Abstract
Cd8a and Cd8b1 coreceptor gene (Cd8) expression is tightly controlled during T-cell development by the activity of five Cd8 enhancers (E8I–E8V). Here we demonstrate a unique transcriptional program regulating CD8 expression during CD8+ effector T-cell differentiation. The Cd8 enhancer E8I and Runx/core-binding factor-β (CBFβ) complexes were required for the establishment of this regulatory circuit, because E8I-, Runx3-, or CBFβ-deficient CD8+ T cells down-regulated CD8α expression during activation. This finding correlated with enhanced repressive histone marks at the Cd8a promoter in the absence of E8I, and the down-regulation of CD8α expression could be blocked by treating E8I-, Runx3-, or CBFβ-deficient CD8+ T cells with the histone deacetylase inhibitor trichostatin A. Moreover, Runx/CBFβ complexes bound the Cd8ab gene cluster in activated CD8+ T cells, suggesting direct control of the Cd8a locus. However, CD8+ effector T cells maintained high levels of CD8α when CBFβ was conditionally deleted after activation. Thus, our data suggest an E8I- and Runx3/CBFβ-dependent epigenetic programming of the Cd8a locus during T-cell activation, leading to Runx/CBFβ complex-independent maintenance of CD8α expression in effector T cells.
Keywords: epigenetic marks, transcriptional control, cytotoxic T lymphocytes
The expression of the CD4 and CD8 coreceptors is linked with the functional phenotype of mature T cells. On conventional T cells, CD8 usually consist of CD8α and CD8β heterodimers (encoded by the closely linked Cd8a and Cd8b1 genes, respectively), and the expression of the Cd8 genes during T-cell development is regulated by the activity of at least five different cis-regulatory elements (1). The first Cd8 enhancer identified, designated E8I, is active in mature CD8 single-positive thymocytes and in CD8+ T cells, and in innate-like CD8αα+ intraepithelial lymphocyte (IEL) of the gut (2, 3). The generation of E8I-deficient mice revealed that E8I is essential for CD8αα expression in γδTCR (T-cell receptor) IEL, while CD8 expression on conventional T cells was not impaired (4, 5). The Cd8 enhancer E8II directs expression of a reporter transgene in double-positive (DP) thymocytes and CD8+ T cells (4), while E8II-deficient mice have normal CD8 expression (6). Combined deletion of E8I and E8II leads to variegated expression of CD8 in DP thymocytes (6), and subsequent studies showed that CD8 variegation correlates with an epigenetic “off” state (7). A similar variegation phenotype is also observed in mice lacking the Cd8 enhancer E8V (8). Another enhancer, E8III, is active in DP thymocytes (4), and combined deletion of E8II and E8III resulted in a mild CD8 variegation phenotype in DP thymocytes, but E8II,E8III-deficient mice have normal levels of CD8 on peripheral T cells (9). Taken together, these studies revealed a complex network of cis-regulatory elements, and link Cd8 enhancer functions with chromatin remodeling of the Cd8ab gene complex.
A new twist in the regulation of Cd8 gene expression and an insight into a novel function of the Cd8 enhancer E8I were obtained from a study showing that subsets of CD8αβ+ T cells transiently express CD8αα homodimers upon activation (10). The expression of CD8αα homodimers on CD8αβ+ T cells was linked to the survival and differentiation of memory precursor cells into memory cells and dependent on E8I, because E8I−/− CD8αβ+ T cells failed to up-regulate CD8αα expression. It was shown that E8I-deficient mice have impaired memory functions (10), although memory cell formation can also occur in the absence of CD8αα homodimer expression on CD8αβ+ T cells in E8I-deficient mice (11, 12). In one of the studies, a decrease in CD8αβ expression on splenic T cells in lymphocyte choriomeningitis virus (LCMV)-infected E8I-deficient mice has been observed, providing the first indication that E8I−/− CD8+ T cells may have a defect in CD8αβ expression upon activation (11).
In this study we investigated whether the expression of CD8 in activated CD8+ T cells is differentially regulated compared with naive CD8+ T cells. We could show that a unique transcriptional program regulates CD8 expression during CD8+ effector T-cell differentiation that is distinct from naive T cells. The Cd8 enhancer E8I and Runx/core-binding factor (CBF)β complexes were required for the establishment of this regulatory circuit, because E8I- or Runx/CBFβ complex-deficient CD8+ T cells down-regulated CD8α expression during activation. The down-regulation was specific for the Cd8a gene and correlated with enhanced repressive histone marks at the Cd8a promoter in the absence of E8I. The down-regulation of CD8α expression could be blocked by treating E8I-deficient CD8+ T cells with the histone deacetylase (HDAC) inhibitor trichostatin A (TSA). This finding demonstrates that CD8 expression can be maintained even in the absence of E8I, and suggests that E8I might protect the Cd8a locus from HDAC-mediated repression upon activation. Moreover, Runx/CBFβ complexes bound the Cd8ab gene cluster in activated CD8+ T cells, suggesting direct control of the Cd8a locus during CD8+ T-cell activation. However, CD8+ effector T cells maintained high levels of CD8α when CBFβ was conditionally deleted after activation. Thus, our data suggest that the induction of this effector T-cell–specific regulatory program for Cd8a gene expression requires E8I-and Runx3/CBFβ-dependent epigenetic programming of the Cd8a locus during T-cell activation, leading to Runx3/CBFβ-independent maintenance of CD8α expression in effector T cells.
