Abstract
Inhibitory interneurons help transform the input of a neural circuit into its output. Such interneurons are diverse, and most have unknown function. To study the function of single amacrine cells in the intact salamander retina, we recorded extracellularly from a population of ganglion cells with a multielectrode array, while simultaneously recording from or injecting current into single Off-type amacrine cells that had linear responses. We measured how visual responses of the amacrine cell interacted both with other visual input to the ganglion cell and with transmission between the two cells. We found that on average, visual responses from Off-type amacrine cells inhibited nearby Off-type ganglion cells. By recording and playing back the light-driven membrane potential fluctuations of amacrine cells during white noise visual stimuli, we found that paradoxically, increasing the light-driven modulations of inhibitory amacrine cells increased the firing rate of nearby Off-type ganglion cells. By measuring the correlations and transmission between amacrine and ganglion cells, we found that, on average, the amacrine cell hyperpolarizes before the ganglion cell fires, generating timed disinhibition just before the ganglion cell spikes. In addition, we found that amacrine to ganglion cell transmission is nonlinear in that increases in ganglion cell activity produced by amacrine hyperpolarization were greater than decreases in activity produced by amacrine depolarization. We conclude that the primary mode of action of this class of amacrine cell is to actively gate the ganglion cell response by a timed release from inhibition.
Keywords: multielectrode recording, computational modeling
In local neural circuits of the brain, interneurons change the output of the circuit by combining their own transmission with inputs to the circuit (1). Inhibitory interneurons throughout the brain have great diversity in their morphology, postsynaptic connections, and biochemistry, but for nearly all of these cells, their functional role in information processing is unknown. In the retina, inhibitory amacrine cells comprise more than 30 classes; most of these cells also have unknown function (2, 3).
Compared with a principal neuron, which represents a circuit's output, understanding the functional role of interneurons is a challenge because the effect of an interneuron on the circuit's output is a combination of multiple circuit properties (4, 5). For example, the effect of an amacrine cell on a retinal ganglion cell is a function of the amacrine cell's light response, its effects of transmission on the retinal ganglion cell, and of other visual input to the ganglion cell. Knowledge of all three of these properties is needed to predict how the amacrine cell will affect the ganglion cell's activity.
The responses of different cells are precisely timed in the retina, with different amacrine and ganglion cells having distinct temporal responses with respect to light (6–9). Inhibitory transmission to ganglion cells is known to have different effects, including reducing activity (6, 10), or increasing activity through disinhibition (11–14). Thus, to understand how a particular amacrine cell changes the ganglion cell response, measurements of both the response and transmission of the interneuron must be temporally precise.
A common approach to understand how interneurons influence a neural circuit is to study their combined inputs by voltage clamping the postsynaptic cell (6, 8, 13). In this regard, recordings from retinal ganglion cells have revealed that inhibitory neurotransmission can suppress activity and that relief from inhibition can generate activity (12–14). This approach, however, measures only the combined effects of many interneurons and not their individual contributions. Thus, it is not clear whether particular amacrine cells suppress or activate ganglion cells, or produce a combination of the two effects.
Here, we take a different approach to understand directly the function of single interneurons. We use simultaneous intracellular recording from an amacrine cell and multielectrode extracellular recording from ganglion cells to characterize the effects of a single amacrine cell on ganglion cell activity. With this configuration, we measured both the amacrine cell's light response and the response of nearby ganglion cells. By injecting timed intracellular current in the amacrine cell while presenting a visual stimulus, we observed how the amacrine cell's transmission changes ganglion cell activity. We found that although an amacrine cell produced inhibitory transmission, during a randomly flickering visual stimulus the effects of disinhibition exceeded those of inhibition, such that the net transmission from the amacrine cell increased ganglion cell activity. Furthermore, by measuring the timing of amacrine and ganglion cell visual responses and the timing of amacrine transmission, we conclude that the primary action of the amacrine cell is to gate the activity of the ganglion cell through the relief of inhibition.
Results
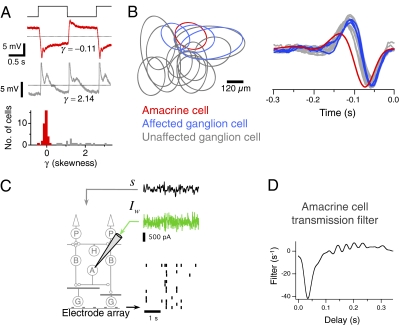
Amacrine cells differ in how much their responses are rectified. They vary from being linear, in that depolarizations and hyperpolarizations are more symmetric about the mean membrane potential (Fig. 1A, Top), to having a high threshold, such as for On-Off cells (Fig. 1A, Middle). To quantify this asymmetry, we measured the third central moment, or skewness γ of the response to a uniform field flash, and found that cells with linear responses formed a cluster near γ = 0, distinguishing these sustained amacrine cells from other cells with a more skewed response (Fig. 1A, Bottom). Here, we study this functional class of amacrine cells with linear responses, although this cluster is likely composed of multiple cell types. In particular, we focus on Off-type amacrine cells to measure how their visually driven responses affect the output of the retina.
