Abstract
We introduce a human retinal pigmented epithelial (RPE) cell-culture model that mimics several key aspects of early stage age-related macular degeneration (AMD). These include accumulation of sub-RPE deposits that contain molecular constituents of human drusen, and activation of complement leading to formation of deposit-associated terminal complement complexes. Abundant sub-RPE deposits that are rich in apolipoprotein E (APOE), a prominent drusen constituent, are formed by RPE cells grown on porous supports. Exposure to human serum results in selective, deposit-associated accumulation of additional known drusen components, including vitronectin, clusterin, and serum amyloid P, thus suggesting that specific protein–protein interactions contribute to the accretion of plasma proteins during drusen formation. Serum exposure also leads to complement activation, as evidenced by the generation of C5b-9 immunoreactive terminal complement complexes in association with APOE-containing deposits. Ultrastructural analyses reveal two morphologically distinct forms of deposits: One consisting of membrane-bounded multivescicular material, and the other of nonmembrane-bounded particle conglomerates. Collectively, these results suggest that drusen formation involves the accumulation of sub-RPE material rich in APOE, a prominent biosynthetic product of the RPE, which interacts with a select group of drusen-associated plasma proteins. Activation of the complement cascade appears to be mediated via the classical pathway by the binding of C1q to ligands in APOE-rich deposits, triggering direct activation of complement by C1q, deposition of terminal complement complexes and inflammatory sequelae. This model system will facilitate the analysis of molecular and cellular aspects of AMD pathogenesis, and the testing of new therapeutic agents for its treatment.
Age-related macular degeneration (AMD) is characterized in its early stages by the presence of extracellular deposits, known as drusen, that accumulate between the basal surface of the retinal pigmented epithelium (RPE) and Bruch's membrane, an extracellular matrix complex that separates the neural retina from the capillary network in the choroid. Early electron microscopic studies suggested that drusen formation may be a consequence of degeneration of the RPE (1–3), initiated by membranous debris shed from its basal surface (4, 5). These early morphological observations have since been confirmed by a number of more recent studies (6–13).
Contemporary investigations of the molecular composition of drusen have provided additional insights into their biogenesis. Immunohistochemical and proteomic studies show that drusen contain a variety of protein and lipid components (14, 15). Among these are several plasma proteins, the presence of which implies a systemic contribution to their genesis. Although the primary biosynthetic source for most of these circulating molecules is the liver, a number of them are also known to be synthesized locally by RPE cells (15–19). The respective contributions of RPE-derived and plasma-derived molecules to the process of drusen biogenesis, as well as the relevant molecular interactions leading to drusen deposition, have not yet been fully elucidated.
During the past decade, compelling evidence has emerged implicating the immune system—and the complement system in particular—in drusen biogenesis and AMD (15, 20, 21). A number of the proteins detected in drusen are either complement components or related molecules. Importantly, variations in several complement-related genes have been shown to be highly significant risk factors for the development of AMD (20, 21). Taken together, these findings are consistent with the general conclusion that chronic local inflammation at the RPE/Bruch's membrane interface contributes to drusen formation and to AMD pathogenesis (12, 14, 22, 23).
Despite these significant advances, the identity of the molecules responsible for triggering activation of the complement cascade, as well as the downstream molecular interactions that promote AMD pathology, remain elusive. This is due, in part, to the dearth of animal and cell-culture models that reproduce the most salient pathologic features of AMD under controlled experimental conditions. We introduce here an RPE cell culture model that mimics numerous aspects of AMD pathology observed in humans, including accumulation of sub-RPE deposits containing known constituents of drusen, activation of the complement system, and deposition of terminal complement complexes. This system provides a unique experimental platform that will facilitate dissection of the cellular and molecular events that lead to drusen formation and contribute to AMD pathogenesis.
Results
Examination of differentiated cultures of primary human RPE cells grown on porous supports led to the identification of a population of globular extracellular deposits that accumulate within the pores of the support material. Initially, we identified these sub-RPE deposits based on their immunoreactivity for apolipoprotein E (APOE) (Fig. 1). Antibodies to several other apolipoproteins, including apolipoproteins A and B, did not show similar immunoreactivity. APOE is a circulating lipid transport protein, a ubiquitous component of human ocular drusen (24–26), and an abundant biosynthetic product of RPE cells (16, 24). The APOE deposits are typically spheroid, sometimes tubular in shape, ranging from 0.5 to 3 μm in diameter. They accumulate within the porous culture matrix and are most concentrated within 20 μm of the basal surface of the RPE. The deposits are nonuniformly distributed along the substratum, with areas of higher density interspersed with areas where the deposits are relatively sparse (Fig. 1). This sporadic distribution of sub-RPE material is consistent with the irregular pattern of drusen development in the eye. The accumulation of APOE from bovine serum that is a constituent of the RPE cell-culture medium does not appear to contribute to the APOE immunoreactivity because cell culture inserts without cells incubated in bovine serum-containing medium and assayed immunocytochemically for APOE show no evidence of immunoreactivity (Fig. S1).
