Abstract
A critical step in neuronal development is the formation of axon/dendrite polarity, a process involving symmetry breaking in the newborn neuron. Local self-amplifying processes could enhance and stabilize the initial asymmetry in the distribution of axon/dendrite determinants, but the identity of these processes remains elusive. We here report that BDNF, a secreted neurotrophin essential for the survival and differentiation of many neuronal populations, serves as a self-amplifying autocrine factor in promoting axon formation in embryonic hippocampal neurons by triggering two nested positive-feedback mechanisms. First, BDNF elevates cytoplasmic cAMP and protein kinase A activity, which triggers further secretion of BDNF and membrane insertion of its receptor TrkB. Second, BDNF/TrkB signaling activates PI3-kinase that promotes anterograde transport of TrkB in the putative axon, further enhancing local BDNF/TrkB signaling. Together, these self-amplifying BDNF actions ensure stable elevation of local cAMP/protein kinase A activity that is critical for axon differentiation and growth.
Keywords: axon initiation and growth, cAMP/protein kinase A elevation, neurotrophin autocrine loops, Trk redistribution
The most distinct cellular feature of the neuron is its polarized structure, consisting of a single long axon and many short and highly branched dendrites. This polarized structure is essential for two primary neuronal functions: the reception and integration of synaptic inputs at the dendrite, and the conduction and delivery of output signals to other cells via the axon. For the formation of this polarity, the postmitotic neuron may have inherited an asymmetry in the activity or distribution of specific cellular components that could trigger local axon/dendrite differentiation. Alternatively, extracellular environment may provide an asymmetric signal in the neuron that specifies its polarity. Such a polarization process may also occur spontaneously, as shown by isolated embryonic hippocampal neurons cultured on a uniform substrate (1). These neurons undergo polarization through distinct stages—the formation of dynamic lamellipodia around the cell periphery (stage 1) within a few hours after plating, the appearance of several neurites of similar morphology (stage 2) after 12–16 h in culture, and differentiation of one axon and multiple dendrites (stage 3) within 1–1.5 d. Recent studies have shown that local elevation of cAMP and protein kinase A (PKA) activity, acting through downstream activation of LKB1 and SAD kinases (2–4), is a critical early event in axon initiation. In cultured developing hippocampal neuron, we also showed that extracellular applied BDNF promotes axon differentiation by elevating cAMP in the undifferentiated neurites (5). In the present study, we further showed that endogenous BDNF in developing hippocampal neurons promotes axon initiation and growth by locally elevating and stabilizing cAMP/PKA activity through self-amplifying autocrine actions.
As a member of the neurotrophin family, BDNF was initially identified as a target-derived factor that promotes the survival of several populations of central neurons (6–9). However, mRNAs of BDNF and its TrkB receptor coexist in the same neuron (10, 11), and antisense BDNF oligonucleotides (12, 13) and anti-BDNF antiserum (14, 15) could reduce the survival of cultured neurons, even as isolated single cells (13). Thus, BDNF may act as an autocrine factor for maintaining neuronal survival during target-independent stages of development (16). However, for rapid modulatory actions of BDNF on nerve growth (17, 18) and synaptic function (19), it remains unclear whether BDNF acts as an autocrine, paracrine, or target-derived factor, and whether the neuronal action of secreted BDNF is local or global. Previous studies have shown that local application of BDNF to the neurite of cultured hippocampal neurons triggers its differentiation into the axon (4). Here, we show that axon development also depends on the secretion of endogenous BDNF, which acts as an autocrine rather than paracrine factor. Using BDNF conjugated to a pH-sensitive probe to monitor BDNF secretion, we found that extracellular BDNF can trigger BDNF secretion from the neurite by local elevation of cAMP/PKA activity. In addition, BDNF also promotes surface insertion of its receptor TrkB through cAMP/PKA activation and anterograde TrkB transport via PI3-kinase (PI3K) activation, further amplifying the BDNF/TrkB signaling. These autocrine actions of BDNF are amplified by nested positive-feedback loops, resulting in stable elevation of local cAMP/PKA activity required for axon development (4).
Results
Secreted BDNF Acts as an Autocrine Factor for Axon Development.
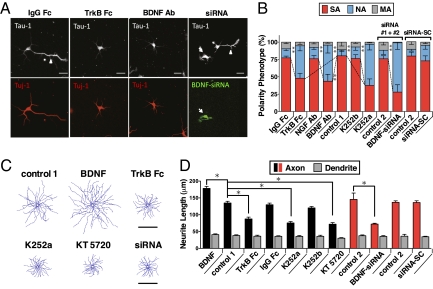
We first tested the hypothesis that the developing neuron secretes BDNF to promote its own axon development. The action of secreted BDNF on cultured rat hippocampal neurons was disrupted by the addition of BDNF antibodies or TrkB-Fc, a soluble ligand for scavenging secreted BDNF, at 6 h after cell plating. Immunostaining of these neurons 2 d later showed that the percentage of neurons exhibiting axon formation was significantly lower than those found in untreated cultures or in cultures treated with IgG-Fc or antibodies against NGF (Fig. 1 A and B). Similar effects on axon differentiation were found when a membrane-permeable trk tyrosine kinase inhibitor K252a or the PKA inhibitor KT5720 was added to the culture, whereas no effect was observed after adding K252b, an analog of K252a with poor membrane permeability. These results based on BDNF antibodies, TrkB-Fc, and K252a together support the notion that BDNF secreted from these neurons facilitates spontaneous axon differentiation via TrkB- and cAMP/PKA-dependent processes.
