Abstract
Monoclonal antibodies are among the most promising therapeutic agents for treating cancer. Therapeutic cancer antibodies bind to tumor cells, turning them into targets for immune-mediated destruction. We show here that this antibody-mediated killing of tumor cells is limited by a mechanism involving the interaction between tumor cell-expressed CD47 and the inhibitory receptor signal regulatory protein-α (SIRPα) on myeloid cells. Mice that lack the SIRPα cytoplasmic tail, and hence its inhibitory signaling, display increased antibody-mediated elimination of melanoma cells in vivo. Moreover, interference with CD47–SIRPα interactions by CD47 knockdown or by antagonistic antibodies against CD47 or SIRPα significantly enhances the in vitro killing of trastuzumab-opsonized Her2/Neu-positive breast cancer cells by phagocytes. Finally, the response to trastuzumab therapy in breast cancer patients appears correlated to cancer cell CD47 expression. These findings demonstrate that CD47–SIRPα interactions participate in a homeostatic mechanism that restricts antibody-mediated killing of tumor cells. This provides a rational basis for targeting CD47–SIRPα interactions, using for instance the antagonistic antibodies against human SIRPα described herein, to potentiate the clinical effects of cancer therapeutic antibodies.
Keywords: antibody-dependent cellular cytotoxicity, neutrophil, immunoreceptor, Fc-receptor
Therapeutic monoclonal antibodies (mAbs) directed against tumor cells have become a valuable alternative or addition to conventional cancer treatment modalities. However, despite the beneficial effects documented for various therapeutic antibodies against different types of cancer, antibodies alone are not curative and methods to improve their efficacy are warranted. Therapeutic cancer antibodies may act by one or more several mechanisms, including immune-mediated effects, such as antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) mechanisms, as well as by direct growth-inhibitory effects on tumor cells (1–3).
Currently, the most widely used examples of therapeutic antibodies are rituximab and trastuzumab. Trastuzumab (Herceptin) is a humanized IgG1 monoclonal antibody approved for the treatment of Her2/Neu-positive breast cancer. Although it is known that trastuzumab binds to the extracellular domain of Her2/Neu, the mechanism(s) of action in patients is not exactly clear. In vitro and in vivo studies in mice suggest that trastuzumab acts by inducing direct G1 growth arrest in breast cancer cells as well as by mediating ADCC (4–6). ADCC can be mediated by Fc receptor-expressing natural killer (NK) cells and phagocytes, including macrophages and granulocytes (7, 8), and a link between FcγRIIa (CD32a) and FcγRIIIa (CD16) polymorphisms and clinical trastuzumab responsiveness in patients with breast cancer suggests an involvement of both types of Fc receptors expressed on phagocytes and NK cells, respectively (3, 9).
NK cell-mediated ADCC is controlled by interactions between “self” MHC class I molecules on (malignant) host cells and inhibitory killer immune receptors (KIRs) expressed on NK cells. Upon ligand binding, inhibitory KIRs recruit and activate the cytosolic tyrosine phosphatases SHP-1 and/or SHP-2 that limit Fc-receptor signaling and, consequently, ADCC toward host cells (7). An inhibitory receptor on myeloid cells, including macrophages and granulocytes, that may potentially act in a similar fashion to restrict antibody-mediated tumor cell elimination is signal regulatory protein (SIRP)α (10–14). The extracellular region of SIRPα interacts with the broadly expressed surface molecule CD47 (15–17). CD47 binding to SIRPα triggers the recruitment and activation of SHP-1 and SHP-2 to immunoreceptor tyrosine-based inhibitory motifs (ITIMs) within the SIRPα cytoplasmic region, and this regulates intracellular signaling pathways and associated downstream functions, usually in a negative fashion (10, 11, 18). It is well-documented, for instance, that SIRPα acts to inhibit in the phagocytosis and in vivo clearance of CD47-expressing host cells, including red blood cells and platelets, by macrophages (19–24). CD47–SIRPα interactions also appear essential for engraftment upon hematopoietic stem cells (25). Based on this, it has been proposed that the broadly expressed CD47 functions, in analogy to MHC class I molecules, as a self signal to control immune effector functions of myeloid cells (19, 26).