Fig. 1.
APOE-containing basal deposits are elaborated by human RPE cells in vitro. Confocal immunofluorescence images of sectioned (A and B) and flat-mounted (C) RPE cell cultures on porous supports. Spherical to amorphous deposits that are immunoreactive for APOE (green, arrows) accumulate within the support matrix. These deposits are nonuniformly distributed with areas of high deposit density (arrows in A and C) interspersed among areas with few to no detectable deposits (asterisk in A). Immunoreactivity for GPNMB (red in A), an abundant RPE membrane protein, is localized to RPE cell surfaces and to particulate sub-RPE material (arrowheads in A) that is distinct from the APOE deposits. Blue, nuclear (“N” in B) DAPI fluorescence in A and B. (Scale bars, 10 μm in A and C; 5 μm in B.)
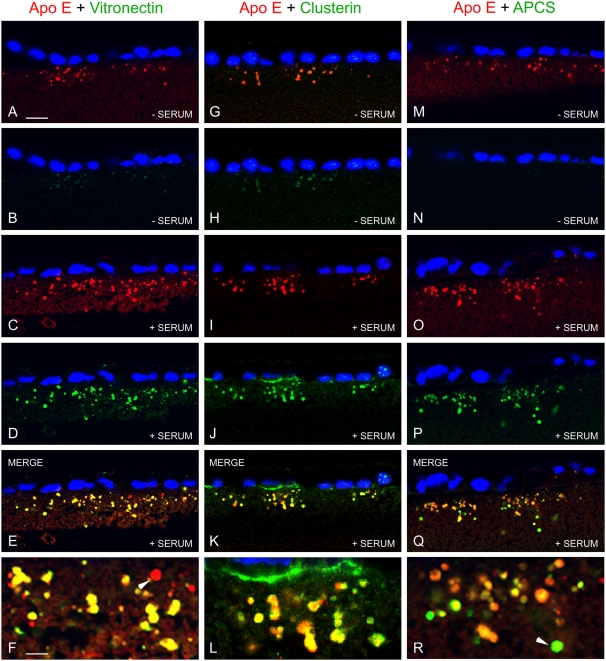
The APOE-containing deposits also show comparatively low levels of immunoreactivity for two other known drusen constituents, clusterin (17, 18, 22) and vitronectin (7, 27), that have been reported to be biosynthetic products of the RPE. However, following exposure to human serum, immunoreactivity for both clusterin and vitronectin is apparent within these deposits (Fig. 2). Serum exposure also facilitates the accumulation of a third plasma protein and known drusen constituent, serum amyloid P component (APCS) (28). Both vitronectin and APCS are also detected in a few deposits with minimal or no APOE immunoreactivity (Fig. 2). In contrast, haptoglobin, another highly abundant plasma protein, but one that is not typically found in drusen, is not detected (Fig. S2), suggesting that there is a selective accumulation of plasma-derived proteins in sub-RPE deposits. The presence of both RPE- and plasma-derived proteins in these sub-RPE deposits is consistent with the compositional profile of human drusen.
Fig. 2.
Association of serum proteins with APOE deposits. Double-label confocal immunofluorescence images showing localization of APOE (red) and vitronectin (green in A–F), clusterin (green in G–L), or serum amyloid P component (APCS, green in M–R) in sub-RPE deposits with or without exposure to human serum (± SERUM). RPE nuclei are indicated by blue DAPI fluorescence. Deposit-associated APOE immunoreactivity is present without human serum exposure (A, G, M); comparatively weak immunoreactivity is detected for vitronectin (B) and clusterin (H), but not for APCS (N). Following exposure to 10% human serum for 24 h, APOE immunoreactivity associated with sub-RPE deposits is marginally increased (C, I, O), whereas there is substantially increased deposit-associated immunoreactivity for vitronectin (D), clusterin (J), and APCS (P). Merged images (E, F, K, L, Q, R) show striking overlap of immunoreactivity (yellow), indicating that serum vitronectin, clusterin, and APCS, all known drusen constituents, selectively associate with APOE-containing sub-RPE deposits. Almost all APOE deposits show evidence of colocalization with these serum proteins; however, there are examples where there is minimal colocalization of vitronectin (arrowhead in F) and some where APCS immunoreactivity appears to be largely independent of APOE (arrowhead in R). (Scale bars, 10 μm in A, applicable to A–E, G–K, and M–Q; 3.5 μm in F, applicable also to L and R.)