Results
Activated E8I−/− CD8+ T Cells Down-Regulate CD8α Expression.
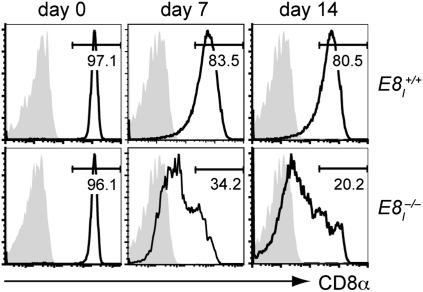
In a previous study it has been reported that Cd8 enhancer E8I-deficient mice express lower levels of CD8αβ on peripheral CD8+ T cells upon infection with LCMV (11). To investigate the role of E8I in regulating CD8α expression in more detail, peripheral wild-type and E8I-deficient CD8+ T cells were isolated and activated with anti-CD3/anti-CD28. Although E8I+/+ cells maintained high-levels of CD8α upon activation over a period of 14 d, E8I-deficient T cells down-regulated CD8α expression already at day 3 and the expression remained low at day 14 (Fig. 1). A similar down-regulation of CD8α expression was also observed upon antigen-specific activation of E8I−/−,OT-I CD8+ T cells in vitro and in vivo (Fig. S1 A and B). However, only E8I−/− CD8+ T cells, but no E8II-deficient or E8II,E8III-deficient CD8+ T cells down-regulated CD8α and CD8β expression on peripheral CD8+ T cells upon activation (Fig. S1 C and D) (6, 9). Moreover, E8I,E8II-deficient CD8+ T cells showed a similar reduction of CD8α expression as E8I−/− CD8+ T cells upon activation, indicating that E8II is not involved in the regulation of CD8α expression upon activation, even in the absence of E8I (Fig. S1C). Collectively, these data show that Cd8 enhancer E8I, but not E8II or E8III, is essential for maintaining CD8α expression at high-levels upon activation.
Fig. 1.
Loss of CD8 expression upon activation of E8I−/− CD8+ T cells. E8I+/+ and E8I−/− CD8+ T cells were activated with anti-CD3/anti-CD28 and CD8α expression was assessed at the indicated time. Numbers show the percentage of cells in the respective region indicated by an interval gate. Filled histograms show CD8α expression levels on naive CD4+ T cells. Data are representative of seven independent experiments.
E8I Regulates Cd8a but Not Cd8b1 Gene Expression.
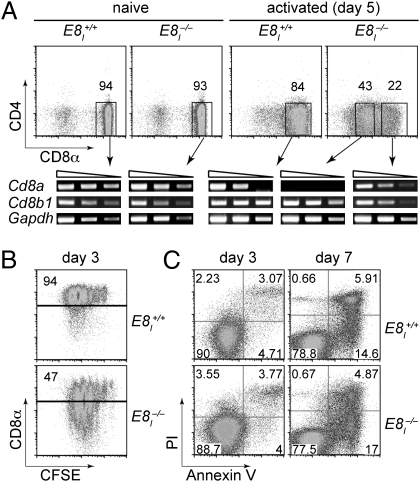
Having determined that CD8α expression is affected in E8I−/− CD8+ T cells, we investigated whether CD8β (encoded by the Cd8b1 gene) expression is impaired by loss of E8I. To test for the expression of CD8β (which requires CD8α for surface expression), CD8+ T cells were activated with anti-CD3/anti-CD28. After 5 d, CD8α− cells from the E8I−/− T-cell cultures were isolated and the expression of the Cd8a and Cd8b1 genes was determined by semiquantitative RT-PCR. As expected, Cd8a gene expression was terminated at the transcriptional level in CD8α– T cells (Fig. 2A). In contrast, CD8α– T cells still expressed normal levels of Cd8b1, indicating that loss of E8I selectively affects Cd8a expression upon activation. Loss of CD8α expression also did not interfere with the proliferation of CD8+ T cells, because CFSE- [5-(and 6)-carboxyfluorescein diacetate succinimidyl ester] labeling experiments revealed a similar proliferation rate of E8I+/+ and E8I−/− CD8+ T cells upon activation (Fig. 2B). In addition, there was no difference in the cell death rate between E8I+/+ and E8I−/− CD8+ T cells (Fig. 2C). However, E8I−/− CD8+ T cells that underwent more cell cycles showed a lower level of CD8α expression at day 3 compared with cells in the same culture that proliferated less (Fig. 2B). In contrast, CD8α expression levels remained high in E8I+/+ CD8+ T cells independent of the degree of proliferation (Fig. 2B). Thus, loss of CD8α expression upon T-cell activation is linked with cell proliferation.
Fig. 2.