Fig. 1.
Secreted BDNF exerts autocrine actions on axon differentiation and growth. (A) Images of hippocampal neurons in 2-d culture, immunostained with axon marker Tau-1 and neuron marker Tuj-1. Cells were incubated with medium containing IgG-Fc (20 μg/ml), TrkB-Fc (20 μg/ml), or BDNF antibodies (5 μg/ml) or transfected with BDNF-siRNA (together with GFP). Arrowhead, axon; arrow: siRNA-transfected cell; *, untransfected cell. (B) Phenotype of 2-d neurons after treatments similar to those in A, including untreated cells (control 1), and untransfected cells in the same cultures (control 2) as those BDNF-siRNA-transfected cells. siRNA-SC, scrambled form of siRNA; SA, single axon; NA, no axon; MA, multiple axons. Data presented as average SEM (n > 50 cells/culture, 3–5 cultures each; **P < 0.01, Tukey test). (C) Composite tracing of axons from 25 randomly sampled neurons from 2-d cultures that were not treated (control 1), treated with BDNF (50 ng/ml), TrkB-Fc (20 μg/ml), K252a (100 nM), K252b (100 nM), or KT5720 (2 μM), or BDNF-siRNA. (D) Axon/dendrite growth in cultures treated with indicated chemicals for 24–36 h (began at 12 h after plating). Data presented as average axon or dendrite length (SEM, n > 50 cells/culture, 3–5 cultures each). *Data significantly different from untreated neurons (control 1) or untransfected neurons in the same culture (control 2) are marked by (P < 0.05, t test).
We then tested whether BDNF acts as an autocrine or paracrine factor in promoting axon differentiation. The BDNF expression was down-regulated in a small population of neurons in these cultures by transfection with two specific siRNAs against BDNF translation [BDNF-siRNA #1 and #2 (20); SI Materials and Methods], together with EGFP as a marker. We found a marked reduction of axon formation in BDNF-siRNA–expressing neurons but not in untransfected neurons in the same culture nor in neurons transfected with a scrambled form of siRNA (Fig. 1 A and B). Furthermore, we found that BDNF also acts as an autocrine factor to promote axon growth in polarized neurons. This was shown by the finding that 12-h treatment with TrkB-Fc, K252a, KT5720, or BDNF-siRNA constructs after axon/dendrite specification (at 24–36 h in culture) resulted in reduced axon growth, without significant effect on dendrite growth. Consistent with previous findings (17, 21), addition of purified recombinant BDNF (50 ng/mL) markedly increased axon growth (Fig. 1 C and D). In contrast, axon/dendrite growth was not affected by the treatment with IgG-Fc, K252b, or a scrambled form of siRNA (Fig. 1 C and D).
cAMP Mediates BDNF-Induced BDNF Secretion.
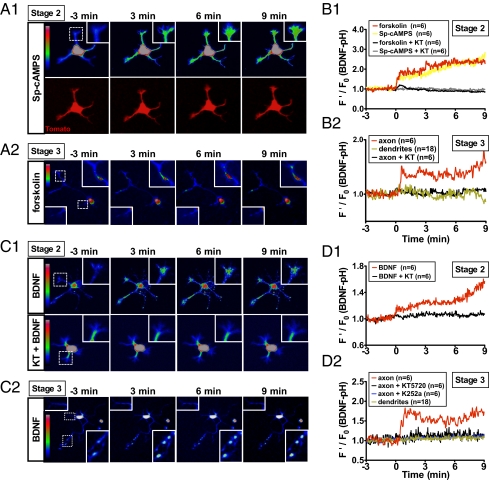
To monitor directly the process of BDNF secretion in developing neurons, we transfected cultured hippocampal neurons at 6 h after plating with a construct coding for BDNF conjugated with a pH-sensitive form of EGFP [BDNF-pHluorin (22); SI Materials and Methods] and monitored the secretion of BDNF-pHluorin by its fluorescence increase upon exocytic secretion to the extracellular space (23). Bath addition of forskolin (20 μM), which elevates cAMP by activating adenylate cyclase, or membrane-permeant cAMP analog Sp-cAMPS (50 μM) resulted in an increase in the BDNF-pHluorin fluorescence surrounding most neurites of unpolarized neurons (stage 2) (1) in 14–24 h cultures (Fig. 2 A1 and B1), consistent with a previous study using ELISA in pituitary adenoma cell line (24). In contrast, the same treatment of polarized neurons (stage 3) (1) in 2-d cultures increased BDNF-pHluorin fluorescence along the axon but not dendrites, with the highest accumulation in the distal axon (Fig. 2 A2 and B2). This increased fluorescence was due to secreted BDNF-pHluorin adhering to the cell surface or culture substrate, because it was completely eliminated by bath-applied membrane-impermeant fluorescence quencher bromophenol blue (Fig. S1) (25). Furthermore, forskolin-induced fluorescence increase was absent when KT5270 was present in the medium, indicating that this secretion was mediated by PKA. Thus, axon/dendrite differentiation is accompanied by a redistribution of cAMP/PKA-sensitive BDNF secretion toward the axon.
Fig. 2.