Strikingly, exposure of the cultures to human serum also enables activation of the complement system, as demonstrated by the deposition of C5b-9 terminal complement complexes in association with the sub-RPE deposits. Anti–C5b-9 immunoreactivity is associated with particulate material that often codistributes with APOE-containing deposits (Fig. 3), but the APOE and C5b-9 immunoreactivity patterns are not strictly colocalized, indicating that they represent spatially distinct elements of the deposits. Notably, C5b-9 immunoreactivity is associated only with the basal deposits, and not with the surfaces of viable RPE cells. Consistent with this observation, these deposits are not typically labeled with an antibody directed against Glycoprotein NMB (GPNMB), an abundant RPE surface protein (29) (Fig. 1A). Cell-culture inserts without cells incubated in bovine serum-containing medium and assayed immunocytochemically for C5b-9 show no evidence of immunoreactivity (Fig. S1), indicating that neither components of the medium nor the support matrix are responsible for complement activation and deposition of C5b-9 complexes.
Fig. 3.
Complement activation by sub-RPE deposits/ultrastructure. (A) Confocal image showing APOE immunofluorescence (green) associated with spheroid to globular sub-RPE deposits and C5b-9 immunofluorescence (red) associated with particulate deposit material that is frequently apposed to the surfaces of the APOE deposits (arrows). Blue, nuclear DAPI fluorescence. (B) Electron microscopic image illustrating the membrane-bounded, multivesicular (arrow) and dense, aggregated (arrowhead) forms of sub-RPE deposits identified ultrastructurally. The deposits occupy anastomotic space among processes that make up the support matrix (M). (Scale bars, 10 μm in A, 400 nm in B.)
By electron microscopy, two morphologically distinct deposit subtypes are identified: nonmembrane-bounded conglomerates of small osmophilic particles, and membrane-bounded, multivesicular structures (Fig. 3). These two subtypes are sometimes segregated, and sometimes comingled within deposits.
We assessed the relative contributions of the different complement activation pathways to deposit-associated formation of C5b-9 terminal complement complexes by exposing cultures to human sera depleted of specific components required for activation of the classical or alternative pathways (Fig. 4 A–F). Initially, we confirmed that C5b-9 immunoreactivity is abolished in the presence of serum depleted of C5, a required element of the terminal complement pathway. When cultures are exposed to serum depleted of Factor B, a component required for activation of the alternative pathway, no significant alteration in C5b-9 labeling levels is detected compared with whole serum controls. In contrast, when cultures are exposed to serum depleted of C1q, a component required for formation of the C1 complex and activation of the classical pathway, C5b-9 immunoreactivity associated with sub-RPE deposits is dramatically reduced. Replenishment of the depleted serum with purified C1q restores C5b-9 immunoreactivity. The presence of C1q in association with APOE-containing deposits following serum exposure was confirmed immunocytochemically (Fig. 4 H–I), indicating that molecular components of these deposits have both C1q-binding and complement-activating properties. Exposure of cells to human serum previously treated with cobra venom factor, in order to irreversibly activate the C3 convertase and generate abundant soluble C5b-9 complexes (30), results in markedly reduced levels of C5b-9 immunoreactivity (Fig. S3). This finding indicates that the association of preformed, soluble C5b-9 complexes with sub-RPE deposit material does not contribute significantly to deposit-associated C5b-9 immunoreactivity. Classical pathway activation is most often triggered by binding of C1q to antigen-antibody complexes. Therefore, we also assessed the ability of human serum depleted in IgG to provoke C5b-9 formation. IgG depletion has no detectable effect on deposit-associated C5b-9 immunoreactivity levels compared with whole serum controls (Fig. S4), thus suggesting that activation of the classical pathway occurs via an antibody-independent mechanism.
Fig. 4.
Deposit-associated complement activation is C1q-dependent. Immunoreactivity for deposit-associated terminal complement complexes (C5b-9 neoepitope, red in A–G). In the absence of human serum exposure, C5b-9 immunoreactivity (A) is indistinguishable from the secondary antibody control (B), confirming the serum-dependence of C5b-9 formation. Abundant C5b-9 immunoreactivity is detected following exposure to complete human serum (C), but only background levels of fluorescence are detected following exposure to sera depleted of C5 (D), or C1q (F). The markedly reduced C5b-9 immunoreactivity in the presence of C1q-depleted serum (F) implicates classical pathway activation, whereas the presence of C5b-9 immunoreactivity on exposure to Factor B-depleted serum (E), at levels similar to that of whole serum (C), indicates a lack of significant contribution by alternative pathway activation. Restoration of complement activation by reintroduction of C1q into C1q-depleted serum (G) confirms dependence on C1q for deposit-associated complement activation. Immunoreactivity for C1q (red in I and J) and APOE (green in H and J) colocalize in many APOE-containing sub-RPE deposits following serum exposure (arrowheads in J). C1q also is associated with basal regions of RPE cells (asterisks in I and J). Blue, nuclear DAPI fluorescence. (Scale bars, 10 μm in A and applicable to A–G; 10 μm in H and applicable to H–J.)