E8I regulates selectively Cd8a gene expression. (A) Dot plots showing CD4 vs. CD8α expression on purified naive (Left) and anti-CD3/anti-CD28 activated (day 5; Right) E8I+/+ and E8I−/− CD8+ T cells. Rectangles indicate sorting gates for cell separation and subsequent isolation of RNA. Activated E8I−/− cytotoxic T cells were sorted for CD8α– and CD8α+ subsets. Semiquantitative RT-PCR analysis shows Cd8a and Cd8b1 expression in the various cell subsets. Gapdh expression was used as loading control. The triangle indicates fivefold dilutions of input. Data are representative of two independent experiments. (B) E8I+/+ and E8I−/− CD8+ T cells were labeled with CFSE and stimulated with anti-CD3/anti-CD28. Dot plots show CD8α expression vs. CFSE at day 3 after stimulation. Data are representative of two independent experiments. (C) E8I+/+ and E8I−/− CD8+ T cells were stimulated with anti-CD3/anti-CD28. Dot plots show Propidium Iodide (PI) uptake and Annexin V staining at day 3 and day 7 after stimulation. Data are representative of two independent experiments.
Altered Histone Marks at the Cd8a Gene-Promoter Region in Activated E8I−/− CD8+ T Cells.
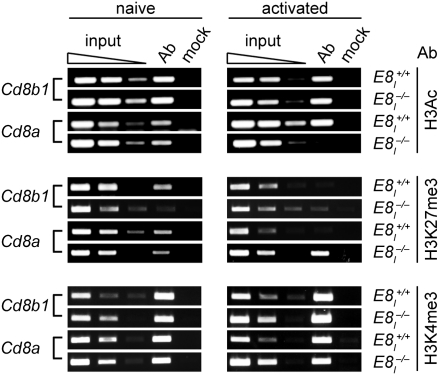
E8I may serve as a recruitment element for a transcription factor that is essential for maintaining CD8α expression after activation. Alternatively, but not mutually exclusive, E8I may be required to keep the Cd8a gene locus epigenetically “ON” to facilitate recruitment of transcription factors required for the continued transcription of the Cd8a gene. A similar role for E8I as a recruitment site for chromatin remodeling factors and epigenetic regulator of the Cd8 loci has been shown already during thymocyte development (6, 7). Thus, we tested whether E8I regulates Cd8a gene expression at the epigenetic level. Naive and activated E8I+/+ and E8I−/− CD8+ T cells (sorted CD8α−cells from activated E8I−/− cells) were isolated and analyzed by ChIP experiments for differences in histone modifications at Cd8a and Cd8b1 promoter regions, including the active marks histone 3 (H3), acetylation (H3Ac), and H3 lysine 4 trimethylation (H3K4me3), as well as the mark for silenced genes H3 lysine 27 trimethylation (H3K27me3) (13, 14). There were similar H3Ac levels at the Cd8a and Cd8b1 promoter regions in nonactivated E8I+/+ and E8I−/− CD8+ T cells, correlating with similar expression levels of Cd8a and Cd8b1 (Fig. 3, Top, and Fig. S2). In contrast, activated E8I-deficient T cells display strongly reduced H3 acetylation levels at the Cd8a promoter compared with activated E8I+/+ CD8+ T cells, and H3Ac levels were readily detected at the Cd8b1 promoter region (Fig. 3, Top, and Fig. S2). Reduced Cd8a expression correlated also with an enhanced appearance of the H3K27me3 repressive mark at the Cd8a gene promoter in activated E8I−/− CD8+ T cells, but there was no difference at the Cd8b1 promoter between E8I+/+ and E8I−/− CD8+ T cells (Fig. 3, Middle, and Fig. S2). Of note, the Cd8a gene promoter remained active H3K4me3 marks in activated E8I−/− CD8+ T cells (Fig. 3, Bottom, and Fig. S2). This finding indicates the presence of active and repressive marks in E8I-deficient effector T cells that have down-regulated CD8α expression, and thus “bivalent” histone marks (15, 16), although it is possible that the marks could be on different alleles or could reflect a spectrum of histone modifications of residual CD8α expression across the population.
Fig. 3.
Epigenetic status of the Cd8a promoter region is E8I−/− CD8+ T cells. ChIP analysis of the Cd8a and Cd8b1 promoter region. Chromatin from naive (Left) or anti-CD3/anti-CD28 activated (day 5, Right) E8I+/+ and E8I−/− CD8+ T cells (sorted CD8α-negative cells from activated E8I−/− cells) was immunoprecipitated with anti-H3Ac (Top), with anti-H3K27me3 (Middle), or with anti-H3K4me3 (Bottom) antibodies followed by PCR with primers specific for the Cd8a and Cd8b1 promoter region. For the mock precipitations, no antibody was added. Input DNA was PCR amplified undiluted or at a dilution of 1:5 or 1:25 (wedges) to ensure PCR quantification in a nonsaturated amplification range. Data are representative of two independent experiments.
