Abstract
Activation-induced cytidine deaminase (AID) is a B lymphocyte-specific DNA deaminase that acts on the Ig loci to trigger antibody gene diversification. Most AID, however, is retained in the cytoplasm and its nuclear abundance is carefully regulated because off-target action of AID leads to cancer. The nature of the cytosolic AID complex and the mechanisms regulating its release from the cytoplasm and import into the nucleus remain unknown. Here, we show that cytosolic AID in DT40 B cells is part of an 11S complex and, using an endogenously tagged AID protein to avoid overexpression artifacts, that it is bound in good stoichiometry to the translation elongation factor 1 alpha (eEF1A). The AID/eEF1A interaction is recapitulated in transfected cells and depends on the C-terminal domain of eEF1A (which is not responsible for GTP or tRNA binding). The eEF1A interaction is destroyed by mutations in AID that affect its cytosolic retention. These results suggest that eEF1A is a cytosolic retention factor for AID and extend on the multiple moonlighting functions of eEF1A.
Functional Ig genes are produced in developing B-lymphocyte precursors by a process of V(D)J gene rearrangement catalyzed by the RAG1/2 recombinase. These rearranged IgV genes are then further diversified by either gene conversion in chicken (using proximal IgV pseudogenes as donors) or by somatic hypermutation in man and mouse (underpinning antibody affinity maturation). The isotype of the antibody can also be changed from IgM to IgG, IgA, or IgE through class-switch recombination.
Ig gene conversion, somatic hypermutation, and class-switch recombination are all initiated by the B lymphocyte-specific enzyme AID, which deaminates cytosine residues within the IgV or switch regions, yielding localized U:G mismatches that are recognized by uracil-DNA glycosylase or MSH2/MSH6, thereby triggering the subsequent gene diversification processes (1).
As an active DNA mutator, AID is a dangerous protein: its abundance appears to be carefully regulated. Ig gene diversification is reduced in cells hemizygous for AID: overexpression or ectopic expression of AID increases the frequency of chromosomal translocations and malignancies. The regulation of AID gene expression occurs both transcriptionally and posttranscriptionally (reviewed in ref. 2).
It is also likely that much regulation of AID occurs posttranslationally. Thus, AID is phosphorylated on several serine/threonine residues, some of which are critical for its function (3–8). Furthermore, although active in the nucleus, the majority of AID is detected in the cytoplasm where it cycles into and out of the nucleus (9–11). Whereas AID's nuclear export is mediated by a Crm1-dependent export sequence (9–11), the mechanism of its nuclear import is still unclear, although the work of Patenaude et al. (12) reveals that dissociation from an unidentified cytosolic retention factor may allow nuclear import with such import depending upon a noncontiguous cluster of basic amino acids in AID.
We have been interested in advancing our understanding of the cytosolic associations of AID and here describe the use of gene-targeting in chicken DT40 B cells to allow tagging of endogenous AID, thereby facilitating the purification of cytosolic AID complexes but avoiding concerns of overexpression artifacts. The results reveal that endogenous cytoplasmic AID partakes in a complex containing stoichiometric quantities of translation elongation factor 1α (eEF1A), with this association likely implicated in the regulation of AID's intracellular trafficking.
Results
Flag-Tagging the Endogenous AID Locus in DT40 Cells.
We generated derivatives of the DT40 B-cell line in which the endogenous AID locus was modified so as to incorporate a single Flag tag at the AID N terminus. To allow targeting of both alleles, one targeting construct contained a puromycin-resistance cassette, whereas the other included a blasticidin-resistance gene. Both cassettes were flanked by LoxP sites. These constructs were sequentially transfected into DT40 cells and homologous recombination events in resistant clones were screened for by Southern blotting on both sides of the homology region (Fig. S1 A and B). After both alleles were targeted, the selection cassettes were removed by transient expression of Cre recombinase.
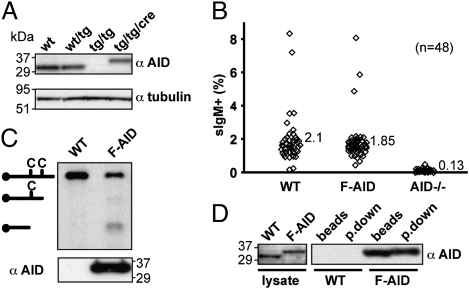
As shown by Western blot (Fig. 1A), AID expression was abolished in cells in which both AID alleles had been targeted, but was recovered at a normal level following Cre-mediated removal of the drug-resistance selection cassettes. The restored AID exhibited a somewhat higher molecular weight, consistent with the inclusion of the N-terminal Flag tag.
Fig. 1.
Endogenously tagged AID retains antibody diversification and DNA deaminase activity. (A) Western blot analysis of AID expression in wild type (WT) DT40 CL18 as well as in derivatives targeted (tg) on one or both AID alleles before or following (Cre) removal of the drug-resistance cassettes. Blots were reprobed with an antibody to tubulin as loading control. The positions of molecular weight markers (which migrate anomalously because they are prestained) are indicated. (B) Antibody diversification through IgV gene conversion monitored by the frequency of surface IgM+ cells in 48 independent subclones of DT40 CL18 homozygous FlagAID knock-in cells (F-AID), as well in control cells [parental DT40 CL18 cells (WT) and in an AID-knockout derivative (AID−/−) (27)] after 21 d of clonal expansion. (C) DNA deaminase activity of purified FlagAID was monitored by oligonucleotide cleavage assay using washed anti-Flag beads that had been incubated with extracts of FlagAID knock-in DT40 cells [or of parental cells as a control (WT)]. Western blot analysis (Lower) revealed that AID protein was only detectable on the beads that had been incubated with the extracts of the knock-in DT40 cells. (D) Adsorption onto anti-Flag beads and elution with 3xFlag peptide brings down Flag-tagged but not untagged AID. Samples of lysates from WT and FlagAID knock-in cells were analyzed by Western blotting with anti-AID antibody (Left), as were samples of the lysates after binding or binding and elution from anti-Flag Dynabeads.