Secretion of BDNF-pHluorin triggered by elevating cAMP or applying BDNF. (A) Fluorescence images of stage 2 (unpolarized, A1) and stage 3 (polarized, A2) hippocampal neurons expressing pH-sensitive fusion protein BDNF-pHluorin, before and at different times after exposure to Sp-cAMPS or forskolin. Insets: Neurite or axon/dendrite tips where pHluorin fluorescence was measured and coded in pseudo colors linearly by the scale shown on the left. (B) Average traces depicting relative pHluorin fluorescence intensity before and after treatment with forskolin and Sp-cAMPS, in the absence or presence of KT7250, for stage 2 and 3 neurons, with the intensity normalized by the mean intensity during the last 3 min before the treatment. n, number of neurons measured. (C and D) Similar to A and B, except that recombinant BDNF (50 ng/mL) was applied.
Previous ELISA measurements have shown that neurotrophins can induce neurotrophin release via the regulated secretory pathway (26, 27). In this study we also performed ELISA on the supernatant of hippocampal cell cultures and found that BDNF secretion was significantly elevated by bath addition of forskolin, Sp-cAMPS, or high K+, and this elevation was abolished by coaddition of KT5720 (Fig. S2). Furthermore, we examined the topography of BDNF-induced BDNF secretion in single developing hippocampal neurons that expressed BDNF-pHluorin. Similar to that observed for Sp-cAMPS and forskolin, bath-applied BDNF (50 ng/mL) increased BDNF-pHluorin fluorescence associated with all neurites in stage-2 neurons (Fig. 2 C1 and D1), but with only the axon in stage-3 neurons (Fig. 2 C2 and D2). No fluorescence increase was observed in the presence of KT5720 in both cases. The above study showed that BDNF-pHluorin fluorescence changes induced by forskolin and BDNF showed similar topography and PKA dependence, suggesting that BDNF may trigger BDNF secretion by increasing the cAMP level. This was tested by directly measuring the level of cAMP and PKA activity with fluorescence resonance energy transfer (FRET) sensors indicator of cAMP using Epac (ICUE) and A-kinase activity reporter (AKAR) (SI Materials and Methods), respectively. We found that bath-applied BDNF induced an elevation of cAMP and PKA activity in neurons expressing ICUE and AKAR (Fig. 3A and Fig. S3), respectively, with a time course and magnitude similar to that induced by forskolin (28). Furthermore, BDNF-induced cAMP/PKA elevation was present in all neurites of stage-2 neurons but became localized to the axon in stage-3 neuron (Fig. 3A2), indicating a redistribution of BDNF-sensitive mechanism during neuronal polarization, similar to that found for forskolin- and BDNF-induced BDNF secretion. Thus, BDNF could trigger BDNF secretion by elevating cAMP/PKA activity.
Fig. 3.
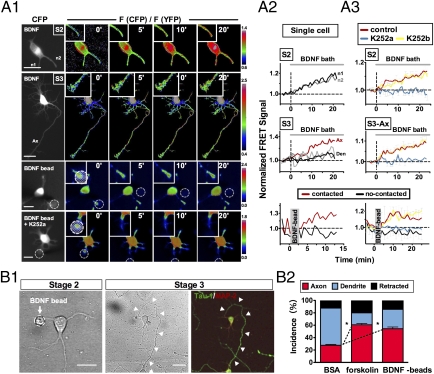
BDNF-induced cAMP elevation and axon differentiation. (A) BDNF induced an elevation of cAMP. (A1), Hippocampal neurons at stage 2 (S2, unpolarized) and 3 (S3, polarized), transfected with FRET indicator for cAMP (ICUE; see SI Materials and Methods). Shown are images of CFP fluorescence and FRET signals at different times after bath-applying of BDNF (50 ng/ml) or contact with a BDNF-coated bead. The FRET signal is the ratio of CFP to YFP fluorescence [F(CFP)/F(YFP)], measured in the boxed regions. Bar, 20 μm. (A2), Traces of cAMP changes at the neurite (or axon/dendrite) tip of individual neurites (normalized by the mean values for 3 min prior to BDNF). (A3) Summary of cAMP changes at the neurite (or axon/dendrite) tip, shown by FRET signals (normalized as in A2), in the absence or presence of K252a (or K252b). Error bar = SEM (n = 5–10 cells each, 1 or 2 neurites/cell). (B) Axon development triggered by local exposure to BDNF or forskolin-induced cAMP elevation. (B1), Images showing local contact of a single BDNF-coated bead with an undifferentiated neurite (10 h after plating, Left), which developed into an axon (arrow heads) 48 h later, as shown by Tau-1 staining (Right). (B2), Percentage (SEM, n > 15 each, *P < 0.05, t test) of neurites that differentiated into axon or dendrite, or retracted at 36–48 h after the contact with a bead coated with BSA (control), forskolin, or BDNF.
BDNF-Induced Local cAMP Elevation Triggers Axon Differentiation.
To determine whether BDNF-induced BDNF secretion operates at a local or global level in the neuron, we applied BDNF to cultured hippocampal neurons with latex beads covalently coated with BDNF (SI Materials and Methods). The beads were removed within a few minutes after the contact to avoid long-term nonspecific effects of the bead, as shown by the induction of ACh receptor clusters by positively charged beads in the muscle fiber (29). We found that the cAMP level, as indicated by FRET signals from ICUE-transfected neurons, was elevated locally and persistently near the contact site within a few minutes after the bead contact with the neurite of stage-2 neurons (Fig. 3 A1 and A2). Consistent with a previous report (28), the cAMP level in noncontacted neurites of the same cell was reduced (Fig. 3A2), indicating long-range inhibitory signaling. Furthermore, the BDNF-coated bead also induced a local elevation of pHluorin fluorescence at the site of bead contact in neurons expressing BDNF-pHluorin, with a time course consistent with cAMP/PKA-dependent BDNF secretion (Fig. S4). The cAMP/PKA elevation induced by either bath- or bead-applied BDNF was reduced by the presence of K252a but not K252b. With the caveat that K252a may exert nonspecific effect, these results do suggest the involvement of Trk signaling (Fig. 3A3). Consistent with the developmental transition in the topography of BDNF secretion (Fig. 2), BDNF-induced cAMP elevation was observed at the axon, not at dendrites (Fig. 3A2). Thus, there were similar developmental changes in the topography of cAMP elevation and BDNF secretion.