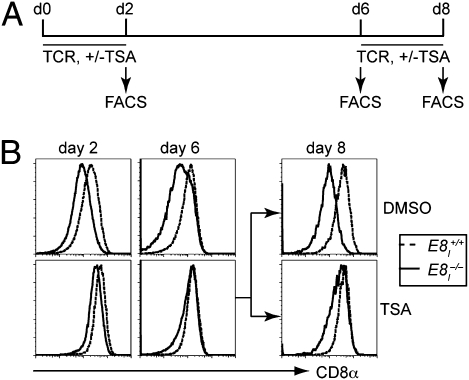
To test whether the impairment of CD8α expression can be overcome by inhibiting HDACs, E8I+/+ and E8I−/− CD8+ T cells were labeled with CFSE and activated for 2 d in the presence of TSA (Fig. 4A), a class I HDAC inhibitor (17). Subsequently, CD8α expression levels were compared at days 2 and 6 between E8I+/+ and E8I−/− cells that underwent a similar number of cell divisions (see Fig. S3A for gating regions). In the presence of TSA, E8I−/− CD8+ T cells did not down-regulate CD8α expression at day 2 (Fig. 4B), but in DMSO-supplemented control cultures CD8α expression was lost in the absence of E8I (Fig. 4B). Moreover, the TSA-mediated rescue of CD8α expression was stable, because CD8α expression in E8I−/− T cells remained high even when the cells were cultured for an additional 4 d (day 6) in the absence of TSA (Fig. 4B). However, the restimulation of “TSA-rescued” E8I-deficient CD8+ T cells led to a down-regulation of CD8α expression, indicating a requirement for E8I if cells are reactivated via the TCR (Fig. 4B) (day 8). In contrast, restimulation of TSA-rescued E8I−/− CD8+ T cells in the presence of TSA maintained high CD8α expression levels (Fig. 4B) (day 8). Of note, TSA could not rescue CD8α expression after E8I−/− T cells had lost already CD8α expression (Fig. S3 B and C). Taken together, these data indicate a TCR-signal–dependent epigenetic programming at the Cd8a gene and that E8I influences the relative acetylation/deacetylation levels and thereby the expression of CD8α.
Fig. 4.
Maintenance of CD8α expression in E8I−/− CD8+ T cells in the presence of TSA. (A) Experimental outline: E8I+/+ and E8I−/− CD8+ T cells were CFSE-labeled and stimulated with anti-CD3/anti-CD28 in the presence of TSA for 2 d, and cultured for an additional 4 d without TSA as described in Materials and Methods. At day 6, E8I+/+ and E8I−/− CD8+ T cells that had been with TSA were restimulated in the presence or absence of TSA for 2 d. CD8α expression was assessed at days 2, 6, and 8. (B) Histograms show CD8α expression on E8I+/+ and E8I−/− CD8+ T cells that were treated as described in A. CD8α expression levels were compared on cell subsets that underwent a similar number of cell divisions (see Fig. S3A for gating regions). Data are representative of two independent experiments.
Runx-Complexes Mediated Control of CD8α Expression Is E8I-Dependent.
The Runx transcription factor family has been implicated in the regulation of CD8 expression, and it has been shown that Runx3 binds to Cd8 enhancer E8I, E8II, and E8V in DP and CD8SP thymocytes (18). We observed that Runx/CBFβ complexes remain bound to the Cd8ab gene complex in activated CD8+ T cells (Fig. S4A). In contrast, binding of Runx/CBFβ complexes to other Cd8 enhancers, such as E8II and E8V, was reduced in the absence of E8I (Fig. S4B). Taken together, the ChIP data suggests a role for Runx/CBFβ complexes in the regulation of CD8α expression also upon activation.
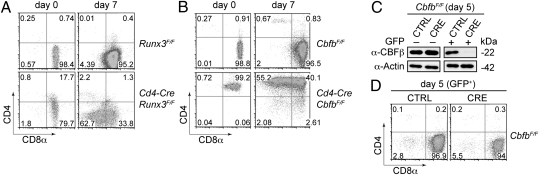
Deletion of Runx3 in the T-cell lineage (using Cd4-Cre × Runx3F/F mice or Runx3−/−:RAG2 chimeras) leads to a partial loss of CD4 silencing in CD8+ T cells. As a consequence, a fraction of peripheral CD8+ T cells expresses CD4, but CD8 expression on peripheral CD8+ T cells remains unchanged in Runx3-deficient T cells (19–21). To directly test whether Runx3 is required for CD8α expression during activation, CD8+ T cells from Runx3F/F and Cd4-Cre × Runx3F/F mice were isolated and activated with anti-CD3/anti-CD28. Similar to E8I-deficient CD8+ T cells, Runx3-deficient CD8+ T cells down-regulated CD8 expression on day 7 upon activation, leading to low-level expression of CD8 (Fig. 5A). To test whether other members of the Runx family, such as Runx1, which is also expressed in CD8+ T cells (19–21), might compensate for loss of Runx3, we determined CD8α expression in CBFβ-deficient CD8+ T cells upon activation. Deletion of CBFβ with the Lck-Cre deleter strain causes a severe positive-selection defect of DP thymocytes, as well as derepression of T-helper-inducing POZ/Krüppel-like factor, leading to a loss of mature CD8+ T cells in the periphery. However, in Cd4-Cre × CbfbF/F mice there is still a significant number of peripheral CD4+CD8+ T cells (i.e., CD8+ T cells which derepress CD4). These CD4+CD8+ T cells develop because of the stability and therefore a low turnover of the CBFβ protein after Cbfb gene inactivation (21). Although CbfbF/F CD8+ T cells displayed normal levels of CD8α expression upon activation, Cd4-Cre × CbfbF/F CD4+CD8+ T cells down-regulated CD8α (Fig. 5B). Because loss of CD8 expression was similar in the absence of CBFβ compared with Runx3-deficient cells, Runx1 appeared not to compensate for loss of Runx3. Moreover, Runx3-deficient or CBFβ-deficient T cells that had down-regulated CD8α expression displayed reduced H3Ac at the Cd8a promoter (Fig. S5A) and the down-regulation could be blocked with TSA treatment (Fig. S5B), suggesting that the E8I and Runx/CBFβ deficiencies impair activation-mediated CD8α maintenance by the same mechanism.
