Abstract
The conservation of crop genetic resources requires understanding the different variables—cultural, social, and economic—that impinge on crop diversity. In small-scale farming systems, seed exchanges represent a key mechanism in the dynamics of crop genetic diversity, and analyzing the rules that structure social networks of seed exchange between farmer communities can help decipher patterns of crop genetic diversity. Using a combination of ethnobotanical and molecular genetic approaches, we investigated the relationships between regional patterns of manioc genetic diversity in Gabon and local networks of seed exchange. Spatially explicit Bayesian clustering methods showed that geographical discontinuities of manioc genetic diversity mirror major ethnolinguistic boundaries, with a southern matrilineal domain characterized by high levels of varietal diversity and a northern patrilineal domain characterized by low varietal diversity. Borrowing concepts from anthropology—kinship, bridewealth, and filiation—we analyzed the relationships between marriage exchanges and seed exchange networks in patrilineal and matrilineal societies. We demonstrate that, by defining marriage prohibitions, kinship systems structure social networks of exchange between farmer communities and influence the movement of seeds in metapopulations, shaping crop diversity at local and regional levels.
Keywords: seed transmission, social reproduction, traditional economic systems
In smallholder farming systems, farmers maintain a large diversity of cultivated species and recognize many different types (landraces) within each of their crops. Farmers dynamically manage this agricultural biodiversity by continually collecting, testing, and selecting new strains with unusual and interesting traits, sourcing “seeds” (here understood as propagules, i.e., true seeds, tubers, rhizomes, or stem cuttings) through different networks, most of which involve exchanging germplasm with other farmers.
Several studies have highlighted the importance of informal seed exchange networks as an essential component of the resilience of local farming systems (1, 2), e.g., in potato (3), maize (4, 5), and manioc (6–8). Exchanging seeds not only allows farmers to obtain new landraces but also to recover lost types or to renew their stock of seeds (1). Through these exchange networks, farmers compile highly diversified portfolios of landraces capable of buffering the effects of unpredictable environmental changes (2). Seed exchange represents a key mechanism in the dynamics of crop genetic diversity (9–11). However, the rules that channel the movement of seeds within and among farming communities have received little attention from geneticists and ethnobotanists alike (12).
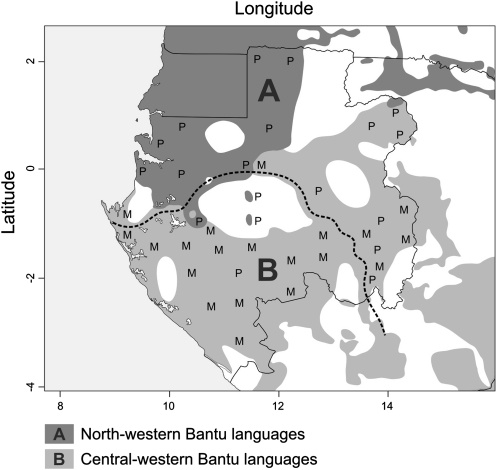
In small-scale farming communities, circulation of crop landraces is often channeled by marriage networks (7, 8, 13, 14). By defining marriage prohibitions, kinship systems structure social networks of exchange between kin (related by descent) and affines (related by marriage). Kinship systems thus determine the connectivity of farmer populations, and, in extenso, by favoring or limiting exchanges between communities, they also determine the connectivity of their crop (15) or livestock (16) populations. Kinship systems are cultural conceptualizations of relationships between individuals, based on the notion of descent and clan membership. Patrilineal systems, in which the clan is transferred through the male line, are the most common type of social organization among human societies (17). In Central Africa, however, matrilineal systems tend to predominate (18). Matrilineal societies are found within an area spreading from Gabon eastward across all of Africa through the Congos, Zambia, Malawi, and Tanzania. In Gabon, the Ogooué River is the line of demarcation between the patrilineal domain and this central African “matrilineal belt” (Fig. 1). The river also corresponds to the boundary between the linguistic zones A and B according to Guthrie's classification of Bantu languages (19). Whereas most populations located south of the Ogooué, in zone B, are organized in matrilineal descent groups, populations of zone A, north of the river, are predominantly patrilineal (17).
Fig. 1.
Major linguistic groups in Gabon and geographical distribution of patrilineal (P) and matrilineal (M) groups on either side of the Ogooué River (dashed line).
This work analyzes how kinship systems impinge on the dynamics of crop genetic diversity by investigating the relationships between marriage exchanges and seed exchange networks. We focus on manioc (Manihot esculenta Crantz), a clonally propagated crop introduced from Brazil into Africa in the 16th century (20). Manioc in Africa is traditionally considered a “woman's crop.” Whereas clearing and burning plots are tasks generally carried out by men, most agricultural tasks and manioc food processing are performed by women, who are also responsible for selecting planting material and managing landraces.
On-farm gender division of labor means that women spend substantially more time than men interacting directly with the plants, learning to recognize, characterize, and classify the diversity of types they maintain in their farms. This gender-asymmetric person–plant relationship widens the gap between men and women in their respective folk taxonomical expertise (21), and examples of women's distinctive, often superior, folk taxonomical knowledge exist not only for manioc (22) but also for bean (23), maize (24), and potato (25). Because knowledge is necessary for the maintenance and management of crop landraces, expertise gives women authority, and women often play a leading role in seed exchanges. Analyzing the social and cultural determinants of seed exchange between farmer communities therefore requires adopting a gender-sensitive approach that recognizes the role of women as the main vectors of diffusion of crop landraces.