The localized cAMP elevation and BDNF secretion triggered by BDNF-coated beads suggests that BDNF-triggered secretion of BDNF could serve as a self-amplifying mechanism for maintaining a stable and localized cAMP elevation, which is required for axon specification via PKA-dependent phosphorylation of LKB1 (4) and subsequent activation of a cascade of downstream effector enzymes [e.g., SAD kinases (2, 3)]. Furthermore, BDNF-coated bead could promote axon differentiation of stage-2 neuron of the contacted neurite. When beads were manipulated into contact with the undifferentiated neurites of stage-2 neurons, and the same neurons were re-examined 2 d later for axon formation (Fig. 3B1), we found a significantly higher incidence of axon formation for neurites contacted by forskolin- or BDNF-coated beads than for those coated with BSA (Fig. 3B2). In contrast, we found no significant effect on the FRET signal and axon initiation after the 3- to 5-min contact of the neurite with uncoated or BSA-coated beads. Thus, local exposure to BDNF or elevation of cAMP indeed promotes local axon differentiation in these neurons.
PKA-Dependent Surface Insertion of TrkB Receptor.
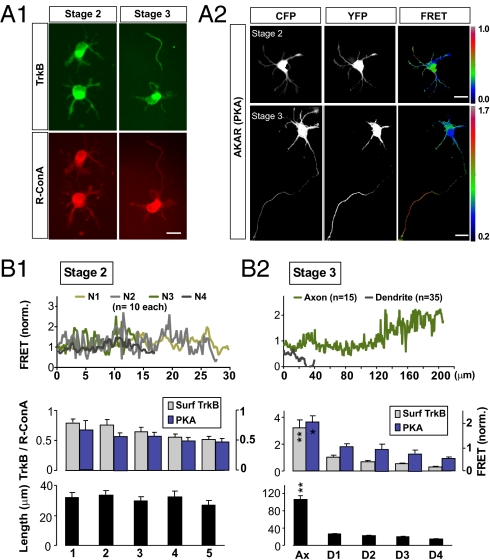
Measurements of BDNF-pHluorin secretion showed a transition from uniform secretion over all neurites to a restricted secretion at the axon as the neuron becomes polarized. Immunostaining of surface TrkB with ectodomain-specific antibodies showed a similar redistribution of TrkB toward the axon (Fig. 4A1). The specificity of the TrkB antibodies used was confirmed by Western blot assays in HEK293 cells, showing higher immunostaining for lysates from cells transfected with TrkB-expressing construct than from nontransfected control cells. To have a control for the membrane area of the neuron, we used the staining with rhodamine-conjugated Con A (R-ConA), a plant lectin that binds to glucose and mannose residues of all cell surface glycoproteins. After normalization of the TrkB staining with that of R-ConA, we found that the highest TrkB staining intensity at the neurite tip of stage-2 neurons was 121% ± 14% (SEM, n = 13) of the mean intensity of tips of the same neuron (as shown for neurites with five highest intensities; Fig. 4B1). For stage-3 neurons, however, the TrkB staining intensity at the axonal tip was 479% ± 50% (SEM, n = 19) of the mean tip intensity (Fig. 4B2). This topographic redistribution of TrkB could be due to either an increased rate of TrkB membrane insertion at the axon or increased trafficking of TrkB precursor vesicles toward the axon, or both. It could also account for the restricted BDNF-induced secretion of BDNF-pHluorin at the distal axon of polarized neurons.
Fig. 4.
Redistribution of TrkB receptor and cAMP toward axon after polarization. (A) Surface TrkB and cytoplasmic cAMP level in stage-2 and stage-3 neurons. (A1) Hippocampal neurons immunostained with ectodomain-specific antibodies for surface TrkB receptors (green). Cell surface was costained with R-ConA (red) for membrane area normalization. (Scale bar, 20 μm.) (A2) FRET images of stage-2 (or stage-3) neurons cotransfected with the FRET indictor for PKA activity (AKAR). (B) Summary graph of stage-2 (B1) or stage-3 (B2) neurons showing mean fluorescence intensity of surface TrkB (±SEM, n = 35–50; **P < 0.01, Tukey test, normalized by ConA staining, left axis) and mean basal PKA activity, as indicated by the AKAR signal (±SEM, n = 10–35; *P < 0.05, Tukey test, right axis) along neurite (Top) or at 5 μm distal of the neurite. The first five neurites of each cell with highest intensities were compared and ranked from left to right by intensity and averaged among all cells. The average lengths of the intensity-ranked neurites are shown below.