Fig. 1.
Measuring the timing of amacrine transmission to multiple ganglion cells. (A) From top to bottom, uniform field flashing stimulus, the membrane potential response of a more linear Off-type amacrine cell, the response of a strongly rectified On-Off amacrine cell, and histogram of the skewness γ of the flash responses of 53 amacrine cells. Dashed line indicates the mean potential. Spikes have been truncated in the rectified amacrine cell. (B, Left) Receptive fields of a single amacrine cell and multiple ganglion cells. Each oval indicates one SD of a 2D Gaussian fit to the receptive field mapped by using a white noise checkerboard stimulus. Colored ovals indicate ganglion cells affected by current injection into the amacrine cell as judged by a peak in the transmission filter. (B, Right) Reverse correlation of the amacrine and ganglion cells to a uniform-field visual stimulus. (C, Left) Diagram of experimental configuration for simultaneous intracellular and multielectrode recording. (C, Right) A 3-s segment of a 300-s recording. (Top) A uniform field visual stimulus with a Gaussian white noise distribution. (Middle) Simultaneously, Gaussian white noise current was injected intracellularly into a linear amacrine cell. (Bottom) Ganglion cell spiking responses were recorded using the multielectrode array. Each row shows the spiking response of a different ganglion cell to a single stimulus trial. (D) Linear transmission filter from an amacrine cell to a single ganglion cell (nearest affected ganglion cell in B) computed by correlating the white noise current injected into the amacrine cell and the firing rate of the ganglion cell over 300 s.
To directly measure the effect of individual amacrine cells on ganglion cell activity, we recorded intracellularly from an amacrine cell while simultaneously recording from a population of ganglion cells with a multielectrode array (Fig. 1 B and C). We studied biphasic Off-type ganglion cells, as classified based on their reverse correlation with a uniform field visual stimulus (Fig. 1B, Right) (9). These cells subdivide into two distinct types with different adaptive properties (15), each of which forms an independent mosaic. Results here are pooled from the two types. To measure the time course and sign of transmission between individual amacrine cells and ganglion cells, we injected white noise current into the amacrine cell with a SD of 500 pA while presenting a white noise visual stimulus (Fig. 1C). Because the inner retina adapts to the stimulus contrast by changing its sensitivity (16, 17), the visual stimulus was included to maintain the retina in a similar state of adaptation in the current injection and control conditions.
With this configuration, we simultaneously performed many paired recordings with one presynaptic cell and many postsynaptic ganglion cells. Using the method of reverse correlation between ganglion cell spiking activity and the injected current, we measured the average time course of amacrine transmission by computing a linear temporal filter between amacrine current and the ganglion cell firing rate (Fig. 1D). This filter represents the average effect on ganglion cell firing rate of a brief impulse of depolarizing current injected into the amacrine cell. Equivalently, this filter can be interpreted as the time-reverse of the amacrine current stimulus feature that the ganglion cell was most sensitive to, on average. Because the injected current was uncorrelated with the visual stimulus, the transmission filter of the amacrine cell could be computed without respect to the visual stimulus. The filter had a negative peak at 37 ± 12 ms (mean ± SD, n = 36 cell pairs), indicating that, on average, a brief impulse of current through the amacrine cell membrane inhibited the activity of nearby ganglion cells. Equivalently, the ganglion cell was most active, on average, when the amacrine cell was hyperpolarized by current according to the time-reverse of the filter. However, because in the white noise stimulus there were equally as many depolarizations as hyperpolarizations, one cannot conclude whether the decrease in ganglion cell firing produced by depolarizing current was the same magnitude as the increase in ganglion cell firing produced by hyperpolarizing current. Note that this approach measures the effects of all pathways between the amacrine and ganglion cells, including chemical monosynaptic, polysynaptic, and electrical synaptic transmission.
In contrast with previous measurements in the catfish of the linear transmission filter between amacrine and ganglion cells, we found that all transmission between Off-type amacrine and Off-type ganglion cells was inhibitory (18). This difference may reflect the larger currents used in the previous study.