Endogenously Tagged AID Is Active in Antibody Diversification.
We had chosen to use an N-terminal Flag tag because such Flag-tagged chicken AID retains biological function, as judged by its ability to restore class-switching to AID-deficient mouse B cells (Fig. S1D). We nevertheless wished to confirm that the endogenously tagged FlagAID in DT40 cells was also active in potentiating antibody diversification. The DT40 cells used in this work are derived from DT40 CL18, which is a DT40 surface IgM (sIgM)-loss variant that contains a frame-shift within its rearranged IgVλ gene. This frame-shift can be repaired by AID-mediated IgV gene conversion, resulting in the appearance of sIgM+ cells; the percentage of sIgM+ cells in the population thus provides a monitor of AID-mediated gene conversion (13).
Flow cytometric analysis of multiple independent DT40 clones revealed that both wild-type CL18 and the FlagAID knock-in cell line gave rise to about 2% of sIgM+ cells after 21 d of clonal expansion (Fig. 1B). Sequence analysis of PCR-amplified IgVλ genes from sorted sIgM+ cells confirmed that most had indeed arisen through IgV gene conversion. In contrast, no significant activity in antibody diversification was evident with the AID−/− line. These findings, taken together with the fact that the knock-in and the wild-type exhibit a similar growth rate (doubling period of about 12 h) (Fig. S1C), indicate that inclusion of the N-terminal Flag tag on the endogenous AID has not significantly affected its functional activity in IgV gene diversification.
Endogenously Tagged FlagAID Is Stoichiometrically Associated with eEF1A.
We wished to purify FlagAID using anti-Flag antibodies to identify any proteins with which it is associated. Our own experience as well as that of others (14) has revealed AID to be sticky, as evidenced, for example, by its nonspecific binding to agarose. We therefore experimented with conditions that would allow us to purify FlagAID effectively and specifically using anti-Flag M2 monoclonal antibody. We found that by using M2 antibody directly conjugated to tosyl-activated Dynabeads (avoiding Sepharose and other sugar-based matrices) we could immunoprecipitate (pull down) FlagAID from cell extracts under physiological salt concentration and elute it by competition with 3xFlag peptide. Using this approach, tagged (but not untagged) AID could be specifically bound to the anti-Flag beads and incubation with the 3xFlag peptide allowed about 50% of the FlagAID to be eluted from the beads (Fig. 1D and Fig. S2).
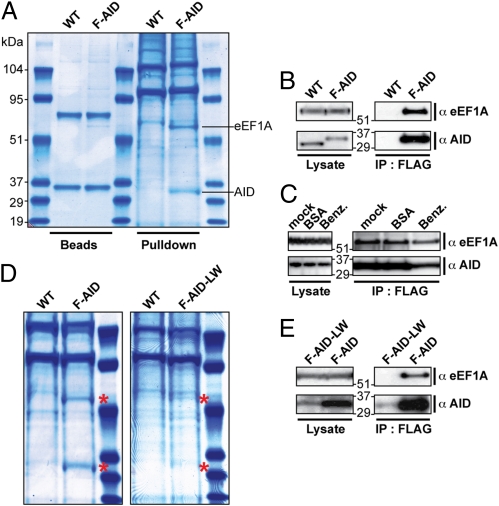
Following this procedure, we managed to obtain a sufficient amount of endogenous FlagAID to detect it by Coomassie staining (Fig. 2A). This FlagAID bound to the anti-Flag beads exhibited DNA deaminase activity, as monitored in an oligonucleotide cleavage assay (Fig. 1C). However, most strikingly, a 50-kDa protein was specifically copurified with AID (Fig. 2A). This band appeared to be stoichiometric with AID on Coomassie-stained gels and was reproducibly copurified at similar levels in at least four independent experiments (Fig. S3).
Fig. 2.
Stoichiometric copurification of eEF1A with endogenously-tagged AID. (A) Coomassie staining of proteins purified from FlagAID knock-in DT40 (F-AID) cells after absorption onto anti-FLAG beads and elution with 3xFlag peptide (Pulldown). A parallel purification from parental DT40 cells (WT) serves as a control. The lanes labeled “Beads” show 3% of the proteins remaining on the beads following the 3xFlag peptide elution. The indicated bands specifically pulled down from F-AID cells were identified by LC-MS-MS as being AID and eEF1A (Fig. S3). (B) Western blot analysis with anti-eEF1A antibodies confirms the presence of eEF1A in the anti-Flag-purified material from F-AID (but not WT) cells. (C) Addition of benzonase (0.16 U/μL; Roche) to the DT40 (F-AID) extracts and its inclusion during the pull-down procedure does not affect the association of eEF1A with Flag-tagged AID. The abundance of eEF1A and AID in total cell lysates (Left) or anti-Flag immunoprecipitates (Right) was monitored by Western blotting. Controls are provided by parallel samples to which benzonase had not been added (mock) or in which BSA (0.016 μg/mL) had been used instead of benzonase. (D) The amount of eEF1A pulled down is diminished using a F-AID DT40 subclone expressing diminished levels of FlagAID (F-AID-LW) as revealed by Coomassie staining of total proteins obtained in parallel purifications. The FlagAID and eEF1A bands are highlighted with red asterisks). (E) Western blot analysis of the abundance of AID and eEF1A in total lysates as well as in anti-Flag immunoprecipitates from the F-AID and F-AID-LW cells analyzed in D.