Discussion
In this study, we introduce an RPE cell-culture model system that mimics facets of drusen formation and complement activation in vivo. Highly differentiated human RPE cells grown on a permeable support produce extracellular sub-RPE deposits that accumulate within the pores of the supporting material over the course of several weeks in culture. Immunocytochemical analyses show that these sub-RPE deposits contain some of the most abundant protein constituents of human drusen. One of these, APOE, is a ubiquitous drusen-associated protein that is synthesized and secreted by the RPE (24). Others, including vitronectin, clusterin, and APCS, selectively accumulate in APOE-containing deposits when exposed to human serum. Haptoglobin, another abundant plasma protein, does not show similar accumulation. Taken together, these findings suggest that specific protein–protein interactions between RPE-derived and plasma-derived proteins are involved in the formation of these in vitro deposits, just as they appear to be in the process of drusen biogenesis in vivo.
Another population of sub-RPE deposits formed in vitro are the target of complement activation and formation of the terminal complement complex [i.e., the membrane attack complex (MAC): a cytolytic, macromolecular complex consisting of the C5b fragment, C6, C7, C8, and multiple C9 molecules (C5b-9n)]. When cultures are incubated with human serum, a complete source of complement system components, C5b-9 immunoreactivity is detected in association with particulate deposits that are distinct from, but in close proximity to, the more globular APOE-containing deposits. C5b-9 immunoreactivity is not detected on intact RPE cell membranes, most likely because of the robust expression of membrane-associated complement inhibitors by the RPE (15, 31, 32).
The substructural morphology of the in vitro deposits (Fig. 3B) is consistent with the fine structure of sub-RPE deposits identified in vivo. The membrane-bounded form has similarities to the RPE-derived, membranous blebs described originally by both Burns and Feeny-Burns (4) and Ishibashi et al. (5) in human tissue sections. This form would appear to be the most likely site of deposit-associated MAC deposition and C5b-9 immunolabeling, since formation of the MAC requires membrane insertion. On the other hand, the particles/vesicles within the enclosing membrane have dimensions similar to the lipoprotein particles previously described as components of drusen and other sub-RPE deposits (9, 26, 33–38). At this point, we have not determined whether APOE immunoreactivity is associated with one or both substructural forms of sub-RPE deposits, and we did not attempt to identify the lipid moieties associated with APOE. Further analyses using protocols designed for enhanced preservation of lipids will be required to more accurately characterize the nature of lipid materials associated with the sub-RPE deposits and expanded, unbiased proteomic profiling will be required to reveal the entire spectrum of proteins comprising the deposits.
RPE cells express transcripts for APOE at levels comparable to those in the liver and brain, the two most abundant biosynthetic sources of APOE (24). APOE is secreted in considerable amounts by RPE cells both apically and basally, and has been implicated in lipid trafficking, facilitating the efflux of lipids from the RPE, and their transit across Bruch's membrane to the choroidal vasculature (16). In human RPE/choroid tissue, we previously documented the presence of APOE-immunoreactive structures of similar size and morphology to those we observe in vitro (24), thus suggesting that the basal shedding of APOE-containing material also occurs in situ. Drusen-associated immunoreactivity for apolipoproteins A-1 and B has been shown in other studies (37, 38); however, we did not observe immunoreactivity for these moieties in the deposits. These results may be related to antibody-dependent issues and/or the nature of the cell culture system employed.
Although AMD risk-conferring gene polymorphisms have been identified for complement Factor H, the major negative regulator of the alternative complement pathway, the alternative pathway does not appear to be the primary activation pathway in this system. Serum depletion of Factor B, a complement component required for alternative pathway activation, does not diminish deposition of C5b-9 complexes. However, when cultures are incubated in serum depleted of C1q, a pattern-recognition molecule required for classical pathway activation, C5b-9 immunoreactivity levels are dramatically reduced. Supplementation with purified C1q restores C5b-9 immunoreactivity. These findings implicate the classical pathway as the principal activation pathway, and suggest that it may be mediated directly via C1q binding. Because the alternative pathway acts as an amplification loop for the classical pathway and can be stimulated by classical pathway activation (39), this conclusion is not inconsistent with a role for the alternative pathway in AMD. Given this interplay between the classical and alternative pathways, some diminution of C5b-9 deposition might have been expected in the absence of Factor B. However, high concentrations of complement Factor H in the human serum may act to effectively repress the alternative pathway in this in vitro system. In vivo, where local Factor H concentrations are likely lower, dysregulation of the amplification loop of the alternative pathway stemming from Factor H dysfunction would be predicted to exacerbate complement-mediated inflammation with pathologic consequences (40).