Fig. 5.
Differential requirement for Runx complexes during activation of CD8+ T cells and in CD8+ effector T cells. (A) CD8+ T cells from Runx3F/F and Cd4-Cre × Runx3F/F mice were activated with anti-CD3/CD28 and cultured for 7 d. Dot plots show CD4 vs. CD8α expression levels on day 0 (after purification) and on activated cells (day 7). Data are representative of four independent experiments. (B) CD8+ T cells from CbfbF/F mice and CD4+CD8+ T cells from Cd4-Cre × CbfbF/F mice were activated and analyzed as described in A. Data are representative of four independent experiments. (C) Immunoblot analysis showing CBFβ expression at day 5 in sorted GFP– and GFP+ M-SCV-pgk-GFP (CTRL) and M-SCV-CRE-pgk-GFP (CRE) transduced CbfbF/F CD8+ T cells. β-Actin was used as a loading control. Data are representative of two independent experiments. (D) CbfbF/F CD8+ T cells were activated with anti-CD3/CD28 and retrovirally infected at day 1 with M-SCV-pgk-GFP (CTRL) or M-SCV-CRE-pgk-GFP (CRE). CD8α expression on GFP+ subsets was assessed at day 5. Gating areas are shown in Fig. S5C. Data are representative of four independent experiments.
To test whether Runx complexes are also required for the maintenance of CD8α expression in effector T cells, we conditionally deleted CBFβ with retroviral Cre after CD8+ T-cell activation. Despite efficient deletion of CBFβ protein in CRE-transduced CD8+ T cells (Fig. 5C), there were similar CD8α expression levels compared with mock-transduced CbfbF/F CD8+ T cells (Fig. 5D and Fig. S5C). Thus, Runx/CBFβ complexes were essential for sustained CD8 expression during activation, but CD8 expression in effector T cells was maintained in a Runx complex-independent manner.
Discussion
Our study reveals a unique transcriptional program that regulates CD8α expression during CD8+ effector T-cell differentiation in a manner distinct from naive T cells, and demonstrates an essential role for Cd8 enhancer E8I and Runx3/CBFβ in this regulatory circuit. It has been shown that E8I−/− CD8+ T cells have normal expression levels of CD8 on DP thymocytes and peripheral CD8+ T cells, demonstrating that E8I is dispensable for the establishment of CD8 expression in thymocytes and in naive T cells (4). However, E8I,E8II-deficient mice display variegated expression of CD8 in DP thymocytes (6), showing that the combined activity of E8I and E8II is necessary for the activation of the Cd8ab gene complex in DP thymocytes. Our current study revealed a unique and nonredundant role of E8I in maintaining Cd8a gene expression during CD8+ T-cell activation. There are two important differences in how E8I is used at different developmental stages. First, the function of E8I in DP thymocytes and naive CD8+ T cells is only revealed in the absence of E8II, but in activated CD8+ T cells deletion of E8I alone is sufficient to alter CD8 expression. Second, in activated CD8+ T cells E8I controls only Cd8a but not Cd8b1 gene expression, but in E8I,E8II-deficient DP thymocytes both Cd8 genes are variegated (6). There are several potential binding sites of the insulator protein CTCF at the Cd8ab gene complex (22). It will be interesting in future studies to investigate whether CTCF recruitment to the Cd8ab gene complex leads to differential regulation of Cd8a and Cd8b1 gene expression in activated T cells.
Another important finding of our study is that Cd8a gene expression in CD8+ effector T cells might be maintained independently of factors required for high-level expression of CD8α during CD8+ T-cell activation. Our data indicate that Runx transcription factors contribute to the regulation of Cd8a gene expression upon activation. Runx/CBFβ complexes have been shown to regulate Cd4 gene silencing (20). In addition, they have been implicated in the activation of CD8 expression during T-cell development, although the molecular details of how Runx factors regulate CD8 expression are not fully understood (18). Among the Runx factors, distal promoter-derived Runx3 is the dominant form expressed in CD8+ T cells (19, 23). However, although conditional deletion of Runx3 in the T-cell lineage leads to a partial loss of CD4 silencing in CD8+ T cells, CD8 expression on peripheral naive CD8+ T cells is not altered in Runx3-deficient T cells, possibly because of the compensatory function of Runx1 (19, 21). This finding clearly demonstrates that Runx3 is not essential for CD8 expression in naive T cells. In contrast, we found that Runx3 and CBFβ are indispensable for high-level expression of CD8 during CD8+ T-cell activation. Because Runx3 expression levels were not changed in activated E8I−/− CD8+ T cells that have down-regulated CD8α expression (Fig. S5D), it is likely that the loss of CD8α expression in the absence of E8I is caused by impaired recruitment of Runx/CBFβ complexes to the Cd8ab gene complex. This theory is supported by the observation that there is less Runx/CBFβ recruited to other Cd8 enhancers in the absence of E8I (Fig. S4B). Interestingly, Runx3/CBFβ recruitment appears to be specific for the CD8 lineage because in Th1 cells (that express Runx/CBFβ complexes) CBFβ did not bind the Cd8 loci (Fig. S4C). In contrast to the initial T-cell activation during CD8+ T-cell differentiation, deletion of CBFβ after T-cell activation (using retroviral Cre-mediated deletion) did not result in loss of CD8α expression. This finding indicates that once Cd8a gene expression is established in CD8+ effector T cells, CD8 expression is maintained independently of Runx/CBFβ complexes and potentially also of Cd8 enhancer E8I.