The sign and delay of transmission is only one component that determines an amacrine cell's effect on the ganglion cell during a visual stimulus. Equally important is how the visual response of the amacrine cell relates to the rest of a ganglion cell's visual input. For example, if inhibition was delivered when the ganglion cell was far below threshold, no effect on activity would result. Thus, we sought to measure the effect of amacrine transmission on ganglion cell activity not when the timing of that transmission was controlled randomly by the experimenter, but when the timing was controlled by the visual stimulus.
To measure the effect of an amacrine cell's visual response on the circuit, we designed an approach that could make a single amacrine cell's voltage fluctuations larger or smaller. During repeated flickering visual stimuli, retinal ganglion cell responses are highly reproducible, with individual spikes generally aligning to within <10 ms (19). We tested whether amacrine cell membrane potential responses to random flicker were also precise by comparing the membrane potential responses between 100-s repeats of an identical stimulus sequence (Fig. S1A). We found that indeed, these responses varied very little between repeats, having a correlation coefficient of 0.96 ± 0.01 (n = 4). Given the high reproducibility of amacrine and ganglion cell responses, we perturbed the output of an amacrine cell at the times determined by its own light-driven membrane fluctuations.
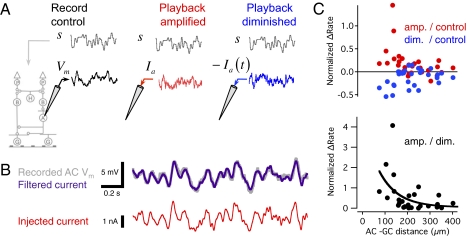
First, we recorded the membrane potential response from an amacrine cell and the spiking responses from a population of ganglion cells (Fig. 2A). Then, we repeated the same visual stimulus while injecting a current into the amacrine cell designed to amplify its voltage fluctuations and, thus, its effect in the circuit. First, we measured the membrane time constant of each amacrine cell by injecting current pulses (Fig. S1B). After recording the amacrine cell's visually driven membrane potential fluctuations, we computed a current to inject by deconvolving the measured membrane potential response with an exponential filter representing the membrane time contrast, which averaged 17 ± 7 ms (n = 6). In this way, we injected a current predicted to generate the measured membrane potential response (Fig. S1 C and D). Although the high resistance of the sharp microelectrode prevents a measurement of voltage when injecting high frequency current, we estimated the resulting voltage change and found it not to require high precision in the measurement of the membrane time constant (Fig. S1 C and D). Because responses in the retina are highly reproducible, this approach serves to amplify the cell's depolarizations and hyperpolarizations, thus increasing its effect in the circuit. Likewise, by injecting a negative version of this same current, we produced changes in membrane potential that would oppose the effect of visual input on the amacrine cell (Fig. 2A, Right).
Fig. 2.
Amplification of inhibitory amacrine cell voltage fluctuations increases ganglion cell firing rate. (A, Left) Control condition. A uniform field stimulus s(t) (Upper) with a Gaussian white noise distribution was presented lasting 300 s. The membrane potential response Vm (Lower) was recorded from an amacrine cell, and spiking responses were recorded from ganglion cells. (A, Center) The visual stimulus s(t) was repeated, and at the same time a current Ia was injected into the amacrine cell such that when Ia was filtered by the membrane time constant, it was predicted to yield the membrane potential Vm recorded in the control condition. (A, Right) The visual stimulus s(t) was again repeated, and a current −Ia was injected into the amacrine cell, diminishing the voltage fluctuations of the cell. (B, Upper) A segment of the recorded membrane potential in the control condition, compared with the injected current filtered through the membrane time constant. Filtered current is displayed scaled in amplitude to match the membrane potential. (B, Lower) The current injected in the amplified condition. (C, Upper) The average change in ganglion cell firing rate in the amplified and diminished conditions normalized by the firing rate in the control condition as a function of distance between the amacrine and ganglion cells. (C, Lower) The average change in firing rate in the amplified condition normalized by the diminished condition. The solid curve is an exponential fit with a space constant of 83 μm. The three conditions were interleaved every 150 s, for a total of 600–900 s. Results are shown for five amacrine and 23 ganglion cells.