Further studies showed that membrane insertion of TrkB could be regulated by BDNF and cytoplasmic cAMP. We found that treatment with either forskolin (20 μM) or BDNF (50 ng/mL) for 3 h at 10 h after cell plating in the presence of the translational inhibitor cycloheximide (25 μg/mL) resulted in an elevated level of surface TrkB in stage-2 neurons (Fig. S5A). The forskolin effect was abolished by the presence of KT5720, whereas the BDNF effect was abolished by the presence of KT5720, K252a (but not K252b), or the PI3K inhibitor LY294002 (Fig. S5B). Similar findings were obtained in the absence of cycloheximide (Fig. S6A). The effectiveness of the cycloheximide treatment in blocking protein synthesis was confirmed by its effect in reducing the total TrkB level in these neurons after 16 h but not 3 h incubation of cycloheximide (Fig. S6B). These results are consistent with those found in retinal ganglion cells (30) and indicate that surface TrkB expression promoted by BDNF depends on cAMP/PKA and PI3 activities but not on protein synthesis. Furthermore, treatment with KT5720, K252a (but not K252b), or LY294002 alone lowered the surface TrkB expression (Fig. S5B), suggesting that constitutive surface TrkB expression is under the regulation of basal PKA and PI3K activities.
Whether BDNF/cAMP signaling may act locally to regulate TrkB surface expression was further examined by plating stage-2 neurons on substrates coated with stripes of membrane-permeable fluorescent cAMP analog (F-cAMP) or BDNF (SI Materials and Methods). For all cells with their soma located at the stripe boundary, we compared the average immunostaining fluorescence of the surface TrkB at neurite tips “on” and “off” the stripe (normalized by that of R-ConA staining) and found a significant preference for the surface TrkB to be expressed on neurites in contact with either F-cAMP or BDNF stripes (Fig. S5C). This preference was abolished by KT5720 and K252a, but not K252b (Fig. S5D). This is consistent with cAMP/PKA-dependent insertion of TrkB induced by F-cAMP or BDNF. By expressing a fusion construct of TrkB-GFP in these neurons, we further found that TrkB surface expression was locally increased within minutes after the contact with a BDNF-coated bead (Fig. S5E). This local TrkB increase was absent when the bead contact was made in the presence of LY294002, TrkB-IgG, and K252a but was unaffected by IgG-Fc, KT5720, or K252b, indicating that BDNF-induced accumulation of TrkB-GFP depends on TrkB and PI3K activity but not PKA activity. This finding suggests that BDNF/TrkB signaling at the neuritic growth cone promotes anterograde transport of TrkB in a PI3K-dependent manner.
PI3K-Dependent Anterograde TrkB Transport.
Previous studies have shown that the retrograde transport of TrkA in sympathetic neurons depends on PI3K/Akt activity (31, 32). In this study, we found that in stage-3 neurons overexpressing TrkB-GFP, bath-application of BDNF or forskolin for 4 h induced a marked preferential increase of TrkB-GFP in the axon (Fig. S7A), a process abolished by the presence of the PI3K inhibitor LY294002, indicating a BDNF/cAMP-dependent anterograde transport of TrkB in the nascent axon. Direct measurements of TrkB transport were made by using the method of fluorescence recovery after photobleaching (FRAP). The neurons were transfected with TrkB-GFP at 16 h after plating and examined 1 d later at stage 3. The GFP fluorescence was photobleached at the axon shaft for a segment of ≈50 μm, and fluorescence recovery at the most proximal and most distal 10-μm regions of the bleached segment was monitored (Fig. S7B1). We found that in the nascent axon, the recovery rate at the proximal end was in general higher than that at the distal end (Fig. S7B1), suggesting net anterograde transport of TrkB. In control neurons expressing TrkB-GFP, the half-time of fluorescence recovery (t1/2) at the proximal end was 57 ± 13 s (SEM, n = 6). Addition of BDNF reduced t1/2 to 18 ± 9 s (SEM, n = 6; Fig. S7B2), indicating accelerated anterograde TrkB transport, and this effect of BDNF remained in the presence of KT5720 (t1/2 = 21 ± 8 s, SEM, n = 6). In contrast, inhibiting PI3K with LY294002 completely prevented the BDNF effect and further prolonged the recovery (t1/2 = 77 ± 15 s, SEM, n = 6; Fig. S7B2). Thus, BDNF/TrkB signaling triggers both PKA-dependent membrane insertion of TrkB as well as PI3K-dependent anterograde transport of TrkB.
Discussion
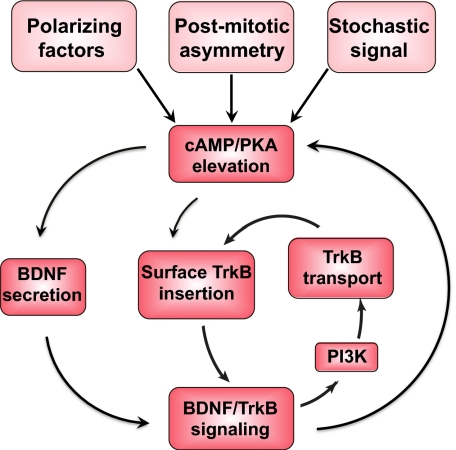
In this work, we found a rapid autocrine action of BDNF in axon development and delineated several positive-feedback mechanisms by which BDNF could elevate and stabilize local neuritic cAMP/PKA activity, which is known to promote axon differentiation and growth. As depicted in Fig. 5, the initial trigger for axon differentiation may come from a gradient of extracellular polarizing factors, an intrinsic cytoplasmic asymmetry in the postmitotic neuron, or stochastic fluctuation of cytoplasmic signals, all of which may cause a local cAMP elevation in one of the neurites of stage-2 neurons. Local cAMP/PKA activity could induce local BDNF secretion and TrkB insertion into the plasma membrane that further enhance the autocrine action of BDNF. In addition, BDNF/TrkB signaling also elevates the PI3K activity that promotes anterograde TrkB transport, which further enhances surface insertion of TrkB and BDNF/TrkB signaling. These nested positive-feedback mechanisms enable the establishment of a stable cAMP/PKA activity locally in the neurite, resulting in its differentiation into axon, with accompanying accumulation of TrkB and BDNF secretion activity toward the nascent axon. This self-amplifying autocrine action of BDNF remains in operation after axon specification, promoting the accelerated growth of the axon. Notably, these autocrine effects of BDNF are rapid posttranslational events independent of protein synthesis and are thus distinct from those that promote neuronal survival (15, 16, 33). Over a longer time scale, feedback mechanisms involving transcriptional and translational regulators could also modulate the level of BDNF and TrkB expression in the neuron (34–36). Whether and to what extent the latter participates in axon formation remain to be determined.