It has been shown that CD8 expression is also regulated at an epigenetic level during thymocyte development (7). Our study also indicates a role for epigenetic regulatory mechanisms during the activation of CD8+ T cells. We observed that the down-regulation of the Cd8a gene in E8I-deficient cells correlated with reduced H3 acetylation. In addition, in E8I−/− cells that had down-regulated CD8α expression, the Cd8a gene promoter contained both active histone H3K4me3 and repressive histone H3K27me3 marks. This finding indicates bivalent chromatin modifications resulting in a silenced state of the Cd8a gene (15, 16), although it remains possible that the active and repressive marks are on different alleles. Remarkably, the addition of the HDAC inhibitor TSA facilitated CD8α expression upon activation, although H3K27me3 marks at the Cd8a promoter are still present in the absence of E8I (Fig. S6). This finding indicates the dominant role of histone acetylation marks over H3K27me3 marks in Cd8a gene regulation upon activation. Because TSA-treated E8I-deficient CD8+ T cells maintained CD8α expression even when cultured for additional 4 to 5 d, it appears that E8I is essential only at the onset of activation. In line with this hypothesis, we found that CD8 expression was not restored by TSA treatment if cells had already down-regulated CD8. Taken together, these data indicate that recruitment of a histone acetyltransferases (HAT) to the Cd8a gene locus is necessary for the maintenance of CD8 expression during T-cell activation, although one cannot formally exclude that the effect of TSA is indirect (e.g., by altering the expression of a chromatin modifying factor). However, restimulated TSA-rescued E8I-deficient CD8+ T cells down-regulated CD8α expression, suggesting that TCR triggering might lead to the induction of an E8I-dependent transcriptional “maintenance” program for CD8α expression. One possible mechanistic explanation for the loss of CD8α expression upon TCR triggering in E8I-deficient CD8+ T cells is that Runx/CBFβ complexes facilitate the recruitment of HATs necessary to keep the Cd8a gene promoter in an open configuration. A similar role for Runx proteins in the recruitment of HATs has been described in Runx1-dependent transcription during myeloid differentiation (24). Thus, in the absence of Runx/CBFβ complexes or E8I, regulatory complexes containing HAT activity and potentially other epigenetic modifying factors cannot assemble properly at the Cd8a gene locus, and as a consequence Cd8a gene expression is lost upon activation. Although the HAT p300 appears to be not recruited to the Cd8ab gene complex (Fig. S7), future studies including ChIP-seq approaches will help to reveal which members of the HAT and HDAC family are recruited to the Cd8a gene locus during T-cell activation.
The observation that E8I is required for Cd8a gene expression upon activation is in part reminiscent to the function of the proximal Cd4 enhancer (E4p) in T cells (25). E4p has been shown to be essential for CD4 expression in DP thymocytes, and E4p is required to establish an epigenetic pattern at the Cd4 locus that allows the propagation of CD4 expression in dividing mature CD4+ T cells, even in the absence of E4p (26). E8I appears to have a very similar function at the Cd8a locus, with respect to its role in the establishment of CD8 expression during TCR stimulation. Thus, both Cd4 and Cd8a gene expression is regulated by cis-regulatory elements that mediate the generation of active histone marks at the respective gene loci to maintain coreceptor expression upon activation.
Our observations raise the interesting question as to why CD8+ effector T cells regulate CD8α expression differently when compared with naive CD8+ T cells. It is conceivable that the switch in the regulation of CD8α expression upon T-cell activation is part of an effector program that is induced during cytotoxic T-lymphocyte (CTL) differentiation. Preliminary results indicate that E8I-deficient CD8+ T cells display reduced CTL activity, most likely because of reduced CD8α expression, despite normal expression of activation markers and CTL effector molecules (Fig. S8). It has been shown that CD8 expression levels can be modulated in vivo upon Vaccinia virus infection (27), and certain cytokines, such as IL-4, can lead to the down-regulation of CD8 expression (28). It will be interesting to investigate whether there is a (patho)physiological condition under which wild-type CD8+ T cells down-regulate CD8α expression via modulation of E8I enhancer (or Runx/CBFβ activity).
Finally, our finding that E8I is essential for Cd8a gene expression in activated CD8+ T cells may also provide an alternative explanation for the observation that E8I-deficient mice show impaired expression of CD8αα homodimers on γδTCR as well as on αβTCR IEL of the gut (4). It has been shown that IEL share characteristics of partially activated lymphocytes (29, 30). Thus, it is tempting to speculate that CD8αα expression on E8I−/− IELs is severely impaired because the cells are at least partially activated, and not because of a distinct mode of Cd8a gene regulation in IELs compared with conventional naive CD8+ T cells.