We compared the firing rate of the ganglion cell under the control condition to when the amacrine cell's transmission was amplified and to when transmission was diminished. Surprisingly, although the amacrine cell's transmission was inhibitory as measured by white noise current injection (Fig. 1D), we found that when we amplified the output of individual amacrine cells, the firing rate of ganglion cells within 200 μm increased by a factor of 1.38 ± 0.12 (n = 11, P = 0.005, paired Student's t test) (Fig. 2D). When we diminished the output of single amacrine cells by injecting an inverted version of this current, activity in ganglion cells within 210 μm decreased by a factor of 0.81 ± 0.05 (n = 15, P = 0.03). In the amplified condition relative to the diminished condition, the firing rate for ganglion cells within 200 μm was greater by a factor of 1.93 ± 0.34 (n = 11, P = 0.02). This change in transmission decayed with a space constant of 83 μm and was not significantly correlated with the ganglion cell firing rate (correlation coefficient r = −0.16, P = 0.28). Thus, even though the transmission of the amacrine cell was inhibitory, on average the effect of the amacrine cell's transmission during a visual stimulus was to increase the firing rate of nearby ganglion cells. To explain this apparent paradox, we compared the timing of the responses of the amacrine cell and ganglion cell.
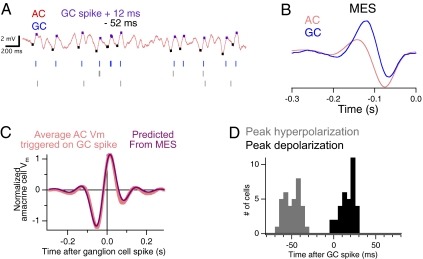
We measured the correlation over time between pairs of amacrine and ganglion cells by computing the average amacrine cell membrane potential surrounding a ganglion cell spike (Fig. 3A). We found that on average, amacrine cells hyperpolarized 40–60 ms before the ganglion cell fired and then reached a peak depolarization within 20 ms after the ganglion cell spiked (Fig. 3 A, C, and D).
Fig. 3.
Linear amacrine cells hyperpolarize just before ganglion cells fire. (A) Simultaneous intracellular recording of an amacrine cell (Upper) and spiking response of three ganglion cells responding to uniform field white noise flicker (Lower). Stimulus duration was 300 s. Ganglion cells were separately measured to be inhibited by the amacrine cell. Tick marks on the amacrine cell response indicate times before and after each ganglion cell spike that correspond to the hyperpolarizing (−52 ms) and depolarizing (+12 ms) peaks of the correlation shown in C. (B) Mean effective visual stimulus (MES) for the amacrine and ganglion cell, computed as the spike-triggered-average of the visual stimulus, or the reverse correlation between the amacrine cell membrane potential and the visual stimulus. (C) Cross-correlation function between the amacrine cell and a ganglion cell, measured as the average amacrine cell membrane potential triggered on a ganglion cell spike, compared with the cross-correlation between the MES of the two cells. The cross-correlations were normalized by the SD of the amacrine cell response. (D) Peak time of hyperpolarizations and depolarizations relative to a ganglion cell spike measured from the cross-correlation function for seven amacrine cells and 35 ganglion cells.
To find the source of the correlation between amacrine and ganglion cell (the response correlation), we examined the response of each cell to the visual stimulus. Using the standard method of reverse-correlation, we computed the average visual stimulus that preceded a spike in the ganglion cell (the spike-triggered average) and the stimulus that preceded a brief depolarization of an amacrine cell (Fig. 3B). For each cell, this function represents the most effective visual stimulus (MES) that activated or depolarized the cell. The MES of the two cells was different, such that the amacrine cell was more monophasic and its peak was more delayed than that of the ganglion cell. We tested whether different MES could explain the response correlation between cells. This need not be the case, for example, if the amacrine and ganglion cell responded to the same type of stimuli on average, but never at the same time, they would have a similar MES although they were anticorrelated with each other. We correlated the two MES and compared the result with the response correlation between amacrine and ganglion cell (Fig. 3C). The MES correlation predicted the response correlation accurately, such that the correlation coefficient between the MES correlation and the response correlation was 0.99 ± 0.002 (n = 22). Thus, the response correlation (Fig. 3C) between the two cells is explained by the different time courses of their visual sensitivity (Fig. 3B).