Drawing from a large cross-cultural comparison of manioc farming systems in Gabon, we combined ethnobotanical and molecular genetic studies to analyze the rules that structure social networks of seed exchange and their impact on manioc genetic diversity. We unravel the intricate relationship between kinship systems, transmission patterns of “heirloom” landraces, and the dynamics of crop genetic diversity.
Results and Discussion
Structure of Manioc Genetic Diversity in Gabon.
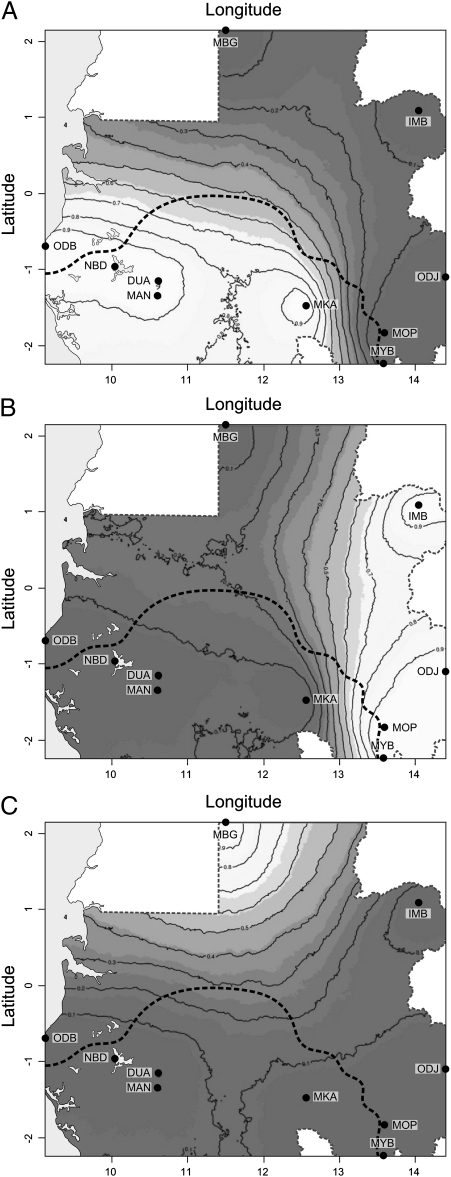
Manioc diversity in Gabon was assessed through a series of village-level studies of manioc farming systems. The study covered 10 communities presenting marked cultural, agroecological, and socioeconomic contrasts (Dataset S1). The number of manioc landraces at the community level was high, with an average of 37 landraces per village, but there were also strong regional disparities, with values ranging from 3 [Mbong-Ete (MBG)] to 60 [Odjouma (ODJ)] (Table 1). To detect whether this regional variability reflected discontinuities in manioc genetic diversity, a Bayesian clustering method was applied to the dataset. Assuming uncorrelated allelic frequencies between sites, Geneland inferred three distinct genetic clusters, with spatial discontinuities reflecting major ethnolinguistic boundaries (Fig. 2): (i) a southwestern cluster, which encompassed all populations located on the left bank of the Ogooué River [Odimba (ODB), Nombedouma (NBD), Douani (DUA), Mandilou (MAN), and Makoula (MKA); languages of group B, predominantly matrilineal]; (ii) an eastern cluster, which encompassed all populations from the B linguistic group located on the right bank of the Ogooué [Imbong (IMB), ODJ, Mopia (MOP), and Mouyabi (MYB); predominantly patrilineal, except for ODJ]; and (iii) a northern cluster, which encompassed populations from the A linguistic group (MBG; patrilineal). All three clusters showed low but significant genetic differentiation, with higher FST values between the northern cluster and each of the other two clusters (Table 2). Allelic diversity was also significantly lower in the northern cluster than in the southwestern and eastern clusters (one-way ANOVA followed by Tukey's post hoc test: F = 311.6, df = 2, P < 10−4).
Table 1.
Characteristics of the 10 communities sampled for manioc diversity in Gabon
| Community (code) | Rules of descent* | H† | L‡ | N§ |
| Douani (DUA) | 3M | 15 | 32 (18) | 55 |
| Imbong (IMB) | 3P | 21 | 26 (12) | 21 |
| Mandilou (MAN) | 2M | 18 | 50 (15) | 54 |
| Mbong-Ete (MBG) | 1P | 28 | 3 (3) | 59 |
| Makoula (MKA) | 1M/1X | 16 | 27 (17) | 41 |
| Mopia (MOP) | 5P/3M | 21 | 46 (10) | 24 |
| Mouyabi (MYB) | 1X | 15 | 36 (14) | 24 |
| Nombedouma (NBD) | 1M | 14 | 48 (34) | 49 |
| Odimba (ODB) | 1P/3M | 12 | 40 (19) | 39 |
| Odjouma (ODJ) | 1M | 31 | 60 (24) | 75 |
*Number of groups with patrilineal (P), matrilineal (M), or mixed (X) descent (details in Dataset S1).
†Number of households surveyed.
‡Number of landraces recorded (number of landraces genotyped). Numbers are corrected for the presence of synonymies and omit ‘unnamed’ landraces (6 recorded in ODB, 3 in NBD, 26 in MOP, 5 in MYB, 3 in IMB, and 2 in ODJ).
§Sample size after removal of clonal replicates.
Fig. 2.
Maps of posterior probabilities of population membership in the southwestern (A), eastern (B), and northern (C) clusters inferred by Geneland. Maps are based on the highest-probability run at a value of K = 3. Contour lines indicate the spatial position of genetic discontinuities. Lighter shading indicates higher probabilities of population membership.
Table 2.