Both the AID and the 50-kDa bands were excised from gels and sequenced by LC-MS-MS. As shown in Fig. S3, the identity of the putative FlagAID was confirmed by identifying five peptides derived from it that could be assigned to the AID sequence. The stoichiometrically copurified band was identified as eEF1A by four independent peptides. Western blot analysis confirmed that the 50-kDa band specifically purified with AID was indeed eEF1A (Fig. 2B). Furthermore, although eEF1A is an abundant protein, we did not observe nonspecific binding to the anti-Flag resin with extracts of wild-type DT40 cells (Fig. 2B).
After extended (>3 mo) subculture, we obtained some subclones of the FlagAID DT40 knock-in line expressing diminished levels of FlagAID protein. The amount of 50-kDa protein obtained from extracts of these subclones by anti-Flag resin was diminished in proportion to the reduction in FlagAID abundance, again consistent with the FlagAID/eEF1A interaction being stoichiometric (Fig. 2 D and E).
Recapitulation of the AID/eEF1A Interaction in Transfected Cells.
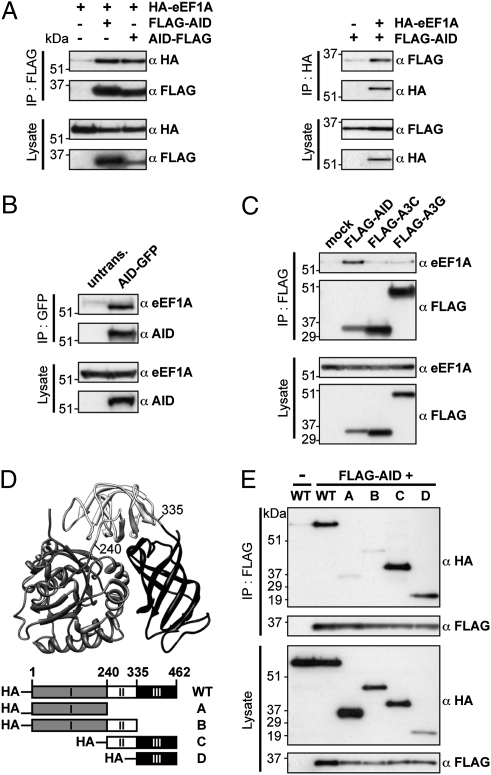
The AID/eEF1A interaction can be recapitulated in human kidney-derived 293T cells following coexpression of human versions of FlagAID and HA-tagged eEF1A. The FlagAID/HA-eEF1A complex can be brought down by antibodies to either the Flag or the HA tags (Fig. 3A). The interaction is similarly detected whether AID has the Flag tag at its N or C terminus (Fig. 3A). The AID/eEF1A association is also observed in human B-cell transfectants, where endogenous eEF1A is immunopreciptated by antibodies to transfected AID-GFP (Fig. 3B). The interaction between eEF1A and tagged AID is therefore independent of the nature or location of the tag.
Fig. 3.
AID/eEF1A interaction is mediated by domain 3 of eEF1A. (A) Human AID with a Flag tag at its N or C terminus (FLAG-AID and AID-FLAG, respectively) associates in 293T cells with cotransfected HA-tagged eEF1A as judged by anti-HA Western blotting of anti-Flag immunoprecipitates (Left) and by anti-Flag Western blotting of anti-HA immunoprecipitates (Right). (B) Stably transfected human AID C-terminally tagged with GFP (AID-GFP) associates with endogenous eEF1A as judged by anti-eEF1A Western blotting of anti-GFP immunoprecipitates of Ramos (GFP-AID) transfectants [RhA2/12B2 cells (28)] using untransfected Ramos as control. (C) Association of endogenous eEF1A with Flag-tagged AID, APOBEC3C, or APOBEC3G transfected into 293T cells was assessed by anti-eEF1A Western blotting of anti-Flag immunoprecipitates. (D) Structure of eEF1A (based on ref. 29) and depiction of truncation variants. (E) Anti-Flag immunoprecipitation of FlagAID from 293T cells cotransfected with various HA-tagged truncation variants of eEF1A reveals that the domain 3 of eEF1A is necessary and sufficient to mediate the interaction with AID.
An interaction with eEF1A is not a general feature of AID/APOBEC family members. Neither of the two cytosolic APOBEC3 family members tested (APOBEC3C and APOBEC3G) showed a similar interaction with eEF1A, as judged by Western blotting for endogenous eEF1A in immunoprecipitates of Flag-tagged APOBEC3s from lysates of 293T cell transfectants (Fig. 3C).
eEF1A Domain 3 Is Implicated in the AID Interaction.
eEF1A can, as judged by the structure of the yeast protein, be divided into three structural domains (Fig. 3D). On the basis of this finding, we designed three truncated variants of eEF1Α and discovered that the third (C-terminal) domain is both necessary and sufficient for interaction with cotransfected AID, as judged by coimmunoprecipitation experiments (Fig. 3E). This third domain of eEF1A is not directly implicated in either GTP or tRNA binding and, indeed, treatment with benzonase (an endonuclease active on both RNA and DNA) does not disrupt the AID/eEF1A interaction (Fig. 2C).
To confirm that the AID/eEF1A interaction takes place within intact cells, we asked whether FRET could be observed in HeLa transfectants between cyan and red fluorescent proteins that had been fused to eEF1A and AID, respectively. FRET was indeed obtained between the eEF1A and AID fusion proteins with the FRET (as well as the individual RFP-EF1A and GFP-AID) signals restricted to the cytoplasm (Fig. S4). Consistent with the coimmunoprecipitation analysis, removal of the third domain of eEF1A abolished the FRET signal.
Residues in AID Necessary for Interaction with eEF1A.