Taken together, our data reveal a unique and unexpected role for the Cd8 enhancer E8I and Runx/CBFβ complexes in the regulation and maintenance of Cd8a gene expression in CD8+ effector T cells, and indicate different mechanisms of how CD8 expression is regulated in naive and effector T cells.
Materials and Methods
Mice.
E8I, E8II, E8I,E8II, and E8II,E8III-deficient mice have been previously described (4, 6, 9). OT-I mice were kindly provided by Maria Sibilia (Medical University of Vienna, Vienna, Austria). All mice were used between 6 and 12 wk of age. All mice were bred and maintained in the animal facility of the Medical University of Vienna, and animal experiments were approved by the animal committee of the Medical University of Vienna and by Federal Ministry for Science and Research.
Antibodies and Flow Cytometry.
The antibodies used for the analysis are described in SI Materials and Methods. Samples were acquired on FACSCalibur and LSRII (BD Biosciences) and data were analyzed with FlowJo software (Treestar).
Isolation of Splenic T Cells and CFSE-Labeling of T Cells.
The purification protocols of the T cells are described in detail in SI Materials and Methods. For some experiments cells were labeled with CFSE, as described in SI Materials and Methods.
CD8+ T-Cell Stimulation.
Purified CD8+ T cells were stimulated as described in SI Materials and Methods.
cDNA Synthesis, Quantitative Real-Time PCR, and Semiquantitative RT-PCR.
Total RNA was isolated from sorted splenic naive or activated (day 5) CD8+ T cells (5 × 105 cells) using TRI reagent (Sigma) and cDNA synthesis was performed as described in SI Materials and Methods.
ChIP Assays.
Chromatin of naive or activated CD8+ T cells was precipitated as described in SI Materials and Methods. The primers used for PCR are shown in Table S1.
TSA Inhibitor Experiments.
Purified CFSE-labeled CD8+ splenic T cells were stimulated with plate-bound anti-CD3ε (1 μg/mL) and anti-CD28 (2 μg/mL) on 48-well plates (0.5 × 106 cells per well) in 500-μL T-cell medium (supplemented with 20 U/mL rhIL-2) in the presence of trichostatin A (16-nM final concentration) or DMSO as carrier for the control group. Two days later, cells were washed once with PBS to remove TSA, and CD8+ T cells were split 1:2 and cultured for additional 4 d in the presence of 100 U/mL rhIL-2. After a total of 6 d in culture, the cells were restimulated on 48-well plates with plate-bound anti-CD3ε (1 μg/mL) and anti-CD28 (2 μg/mL) for an additional 2 d, either in the presence or absence of TSA (or DMSO). In one experimental approach, CD8+ T cells were stimulated with anti-CD3/CD28 for 2 d, split 1:2 and cultured for an additional 4 d in the presence of 100 U/mL rhIL-2. At day 6, cells were treated with TSA or DMSO for 2 d. The cells were analyzed by flow cytometry at the indicated time points.
Retroviral Infection of CD8+ T Cells.
Retroviral infection was performed as described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dagmar Stoiber-Sakaguchi, Olivia Simma, and Winfried Pickl (Medical University of Vienna) for technical help. This study was supported by the Austrian Science Fund (research Grants P16708, P19930, and I698), and by the START Program (Project Y-163) of the Austrian Ministry of Science and Research (BM:WF) (to W.E.); a PhD fellowship of the High Education Commission of Pakistan (to H.H.); a Leukemia and Lymphoma Society Special Fellowship (to T.E.); and a RIKEN Research Center for Allergy and Immunology International collaboration award (to I.T. and W.E.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.M. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105835108/-/DCSupplemental.
References
- 1.Kioussis D, Ellmeier W. Chromatin and CD4, CD8A and CD8B gene expression during thymic differentiation. Nat Rev Immunol. 2002;2:909–919. doi: 10.1038/nri952. [DOI] [PubMed] [Google Scholar]
- 2.Ellmeier W, Sunshine MJ, Losos K, Hatam F, Littman DR. An enhancer that directs lineage-specific expression of CD8 in positively selected thymocytes and mature T cells. Immunity. 1997;7:537–547. doi: 10.1016/s1074-7613(00)80375-1. [DOI] [PubMed] [Google Scholar]
- 3.Hostert A, et al. A region in the CD8 gene locus that directs expression to the mature CD8 T cell subset in transgenic mice. Immunity. 1997;7:525–536. doi: 10.1016/s1074-7613(00)80374-x. [DOI] [PubMed] [Google Scholar]
- 4.Ellmeier W, Sunshine MJ, Losos K, Littman DR. Multiple developmental stage-specific enhancers regulate CD8 expression in developing thymocytes and in thymus-independent T cells. Immunity. 1998;9:485–496. doi: 10.1016/s1074-7613(00)80632-9. [DOI] [PubMed] [Google Scholar]
- 5.Hostert A, et al. Hierarchical interactions of control elements determine CD8alpha gene expression in subsets of thymocytes and peripheral T cells. Immunity. 1998;9:497–508. doi: 10.1016/s1074-7613(00)80633-0. [DOI] [PubMed] [Google Scholar]
- 6.Ellmeier W, Sunshine MJ, Maschek R, Littman DR. Combined deletion of CD8 locus cis-regulatory elements affects initiation but not maintenance of CD8 expression. Immunity. 2002;16:623–634. doi: 10.1016/s1074-7613(02)00309-6. [DOI] [PubMed] [Google Scholar]