Chao et al. (27) have recently reported that antibodies against CD47 synergize with the therapeutic cancer antibody rituximab in the phagocytosis of non-Hodgkin lymphoma by macrophages in immunodeficient mice. However, this study does not provide conclusive evidence for the role of CD47–SIRPα interactions in the context of antibody therapy against cancer. In the present study, we demonstrate that CD47–SIRPα interactions and SIRPα signaling negatively regulate trastuzumab-mediated ADCC in vitro and antibody-dependent elimination of tumor cells in vivo. These findings support the idea that CD47–SIRPα interactions create a barrier for antibody-mediated tumor cell elimination and provide a rational basis for targeting CD47–SIRPα interactions to potentiate the clinical effects of cancer therapeutic antibodies.
Results
Antibody-Mediated Cancer Elimination in Vivo Is Restricted by SIRPα Signaling.
We postulated that interactions between CD47, expressed broadly on normal and tumor cells, and the myeloid inhibitory immunoreceptor SIRPα would negatively regulate phagocyte-mediated ADCC induced by cancer therapeutic antibodies, and that targeting of CD47–SIRPα interactions would comprise a generic strategy to improve antibody therapy against cancer. In line with this, Chao et al. (27) have recently shown that antibodies against human CD47 synergize with rituximab in the elimination of non-Hodgkin lymphoma cells in immunodeficient mice and in in vitro phagocytosis experiments. Instead, we used mutant mice lacking the SIRPα cytoplasmic tail (21) to investigate whether inhibitory signaling via SIRPα could regulate the antibody-mediated elimination of syngeneic tumor cells in immunocompetent mice. In particular, we used the well-established mouse metastatic B16 melanoma model, in which the therapeutic antibody TA99, directed against the melanoma gp75 tumor antigen, has shown prominent beneficial effects in tumor cell clearance (28). First, B16F10 cells that expressed surface CD47 (Fig. 1A) were injected i.v., in the absence of therapeutic TA99 antibody, into wild-type and SIRPα-mutant mice, and this resulted in a similar tumor formation in both strains of mice (Fig. 1B), indicating that SIRPα signaling did not affect tumor cell metastasis and outgrowth per se. Next, these experiments were performed in mice that were treated with suboptimal concentrations of TA99 antibody. TA99 antibody treatment resulted only in a minimal reduction in tumor cell outgrowth in wild-type mice, but tumor formation was essentially abrogated in SIRPα-mutant animals under these conditions (Fig. 1C). This demonstrated directly that SIRPα-derived signals can form a limitation for antibody-dependent tumor cell elimination in vivo.
Fig. 1.
SIRPα signaling limits antibody-mediated destruction of melanoma cells in vivo. (A) CD47 expression on B16F10 mouse melanoma cells as demonstrated by flow cytometry using anti-mouse CD47 antibody (Miap301) and phycoerythrin-labeled anti-mouse IgG (filled histogram). The open histogram represents the isotype control. (B) Comparable outgrowth of B16 melanoma in wild-type and SIRPα-mutant mice in the absence of therapeutic antibody. Wild-type and SIRPα-mutant mice were injected i.v. with 1.5 × 105 B16F10 tumor cells. After 21 d, mice were killed, lungs were excised and photographed (representative examples are shown), and tumor loads were determined and expressed as the sum of the following scores: metastases less than 1 mm were scored as 1; metastases between 1 and 2 mm were scored as 3; and metastases larger than 2 mm were scored as 10. Measurements from individual mice are shown, with means indicated by bars, and statistical differences between groups (n = 10) were determined by ANOVA. Note that comparable tumor loads occur in wild-type (34.7 ± 9.5) (mean ± SEM) and SIRPα-mutant mice (35.9 ± 5.2). Data are from one representative experiment out of three. (C) Enhanced antibody-mediated clearance of B16 melanoma cells in SIRPα-mutant mice. Wild-type and SIRPα-mutant mice were challenged i.v. with 1.5 × 105 B16F10 tumor cells and, where indicated, with a suboptimal dose of 10 μg of TA99 antibody (or PBS as control) on days 0, 2, and 4. After 21 d, mice were killed and analyzed as in B. Measurements from individual mice are shown, with means indicated by bars, and statistical differences between groups (n = 8) were determined by ANOVA. Note the black nodules of melanoma lung metastases in B and C. Note in the graph in C that TA99 antibody treatment resulted only in a minimal nonsignificant reduction in tumor cell outgrowth in wild-type animals [47.9 ± 9.4 (mean ± SEM) in PBS-treated mice compared with 29.0 ± 7.8 in TA99-treated mice], but tumor formation was essentially absent in SIRPα-mutant animals treated with TA99 antibody (4.5 ± 1.0). Data are from one representative experiment out of three.