Pairwise FST and summary statistics for the northern, eastern, and southwestern clusters inferred by Geneland
| Pairwise FST‡ |
|||||||
| Cluster | N* | Heterozygosity† (HE/HO) | FIS‡ | § | Private alleles | Eastern | Northern |
| Southwestern | 238 | 0.645/0.701 | −0.048 | 5.29 (0.32) | 2 | 0.018 | 0.062 |
| Eastern | 144 | 0.656/0.736 | −0.118 | 5.59 (0.25) | 2 | 0.058 | |
| Northern | 59 | 0.590/0.658 | −0.106 | 4.81 (0.07) | 0 | ||
*Number of distinct multilocus genotypes (MLGs).
†Expected heterozygosity (HE)/observed heterozygosity (HO).
‡All P < 0.01.
§Allelic richness (SD).
Results of the Bayesian approach support a three-pronged introduction of manioc into Gabon: from Cameroon (northern cluster), from Congo (eastern cluster), and from the coast, spreading along the Ogooué River (southwestern cluster). Patterns are congruent with linguistic data (26) and suggest that spatial discontinuities of manioc genetic diversity may be the signature of the different historical routes of manioc diffusion into Gabon. However, the persistence of these geographical patterns indicates that barriers must have impeded seed flow, possibly by limiting connectivity among farmer communities.
In Gabon, the Fang represent approximately one-third (∼600,000) of the total population. They form a large, homogeneous, patrilineal cluster that extends over most of northern Gabon, Equatorial Guinea, and southern Cameroon. Gabon is home to ∼50 different Bantu tribes, and although most of the country is characterized by a high cultural diversity, northern Gabon has remained exclusively Fang since the late 18th century (27), constituting an area of strong ethnic homogeneity with low to no penetration by other cultures. Approximately 99% of the population is Fang (28), suggesting that marriages are preferentially endogamous. Alexandre (27) wrote about an “ethnological boundary” between the Fang and their southern matrilineal neighbors. This line of demarcation between distinct cultural areas was also reflected in regional patterns of manioc genetic diversity. Northern Gabon subsumed not only the lowest ethnic diversity but also the lowest manioc varietal diversity, with almost a 10-fold variation in varietal diversity between the northern cluster and the eastern and southwestern clusters (Fig. S1).
Deciphering Regional Patterns of Diversity.
Several factors may influence farmers’ choice to maintain high or low numbers of landraces in their farms. Low diversity in northern Gabon may reflect a stern selection of the most productive landraces dictated by strong market orientations, a cultural choice, or simply a lack of useful diversity. In MBG, IMB, ODB, and, to a lesser extent, MAN and ODJ, manioc farming was the main economic activity, but levels of varietal diversity were not related to the local economic importance of manioc (Dataset S1). Diachronic data, conversely, show that there has been little increase of the regional varietal portfolio since colonial administrations encouraged manioc farming in northern Gabon in the 1890s–1910s (SI Text), suggesting that the narrow range of named diversity stems in part from historical contingencies. Given the considerable diversity available at the country scale [between 300 and 400 landraces (29)], the strong regional contrast between the northern cluster and the southwestern and eastern clusters suggests that cultural factors have curbed the inflow of manioc landraces.
To distinguish between the remaining hypotheses—a cultural choice or a lack of useful diversity—we investigated local seed exchange networks, asking farmers to specify how, where, and from whom they acquired their manioc landraces (Table 3). In most communities, we found that farmers grew a “core” collection of landraces bequeathed by their mother or mother-in-law, which they frequently enriched by soliciting cuttings from relatives or neighbors (horizontal exchanges). However, whereas vertical transmission (mother to daughter) was predominant in matrilineal societies, affinal transmission (i.e., the transfer of landraces from mother-in-law to daughter-in-law) was characteristic of patrilineal societies and predominant among the Fang (MBG), where 80% of the farmers interviewed had received their manioc cuttings from their mother-in-law; the only farmers to have received cuttings from their mother were women born in MBG and who were either unmarried or derogated to the rule of virilocal postmarital residence (Table S1).
Table 3.
Rules of descent and seed transmission strategies
| Modes of transmission† |
||||||||||||
| Community | Rules of descent* | M | MM | Z | HM | HMM | HZ | R | C | N | O | Pg |
| DUA | 3M | 0.93 | 0.07 | |||||||||
| IMB | 3P | 0.24 | 0.43 | 0.10 | 0.14 | 0.29 | 0.05 | |||||
| MAN | 2M | 0.44 | 0.06 | 0.06 | 0.17 | 0.39 | ||||||
| MBG | 1P | 0.21 | 0.79 | |||||||||
| MKA | 1M/1X | 0.94 | 0.06 | |||||||||
| MOP | 5P/3M | 0.24 | 0.05 | 0.10 | 0.19 | 0.05 | 0.10 | 0.05 | 0.10 | 0.24 | 0.14 | |
| MYB | 1X | 0.40 | 0.33 | 0.13 | 0.20 | |||||||
| NBD | 1M | 0.93 | 0.07 | 0.29 | 0.21 | 0.21 | 0.36 | |||||
| ODB | 1P/3M | 0.33 | 0.25 | 0.42 | 0.67 | |||||||
| ODJ | 1M | 0.87 | 0.68 | 0.32 | ||||||||
*Number of groups with patrilineal (P), matrilineal (M), or mixed (X) descent (details in Dataset S1).
†M, mother; MM, grandmother (mother's mother); Z, sister; HM, mother-in-law (husband's mother); HMM, grandmother-in-law (husband's mother's mother); HZ, sister-in-law (husband's sister); R, other relatives; C, Rivale (concubine in polygynous households); N, neighborhood; O, other villages; Pg, development program. Values reported in the table indicate the percentage of farmers mentioning each source (several sources are possible).