The classical pathway is typically triggered by binding of C1q to the Fc fragment of IgG or IgM in clusters of antigen-antibody complexes on the surfaces of microbial pathogens. When exposed to serum depleted of IgG, C5b-9 immunoreactivity levels are unaffected, thus suggesting that activation is not triggered by an antibody-mediated mechanism. However, the classical pathway can also be activated by direct binding of C1q to a variety of other molecules, such as C-reactive protein, APCS, amyloid-β, and modified LDL cholesterol (41, 42). In addition, C1q can bind apoptotic cells and cell membrane blebs with consequent activation of the classical complement pathway (43–45). Collectively, these findings are consistent with the general conclusion that one or more C1q-binding molecules are the most likely triggers of the complement cascade in this system and perhaps in the eye as well. The identification of C1q in association with APOE-containing deposits following serum exposure suggests that they contain a molecular ligand for C1q, and thus a potential activator of the complement cascade. Membranous deposits that lie in close proximity to the APOE/C1q-containing deposits would then be predicted to provide the surface required for MAC formation. Further analyses will be required to identify specific C1q binding partners that may be responsible.
Previous studies of sub-RPE deposit formation in vitro used ARPE-19 cells grown on impermeable tissue-culture plastic. Under these conditions, sub-RPE deposits with drusen-like properties were not apparent, nor were known drusen constituents identified (46, 47). Interestingly, Amin et al. (46) did note an increase in total membranous and condensed deposits when cells were cultured on porous supports as opposed to tissue-culture plastic. In the in vitro system we describe here, the macromolecular material shed at the basal RPE surface tends to accumulate in the pores of the culture support. The size and shape of the deposits are likely to be influenced by the physical dimensions of the substrate pores and, to some extent, this porous meshwork may also act to retain deposit material, limiting its diffusion into the basal medium compartment.
In situ, it is probable that a mechanism exists for the removal of basally shed RPE debris because, early in life, it does not typically accumulate in the sub-RPE compartment. However, disruption of the normal clearance mechanism, perhaps as a result of age-related changes in phagocytic activity, immune function, and/or the structure and composition of Bruch's membrane, would be predicted to lead to the accumulation of sub-RPE debris and eventually to the formation of human drusen. If this model is correct, the use of experimental substrates with biochemical and diffusional properties that mimic the lipid-rich, hydrophobic environment that characterizes the aged Bruch's membrane might result in the buildup of deposit aggregates that more closely resemble human drusen. Alternatively, interactions among drusen-associated proteins and lipids that are not present in our in vitro system may be required for the establishment of characteristic drusen morphology.
It is noteworthy that complement-activating sub-RPE debris is formed by cultured human RPE cells that are not exposed to shed photoreceptor outer segments and, thus, are not impacted by the metabolic derivatives of membrane lipids that have been proposed as complement-activating and immunogenic elements in AMD (48, 49). However, such factors, in conjunction with aging, environmental influences, and genetic predisposition could play a role in promoting the susceptibility of RPE cells to directed or bystander complement attack in vivo. We do not find evidence for RPE-directed complement attack (i.e., C5b-9 complexes associated with the RPE cell surface), most likely because the cells are chronologically young (derived from fetal sources), express membrane-associated negative regulators of complement including CD46, CD55, and CD59 (15, 31, 32), and have not been affected by senescence or exposed to environmental “stressors.”
Importantly, the cell-based system we have used in these studies recapitulates some of the key pathologic processes at play in AMD. As such, this system should facilitate rigorous experimental analysis of the molecular basis of drusen formation and complement activation in AMD, the evaluation of known AMD risk factors at cellular and molecular levels, and the testing of novel therapeutic strategies for the treatment of AMD in its earliest stages.
Methods
RPE Cell Culture.
