reagan LP, McEwen BS. Controversies surrounding glucocorticoid-mediated cell death in the hippocampus. J Chem Neuroanat. 1997;13:149–167. doi: 10.1016/s0891-0618(97)00031-8. [DOI] [PubMed] [Google Scholar]
- 34.Komatsuzaki Y, et al. Rapid spinogenesis of pyramidal neurons induced by activation of glucocorticoid receptors in adult male rat hippocampus. Biochem Biophys Res Commun. 2005;335:1002–1007. doi: 10.1016/j.bbrc.2005.07.173. [DOI] [PubMed] [Google Scholar]
- 35.Shors TJ, Falduto J, Leuner B. The opposite effects of stress on dendritic spines in male vs. female rats are NMDA receptor-dependent. Eur J Neurosci. 2004;19:145–150. doi: 10.1046/j.1460-9568.2003.03065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michelsen KA, et al. Prenatal stress and subsequent exposure to chronic mild stress influence dendritic spine density and morphology in the rat medial prefrontal cortex. BMC Neurosci. 2007;8:107. doi: 10.1186/1471-2202-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol. 2007;17:381–386. doi: 10.1016/j.conb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Radley JJ, Morrison JH. Repeated stress and structural plasticity in the brain. Ageing Res Rev. 2005;4:271–287. doi: 10.1016/j.arr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci USA. 2010;107:2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dumitriu D, et al. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci. 2010;30:7507–7515. doi: 10.1523/JNEUROSCI.6410-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Devireddy LR, Gazin C, Zhu X, Green MR. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 2005;123:1293–1305. doi: 10.1016/j.cell.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 42.Cingolani LA, et al. Activity-dependent regulation of synaptic AMPA receptor composition and abundance by beta3 integrins. Neuron. 2008;58:749–762. doi: 10.1016/j.neuron.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crichton RR, Dexter DT, Ward RJ. Brain iron metabolism and its perturbation in neurological diseases. J Neural Transm. 2011;118:301–314. doi: 10.1007/s00702-010-0470-z. [DOI] [PubMed] [Google Scholar]
- 44.Pawlak R, et al. Tissue plasminogen activator and plasminogen mediate stress-induced decline of neuronal and cognitive functions in the mouse hippocampus. Proc Natl Acad Sci USA. 2005;102:18201–18206. doi: 10.1073/pnas.0509232102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim BG, Dai HN, McAtee M, Vicini S, Bregman BS. Labeling of dendritic spines with the carbocyanine dye DiI for confocal microscopic imaging in lightly fixed cortical slices. J Neurosci Methods. 2007;162:237–243. doi: 10.1016/j.jneumeth.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.