Neither measurements of correlation alone (Fig. 3C) nor isolated measurements of transmission (Fig. 1D) are sufficient to predict how an interneuron will affect the circuit under normal operation. To estimate the combined effect of the amacrine cell's response and its transmission on ganglion cell activity, we predicted the transmission of the amacrine cell as a function of time during the control visual stimulus. We made the assumption that the transmission filter measured during white noise current injection was unchanged in the control condition. We then predicted the amacrine cell's transmission by convolving the transmission filter with the membrane potential response to a uniform flickering random stimulus (Fig. 4A). The transmission filter measured with injected current (Fig. 1D) is a combination of both the amacrine cell's membrane time constant and its transmission as driven by the membrane potential. Thus, to compensate for the discrepancy in the measured transmission filter, we deconvolved the resulting predicted transmission gT(t) by this membrane time constant. To predict the average transmission from an amacrine cell near the time of a ganglion cell spike, we then computed the spike-triggered average of gT(t) (Fig. 4B). We found that the net effect of the amacrine hyperpolarization (Fig. 3C) and the delay in inhibitory transmission (Fig. 1D) was to cause disinhibition to peak close to the time of a ganglion cell spike. Note that the two measurements of correlation and transmission need not combine to match the time of ganglion cell spiking, because an amacrine cell that is correlated with a ganglion cell by common visual input need not even connect to that ganglion cell. Furthermore, a biphasic filter could shift the peak of the predicted transmission to an earlier time. Nonetheless, nearly all amacrine and ganglion cell pairs were similarly timed, such that on average, the amacrine cell had a positive (disinhibitory) contribution to ganglion cell activity, peaking 15 ± 8 ms (mean ± SD) before a ganglion cell spiked (Fig. 4C).
Fig. 4.
Estimated timing of amacrine contribution to ganglion cell activity. (A, Top Left) Amacrine cell potential response to a uniform field visual stimulus. Stimulus duration was 300 s. (A, Top Right) Linear transmission filter between the amacrine cell and a ganglion cell. (A, Middle Left) Linear prediction of amacrine transmission gT(t), computed by convolving the membrane potential recorded without current injection with the transmission filter measured using white noise current injection (Fig. 1), and then deconvolving with the measured membrane time constant of the amacrine cell. Ticks indicate the value of the predicted transmission gT(t) at the times of ganglion cell spikes. Because the amplitude of the transmission filter was normalized, the predicted transmission is displayed in units of SD. (A, Bottom) The spiking response of the ganglion cell, recorded without current injection simultaneously with the response of the amacrine cell. (B) Cross-correlation function between gT(t) and the spiking response of the ganglion cell. The cross-correlation was normalized by the SD of gT(t). Because the filter has a negative peak, values of the predicted transmission below the mean represent inhibition, and values above the mean represent disinhibition. (C) The peak timing of the cross-correlation function in B for 10 amacrine cells and 30 ganglion cells. (D) The average ganglion cell firing rate displayed as a function of the amacrine cell predicted transmission gT(t). The dotted line indicates the point corresponding to one-half of the maximal firing rate.
We next examined how well the predicted magnitude of disinhibition matched ganglion cell activity by computing a nonlinear function that mapped the predicted amacrine transmission to the measured ganglion cell firing rate (Fig. 4D). We found that this function had a very high threshold such that ganglion cell activity occurred only when the predicted transmission was well above the mean, corresponding to when inhibition was lowest. On average, the half-maximal value of ganglion cell activity occurred at a value 1.87 ± 0.13 (n = 29) SDs above the mean of the predicted amacrine transmission. The effect of the high threshold between predicted amacrine transmission and ganglion cell activity was that when inhibitory transmission was low (gT (t) > 0), a greater modulation of activity occurred than when inhibitory transmission was high (gT (t) < 0). This finding suggests an explanation as to why amplifying amacrine cell transmission increased ganglion cell firing rate (Fig. 2). Amacrine hyperpolarizations caused a greater change in ganglion cell activity than did amacrine depolarizations, producing a net increase in firing rate.
This analysis examining the threshold relationship between amacrine transmission and ganglion cell activity was computed when no current was injected and was based on the assumption that the transmission filter was representative of transmission in the control condition. We confirmed this threshold independently by injecting white noise current into the amacrine cell as in Fig. 1. We directly measured the threshold between amacrine and ganglion cell by computing a linear–nonlinear model (20) consisting of the linear temporal transmission filter followed by a static nonlinearity (Fig. S2). In addition to capturing the sensitivity of the cell, the nonlinearity captures threshold and saturation, properties which are known to occur in synaptic transmission (21–23). We found that the nonlinearity, in nearly all cases, was accelerating, meaning that the slope above the mean level of transmission was greater than the slope below the mean level, on average by a factor of 6.7 ± 1.9 (n = 28 cell pairs). This result confirms that the effect of disinhibition when the amacrine cell hyperpolarized was greater than the effect of inhibition when the amacrine cell depolarized.