RPE cells were derived from human fetal eyes (Advanced Bioscience Resources) as described by Hu and Bok (50). Early passage (p1–p2) cells were seeded onto laminin-coated porous supports (Millipore Millicell-HA Culture Plate Inserts, PIHA 01250) and cultured in “Miller medium” (51). One- to 3-mo postseeding, cultures were exposed to complement-competent human sera, human sera depleted of C1q, C5, or Factor B (Table S1), or human serum depleted of IgG by absorption with protein A/G coupled to agarose beads (Pierce) (Fig. S4) at 10% (vol/vol) in serum-free Miller medium for 24 h. The specimens were then rinsed in PBS (3×, 15 min), fixed for 10 min in 4% paraformaldehyde in PBS, and stored in 0.4% paraformaldehyde pending sectioning and immunohistochemical analyses.
Immunohistochemistry.
Confocal laser scanning immunofluorescence imaging was performed as described previously (52) on 100-μm vibratome sections or flat mounts of fixed RPE cell cultures. Flat mounts were bleached (Melanin Bleach Kit; Polysciences) for 10 min to allow visualization of sub-RPE deposits that would otherwise be obscured by RPE pigment. Antibodies utilized are listed in Table S2.
Electron Microscopy.
RPE cultures were fixed in 2% glutaraldehyde and 2% formaldehyde, postfixed in 2% osmium tetroxide, dehydrated in a graded ethanol series up to 100% and embedded in LX-112 resin (Ladd Research). Thin sections were stained with 2% uranyl acetate and lead citrate and imaged on a JEOL JEM-1230 transmission electron microscope.
Supplementary Material
Acknowledgments
We thank Drs. Catherine Bowes Rickman and Gregory S. Hageman for insightful comments on the manuscript. This work was supported by Grant EY R24 EY017404 from the National Eye Institute and by generous benefactors of the Center for the Study of Macular Degeneration at the University of California, Santa Barbara.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109703108/-/DCSupplemental.
References
- 1.Farkas TG, Sylvester V, Archer D. The ultrastructure of drusen. Am J Ophthalmol. 1971;71:1196–1205. doi: 10.1016/0002-9394(71)90963-9. [DOI] [PubMed] [Google Scholar]
- 2.Sarks SH. Ageing and degeneration in the macular region: A clinico-pathological study. Br J Ophthalmol. 1976;60:324–341. doi: 10.1136/bjo.60.5.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green WR, Key SN., 3rd Senile macular degeneration: A histopathologic study. 1977. Retina. 2005;25(5, Suppl):180–250. discussion 250–254. [PubMed] [Google Scholar]
- 4.Burns RP, Feeney-Burns L. Clinico-morphologic correlations of drusen of Bruch's membrane. Trans Am Ophthalmol Soc. 1980;78:206–225. [PMC free article] [PubMed] [Google Scholar]
- 5.Ishibashi T, Patterson R, Ohnishi Y, Inomata H, Ryan SJ. Formation of drusen in the human eye. Am J Ophthalmol. 1986;101:342–353. doi: 10.1016/0002-9394(86)90830-5. [DOI] [PubMed] [Google Scholar]
- 6.Kliffen M, van der Schaft TL, Mooy CM, de Jong PT. Morphologic changes in age-related maculopathy. Microsc Res Tech. 1997;36:106–122. doi: 10.1002/(SICI)1097-0029(19970115)36:2<106::AID-JEMT4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 7.Hageman GS, Mullins RF. Molecular composition of drusen as related to substructural phenotype. Mol Vis. 1999;5:28. [PubMed] [Google Scholar]
- 8.Mullins RF, Aptsiauri N, Hageman GS. Structure and composition of drusen associated with glomerulonephritis: implications for the role of complement activation in drusen biogenesis. Eye (Lond) 2001;15:390–395. doi: 10.1038/eye.2001.142. [DOI] [PubMed] [Google Scholar]
- 9.Sarks SH, Arnold JJ, Killingsworth MC, Sarks JP. Early drusen formation in the normal and aging eye and their relation to age related maculopathy: A clinicopathological study. Br J Ophthalmol. 1999;83:358–368. doi: 10.1136/bjo.83.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarks S, Cherepanoff S, Killingsworth M, Sarks J. Relationship of Basal laminar deposit and membranous debris to the clinical presentation of early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007;48:968–977. doi: 10.1167/iovs.06-0443. [DOI] [PubMed] [Google Scholar]
- 11.Russell SR, Mullins RF, Schneider BL, Hageman GS. Location, substructure, and composition of basal laminar drusen compared with drusen associated with aging and age-related macular degeneration. Am J Ophthalmol. 2000;129:205–214. doi: 10.1016/s0002-9394(99)00345-1. [DOI] [PubMed] [Google Scholar]