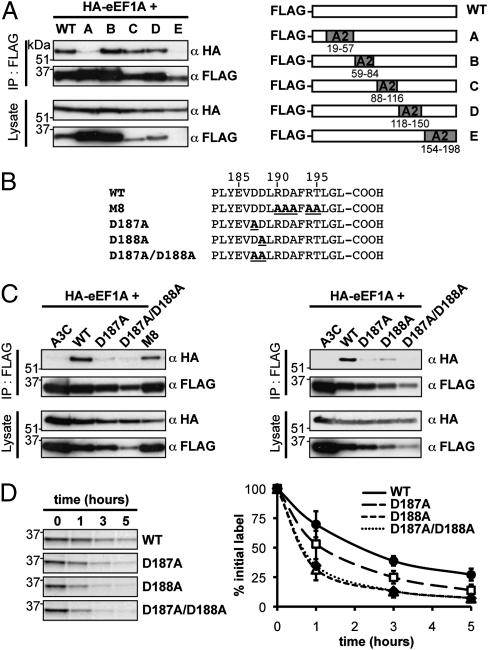
To gain insight into residues in AID necessary for eEF1A interaction, we analyzed a set of chimeric proteins in which selected regions of AID have been replaced by the homologous regions of APOBEC2. Chimeras B, C, and D, although expressed at different levels, all retain the ability to interact with eEF1A, whereas chimeras A and E have lost eEF1A interaction (Fig. 4A). Chimera A is well expressed and, like eEF1A, is found in the cytosol, consistent with previous findings that have identified residues in AID's N-terminal region necessary for its nuclear import (12). The N-terminal portion of AID therefore appears necessary (either indirectly or directly) for the eEF1A interaction. The interpretation of the result with chimera E is slightly less straightforward because the removal of the C-terminal region of AID [which also contains its nuclear export sequence (NES) as well as residues implicated in AID's cytosolic retention] results in AID destabilization (9, 12, 15).
Fig. 4.
Effects of mutations in AID that disrupt the eEF1A interaction. (A) Anti-Flag immunoprecipitation of FlagAID/APOBEC2 chimeras (12, 26) from 293T cells cotransfected with HA-eEF1A reveals that intact regions A and E are required for interaction with eEF1A. (B) Amino acid sequences of the C-terminal regions of the different FlagAID mutants used in this study. (C) Anti-Flag immunoprecipitation of mutant FlagAIDs from 293T cells cotransfected with HA-eEF1A shows that AID residues D187 and D188 are implicated in eEF1A interaction. (D) Pulse-chase analysis of the stability of FlagAID mutants in transfected 293T cells with each curve (Right) showing the mean and SD of at least two independent experiments.
To investigate the possible involvement of AID's specific NES in the eEF1A interaction, we asked whether the interaction was retained with a multiply mutated NES mutant (M8) that nevertheless confers nuclear export function and protein stabilization (15). The results reveal that AID's NES can be substantially altered without seriously jeopardizing the eEF1A interaction (Fig. 4 B and C).
We next focused on a pair of aspartate residues (D187 and D188) located immediately adjacent to AID's NES that have been implicated in AID's cytosolic retention (12). Mutation in either D187 or D188 led to a substantial reduction in the interaction with eEF1A (Fig. 4C). Although these mutations led to a destabilization of AID (which is particularly marked with the D188A and D187A/D188A double-mutants) (Fig. 4D), the reduced abundance of these mutants is not itself sufficient to account for the loss of detectable eEF1A in the immunoprecipitates. Rather, the mutations appear to disrupt the interaction with eEF1A, with diminished AID stability being a likely consequence.
Effect of AID (D187A) Mutation on AID Function.
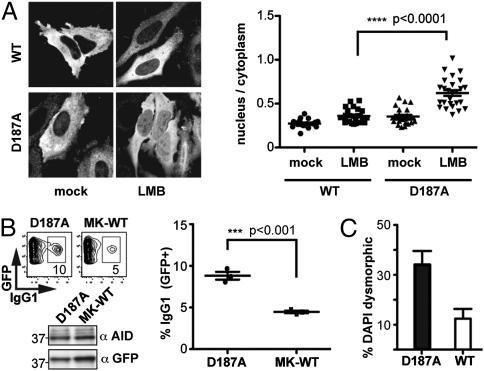
We were interested in ascertaining whether the disruption of the eEF1A interaction caused by the D187A mutation correlated with any alteration in AID function. Although Flag-tagged wild-type AID is normally nearly entirely cytoplasmic as judged by immunofluorescence, the tagged D187A mutant by comparison [and consistent with previous studies (12)] shows a significantly increased presence in the nucleus with the difference between the wild-type and D187A mutant being especially apparent following treatment with the export inhibitor leptomycin B (Fig. 5A).
Fig. 5.
Effect of AID [D187A] mutation on AID function. (A) Immunofluorescence analysis of HeLa cells transfected with FlagAID (after 3 h of mock or leptomycin B treatment) reveals increased nuclear localization of the D187A mutant. Representative images are shown on the left with quantification of the nuclear:cytoplasmic fluorescence signal from multiple images presented on the right. (B) Class-switching to IgG1 of B cells that have been transduced to express either AID(D187A), or a wild-type AID, with the latter translated from an mRNA with a mutated Kozak sequence (7) (MK-WT). The percentage of GFP+ cells that have switched to IgG1 is indicated within the FACS plots and is representative of three independent experiments with an average of 4.5 ± 0.15% for MK-WT and 8.8 ± 0.5% for the D187A mutant. Wild-type AID when more highly expressed (by use of an unmutated Kozak sequence) gave 14.0 ± 0.7% IgG1+ cells. The proportion of cells transduced to GFP+ was between 10% and 20% in all experiments. AID abundance was monitored by Western blotting. (C) Cytotoxicity of wild-type or D187A mutant AID expressed in HeLa cells as monitored 24 h after transfection. The histogram indicates the percentage of AID+ cells (identified by immunofluorescence) which show abnormal nuclear morphology (as revealed by DAPI staining). Numbers of blind scored AID+ cells per construct: wild-type (189), D187A mutant (138). Error bars indicate ± SEM.