Discussion
Here we have shown that increasing the light driven fluctuations of inhibitory Off-type amacrine cells increases the firing rate of nearby Off-type ganglion cells (Fig. 5). These effects can be explained by the relative timing of the amacrine and ganglion cell's visual response (Fig. 5B) and the timing of the amacrine cell's transmission. In response to common visual input, on average, the amacrine cell hyperpolarizes before the ganglion cell fires, and the timing of inhibitory transmission is such that the hyperpolarization of the amacrine cell causes disinhibition to peak just before the ganglion cell fires (Fig. 5C). Furthermore, transmission from the amacrine cell crosses an increasing nonlinearity, such that disinhibitory effects on ganglion cell firing are greater than inhibitory effects. The finding that disinhibitory effects exceed inhibitory effects is consistent with recently measured examples of sustained off-type amacrine transmission using steady positive and negative current pulses (24).
Fig. 5.
Disinhibitory gating from amacrine cell transmission. (A) Simplified circuit of an amacrine cell with a linear synaptic terminal making a synapse on the rectifying synaptic terminal of a bipolar cell, or directly onto the ganglion cell. (B) Filters of bipolar, amacrine, and ganglion cells. Bipolar cell filters (dotted line) were not measured in this study, but have been shown to be biphasic (10). For purposes of illustration, the bipolar cell is taken to have similar time course as the ganglion cell, but advanced by 10 ms. Filters of amacrine and ganglion cells are taken from the time-reverse of the mean effective stimuli in Fig. 3B. (C) The stimulus is the mean effective stimulus of the ganglion cell. Bipolar, amacrine, and ganglion cell responses were computed by convolving the stimulus with the response filters in B. For the ganglion cell, an additional threshold was applied. The time course of inhibitory neurotransmitter was computed by convolving the amacrine cell response and the negative of the measured transmission filter to the ganglion cell. Thus, transmission is shown to represent inhibitory neurotransmitter, which decreases when the ganglion cell is active.
These studies measure the combined effects of multiple components of dynamic activity in a neural circuit, including the interneuron's response, the response of the circuit's output, and the transmission of the interneuron. Interactions between these properties illustrate that all of these measurements are needed to understand the contribution of a cell to the circuit. For example, without knowing the relative timing of amacrine and ganglion cell input, one would conclude that the amacrine cell primarily inhibits, reducing ganglion cell firing, instead of primarily disinhibiting. Because neural responses have multiple phases, hyperpolarizing and depolarizing at different times, failure to account for the timing of responses and transmission may yield a conclusion opposite to the neuron's true function. This notion is particularly relevant to newer techniques using optical stimulation of the brain (25), which can be applied without measurements of the neural response.
Given the LN model of inhibitory amacrine transmission, we can consider the origin of the intervening threshold between amacrine and ganglion cell. One possible location is at a presynaptic terminal because of the voltage dependence of calcium currents (23). However, if the source of this threshold were the amacrine cell's presynaptic terminal, the sign inversion of inhibitory transmission would occur after this threshold (Fig. S3A). This location is inconsistent with the LN model of a negative filter followed by an accelerating nonlinearity (Fig. S2). Instead, such a system would produce an LN model with an inhibitory filter followed by a saturating nonlinearity (Fig. S3A). This circuit would cause amacrine cell fluctuations to produce a net inhibition of ganglion cell activity, the opposite of what we observed. An alternative is that the threshold lies not at the amacrine cell synaptic terminal, but at an intervening bipolar cell synaptic terminal. In this case, the threshold of the bipolar cell terminal calcium current would lie after the sign inversion produce by the inhibitory receptor, consistent with the LN model (Fig. S3B). This arrangement implies more linear transmission from the amacrine cell itself. Another possibility is that the threshold is produced by the spiking threshold of the ganglion cell, which also lies after the inhibitory receptor (Fig. S3B).However, recent measurements of the spatial effects of sustained amacrine cells on ganglion cell receptive fields indicate that inhibition is presynaptic (24).
These results extend recent studies of sustained Off amacrine cell transmission that showed a modulatory effect on ganglion cell visual sensitivity (24). The timing of transmission measured here with white noise current is faster than that estimated previously using steady pulses injected in the dark, and thus these results make more precise the timing of the amacrine pathway.
Studies here were conducted with uniform field stimuli. Because the amacrine cell was very near to the ganglion cell (≈200 μm), these results apply to the case where amacrine and ganglion cell receive similar visual input. In the case of spatiotemporal such as edges, if the amacrine and ganglion cell received differentially timed visual input, disinhibition may not occur with the same timing as we have measured, and the amacrine cell may act in a inhibitory manner to suppress firing, or may not affect ganglion cell firing. Studying the effects of spatiotemporal stimuli when amacrine and ganglion cells receive different input will be an important direction of future study.