- 12.Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134:411–431. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 13.Anderson DH, et al. Characterization of beta amyloid assemblies in drusen: The deposits associated with aging and age-related macular degeneration. Exp Eye Res. 2004;78:243–256. doi: 10.1016/j.exer.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Hageman GS, et al. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20:705–732. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 15.Anderson DH, et al. The pivotal role of the complement system in aging and age-related macular degeneration: Hypothesis re-visited. Prog Retin Eye Res. 2010;29:95–112. doi: 10.1016/j.preteyeres.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishida BY, et al. Regulated expression of apolipoprotein E by human retinal pigment epithelial cells. J Lipid Res. 2004;45:263–271. doi: 10.1194/jlr.M300306-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.An E, et al. Secreted proteome profiling in human RPE cell cultures derived from donors with age related macular degeneration and age matched healthy donors. J Proteome Res. 2006;5:2599–2610. doi: 10.1021/pr060121j. [DOI] [PubMed] [Google Scholar]
- 18.Suuronen T, Nuutinen T, Ryhänen T, Kaarniranta K, Salminen A. Epigenetic regulation of clusterin/apolipoprotein J expression in retinal pigment epithelial cells. Biochem Biophys Res Commun. 2007;357:397–401. doi: 10.1016/j.bbrc.2007.03.135. [DOI] [PubMed] [Google Scholar]
- 19.Kim YH, et al. Regulated secretion of complement factor H by RPE and its role in RPE migration. Graefes Arch Clin Exp Ophthalmol. 2009;247:651–659. doi: 10.1007/s00417-009-1049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gehrs KM, Jackson JR, Brown EN, Allikmets R, Hageman GS. Complement, age-related macular degeneration and a vision of the future. Arch Ophthalmol. 2010;128:349–358. doi: 10.1001/archophthalmol.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hecker LA, Edwards AO. Genetic control of complement activation in humans and age related macular degeneration. Adv Exp Med Biol. 2010;703:49–62. doi: 10.1007/978-1-4419-5635-4_4. [DOI] [PubMed] [Google Scholar]
- 22.Johnson LV, Ozaki S, Staples MK, Erickson PA, Anderson DH. A potential role for immune complex pathogenesis in drusen formation. Exp Eye Res. 2000;70:441–449. doi: 10.1006/exer.1999.0798. [DOI] [PubMed] [Google Scholar]
- 23.Johnson LV, Leitner WP, Staples MK, Anderson DH. Complement activation and inflammatory processes in drusen formation and age related macular degeneration. Exp Eye Res. 2001;73:887–896. doi: 10.1006/exer.2001.1094. [DOI] [PubMed] [Google Scholar]
- 24.Anderson DH, et al. Local cellular sources of apolipoprotein E in the human retina and retinal pigmented epithelium: Implications for the process of drusen formation. Am J Ophthalmol. 2001;131:767–781. doi: 10.1016/s0002-9394(00)00961-2. [DOI] [PubMed] [Google Scholar]
- 25.Crabb JW, et al. Drusen proteome analysis: An approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci USA. 2002;99:14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, et al. Abundant lipid and protein components of drusen. PLoS One. 2010;5:e10329. doi: 10.1371/journal.pone.0010329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wasmuth S, Lueck K, Baehler H, Lommatzsch A, Pauleikhoff D. Increased vitronectin production by complement-stimulated human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2009;50:5304–5309. doi: 10.1167/iovs.08-3326. [DOI] [PubMed] [Google Scholar]
- 28.Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14:835–846. [PubMed] [Google Scholar]
- 29.Alcazar O, et al. Proteomics characterization of cell membrane blebs in human retinal pigment epithelium cells. Mol Cell Proteomics. 2009;8:2201–2211. doi: 10.1074/mcp.M900203-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tylman M, Bengtson JP, Bengtsson A. Activation of the complement system by different autologous transfusion devices: An in vitro study. Transfusion. 2003;43:395–399. doi: 10.1046/j.1537-2995.2003.00311.x. [DOI] [PubMed] [Google Scholar]
- 31.Yang P, Tyrrell J, Han I, Jaffe GJ. Expression and modulation of RPE cell membrane complement regulatory proteins. Invest Ophthalmol Vis Sci. 2009;50:3473–3481. doi: 10.1167/iovs.08-3202. [DOI] [PubMed] [Google Scholar]
- 32.Vogt SD, Barnum SR, Curcio CA, Read RW. Distribution of complement anaphylatoxin receptors and membrane-bound regulators in normal human retina. Exp Eye Res. 2006;83:834–840. doi: 10.1016/j.exer.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Killingsworth MC. Age-related components of Bruch's membrane in the human eye. Graefes Arch Clin Exp Ophthalmol. 1987;225:406–412. doi: 10.1007/BF02334166. [DOI] [PubMed] [Google Scholar]