We wondered if the diminished cytosolic retention of the D187A mutant might affect its efficacy in mediating class-switch recombination. Because this mutant is more rapidly degraded than the wild-type enzyme, we compared its ability to restore class-switching to AID-deficient B cells with that achieved using wild-type AID, but with the expression of the latter being reduced to match that of the D187A mutant by use of a mutated Kozak sequence (7). The results reveal that, consistent with its increased nuclear localization, the D187A mutant is more effective in mediating class-switching than the wild-type enzyme when expressed at similar levels (Fig. 5B). Furthermore, when overexpressed in HeLa cells, the D187A mutant also exhibits greater cytotoxicity than the wild-type enzyme, as judged by cellular nuclear morphology (Fig. 5C).
Nature of the Cytosolic AID Complex.
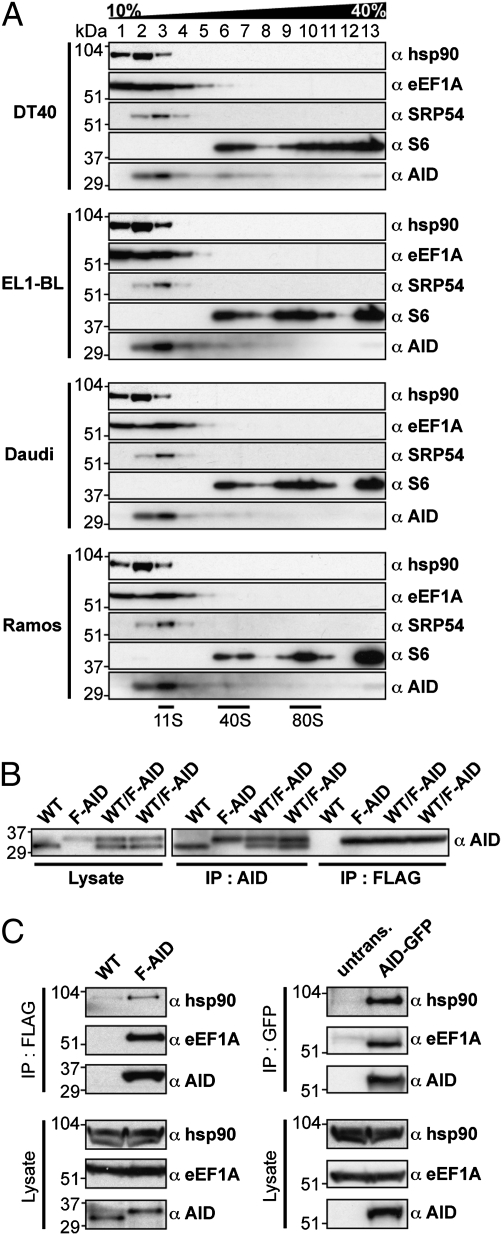
Whereas the results reveal a stable interaction between cytosolic AID and eEF1A, which is important for AID cytosolic retention, they do not allow us to conclude about the extent of the cytosolic AID complex. We therefore performed a sucrose gradient centrifugation of extracts from DT40 and human B-cell lines to gain insight into its size. The majority of AID is found in relatively low molecular weight fractions, consistent with a sedimentation coefficient of 10–11S (Fig. 6A). Some eEF1A is also found in this fraction, although most sediments are at a lower rate. Given that the signal recognition particle sediments at 11S and has a molecular weight around 340 kDa (16), it is likely that cytoplasmic AID is part of a complex that includes more than a single AID and single eEF1A molecule. We suspect however that the major endogenous cytoplasmic complex does not contain multiple AID subunits because immunoprecipitation of FlagAID from DT40 cells in which only one of the two AID alleles has been tagged does not also bring the wild-type (untagged) AID polypeptide down at the same time (Fig. 6B).
Fig. 6.
Sedimentation and association analysis of the cytosolic AID complex in cultured B cells. (A) Cell extracts from DT40, EL1-BL, Daudi, and Ramos cells were fractionated by centrifugation on 10–40% sucrose gradient and fractions subjected to Western blotting using antibodies to AID, SRP54, eEF1A, hsp90 and ribosomal protein S6. (B) Association between wild-type (WT) and endogenously-tagged AID (FlagAID) was monitored by immunoprecipitating FlagAID from WT/FlagAID heterozygous DT40 cells in which only one of the two AID alleles was Flag-tagged (Fig. S1). Extracts from WT/FlagAID heterozygous cells [as well as from wild-type DT40 CL18 (WT) and homozygous FlagAID knock-in (F-AID) control cells] were subjected to immunoprecipitation with either anti-Flag or anti-AID followed by Western blotting with anti-AID. (The two WT/F-AID samples represent two independent heterozygote clones). (C) Hsp90 is associated with FlagAID in DT40 cells (Left) and with AID-GFP in Ramos cells (Right).
The likelihood, therefore, is that there are other constituents of the cytoplasmic AID complex. One possible candidate is hsp90, which Orthwein et al. (17) have recently shown to associate with AID in Ramos transfectants. Indeed, we also find hsp90 associated with the endogenously-tagged AID in DT40 cells as well as transfected AID-GFP from Ramos by coimmunoprecipitation analysis (Fig. 6C) and, like eEF1A, some hsp90 is also found in the sucrose gradient fractions that contain AID (Fig. 6A). However, at present we have little insight into the stoichiometry of the hsp90/AID interaction and a description of the complete composition of the 11S cytoslic AID/eEF1A complex is clearly a topic for further investigation.