Other disinhibitory effects have been described in the retina, measuring the combined effects of many amacrine cells. In the guinea pig, pharmacological measurements have suggested that AII amacrine cells carrying signals from cone photoreceptors hyperpolarize to disinhibit Off-type ganglion cells (12). In the salamander retina, disinhibition has been shown in the context of a flash of light (11), where hyperpolarization of GABAergic amacrine cells is thought to delay the truncation of the Off ganglion cell light response. Here, we have directly measured the overall output from single linear amacrine cells, confirming that these cells gate the ganglion cell response primarily through disinhibition.
The disinhibitory effects in linear amacrine can be contrasted with those of rectified amacrine cells. For example, polyaxonal amacrine cells have been shown to generate selectivity for differential motion by inhibiting object motion sensitive (OMS) ganglion cells (10, 26). In contrast to the amacrine and ganglion cell pairs studied here, polyaxonal amacrine cells and OMS ganglion cells receive highly correlated excitatory input, such that the amacrine cell depolarizes just before the ganglion cell spikes. In addition, polyaxonal amacrine cells are silent most of the time and produce temporally sparse depolarizations from the resting potential. When the entire visual field moves coherently as in the case of fixational eye movements, transmission from these amacrine cells suppresses activity in the OMS ganglion cell population.
By comparing these two classes of cells, one can outline broadly two functions of inhibitory signaling in the retina. Strongly rectified amacrine cells are restricted to signaling by an increase in inhibition, which may lead to suppression of rejected visual features.
In other cells, including sustained Off-type amacrine cells and the AII amacrine cell (24, 27), a significant amplitude of the light response is symmetric above and below the mean potential. Although this group of linear amacrine cells likely comprises multiple types, our results within this set are consistent and, thus, provide an indication of a general rule concerning this group of cells. The strong threshold that we observe after the amacrine cell (Fig. S2) implies that the baseline inhibition is near its maximum effect, thus causing the primary signaling mode to be restricted to a decrease in inhibition. Rather than suppressing unwanted features, these cells may serve to create or amplify a visual feature. Thus, observing the timing and nonlinearity of an amacrine cell's response and transmission reveals how these properties interact to control whether the cell conveys or suppresses visual information in the retinal circuit.
Methods
Simultaneous intracellular and multielectrode array recording was performed in the intact isolated retina as described (16). Current was injected through sharp microelectrodes (150–250 MΩ) in bridge mode. Visual stimuli were uniform field and drawn from a Gaussian distribution with a constant mean intensity. Linear–nonlinear models of visual responses and of responses to current injection were computed as described (16). Detailed methods are included in SI Methods.
Supplementary Material
Acknowledgments
We thank D. B. Kastner for comments on the manuscript. This work was supported by grants from the National Institutes of Health (National Eye Institute), the Pew Charitable Trust, the McKnight Foundation, and the E. Matilda Ziegler Foundation (to S.A.B.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107994108/-/DCSupplemental.
References
- 1.Buzsáki G, Geisler C, Henze DA, Wang XJ. Interneuron Diversity series: Circuit complexity and axon wiring economy of cortical interneurons. Trends Neurosci. 2004;27:186–193. doi: 10.1016/j.tins.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Masland RH. The fundamental plan of the retina. Nat Neurosci. 2001;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- 3.Gollisch T, Meister M. Eye smarter than scientists believed: Neural computations in circuits of the retina. Neuron. 2010;65:150–164. doi: 10.1016/j.neuron.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McBain CJ, Fisahn A. Interneurons unbound. Nat Rev Neurosci. 2001;2:11–23. doi: 10.1038/35049047. [DOI] [PubMed] [Google Scholar]
- 5.Moore CI, Carlen M, Knoblich U, Cardin JA. Neocortical interneurons: From diversity, strength. Cell. 2010;142:189–193. doi: 10.1016/j.cell.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roska B, Werblin F. Vertical interactions across ten parallel, stacked representations in the mammalian retina. Nature. 2001;410:583–587. doi: 10.1038/35069068. [DOI] [PubMed] [Google Scholar]