- 34.Ulshafer RJ, Allen CB, Nicolaissen B, Jr, Rubin ML. Scanning electron microscopy of human drusen. Invest Ophthalmol Vis Sci. 1987;28:683–689. [PubMed] [Google Scholar]
- 35.Curcio CA, Millican CL, Bailey T, Kruth HS. Accumulation of cholesterol with age in human Bruch's membrane. Invest Ophthalmol Vis Sci. 2001;42:265–274. [PubMed] [Google Scholar]
- 36.Curcio CA, Johnson M, Huang JD, Rudolf M. Apolipoprotein B-containing lipoproteins in retinal aging and age-related macular degeneration. J Lipid Res. 2010;51:451–467. doi: 10.1194/jlr.R002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malek G, Li CM, Guidry C, Medeiros NE, Curcio CA. Apolipoprotein B in cholesterol-containing drusen and basal deposits of human eyes with age-related maculopathy. Am J Pathol. 2003;162:413–425. doi: 10.1016/S0002-9440(10)63836-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li C-M, et al. Lipoprotein-like particles and cholesteryl esters in human Bruch's membrane: Initial characterization. Invest Ophthalmol Vis Sci. 2005;46:2576–2586. doi: 10.1167/iovs.05-0034. [DOI] [PubMed] [Google Scholar]
- 39.Meri S, Pangburn MK. A mechanism of activation of the alternative complement pathway by the classical pathway: Protection of C3b from inactivation by covalent attachment to C4b. Eur J Immunol. 1990;20:2555–2561. doi: 10.1002/eji.1830201205. [DOI] [PubMed] [Google Scholar]
- 40.Holers VM. The spectrum of complement alternative pathway-mediated diseases. Immunol Rev. 2008;223:300–316. doi: 10.1111/j.1600-065X.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- 41.Gaboriaud C, et al. Structure and activation of the C1 complex of complement: Unraveling the puzzle. Trends Immunol. 2004;25:368–373. doi: 10.1016/j.it.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 42.Bohlson SS, Fraser DA, Tenner AJ. Complement proteins C1q and MBL are pattern recognition molecules that signal immediate and long-term protective immune functions. Mol Immunol. 2007;44:33–43. doi: 10.1016/j.molimm.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 43.Navratil JS, Watkins SC, Wisnieski JJ, Ahearn JM. The globular heads of C1q specifically recognize surface blebs of apoptotic vascular endothelial cells. J Immunol. 2001;166:3231–3239. doi: 10.4049/jimmunol.166.5.3231. [DOI] [PubMed] [Google Scholar]
- 44.Nauta AJ, et al. Direct binding of C1q to apoptotic cells and cell blebs induces complement activation. Eur J Immunol. 2002;32:1726–1736. doi: 10.1002/1521-4141(200206)32:6<1726::AID-IMMU1726>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 45.Païdassi H, et al. C1q binds phosphatidylserine and likely acts as a multiligand-bridging molecule in apoptotic cell recognition. J Immunol. 2008;180:2329–2338. doi: 10.4049/jimmunol.180.4.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amin S, et al. Modulation of Sub-RPE deposits in vitro: A potential model for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004;45:1281–1288. doi: 10.1167/iovs.03-0671. [DOI] [PubMed] [Google Scholar]
- 47.Marin-Castaño ME, et al. Repetitive nonlethal oxidant injury to retinal pigment epithelium decreased extracellular matrix turnover in vitro and induced sub-RPE deposits in vivo. Invest Ophthalmol Vis Sci. 2006;47:4098–4112. doi: 10.1167/iovs.05-1230. [DOI] [PubMed] [Google Scholar]
- 48.Hollyfield JG, Perez VL, Salomon RG. A hapten generated from an oxidation fragment of docosahexaenoic acid is sufficient to initiate age-related macular degeneration. Mol Neurobiol. 2010;41:290–298. doi: 10.1007/s12035-010-8110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sparrow JR. Bisretinoids of RPE lipofuscin: trigger for complement activation in age-related macular degeneration. Adv Exp Med Biol. 2010;703:63–74. doi: 10.1007/978-1-4419-5635-4_5. [DOI] [PubMed] [Google Scholar]
- 50.Hu J, Bok D. A cell culture medium that supports the differentiation of human retinal pigment epithelium into functionally polarized monolayers. Mol Vis. 2001;7:14–19. [PubMed] [Google Scholar]
- 51.Maminishkis A, et al. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest Ophthalmol Vis Sci. 2006;47:3612–3624. doi: 10.1167/iovs.05-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anderson DH, et al. Vitronectin gene expression in the adult human retina. Invest Ophthalmol Vis Sci. 1999;40:3305–3315. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.