Discussion
The results reveal that AID in the cytoplasm forms part of a low molecular weight complex in which it is stoichiometrically associated with eEF1A, this interaction being mediated by eEF1A domain 3. Mutation of AID residue 187 disrupts the interaction with eEF1A and leads to the destabilization of AID as well as its increased translocation to the nucleus. Therefore, the results suggest that interaction with eEF1A likely contributes to AID's cytosolic retention and stabilization.
The abundance of AID in the nucleus appears to be under tight control. It will be interesting to discover whether the interaction of AID with eEF1A, as well as possibly with other components of the cytosolic retention complex, is regulated (e.g., by posttranslational modifications) so as to allow for control of its release for nuclear import. Perturbation of the interaction between AID and components of the cytosolic retention complex could obviously have implications for both antibody diversification and oncogenesis.
The findings described here extend on the moonlighting functions of eEF1A. Although its primary and ancestral role is in protein synthesis (where it delivers aminoacyl-tRNAs to the translating ribosome), eEF1A is abundant in the cytosol and has frequently been detected during purification of other cytosolic proteins, where it is often viewed as a contaminant. Indeed, Okazaki et al. (18) very recently noted the presence of eEF1A along with other proteins in a partially purified sample of tagged AID obtained from AID-overexpressing cells; bands with a mobility corresponding to that of eEF1A can also be discerned in other studies of overexpressed AID (17, 19). However, the results obtained in this work showing the presence of eEF1A at stoichiometric abundance in samples of AID prepared without overexpression along with the mutagenesis experiments reveal that the AID/eEF1A interaction is indeed physiological. Other functions proposed for eEF1A outside its role in the translation machinery (reviewed in refs. 20 and 21) include nuclear transport, protein turnover/quality control, and apoptosis, as well as in the replication of several positive-strand RNA viruses. eEF1A has also been found to bind actin, where it functions in the regulation of the actin cytoskeleton and cell morphology, with more than 60% of eEF1A being estimated to be bound to actin in living cells (22, 23). It could be that eEF1A's association with the cytoskeleton has significance with regard to the regulation of the intracellular trafficking of AID.
There is clearly more to be learned about the definition of the components of the 10S–11S AID/eEF1A complex. We believe that the major cytosolic AID complex likely contains only a single AID molecule because anti-Flag immunoprecipitation from cells carrying one Flag-tagged AID allele and one untagged allele only brings down FlagAID. This is not a definitive interpretation because the anti-Flag mAb could conceivably perturb the FlagAID/AID (or even FlagAID/eEF1A) interaction or dimerization. Furthermore, the interpretation need not extend to nuclear AID. Nevertheless, the most straightforward explanation is that the major cytosolic AID complex contains a single AID subunit, raising the issue of what the complex contains apart from AID and eEF1A. Assays of recombinant AID have suggested that it is likely associated with RNA (24). So far, using photocross-linking we have been unable to identify a specific AID-associated RNA, but this clearly does not rule out the existence of such an RNA. Orthwein et al. (17) have provided compelling evidence for an association of AID with hsp90. We certainly see hsp90 in immunoprecipitates of FlagAID (Fig. 6C), although a major hsp90 band does not stand out in our Coomassie stained SDS/PAGE analysis of immunopurified AID (Fig. 2A). It could be that an hsp90 band is obscured by other background (nonspecific) bands proteins present in the sample, that hsp90 has been substantially depleted during the washing steps, or simply that the hsp90 association is more transient or not one of high stoichiometry. Indeed, Orthwein et al. propose that the hsp90/AID complex is distinct from the complex that mediates AID's cytosolic retention. Thus, it could well be that AID shuttles between an eEF1A-containing complex (which contains the majority of cytosolic AID and which confers stabilization and cytosolic retention) and an hsp90-based chaperone complex as part of its course of intracellular trafficking. This theory is obviously speculative but emphasizes the importance in future work of elucidating the full nature of the 11S AID complex as well as identifying the mechanism by which cytosolic AID is released from its eEF1A-retention complex.
Materials and Methods
Generating DT40 Clones with Flag-Tagged AID Loci.
The AID genomic region, including 3 kb of flanking sequence on each side, was amplified from chicken genomic DNA to generate targeting constructs that included an in-frame Flag tag at an NcoI site created at the initiator ATG codon, as well as either LoxP-flanked puromycin or blasticidin expression cassettes inserted in a BamHI site created within the AID first intron (Fig. S1). NotI-linearized constructs were transfected into DT40 CL18 cells (BioRad GenePulser II, 25 μF, 550V) and correct targeting in drug-resistant clones analyzed by Southern blotting with 5′- and 3′-probes (Fig. S1). Drug-resistance cassettes were removed by transient Cre expression (Amaxa Nucleofector T).
Monitoring AID Activity.
AID activity in IgVλ gene conversion was assayed by determining the frequency of reversion of the targeted DT40 CL18 derivatives to a surface-IgM+ phenotype as previously described (13), analyzing 48 independent clones of each cell type after 21 d of clonal expansion. Class-switching was analyzed after 3 d of culture with LPS+IL4 following retroviral AID delivery to AID−/− B cells (7, 15). Cytosine-DNA deaminase activity of tagged AID purified on anti-Flag resin from DT40 cell extracts (5 × 108 cells) was assayed by incubation with a 5-fluoresceinated oligonucleotide substrate and gel electrophoresis following treatment with uracil-DNA glycosylase as previously described (25).
Purification of Flag-AID from DT40.