- 7.Baccus SA. Timing and computation in inner retinal circuitry. Annu Rev Physiol. 2007;69:271–290. doi: 10.1146/annurev.physiol.69.120205.124451. [DOI] [PubMed] [Google Scholar]
- 8.Pang JJ, Gao F, Wu SM. Segregation and integration of visual channels: Layer-by-layer computation of ON-OFF signals by amacrine cell dendrites. J Neurosci. 2002;22:4693–4701. doi: 10.1523/JNEUROSCI.22-11-04693.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Segev R, Puchalla J, Berry MJ., 2nd Functional organization of ganglion cells in the salamander retina. J Neurophysiol. 2006;95:2277–2292. doi: 10.1152/jn.00928.2005. [DOI] [PubMed] [Google Scholar]
- 10.Baccus SA, Olveczky BP, Manu M, Meister M. A retinal circuit that computes object motion. J Neurosci. 2008;28:6807–6817. doi: 10.1523/JNEUROSCI.4206-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roska B, Nemeth E, Werblin FS. Response to change is facilitated by a three-neuron disinhibitory pathway in the tiger salamander retina. J Neurosci. 1998;18:3451–3459. doi: 10.1523/JNEUROSCI.18-09-03451.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manookin MB, Beaudoin DL, Ernst ZR, Flagel LJ, Demb JB. Disinhibition combines with excitation to extend the operating range of the OFF visual pathway in daylight. J Neurosci. 2008;28:4136–4150. doi: 10.1523/JNEUROSCI.4274-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pang JJ, Gao F, Wu SM. Light-evoked excitatory and inhibitory synaptic inputs to ON and OFF alpha ganglion cells in the mouse retina. J Neurosci. 2003;23:6063–6073. doi: 10.1523/JNEUROSCI.23-14-06063.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margolis DJ, Detwiler PB. Different mechanisms generate maintained activity in ON and OFF retinal ganglion cells. J Neurosci. 2007;27:5994–6005. doi: 10.1523/JNEUROSCI.0130-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kastner DB, Baccus SA. Coordinated dynamic encoding in the retina using opposing forms of plasticity. Nat Neurosci. 2011;14:1317–1322. doi: 10.1038/nn.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baccus SA, Meister M. Fast and slow contrast adaptation in retinal circuitry. Neuron. 2002;36:909–919. doi: 10.1016/s0896-6273(02)01050-4. [DOI] [PubMed] [Google Scholar]
- 17.Kim KJ, Rieke F. Temporal contrast adaptation in the input and output signals of salamander retinal ganglion cells. J Neurosci. 2001;21:287–299. doi: 10.1523/JNEUROSCI.21-01-00287.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakai HM, Naka K. Dissection of the neuron network in the catfish inner retina. I. Transmission to ganglion cells. J Neurophysiol. 1988;60:1549–1567. doi: 10.1152/jn.1988.60.5.1549. [DOI] [PubMed] [Google Scholar]
- 19.Berry MJ, Warland DK, Meister M. The structure and precision of retinal spike trains. Proc Natl Acad Sci USA. 1997;94:5411–5416. doi: 10.1073/pnas.94.10.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chichilnisky EJ. A simple white noise analysis of neuronal light responses. Network. 2001;12:199–213. [PubMed] [Google Scholar]
- 21.Field GD, Rieke F. Nonlinear signal transfer from mouse rods to bipolar cells and implications for visual sensitivity. Neuron. 2002;34:773–785. doi: 10.1016/s0896-6273(02)00700-6. [DOI] [PubMed] [Google Scholar]
- 22.Sampath AP, Rieke F. Selective transmission of single photon responses by saturation at the rod-to-rod bipolar synapse. Neuron. 2004;41:431–443. doi: 10.1016/s0896-6273(04)00005-4. [DOI] [PubMed] [Google Scholar]
- 23.Burrone J, Lagnado L. Synaptic depression and the kinetics of exocytosis in retinal bipolar cells. J Neurosci. 2000;20:568–578. doi: 10.1523/JNEUROSCI.20-02-00568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Vries SE, Baccus SA, Meister M. The projective field of a retinal amacrine cell. J Neurosci. 2011;31:8595–8604. doi: 10.1523/JNEUROSCI.5662-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiala A, Suska A, Schlüter OM. Optogenetic approaches in neuroscience. Curr Biol. 2010;20:R897–R903. doi: 10.1016/j.cub.2010.08.053. [DOI] [PubMed] [Google Scholar]
- 26.Olveczky BP, Baccus SA, Meister M. Segregation of object and background motion in the retina. Nature. 2003;423:401–408. doi: 10.1038/nature01652. [DOI] [PubMed] [Google Scholar]
- 27.Nelson R. AII amacrine cells quicken time course of rod signals in the cat retina. J Neurophysiol. 1982;47:928–947. doi: 10.1152/jn.1982.47.5.928. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.