Fig. 5.
Nested positive-feedback mechanisms in autocrine BDNF/TrkB signaling. First, local elevation of cAMP/PKA activity resulting from intrinsic or extrinsic signals causes local secretion, which in turn further elevates local cAMP/PKA activity. Second, local cAMP/PKA activity causes local surface insertion of TrkB, further amplifying BDNF/TrkB signaling and enhancing local cAMP/PKA activity. Third, elevating BDNF/TrkB signaling activates PI3K activity, which promotes anterograde trafficking of TrkB, further enhancing local surface insertion of TrkB and BDNF/TrkB signaling.
As first pointed out by Ramon y Cajal more than a century ago (37), developing neuritic processes resemble migrating cells, which can sense gradients of extracellular cues and become polarized as they undergo chemotactic migration. Such gradient sensing and cell polarization could be enhanced by local excitation via positive-feedback loops and global inhibition via long-range diffusible inhibitor (38). These two processes could also stabilize cytoplasmic asymmetry originated from stochastic fluctuation of polarity determinants, leading to spontaneous polarization of migrating cells in the absence of chemoattractants (39). Positive-feedback loops mediated by PIP3 and Rho GTPase has been demonstrated in neutrophil polarization during chemotaxis (40, 41). In cultured hippocampal neurons, PI3K activity was highly localized to the tip of the newly specified axon, and inhibitors of PI3K disrupted such localization and prevented neuronal polarization (42). Because PI3K is a major downstream effector of BDNF (8), the highly localized PI3K at the axonal tip could initially be triggered by the localized autocrine action of BDNF, and the PI3K/PIP3/RhoGTPase feedback loop thus represents another positive-feedback loop in promoting PI3K-dependent TrkB anterograde transport shown in the present study (Fig. S7). Indeed, asymmetric PIP3 and Akt signaling was found to mediate chemotaxis of neuritic growth cones of Xenopus spinal neurons induced by a gradient of BDNF (43). Other activators of PI3K [e.g., shootin1 and Singar1/2 (44, 45)] resulting from asymmetric intrinsic or extrinsic factors could also serve as the initial trigger for the activation of PI3K. In the latter scenario, local elevation of PI3K could then induce local accumulation of TrkB in the neurite, leading to elevated BDNF/TrkB signaling and subsequent amplification via both PI3K and cAMP/PKA pathways. The autocrine BDNF action links these two major signaling pathways via nested positive-feedback loops and ensures their activation for axon differentiation and growth. The initial trigger could occur as an induced or spontaneous activation of one component in these nested positive-feedback loops, and blocking the critical catalytic component in each loop (TrkB, PKA, or PI3K) could reduce (but not completely abolish) axon formation.
We have observed a striking reorganization of BDNF/TrkB signaling toward the axon during the process of axon formation. This was shown by the transition of global BDNF secretion in stage-2 neurons to localized BDNF secretion at the axon in stage-3 neurons (Fig. 2), together with similar redistribution of surface TrkB (Fig. 4A1). These are accompanied by spatially correlated changes in the constitutive cAMP/PKA activity toward the distal axon (Fig. 4A2). Using FRET imaging of the PKA activity in AKAR-expressing hippocampal neurons, we found that in stage-2 neurons the variation of FRET signals at the neurite tips was small, and the neurite lengths were similar (Fig. 4B1). In contrast, in stage-3 polarized neurons, the PKA signals at the axon tip were much higher than those at dendritic tips, with the axon tip signal 330% ± 7% (n = 10) of the mean value at dendrite tips (Fig. 4B2). This indicates that neuronal polarization undergoes a transition from relatively uniform cAMP/PKA activities in undifferentiated neurites to a much higher cAMP/PKA activity at the distal axon, in line with the topographic transition of BDNF-induced BDNF secretion and TrkB surface expression during axon formation. The localization of these cellular activities could be the consequence of the self-amplifying autocrine signaling of BDNF and directly associated with the process of axon specification and the accelerated growth of nascent axons.