Cells (5 × 109) that had been grown for 60 h at 37 °C in RPMI containing 9% FBS, 1% chicken serum, 100 μM β-mercaptoethanol, and antibiotics were lysed for 30 min on ice in Lysis Buffer [20 mM Hepes (pH 7.5), 150 mM NaCl, 10% Glycerol, 0.2% Triton X-100, Complete proteinase inhibitors (Roche) and PhosStop (Roche)]. Following clarification, the lysate was incubated for 2 h at 4 °C with 200 μL tosyl-activated Dynabeads that had been coated with M2 anti-FLAG antibody (Sigma) according to the manufacturer's instructions. After washing five times in Wash150 buffer [20 mM Hepes (pH 7.5), 150 mM NaCl, 0.1% Triton X-100, complete proteinase inhibitors], bound proteins were eluted in 3xFlag peptide (200 ng/μL; Sigma). The eluted fraction was TCA-precipitated before being run on NuPage 4–12% Bis-Tris gel [along with prestained molecular weight markers (BioRad)] and transferred on to PVDF membrane or stained with InstantBlue (Expedeon). For experiments involving benzonase (Roche) treatment, the lysis and wash buffers were supplemented to 5 mM in MgCl2.
Monitoring Expression and Interactions in Transfected Cells.
For overexpression in mammalian cells, cDNAs were amplified using the appropriate oligonucleotides (Fig. S5) and inserted into pCi (Promega) -NheI/NotI. Expression constructs were transfected into 293T or HeLa cells (GeneJuice; Merck) and analyzed after 24 h. For protein association studies, cells were lysed in Lysis Buffer and clarified lysates incubated (2 h, 4 °C) with M2-coated Dynabeads for anti-Flag purification or with Protein A Dynabeads coated with either anti-HA (Abcam; ab16918), anti-GFP (ab290), or anti-AID (ab59361) antibodies. After five washes in Wash150 buffer for anti-AID and anti-GFP pulldowns, or in Wash500 buffer (as Wash150 but with 500 mM NaCl) for anti-Flag and anti-HA pulldowns, samples were resuspended in loading buffer and subjected to SDS/PAGE. For Western blotting, AID was detected either rabbit polyclonal (ab59361) or rat monoclonal (EK2 5G9; Cell Signaling) antibodies or, in the case of retrovirally transduced mouse B cells, with a mAb directed against an AID N-terminal peptide (26). eEF1A was detected with either rabbit polyclonal (ab37969) or mouse monoclonal (Millipore; CBP-KK1) antibodies, tubulin with antitubulin-HRP (ab40742), Flag tag with M2-HRP (Sigma), and HA tag with anti-HA-HRP (3F10; Roche). SRP54 was detected with monoclonal anti-SRP54 (610940; BD), ribosomal protein S6 with RPS6 antibody (A300-557A, Bethyl Laboratories), GFP with a goat polyclonal antibody (ab6663) and hsp90 with a mouse monoclonal antibody (ab13492).
For pulse–chase experiments, 293T cells that had been transiently transfected 24 h before were transferred into methionine-free DMEM for 1 h before a 1-h pulse with l-[35S]methionine (106 cells + 20 μCi per time point). Cells were then washed and incubated for the indicated times in DMEM supplemented to 1 mM in methionine. Clarified lysate was subjected to anti-Flag immunoprecipitation followed by SDS/PAGE and autoradiography. Signals were quantified on a Typhoon phosphorimager.
For immunofluorescence analysis, cells grown on cover-slips—and treated or not for 3 h in 20 ng/mL Leptomycin B (LC Laboratories)—were fixed with 4% paraformaldehyde, permeabilized in 0.5% Triton, stained with anti-Flag and goat anti mouse IgG-Alexa568 (Invitrogen) antibodies, counterstained with DAPI, and permanently mounted in Dabco-PVA. Slides were imaged in a Nikon Cl-si scanning microscope with 60× Plan-Apo Uv-corrected N.A. 1.4 objective and 3× digital zoom. The ratio of nuclear:cytoplasmic mean fluorescence was quantified from raw images using Fuji imaging software.
Velocity Sedimentation.
Clarified cell extracts (500 μL) in Lysis buffer supplemented to 5 mM in MgCl2 were layered onto a 12 mL continuous 10–40% sucrose gradient prepared in Wash150 buffer supplemented to 5 mM in MgCl2 and subjected to centrifugation (25,000 rpm; 16 h; 4 °C) in a SW40 rotor (Beckman Coulter). Thirteen 1-mL fractions were collected manually and aliquots subjected to SDS/PAGE and Western blotting.
Supplementary Material
Acknowledgments
We thank Julian Sale for his help in designing targeting constructs and FRET analysis, and Farida Begum and Maria Daly for assistance with MS protein identification and cell sorting, respectively. J.H. is supported by fellowships from the Swiss National Science Foundation (PBGEA-119331 and PA0033-121425) and from the Lady Tata Memorial Trust.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1106729108/-/DCSupplemental.