Expression of CD47 in Breast Cancer Correlates with Adverse Features and Resistance to Trastuzumab.
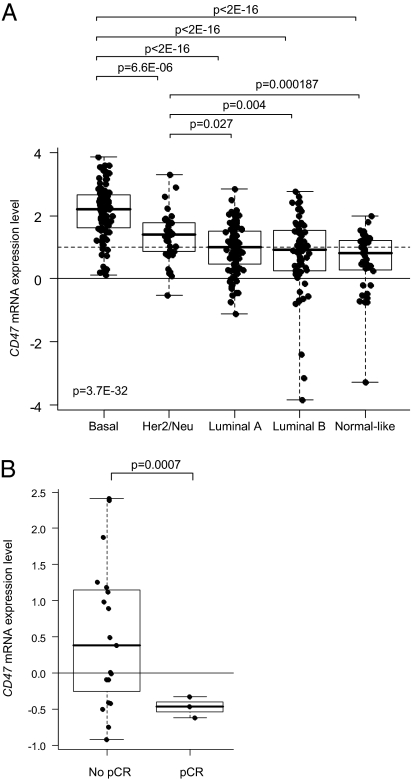
In line with the above, we hypothesized that CD47–SIRPα interactions were restricting the clinical efficacy of trastuzumab in the treatment of patients with Her2/Neu-positive breast cancer. To test this hypothesis, we explored a possible relationship between CD47 expression and breast cancer pathological features and clinical trastuzumab responsiveness. To do so, we analyzed breast cancer tissue CD47 mRNA expression in our cohort of 353 breast cancer patients as well as in a public data set (29). CD47 mRNA was overexpressed in many tumors, and expression correlated with poor-prognosis molecular subtypes (i.e., basal, Her2/Neu+) (Fig. 2A) and with adverse pathological features [i.e., high-grade, estrogen receptor (ER)−, progesterone receptor (PR)−]. Furthermore, analysis of a relatively small public data set (29) of Her2/Neu-positive breast cancer patients treated with trastuzumab plus vinorelbine revealed an inverse correlation between CD47 expression level and pathological response to the therapy (Fig. 2B), with significantly lower CD47 expression in complete responders. Although the latter finding clearly requires confirmation in a larger and independent patient cohort, it is consistent with an adverse role of CD47 in the trastuzumab-mediated elimination of breast cancer cells.
Fig. 2.
CD47 mRNA expression in breast cancer. (A) Correlation with molecular subtypes: basal, Her2/Neu-positive, luminal A, luminal B, and normal-like (Institut Paoli-Calmettes series; n = 353). Log2-transformed expression levels in tumors are reported as box plots relative to expression in normal breast (NB; horizontal solid line). Overexpression (ratio T:NB ≥2; horizontal dashed line) of CD47 was found in 63% of tumors. Note that the poor-prognosis subtypes (i.e., basal and Her2/Neu+) have the highest CD47 expression levels. Differences in expression levels between the five subtypes were tested for significance using one-way ANOVA, and between two subtypes using Student's t test. (B) Correlation with pathological response to trastuzumab plus vinorelbine treatment [public data set (29); n = 22]. Log2-transformed expression levels in tumors are reported as box plots relative to median expression in all samples (median; horizontal solid line). Note that patients with a pathological complete response (pCR; n = 3) have significantly lower CD47 expression than patients with an incomplete response (no pCR; n = 19).
Targeting CD47–SIRPα Interactions Potentiates Trastuzumab-Mediated ADCC Against Breast Cancer Cells.
To directly investigate whether CD47–SIRPα interactions play a role in the trastuzumab-dependent destruction of breast cancer cells by phagocytes, we established an in vitro ADCC assay using trastuzumab-opsonized human SKBR-3 breast cancer cells expressing surface Her2/Neu and CD47 (Fig. 3A) as targets and human neutrophils as effector cells. Trastuzumab-mediated ADCC by neutrophils was potently and synergistically enhanced by F(ab′)2 fragments of the B6H12 mAb that blocks CD47 binding to SIRPα (30) (Fig. 3 B–E). The enhancing effect of blocking anti-CD47 F(ab′)2 was observed at different effector:target (E:T) ratios (Fig. 3C) and appeared to act by both decreasing the threshold as well as by increasing the magnitude of killing (Fig. 3D). Importantly, in the absence of trastuzumab, no detectable tumor killing effect of anti-CD47 F(ab′)2 was observed, suggesting that CD47–SIRPα interactions do not control antibody-independent mechanisms of killing. This observation is in apparent contrast with the results of Chao et al. (27, 31), who also reported significant effects on lymphoma phagocytosis with the anti-CD47 mAb B6H12 alone. The latter may possibly relate, at least in part, to their use of intact B6H12 mAb that according to our own results can indeed cause direct ADCC in SKBR-3 cells (Fig. S1).