A critical property of BDNF as an autocrine factor is that it is highly positively charged (pI ≈9). This allows immediate binding of secreted BDNF to the cell surface (26) or extracellular matrix near the secretion site. Localized action of secreted BDNF at the synapse plays a critical role in synapse-specific long-term modification (46). In developing neuron, localized secretion allows local amplification of autocrine signals, a critical step in breaking the cellular symmetry during axon/dendrite formation. Besides BDNF, other neurotrophin family members (e.g., NT3) may also serve similar self-amplifying autocrine functions in axon development. Mice lacking the gene that encodes CAPS2 (Ca2+-dependent activator proteins for secretion 2), a protein participating in dense-core vesicle exocytosis and BDNF/NT-3 release from parallel fibers of cerebellar granule cells, have shown developmental deficits, including fewer branched dendrites in Purkinje cells and loss of the intercrural fissure (47). This supports the notion that impairment of neurotrophin secretion causes differentiation defects in neurons. However, paracrine action of neurotrophin may also contribute in part to axon/dendrite development. The variability and limitation of the present experiments make it rather difficult to clearly differentiate between the autocrine and paracrine effects. In addition, no abnormality in axon development has been reported in mice with bdnf gene deletion (48); this could be attributed to compensatory effects of other factors or other neurotrophin family members of similar autocrine function. Further studies using primary neuron cultures from these knockout animals would help to determine whether such compensatory mechanisms exist in these neurons. Redundant actions of multiple factors may help to ensure the presence of local positive-feedback mechanisms for symmetry breaking during neuronal polarization.
Materials and Methods
SI Materials and Methods provides information for antibodies, reagents, and materials. Experimental procedures of cell cultures, immunostaining, time-lapse imaging, FRET imaging, and photobleaching studies are also included. For further information on analysis of polarity phenotype, refer also to the SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Z.-Y. Chen (Shandong University) for the Flag-TrkB-GFP construct, J. Zhang for the indicator of cAMP using Epac (ICUE) and A-kinase activity reporter fluorescence resonance energy transfer probes, M. Kojima (National Institute of Advanced Industrial Science and Technology) for the cDNA for BDNF–EGFP construct, and G. Miesenboeck (Yale University) for superecliptic pHluorin construct. This work was supported in part by grants from National Institutes of Health (EY014979 and PN2EY018228).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1115907108/-/DCSupplemental.
References
- 1.Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kishi M, Pan YA, Crump JG, Sanes JR. Mammalian SAD kinases are required for neuronal polarization. Science. 2005;307:929–932. doi: 10.1126/science.1107403. [DOI] [PubMed] [Google Scholar]
- 3.Barnes AP, et al. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell. 2007;129:549–563. doi: 10.1016/j.cell.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 4.Shelly M, Cancedda L, Heilshorn S, Sumbre G, Poo MM. LKB1/STRAD promotes axon initiation during neuronal polarization. Cell. 2007;129:565–577. doi: 10.1016/j.cell.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Shelly M, et al. Semaphorin3A regulates neuronal polarization by suppressing axon formation and promoting dendrite growth. Neuron. 2011;71:433–446. doi: 10.1016/j.neuron.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1:549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang EJ, Reichardt LF. Neurotrophins: Roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang EJ, Reichardt LF. Trk receptors: Roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 9.Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 10.Miranda RC, Sohrabji F, Toran-Allerand CD. Neuronal colocalization of mRNAs for neurotrophins and their receptors in the developing central nervous system suggests a potential for autocrine interactions. Proc Natl Acad Sci USA. 1993;90:6439–6443. doi: 10.1073/pnas.90.14.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kokaia Z, et al. Coexpression of neurotrophins and their receptors in neurons of the central nervous system. Proc Natl Acad Sci USA. 1993;90:6711–6715. doi: 10.1073/pnas.90.14.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright EM, Vogel KS, Davies AM. Neurotrophic factors promote the maturation of developing sensory neurons before they become dependent on these factors for survival. Neuron. 1992;9:139–150. doi: 10.1016/0896-6273(92)90229-7. [DOI] [PubMed] [Google Scholar]
- 13.Acheson A, et al. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374:450–453. doi: 10.1038/374450a0. [DOI] [PubMed] [Google Scholar]
- 14.Hyman C, et al. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350:230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh A, Carnahan J, Greenberg ME. Requirement for BDNF in activity-dependent survival of cortical neurons. Science. 1994;263:1618–1623. doi: 10.1126/science.7907431. [DOI] [PubMed] [Google Scholar]
- 16.Davies AM, Wright EM. Neurotrophic factors. Neurotrophin autocrine loops. Curr Biol. 1995;5:723–726. doi: 10.1016/s0960-9822(95)00144-8. [DOI] [PubMed] [Google Scholar]
- 17.Song HJ, Ming GL, Poo MM. cAMP-induced switching in turning direction of nerve growth cones. Nature. 1997;388:275–279. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- 18.Arimura N, Kaibuchi K. Neuronal polarity: From extracellular signals to intracellular mechanisms. Nat Rev Neurosci. 2007;8:194–205. doi: 10.1038/nrn2056. [DOI] [PubMed] [Google Scholar]