- 7.Bilic I, et al. Negative regulation of CD8 expression via Cd8 enhancer-mediated recruitment of the zinc finger protein MAZR. Nat Immunol. 2006;7:392–400. doi: 10.1038/ni1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garefalaki A, et al. Variegated expression of CD8 alpha resulting from in situ deletion of regulatory sequences. Immunity. 2002;16:635–647. doi: 10.1016/s1074-7613(02)00308-4. [DOI] [PubMed] [Google Scholar]
- 9.Feik N, et al. Functional and molecular analysis of the double-positive stage-specific CD8 enhancer E8III during thymocyte development. J Immunol. 2005;174:1513–1524. doi: 10.4049/jimmunol.174.3.1513. [DOI] [PubMed] [Google Scholar]
- 10.Madakamutil LT, et al. CD8alphaalpha-mediated survival and differentiation of CD8 memory T cell precursors. Science. 2004;304:590–593. doi: 10.1126/science.1092316. [DOI] [PubMed] [Google Scholar]
- 11.Chandele A, Kaech SM. Cutting edge: memory CD8 T cell maturation occurs independently of CD8alphaalpha. J Immunol. 2005;175:5619–5623. doi: 10.4049/jimmunol.175.9.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong W, Reinherz EL. CD8 alpha alpha homodimer expression and role in CD8 T cell memory generation during influenza virus A infection in mice. Eur J Immunol. 2005;35:3103–3110. doi: 10.1002/eji.200535162. [DOI] [PubMed] [Google Scholar]
- 13.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 14.Ruthenburg AJ, Li H, Patel DJ, Allis CD. Multivalent engagement of chromatin modifications by linked binding modules. Nat Rev Mol Cell Biol. 2007;8:983–994. doi: 10.1038/nrm2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azuara V, et al. Chromatin signatures of pluripotent cell lines. Nat Cell Biol. 2006;8:532–538. doi: 10.1038/ncb1403. [DOI] [PubMed] [Google Scholar]
- 16.Bernstein BE, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida M, Kijima M, Akita M, Beppu T. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J Biol Chem. 1990;265:17174–17179. [PubMed] [Google Scholar]
- 18.Sato T, et al. Dual functions of Runx proteins for reactivating CD8 and silencing CD4 at the commitment process into CD8 thymocytes. Immunity. 2005;22:317–328. doi: 10.1016/j.immuni.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Egawa T, Tillman RE, Naoe Y, Taniuchi I, Littman DR. The role of the Runx transcription factors in thymocyte differentiation and in homeostasis of naive T cells. J Exp Med. 2007;204:1945–1957. doi: 10.1084/jem.20070133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taniuchi I, et al. Differential requirements for Runx proteins in CD4 repression and epigenetic silencing during T lymphocyte development. Cell. 2002;111:621–633. doi: 10.1016/s0092-8674(02)01111-x. [DOI] [PubMed] [Google Scholar]
- 21.Naoe Y, et al. Repression of interleukin-4 in T helper type 1 cells by Runx/Cbf beta binding to the Il4 silencer. J Exp Med. 2007;204:1749–1755. doi: 10.1084/jem.20062456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parelho V, et al. Cohesins functionally associate with CTCF on mammalian chromosome arms. Cell. 2008;132:422–433. doi: 10.1016/j.cell.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Egawa T, Littman DR. ThPOK acts late in specification of the helper T cell lineage and suppresses Runx-mediated commitment to the cytotoxic T cell lineage. Nat Immunol. 2008;9:1131–1139. doi: 10.1038/ni.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitabayashi I, Yokoyama A, Shimizu K, Ohki M. Interaction and functional cooperation of the leukemia-associated factors AML1 and p300 in myeloid cell differentiation. EMBO J. 1998;17:2994–3004. doi: 10.1093/emboj/17.11.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taniuchi I, Ellmeier W. Transcriptional and epigenetic regulation of CD4/CD8 lineage choice. Adv Immunol. 2011;110:71–110. doi: 10.1016/B978-0-12-387663-8.00003-X. [DOI] [PubMed] [Google Scholar]
- 26.Chong MM, et al. Epigenetic propagation of CD4 expression is established by the Cd4 proximal enhancer in helper T cells. Genes Dev. 2010;24:659–669. doi: 10.1101/gad.1901610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao Z, Mescher MF, Jameson SC. Detuning CD8 T cells: Down-regulation of CD8 expression, tetramer binding, and response during CTL activation. J Exp Med. 2007;204:2667–2677. doi: 10.1084/jem.20062376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kienzle N, et al. Progressive differentiation and commitment of CD8+ T cells to a poorly cytolytic CD8low phenotype in the presence of IL-4. J Immunol. 2005;174:2021–2029. doi: 10.4049/jimmunol.174.4.2021. [DOI] [PubMed] [Google Scholar]
- 29.Montufar-Solis D, Garza T, Klein JR. T-cell activation in the intestinal mucosa. Immunol Rev. 2007;215:189–201. doi: 10.1111/j.1600-065X.2006.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Wijk F, Cheroutre H. Intestinal T cells: Facing the mucosal immune dilemma with synergy and diversity. Semin Immunol. 2009;21(3):130–138. doi: 10.1016/j.smim.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.