References
- 1.Maul RW, Gearhart PJ. AID and somatic hypermutation. Adv Immunol. 2010;105:159–191. doi: 10.1016/S0065-2776(10)05006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stavnezer J. Complex regulation and function of activation-induced cytidine deaminase. Trends Immunol. 2011;32:194–201. doi: 10.1016/j.it.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basu U, et al. The AID antibody diversification enzyme is regulated by protein kinase A phosphorylation. Nature. 2005;438:508–511. doi: 10.1038/nature04255. [DOI] [PubMed] [Google Scholar]
- 4.Pasqualucci L, Kitaura Y, Gu H, Dalla-Favera R. PKA-mediated phosphorylation regulates the function of activation-induced deaminase (AID) in B cells. Proc Natl Acad Sci USA. 2006;103:395–400. doi: 10.1073/pnas.0509969103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McBride KM, et al. Regulation of hypermutation by activation-induced cytidine deaminase phosphorylation. Proc Natl Acad Sci USA. 2006;103:8798–8803. doi: 10.1073/pnas.0603272103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatterji M, Unniraman S, McBride KM, Schatz DG. Role of activation-induced deaminase protein kinase A phosphorylation sites in Ig gene conversion and somatic hypermutation. J Immunol. 2007;179:5274–5280. doi: 10.4049/jimmunol.179.8.5274. [DOI] [PubMed] [Google Scholar]
- 7.McBride KM, et al. Regulation of class switch recombination and somatic mutation by AID phosphorylation. J Exp Med. 2008;205:2585–2594. doi: 10.1084/jem.20081319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng HL, et al. Integrity of the AID serine-38 phosphorylation site is critical for class switch recombination and somatic hypermutation in mice. Proc Natl Acad Sci USA. 2009;106:2717–2722. doi: 10.1073/pnas.0812304106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brar SS, Watson M, Diaz M. Activation-induced cytosine deaminase (AID) is actively exported out of the nucleus but retained by the induction of DNA breaks. J Biol Chem. 2004;279:26395–26401. doi: 10.1074/jbc.M403503200. [DOI] [PubMed] [Google Scholar]
- 10.McBride KM, Barreto V, Ramiro AR, Stavropoulos P, Nussenzweig MC. Somatic hypermutation is limited by CRM1-dependent nuclear export of activation-induced deaminase. J Exp Med. 2004;199:1235–1244. doi: 10.1084/jem.20040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito S, et al. Activation-induced cytidine deaminase shuttles between nucleus and cytoplasm like apolipoprotein B mRNA editing catalytic polypeptide 1. Proc Natl Acad Sci USA. 2004;101:1975–1980. doi: 10.1073/pnas.0307335101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patenaude AM, et al. Active nuclear import and cytoplasmic retention of activation-induced deaminase. Nat Struct Mol Biol. 2009;16:517–527. doi: 10.1038/nsmb.1598. [DOI] [PubMed] [Google Scholar]
- 13.Buerstedde JM, et al. Light chain gene conversion continues at high rate in an ALV-induced cell line. EMBO J. 1990;9:921–927. doi: 10.1002/j.1460-2075.1990.tb08190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metzner M, Schuh W, Roth E, Jäck HM, Wabl M. Two forms of activation-induced cytidine deaminase differing in their ability to bind agarose. PLoS ONE. 2010;5:e8883. doi: 10.1371/journal.pone.0008883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geisberger R, Rada C, Neuberger MS. The stability of AID and its function in class-switching are critically sensitive to the identity of its nuclear-export sequence. Proc Natl Acad Sci USA. 2009;106:6736–6741. doi: 10.1073/pnas.0810808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walter P, Blobel G. Purification of a membrane-associated protein complex required for protein translocation across the endoplasmic reticulum. Proc Natl Acad Sci USA. 1980;77:7112–7116. doi: 10.1073/pnas.77.12.7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orthwein A, et al. Regulation of activation-induced deaminase stability and antibody gene diversification by Hsp90. J Exp Med. 2010;207:2751–2765. doi: 10.1084/jem.20101321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okazaki IM, et al. Histone chaperone Spt6 is required for class switch recombination but not somatic hypermutation. Proc Natl Acad Sci USA. 2011;108:7920–7925. doi: 10.1073/pnas.1104423108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeevan-Raj BP, et al. Epigenetic tethering of AID to the donor switch region during immunoglobulin class switch recombination. J Exp Med. 2011;208:1649–1660. doi: 10.1084/jem.20110118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ejiri S. Moonlighting functions of polypeptide elongation factor 1: From actin bundling to zinc finger protein R1-associated nuclear localization. Biosci Biotechnol Biochem. 2002;66:1–21. doi: 10.1271/bbb.66.1. [DOI] [PubMed] [Google Scholar]
- 21.Mateyak MK, Kinzy TG. eEF1A: Thinking outside the ribosome. J Biol Chem. 2010;285:21209–21213. doi: 10.1074/jbc.R110.113795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edmonds BT, Murray J, Condeelis J. pH regulation of the F-actin binding properties of Dictyostelium elongation factor 1 alpha. J Biol Chem. 1995;270:15222–15230. doi: 10.1074/jbc.270.25.15222. [DOI] [PubMed] [Google Scholar]
- 23.Gross SR, Kinzy TG. Translation elongation factor 1A is essential for regulation of the actin cytoskeleton and cell morphology. Nat Struct Mol Biol. 2005;12:772–778. doi: 10.1038/nsmb979. [DOI] [PubMed] [Google Scholar]
- 24.Bransteitter R, Pham P, Scharff MD, Goodman MF. Activation-induced cytidine deaminase deaminates deoxycytidine on single-stranded DNA but requires the action of RNase. Proc Natl Acad Sci USA. 2003;100:4102–4107. doi: 10.1073/pnas.0730835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang M, Yang Z, Rada C, Neuberger MS. AID upmutants isolated using a high-throughput screen highlight the immunity/cancer balance limiting DNA deaminase activity. Nat Struct Mol Biol. 2009;16:769–776. doi: 10.1038/nsmb.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conticello SG, et al. Interaction between antibody-diversification enzyme AID and spliceosome-associated factor CTNNBL1. Mol Cell. 2008;31:474–484. doi: 10.1016/j.molcel.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Arakawa H, Hauschild J, Buerstedde JM. Requirement of the activation-induced deaminase (AID) gene for immunoglobulin gene conversion. Science. 2002;295:1301–1306. doi: 10.1126/science.1067308. [DOI] [PubMed] [Google Scholar]
- 28.Rada C, Jarvis JM, Milstein C. AID-GFP chimeric protein increases hypermutation of Ig genes with no evidence of nuclear localization. Proc Natl Acad Sci USA. 2002;99:7003–7008. doi: 10.1073/pnas.092160999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersen GR, et al. Structural basis for nucleotide exchange and competition with tRNA in the yeast elongation factor complex eEF1A:eEF1Balpha. Mol Cell. 2000;6:1261–1266. doi: 10.1016/s1097-2765(00)00122-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.