Fig. 3.
Interference with CD47–SIRPα interactions using blocking anti-CD47 antibody B6H12 potentiates trastuzumab-mediated ADCC of neutrophils toward Her2/Neu-positive SKBR-3 breast cancer cells. (A) Flow cytometric analysis of Her2/Neu and CD47 surface expression on SKBR-3 breast cancer cells (filled histograms), using trastuzumab and B6H12 mAb, respectively, against CD47. Isotype controls are shown in the open histograms. (B) ADCC of neutrophils against trastuzumab-opsonized SKBR-3 cells (E:T ratio, 50:1) in the absence or presence of B6H12 anti-CD47 F(ab′)2. Shown is a representative example. Results are expressed as means ± SD of triplicate measurements, and statistical differences were shown by Student's t test. Note that anti-CD47 F(ab′)2 fragments do not affect cytotoxicity alone, but do synergize with trastuzumab. (C and D) Blocking CD47–SIRPα interactions using anti-CD47 F(ab′)2 enhances the ADCC of neutrophils against trastuzumab-opsonized SKBR-3 cells at different E:T ratios (C) and trastuzumab concentrations (D). Shown is a representative experiment out of three. (E) The effects of anti-CD47 F(ab′)2 on ADCC toward trastuzumab-opsonized SKBR-3 cells using neutrophils from different donors in multiple independent experiments (n = 53). For clarity, only the values in the presence of trastuzumab ± anti-CD47 F(ab′)2 are shown, with the matched values of the two conditions for each donor connected by lines. Killing in the absence of trastuzumab ± anti-CD47 F(ab′)2 was always below 5%. P values of statistically significant differences, as determined by Student's t test, are indicated.
In the numerous independent experiments (n > 50) that were performed with neutrophils as effector cells for killing of trastuzumab-opsonized SKBR-3 cells a consistent enhancing effect of anti-CD47 F(ab′)2 was observed, although the degree of killing (with trastuzumab alone) varied considerably for different effector cell donors (Fig. 3B). The latter appeared to be related to a factor(s) intrinsic to the effector cells, including individual differences in the expression of FcγRI and FcγRIIIb receptors that is pivotal for the induction of ADCC (Fig. S2).
Reduction of CD47 in Breast Cancer Cells Promotes Trastuzumab-Mediated ADCC.
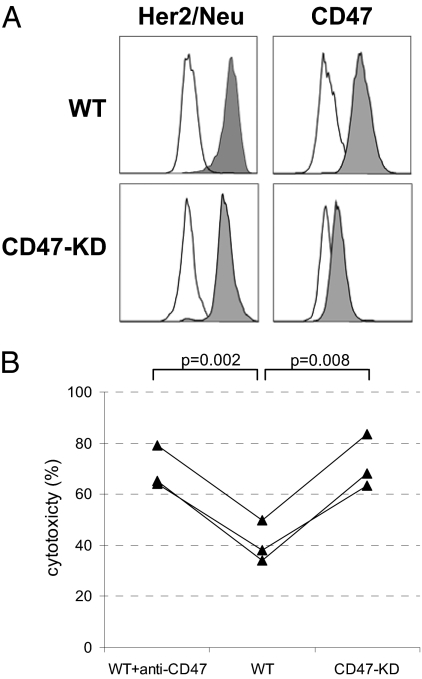
To further study a regulatory role of CD47–SIRPα interactions in ADCC, siRNA-mediated knockdown of CD47 expression was performed in SKBR-3 target cells. This yielded cells with 80–90% reduced surface CD47 expression (Fig. 4A). These cells were significantly more sensitive toward neutrophil-mediated ADCC, consistent with a role for CD47–SIRPα interactions in restricting tumor cell killing (Fig. 4B). The increase was comparable to levels seen with wild-type SKBR-3 cells in the presence of blocking anti-CD47 F(ab′)2.
Fig. 4.