- 19.Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 20.Zhou P, et al. Polarized signaling endosomes coordinate BDNF-induced chemotaxis of cerebellar precursors. Neuron. 2007;55:53–68. doi: 10.1016/j.neuron.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshimura T, et al. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120:137–149. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Matsuda N, et al. Differential activity-dependent secretion of brain-derived neurotrophic factor from axon and dendrite. J Neurosci. 2009;29:14185–14198. doi: 10.1523/JNEUROSCI.1863-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miesenböck G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 24.Goodman LJ, et al. Regulated release and polarized localization of brain-derived neurotrophic factor in hippocampal neurons. Mol Cell Neurosci. 1996;7:222–238. doi: 10.1006/mcne.1996.0017. [DOI] [PubMed] [Google Scholar]
- 25.Song AH, et al. A selective filter for cytoplasmic transport at the axon initial segment. Cell. 2009;136:1148–1160. doi: 10.1016/j.cell.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 26.Blöchl A, Thoenen H. Characterization of nerve growth factor (NGF) release from hippocampal neurons: Evidence for a constitutive and an unconventional sodium-dependent regulated pathway. Eur J Neurosci. 1995;7:1220–1228. doi: 10.1111/j.1460-9568.1995.tb01112.x. [DOI] [PubMed] [Google Scholar]
- 27.Krüttgen A, Möller JC, Heymach JV, Jr, Shooter EM. Neurotrophins induce release of neurotrophins by the regulated secretory pathway. Proc Natl Acad Sci USA. 1998;95:9614–9619. doi: 10.1073/pnas.95.16.9614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shelly M, et al. Local and long-range reciprocal regulation of cAMP and cGMP in axon/dendrite formation. Science. 2010;327:547–552. doi: 10.1126/science.1179735. [DOI] [PubMed] [Google Scholar]
- 29.Peng HB, Cheng PC. Formation of postsynaptic specializations induced by latex beads in cultured muscle cells. J Neurosci. 1982;2:1760–1774. doi: 10.1523/JNEUROSCI.02-12-01760.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer-Franke A, et al. Depolarization and cAMP elevation rapidly recruit TrkB to the plasma membrane of CNS neurons. Neuron. 1998;21:681–693. doi: 10.1016/s0896-6273(00)80586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuruvilla R, Ye H, Ginty DD. Spatially and functionally distinct roles of the PI3-K effector pathway during NGF signaling in sympathetic neurons. Neuron. 2000;27:499–512. doi: 10.1016/s0896-6273(00)00061-1. [DOI] [PubMed] [Google Scholar]
- 32.Miller FD, Kaplan DR. On Trk for retrograde signaling. Neuron. 2001;32:767–770. doi: 10.1016/s0896-6273(01)00529-3. [DOI] [PubMed] [Google Scholar]
- 33.Lindholm D, Carroll P, Tzimagiogis G, Thoenen H. Autocrine-paracrine regulation of hippocampal neuron survival by IGF-1 and the neurotrophins BDNF, NT-3 and NT-4. Eur J Neurosci. 1996;8:1452–1460. doi: 10.1111/j.1460-9568.1996.tb01607.x. [DOI] [PubMed] [Google Scholar]
- 34.Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 35.Shieh PB, Hu SC, Bobb K, Timmusk T, Ghosh A. Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron. 1998;20:727–740. doi: 10.1016/s0896-6273(00)81011-9. [DOI] [PubMed] [Google Scholar]
- 36.Deogracias R, Espliguero G, Iglesias T, Rodríguez-Peña A. Expression of the neurotrophin receptor trkB is regulated by the cAMP/CREB pathway in neurons. Mol Cell Neurosci. 2004;26:470–480. doi: 10.1016/j.mcn.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Cajal SRY. In: Histology of the Nervous System of Man and Vertebrates. Swanson N, Swanson L, translators. New York: Oxford Univ Press; 1995. [Google Scholar]
- 38.Xiong Y, Huang CH, Iglesias PA, Devreotes PN. Cells navigate with a local-excitation, global-inhibition-biased excitable network. Proc Natl Acad Sci USA. 2010;107:17079–17086. doi: 10.1073/pnas.1011271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wedlich-Soldner R, Li R. Spontaneous cell polarization: Undermining determinism. Nat Cell Biol. 2003;5:267–270. doi: 10.1038/ncb0403-267. [DOI] [PubMed] [Google Scholar]
- 40.Weiner OD, et al. A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat Cell Biol. 2002;4(7):509–513. doi: 10.1038/ncb811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang F, et al. Lipid products of PI(3)Ks maintain persistent cell polarity and directed motility in neutrophils. Nat Cell Biol. 2002;4(7):513–518. doi: 10.1038/ncb810. [DOI] [PubMed] [Google Scholar]
- 42.Shi SH, Jan LY, Jan YN. Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell. 2003;112:63–75. doi: 10.1016/s0092-8674(02)01249-7. [DOI] [PubMed] [Google Scholar]
- 43.Henle SJ, et al. Asymmetric PI(3,4,5)P3 and Akt signaling mediates chemotaxis of axonal growth cones. J Neurosci. 2011;31:7016–7027. doi: 10.1523/JNEUROSCI.0216-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toriyama M, et al. Shootin1: A protein involved in the organization of an asymmetric signal for neuronal polarization. J Cell Biol. 2006;175:147–157. doi: 10.1083/jcb.200604160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mori T, Wada T, Suzuki T, Kubota Y, Inagaki N. Singar1, a novel RUN domain-containing protein, suppresses formation of surplus axons for neuronal polarity. J Biol Chem. 2007;282:19884–19893. doi: 10.1074/jbc.M700770200. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka J, et al. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science. 2008;319:1683–1687. doi: 10.1126/science.1152864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sadakata T, et al. Impaired cerebellar development and function in mice lacking CAPS2, a protein involved in neurotrophin release. J Neurosci. 2007;27:2472–2482. doi: 10.1523/JNEUROSCI.2279-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klein R. Role of neurotrophins in mouse neuronal development. FASEB J. 1994;8:738–744. doi: 10.1096/fasebj.8.10.8050673. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.