All populations in Gabon are structured in virilocal exogamous lineages, forcing men to seek wives outside their community while women move out after marriage to settle in the village of their husband. In matrilineal societies, where transmission of manioc clones is vertical, virilocality stimulates the exchange of manioc landraces between villages. When they move to the village of their husband, in-marrying women bring along with them a few cuttings from their mother's farm, thereby contributing to enrich varietal diversity at the community level. Women thus become the main vectors of the diffusion of manioc landraces, and by exchanging wives, communities also exchange clones. Among the Fang, however, the bride moves empty-handed to the village of her husband. Affinal transmission, as opposed to vertical transmission, thus means that in patrilineal virilocal societies, there is no inflow of manioc cuttings accompanying the inflow of women.
Seed Transmission as a Risk-Aversion Strategy.
Divergences in rules of generational transmission of clones therefore seem likely to have played an important role in shaping regional disparities in levels of manioc genetic diversity in Gabon. Exchanging cuttings is common practice among manioc farmers (6, 7, 13). Farmers seek new landraces to try, and when they spot an interesting type in a neighbor's garden, they ask for a few cuttings to experiment with. Although the majority of exchanges of germplasm occurred within the family, horizontal exchanges represented an important alternative source of diversity in most communities in Gabon, except in MBG, where farmers depended exclusively on the landraces they inherited from their mother-in-law or, for a minority, from their mother.
Analyses of the distribution of genotypic diversity among farmers in MBG clearly illustrated the predominance of affinal ties in local networks of seed exchange. Because manioc is propagated by stem cuttings, with the occasional (conscious or accidental) recruitment of self-sown manioc volunteer seedlings (30), manioc landraces tend to consist, at the community level, of one largely dominant clone and few to several minor multilocus genotypes (MLGs). In MBG, all farmers grew the same set of three landraces (“Adzoro”, “Esobo-Nku”, and “Afouba-Mbõng”). Although we found only one major clone of Esobo-Nku (G22) and one major clone of Afouba-Mbõng (G10), we found that two major clones of Adzoro, G5 and G14, coexisted in the village (Fig. S2). The two clones were not randomly distributed among farmers, and farmers grew either G5 or G14, but never a mixture of the two (Fig. S3). In fact, the distribution of G5 and G14 closely paralleled farmers’ kin relationships, with groups of genotypic uniformity corresponding to affinal chains of transmission (Fig. S4).
Risk aversion is a major force driving farmers’ behavior and decisions concerning varietal diversity in smallholder farming systems. Trust, in particular, is an important factor in social networks of seed exchange (1, 9). Trust orientates farmers’ choice of seed providers, and farmers tend to choose them among relatives or people with whom they are socially connected (9) or whose folk taxonomical expertise they recognize. In Peru, Boster (6, 22) gave a clear illustration of the interdependence of these two components of the exchange—material and cultural—among the Aguaruna Jívaro. Comparing farmers’ aptitudes in identifying manioc landraces, Boster showed that agreement between farmers is dependent upon the “social context of learning.” The predominance of uxorilocality among the Aguaruna favors vertical transmission of folk taxonomical knowledge and clones, and Boster found that agreement among kin-related farmers was highest between mother and daughter (22). Daughters learn to differentiate manioc landraces from their mothers, and at marriage, the first clones a woman cultivates are those she took from her mother's garden. However, Boster also showed that where the spouses chose instead a virilocal residence, the incoming bride received most of her clones from female in-law kin, and Boster found highest agreement in naming manioc landraces between sisters-in-law (22). By fostering cultural consensus, seed transmission creates social bonds that reinforce the relationship between the bride and her in-laws and strengthen social cohesion.
A lack of accessible and reliable information on seeds, conversely, can deter farmers from sourcing landraces outside the domestic sphere. Initial access to seed but also to information is especially critical for young farmers. By favoring the continuation of heirloom landraces within the clan through a codified system of transmission of manioc clones, farmers ensure access to seeds and knowledge and circumvent potential risks, such as viruses, pests, and other pathogens, associated with external sources of germplasm. In a virilocal system, affinal transmission allows farmers to easily control the seed system. In matrilineal societies, the continuous inflow of manioc landraces through marriage networks contributes to increase varietal diversity at the community level, but farmers may have little control over the seeds that their daughters-in-law bring into the community. The choice of Fang farmers to opt for affinal transmission, therefore, could reflect a strategy to control seed exchange networks. On the downside, affinal transmission deprives farmers of an important source of diversity. By constraining manioc genetic diversity within the boundaries of the village and by disconnecting villages from regional exchange webs, affinal transmission cancels the “buffer effect” (31) of dynamic exchanges of planting material, putting manioc genetic diversity at risk of erosion.
Compounding the limited inflow of new landraces at the local level, the strong ethnic homogeneity and prevalent endogamy of the Fang probably contributed to the isolation of manioc from northern Gabon as an independent genetic cluster. The attitude of the Fang to sourcing diversity contrasts with that of other communities, not only in that the Fang show little interest in trying new landraces, but also in that their practices prevent any exchanges of germplasm between clans. Similar patterns of restricted exchanges between clans were reported for peanut seeds among the Ntumu in Cameroon (32). This form of “endo-agriculture” is reminiscent of Melanesian societies who “exchange” wives but proscribe exchanges of yam seeds between matrilines, as a way to compensate for clanic exogamy (33). Hence, rather than a form of control of the seed system, affinal transmission may instead represent a form of social control exerted by women on their daughters-in-law. Understanding divergences in rules of seed transmission between matrilineal and patrilineal societies, therefore, requires also understanding the social significance of seed transmission.
From Clonal Propagation to Social Reproduction.
In matrilineal societies, lineage and manioc clones are both transmitted directly along the female line. In patrilineal societies, however, the reproduction of the lineage depends on women who are not related to the clan they perpetuate. Descent group membership thus results from a social construct of relatedness rather than from an actual genetic bond. The challenge, therefore, is to maintain the coherence of the group while recruiting women from outside the community and relying on these “alien” elements to ensure the reproduction of the lineage.
Affinal transmission of manioc clones follows directly from this “patrilineal puzzle” (34). Because manioc belongs to the feminine realm, a man cannot inherit manioc clones; however, neither can clones be transmitted to his sister, because when she marries, she leaves the community and detaches from her paternal lineage to attach herself to the lineage of her husband. Affinal transmission is the result of this instability of the female lineage in patrilineal societies; to maintain clones within the clan through the female line, clones must be transmitted along affinal chains, from mother-in-law to daughter-in-law.
Entrusting manioc clones to the younger generation, however, involves more than sharing germplasm or knowledge, and when a newly wed woman plants the clones she received from her mother or mother-in-law, she reproduces a family scheme, assuming her new role and taking on her share in the sexual division of domestic chores. The transmission of manioc clones, as the basic elements of the production system that structures the household economy, becomes the medium through which the older generation teaches the new wife her role and position in the society; with manioc clones come responsibilities—to one's husband, to one's children, and to one's clan. In other words, through the transmission and multiplication of manioc clones, it is not only the genotypes that are reproduced, but also the structure of society. Through this form of social reproduction (35), the transmission of manioc cuttings thus appears to be intrinsically connected with the social conventions that govern the transmission of lineage.
Among the Fang, the arrival of a new bride in the village is traditionally supervised by her mother-in-law, who sees to her integration into the community. This “tutelage” by the mother-in-law is a regular feature of patrilineal virilocal societies (36) and reflects the transfer of knowledge and skills that accompanies the initiation of the in-marrying woman to her new role and position in the clan of her husband. Manioc clones, but also peanut seeds (32), are only some of the elements that women transmit to their daughters-in-law in Fang societies. Their transmission denotes a change in the social and jural status of the bride. With the clones she received from her mother-in-law, the young bride will cultivate the land owned by her husband. Affinal transmission of manioc clones thus becomes a form of devolution to the young wife of cultivation rights over the land owned by her husband, thereby evading the paradoxical situation where women control the crops but not the land they cultivate (37). Here, the transmission of manioc clones enacts a transfer of clan membership.
By transforming affines into consanguines, affinal transmission thus participates in preserving social coherence. When marriage involves members of distinct patrilinear virilocal ethnic groups, however, this transfer of clan membership can eventually result in the bride cutting off all ties with her kinsmen, and vertical transmission may be favored instead to preserve the maternal social bond. The substantial levels of vertical transmission we found in mixed communities of patrilineal descent (IMB, MOP, MYB; Table 3) suggest that alternative strategies of social reproduction prevail where cultural diversity and geographic proximity favor intermarriage. Marriages between patrilineal and matrilineal societies, in particular, pose important problems in terms of clan membership and inheritance. Changes in descent rules resulting from patrilineal/matrilineal (P/M) boundary issues have been documented in Ghana (38), but also in the past in Gabon (39, 40). Because of the potential problems they raise, marriages between patrilineal and matrilineal groups are usually avoided, resulting in “inheritance boundaries” (38). In Gabon, the Ogooué River forms a natural barrier between the patrilineal and matrilineal domains, but around Libreville, La Lopé, and Franceville, societies with opposite systems of inheritance cohabit. Restricted marriage exchanges between patrilineal and matrilineal communities could have contributed to limit seed exchanges across P/M boundaries, maintaining geographical patterns of manioc diversity that reflect preferential marriages among populations with compatible rules of inheritance.
Seed Transmission as a Grand-Maternal Strategy.
In most patrilineal societies, the family-in-law must pay a “bride-price” in return for the transfer of jural authority over the bride. Although seeds do not enter into the composition of this bridewealth, divergences in rules of seed transmission between patrilineal and matrilineal systems reflect a difference in the investment of grandparents in the offspring of the marriage. In matrilineal virilocal societies, marriage payments tend to be rare or absent. Because filiation is handed down through the female line, daughters remain attached to their maternal kin after marriage, and children belong to their maternal clan. In patrilineal virilocal societies, in contrast, the parents of the groom must repay the parents of the bride for the acquisition of rights in genetricem—that is, the right to filiate the children to be born to their daughter-in-law (41). For mothers, however, the corollary is the loss of rights over their own daughters’ children. Seen from the mothers’ point of view, it is therefore better to “invest” in a daughter-in-law than in a daughter. In other words, it is the investment in grandchildren that determines the sense of the flow of seeds.
Seeds thus indirectly enter into the equation that determines economic reciprocity in marital transaction. Because they are necessary to convert land into wealth, seeds, like land, are a key element of the system of production and form part of the “capital” that farmers want to transmit to their (grand)children. Rules of seed transmission have emerged from the necessity to ensure the perpetuation of the clan, through the replication of the family as a productive and reproductive unit. This intricate connection between production and reproduction forms the basis of economic systems of traditional societies (35) and structures social organization in many societies, not only in Africa (42) but also in Amazonia (43) and Melanesia (44), explaining why seed exchange is often interrelated with marriage exchange (7, 8, 13, 14, 32, 33, 45).
Affinal transmission has been described among other patrilineal societies, e.g., the Makina and the Shake in Gabon (46), but also among the Baniwa, Baré, and Tukano Amazonian tribes (45). Compared with African societies, however, Amerindian societies present a greater variety of social structures. Cognatic systems, where descent is traced through both paternal and maternal lineages, are more widespread in Amazonia. Amerindians also have different conceptualizations of filiation and different material cultures of matrimonial exchange (47, 48). Unlike in Africa or in Melanesia, there is no principle of substitutability of women for wealth; the payment of a bride-price, through which a man “buys a woman's womb” (49), is seldom practiced in Amazonia, and generally bride service is substituted to the payment of bridewealth (47). Associated with intervals of postmarital uxorilocal residence, bride-service favors mixed-inheritance systems where manioc clones are inherited from both the mother and the mother-in-law (43).
Given the complexity and diversity of social structures, the influence of marriage exchanges on regional seed flow is likely to vary with the social significance of the crop, i.e., its local cultural and economic importance, but also its “gender”—that is, whether the plant is socially considered a men's or a women's crop. Interpreting seed transmission as an epiphenomenon of the economic system of traditional societies, however, provides a conceptual framework that can help untangle the intricate relationship between marriage exchanges and seed exchanges and predict the direction and impact of seed movements among and within communities.
Conclusions
Little attention has been paid to the central role of strategies of social reproduction in shaping crop genetic diversity at local and regional levels. However, by connecting or disconnecting human populations, kinship systems can influence the genetic structure of crop populations just as they influence genetic diversity in human populations.
In traditional societies, the continuity of ethnic identity depends upon the maintenance of cultural boundaries that define ascription of a member to the group and exclusion of others (50). The continuation of these boundaries in time implies, however, the preservation of a set of cultural norms and values that mark out the difference between members and nonmembers—i.e., rules of cultural transmission. While maintaining genetic boundaries between ethnic groups (51–53), kinship systems also ensure the perpetuation of these cultural norms and values.
Manioc clones and, more generally, seeds (e.g., potato tubers, sorghum panicles, maize kernels, or sweet potato vines), are socially salient items that participate in the construction of cultural identity by assigning gender roles and normalizing social interactions. By promoting the maintenance of manioc heirloom landraces within the bounds of the lineage, seed inheritance systems contribute, symbolically and materially, to strategies of social reproduction. The key to understanding the dynamics of crop genetic diversity, therefore, lies not only in the factors that influence connectivity between farmer communities, but also in the rules that govern the transmission of lineage within communities.
Drawing a parallel between clones of yam and the origin of clans among Kanak people in New Caledonia, Haudricourt (54) stressed the input of botany to the general understanding of social interactions and the construction of identity. In turn, by showing how manioc clones “hitchhike” along bloodlines, we demonstrate that anthropology offers powerful conceptual tools to help unravel the dynamics of crop genetic diversity.
Materials and Methods
Ethnographic Surveys and Plant Collections.
In each of the 10 communities surveyed, we conducted a series of 15–30 independent, semistructured, on-farm interviews. A total of 191 farmers, 183 of whom were women, were interviewed. Informants were randomly selected from among farmers willing to participate. Rarefaction curves were used to determine the minimum number of interviews required to ensure that the sample size was sufficient to record all landraces present at the village level. Age, village of birth, and parental lineages were recorded for each farmer, and social networks describing farmer kinship relations were drawn. Farmers were also asked to name all manioc landraces they grow in their farms. Following their directions, five plants per landrace per farmer were collected.
Genetic and Statistical Analysis.
Genetic diversity was assessed by using six simple sequence repeat (SSR) markers (SI Materials and Methods). Only landraces for which at least 10 plants were collected were characterized. The Bayesian clustering method implemented in the R package Geneland 3.1.4 (55) was used to detect geographical discontinuities in manioc genetic diversity at the scale of the entire country. Geneland does not assume admixture (i.e., individuals are discretely distributed among inferred populations) and uses the genetic and geographic information of each sampling unit, with no prior assumptions about population groups or boundaries, to infer K, the number of clusters in the data. Because the method implemented in Geneland assumes that populations are panmictic, clonal replicates were removed from the dataset (treating each study site independently), leading to the identification of 441 distinct MLGs.
Five independent runs were performed with 100,000 Markov-chain Monte Carlo iterations, of which every 100th one was saved. K, the number of genetic clusters tested, was set to vary between 1 and 10. The Dirichlet spatial model for allelic frequencies (D model), which assumes uncorrelated allelic frequencies across sites, was used as a prior for allele frequencies. To refine the geographic map of the genetic clusters inferred by Geneland, the model was run five more times, treating the number of clusters as known, based on the number of clusters, K, inferred from the previous runs. Posterior probabilities of population membership for each pixel of the spatial domain were computed by using a burn-in period length of 500 iterations. Each cluster determined by Geneland was characterized by using standard population genetic statistics. Allelic richness (Â), F statistics, and observed (HE) and expected (HO) heterozygosity were computed by using Fstat 2.9.3 (56).
Supplementary Material
Acknowledgments
We thank S. Le Bomin, E. Heyer, M. Hochberg, and two anonymous referees for insightful comments on the manuscript; and the people of Douani, Imbong, Makoula, Mandilou, Mbong-Ete, Mopia, Mouyabi, Nombedouma, Odimba, and Odjouma for their time and hospitality. This study is part of M.D.'s doctoral thesis at Trinity College, University of Dublin. This work was funded by Irish Research Council for Science, Engineering, and Technology (IRCSET; funded under the National Development Plan) Grant RS/2005/44. Additional funding was received from IRCSET and Teagasc under the Ulysses France–Ireland Exchange Scheme. Research was hosted in Gabon by the Laboratoire Universitaire des Traditions Orales et Dynamiques Contemporaines (Université Omar Bongo) and in France by the Centre d'Ecologie Fonctionnelle et Evolutive (Unité Mixte de Recherche 5175, Centre National de la Recherche Scientifique), where part of the genetic work was undertaken.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1106259108/-/DCSupplemental.
References
- 1.Almekinders CJM, Louwaars NP, de Bruijn GH. Local seed systems and their importance for an improved seed supply in developing countries. Euphytica. 1994;78:207–216. [Google Scholar]
- 2.Berkes F, Folke C. In: Linking Ecological and Social Systems: Management Practices and Social Mechanisms for Building Resilience. Folke C, Berkes F, editors. Cambridge, UK: Cambridge University Press; 1998. pp. 1–25. [Google Scholar]
- 3.Brush SB, Carney HJ, Huamán Z. Dynamics of Andean potato agriculture. Econ Bot. 1981;35:70–88. [Google Scholar]
- 4.Louette D, Charrier A, Berthaud J. In situ conservation of maize in Mexico: Genetic diversity and maize seed management in a traditional community. Econ Bot. 1997;51:20–38. [Google Scholar]
- 5.Bellon MR, Hodson D, Hellin J. Assessing the vulnerability of traditional maize seed systems in Mexico to climate change. Proc Natl Acad Sci USA. 2011;108:13432–13437. doi: 10.1073/pnas.1103373108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boster JS. Exchange of varieties and information between Aguaruna manioc cultivators. Am Anthropol. 1986;88:428–436. [Google Scholar]
- 7.Chernela JM. In: SUMA: Etnológica Brasilieira. Ribeiro B, editor. Petrópolis, Brazil: FINEP; 1986. pp. 151–158. [Google Scholar]
- 8.Emperaire L, Peroni N. Traditional management of agrobiodiversity in Brazil: A case study of manioc. Hum Ecol. 2007;35:761–768. [Google Scholar]
- 9.Badstue LB, et al. The dynamics of farmers’ maize seed supply practices in the central valleys of Oaxaca, Mexico. World Dev. 2007;35:1579–1593. [Google Scholar]
- 10.Coomes OT. Of stakes, stems and cuttings: The importance of local seed systems in traditional Amazonian societies. Prof Geogr. 2010;62:323–334. [Google Scholar]
- 11.Stromberg PM, Pascual U, Bellon MR. Seed systems and farmers’ seed choices: The case of maize in the Peruvian Amazon. Hum Ecol. 2010;38:539–553. [Google Scholar]
- 12.Eyzaguirre P. In: Researching the Culture in Agriculture. Cernea M, Kassam A, editors. Wallingford, UK: CABI; 2006. pp. 264–284. [Google Scholar]
- 13.Emperaire L, Pinton F, Second G. Gestion dynamique de la diversité variétale du manioc en Amazonie du Nord-Ouest. Nat Sci Soc. 1998;6:27–42. [Google Scholar]
- 14.Sirbanchongkran A, et al. Varietal turnover and seed exchange: Implications for conservation of rice genetic diversity on farm. Int Rice Res Notes. 2004;29:12–14. [Google Scholar]
- 15.Perales HR, Benz BF, Brush SB. Maize diversity and ethnolinguistic diversity in Chiapas, Mexico. Proc Natl Acad Sci USA. 2005;102:949–954. doi: 10.1073/pnas.0408701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berthouly C, et al. How does farmer connectivity influence livestock genetic structure? A case-study in a Vietnamese goat population. Mol Ecol. 2009;18:3980–3991. doi: 10.1111/j.1365-294X.2009.04342.x. [DOI] [PubMed] [Google Scholar]
- 17.Murdock GP. Ethnographic Atlas. Pittsburgh: University of Pittsburgh Press; 1967. [Google Scholar]
- 18.Aberle DF. In: Matrilineal Kinship. Schneider DM, Gough K, editors. Berkeley, CA: University of California Press; 1961. pp. 655–727. [Google Scholar]
- 19.Guthrie M. The Classification of the Bantu Languages. London: Oxford University Press for the International African Institute; 1948. [Google Scholar]
- 20.Jones WO. Manioc in Africa. Palo Alto, CA: Stanford University Press; 1959. [Google Scholar]
- 21.Howard PL. Women and Plants: Gender Relations in Biodiversity Management and Conservation. London: Zed Press; 2003. [Google Scholar]
- 22.Boster JS. In: Directions in Cognitive Anthropology. Dougherty J, editor. Urbana, IL: University of Illinois Press; 1985. pp. 177–197. [Google Scholar]
- 23.Ferguson AE. Gendered science: A critique of agricultural development. Am Anthropol. 1994;96:540–552. [Google Scholar]
- 24.Chambers KJ, Momsen JH. From the kitchen and the field: Gender and maize diversity in the Bajo region of Mexico. Singap J Trop Geogr. 2007;28:39–56. [Google Scholar]
- 25.Tapia ME, De la Torre A. Women Farmers and Andean Seeds. Rome: FAO/IPGRI; 1998. [Google Scholar]
- 26.Rossel G. Wageningen, The Netherlands: Wageningen Agricultural University; 1987. Gewas innovaties in Gabon van prehistorie tot koloniale tijd. MSc dissertation. [Google Scholar]
- 27.Alexandre P. Proto-histoire du groupe Beti-Bulu-Fang: Essai de synthèse provisoire. Cah Etud Afr. 1965;4:503–560. [Google Scholar]
- 28.Joiris DV, Bahuchet S. In: Situation des Populations Indigènes des Forêts Denses Humides. Bahuchet S, de Maret P, editors. Luxembourg: Office des Publications Officielles des Communautés Européennes; 1994. pp. 387–441. [Google Scholar]
- 29.Delêtre M. Dublin, Ireland: Trinity College, University of Dublin; 2010. The ins and outs of manioc diversity in Gabon, Central Africa: A pluridisciplinary approach to the dynamics of genetic diversity of Manihot esculenta Crantz (Euphorbiaceae). PhD dissertation. [Google Scholar]
- 30.McKey D, Elias M, Pujol B, Duputié A. The evolutionary ecology of clonally propagated domesticated plants. New Phytol. 2010;186:318–332. doi: 10.1111/j.1469-8137.2010.03210.x. [DOI] [PubMed] [Google Scholar]
- 31.Peroni N, Hanazaki N. Current and lost diversity of cultivated varieties, especially cassava, under swidden cultivation systems in the Brazilian Atlantic Forest. Agric Ecosyst Environ. 2002;92:171–183. [Google Scholar]
- 32.Cogels S. Brussels, Belgium: Université Libre de Bruxelles; 2002. Les Ntumu du sud-Cameroun forestier: Une société de non-spécialistes. Système de production, stratégies d'usage des ressources et enjeux du changement. PhD dissertation. [Google Scholar]
- 33.Lévi-Strauss C. The Savage Mind. London: Weidenfeld & Nicolson; 1966. [Google Scholar]
- 34.Karp I. Laughter at marriage: Subversion in performance. J Folklore Res. 1988;25:35–52. [Google Scholar]
- 35.Meillassoux C. Essai d'interprétation du phénomène économique dans les sociétés traditionnelles d'autosubsistance. Cah Etud Afr. 1960;1:38–67. [Google Scholar]
- 36.Herbich I, Dietler M. In: Breaking Down Boundaries: Anthropological Approaches to Cultural Transmission and Material Culture. Stark MT, Bowser BJ, Horne L, editors. Tucson, AZ: University of Arizona Press; 2008. pp. 223–244. [Google Scholar]
- 37.Goheen M. Men Own the Fields, Women Own the Crops: Gender and Power in the Cameroon Grassfields. Madison, WI: University of Wisconsin Press; 1996. [Google Scholar]
- 38.Goody J. Comparative Studies in Kinship. London: Routledge and Kegan Paul; 1969. pp. 120–146. [Google Scholar]
- 39.Perrois L. Chroniques du pays Kota (Gabon) Cah Orstom (Sci Hum) 1970;7:15–119. [Google Scholar]
- 40.Bucher HH. Mpongwe origins: Historiographical perspectives. Hist Afr. 1975;2:59–89. [Google Scholar]
- 41.Bohannan L. Dahomean marriage: A revaluation. Africa. 1949;19:273–287. [Google Scholar]
- 42.Goody J, Buckley J. Inheritance and women's labour in Africa. Africa. 1973;43:108–121. [Google Scholar]
- 43.Rival L. In: Gender in Amazonia and Melanesia: An Exploration of the Comparative Method. Gregor T, Tuzin D, editors. Berkeley, CA: University of California Press; 2001. pp. 57–79. [Google Scholar]
- 44.Weiner AB. Trobriand kinship from another view: The reproductive power of women and men. Man (Lond) 1979;14:328–348. [Google Scholar]
- 45.Pinton F, Emperaire L. Le manioc en Amazonie brésilienne: Diversité variétale et marché. Genet Sel Evol. 2001;33:S491–S512. [Google Scholar]
- 46.Binot A. Brussels, Belgium: Université Libre de Bruxelles; 1998. Particularités de l'agriculture et approche de la dynamique postculturale en périphérie de la réserve de la Lopé, Gabon. MSc dissertation. [Google Scholar]
- 47.Descola P. In: Gender in Amazonia and Melanesia: An Exploration of the Comparative Method. Gregor T, Tuzin D, editors. Berkeley, CA: University of California Press; 2001. pp. 91–114. [Google Scholar]
- 48.Hugh-Jones S. In: Gender in Amazonia and Melanesia: An Exploration of the Comparative Method. Gregor T, Tuzin D, editors. Berkeley, CA: University of California Press; 2001. pp. 245–278. [Google Scholar]
- 49.Biersack A. In: Gender in Amazonia and Melanesia: An Exploration of the Comparative Method. Gregor T, Tuzin D, editors. Berkeley, CA: University of California Press; 2001. pp. 69–90. [Google Scholar]
- 50.Barth F. Ethnic Groups and Boundaries: The Social Organization of Culture Difference. Boston: Little Brown & Co; 1969. [Google Scholar]
- 51.Hamilton G, Stoneking M, Excoffier L. Molecular analysis reveals tighter social regulation of immigration in patrilocal populations than in matrilocal populations. Proc Natl Acad Sci USA. 2005;102:7476–7480. doi: 10.1073/pnas.0409253102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chaix R, et al. From social to genetic structures in central Asia. Curr Biol. 2007;17:43–48. doi: 10.1016/j.cub.2006.10.058. [DOI] [PubMed] [Google Scholar]
- 53.Heyer E, et al. Genetic diversity and the emergence of ethnic groups in Central Asia. BMC Genet. 2009;10:49. doi: 10.1186/1471-2156-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haudricourt AG. Nature et culture dans la civilisation de l'igname: L'origine des clones et des clans. Homme. 1964;4:93–104. [Google Scholar]
- 55.Guillot G, Mortier F, Estoup A. Geneland: A computer package for landscape genetics. Mol Ecol Notes. 2005;5:712–715. [Google Scholar]
- 56.Goudet J. FSTAT (version 1.2): A computer program to calculate F-statistics. J Hered. 1995;86:485–486. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.