Knockdown of CD47 in SKBR-3 breast cancer target cells enhances trastuzumab-dependent neutrophil-mediated ADCC. (A) Flow cytometric analysis for Her2/Neu and CD47 surface expression in SKBR-3 cells transfected with empty vector (control) or CD47 shRNA (CD47-KD). Note that CD47 expression is strongly decreased in the CD47-KD cells (mean fluorescence intensity (MFI) = 358 in CD47-KD cells vs. MFI = 4.187 in control), but Her2/Neu levels are unaltered (MFI = 18.638 in CD47-KD cells and MFI = 18.993 in control). (B) Neutrophil-mediated ADCC using control and CD47-KD SKBR-3 cells opsonized with trastuzumab in three independent experiments with three different effector cell donors. Note that a similar level of enhancement occurs with anti-CD47 F(ab′)2-mediated blocking and CD47 knockdown. P values of statistically significant differences, as determined by Student's t test, are indicated.
Unique mAb Against SIRPα Potentiates Trastuzumab-Mediated ADCC Against Breast Cancer Cells.
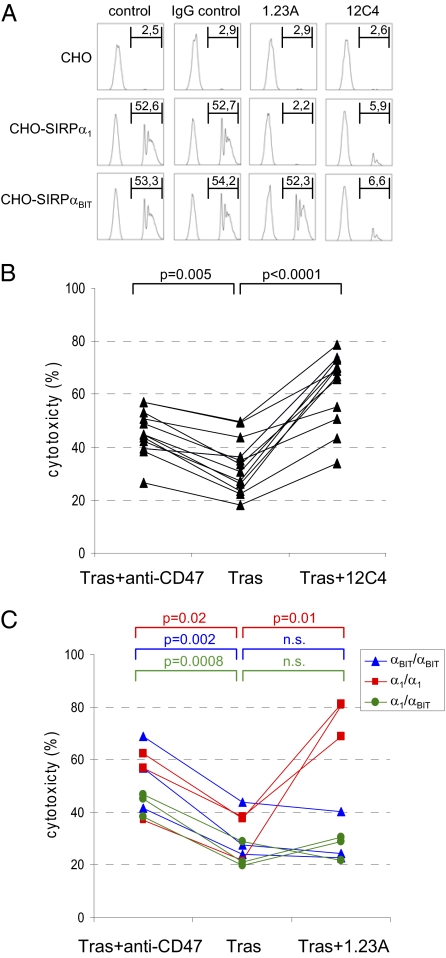
Although the above strongly supported the idea that CD47–SIRPα interactions regulate ADCC in vitro and tumor elimination in vivo, it was important to confirm these findings with blocking antibodies against SIRPα. In fact, because of its much more restricted expression (12, 16), we anticipate that SIRPα, rather than the ubiquitous CD47, constitutes the preferred target for potential future therapeutic intervention. Because the previously reported antibodies against human SIRPα available to us either lacked the proper specificity or the ability to block interactions with CD47, we generated unique blocking mAbs against SIRPα1. One antibody, designated 1.23A, was generated by electrofusion technology following negative selection on CHO cells expressing the myeloid-specific SIRP family member SIRPβ1, whereas the other, designated 12C4, was generated by conventional hybridoma technology. Both of the two SIRPα polymorphic variants predominating in the Caucasian population, SIRPα1 and SIRPαBIT, as well as the highly homologous myeloid SIRPβ1 and nonmyeloid SIRPγ family members were recognized by 12C4, but the 1.23A mAb exclusively recognized the SIRPα1 variant (Fig. S3 A and B). Staining of leukocytes from SIRPα-genotyped individuals was consistent with this specificity (Fig. S3C), with the mAb 1.23A selectively recognizing monocytes and neutrophils from both α1/α1-homozygous and α1/αBIT-heterozygous individuals. Both mAbs effectively inhibited the binding of CD47-coated beads to CHO cells expressing SIRPα1 and/or SIRPαBIT (Fig. 5A) and promoted trastuzumab-mediated ADCC toward SKBR-3 cells by neutrophils from individuals with different genotypes (Fig. 5 B and C). For the 1.23A mAb, enhanced killing was only observed when neutrophils from α1/α1-homozygous individuals were used. When αBIT/αBIT-homozygous or α1/αBIT-heterozygous donor cells were used, 1.23A did not enhance SKBR-3 killing by trastuzumab, suggesting that the presence of a single functional allele of SIRPα is sufficient to restrict ADCC and that both alleles have to be inhibited simultaneously to achieve a beneficial effect accordingly.
Fig. 5.
Monoclonal antibodies against SIRPα that block CD47–SIRPα interactions enhance ADCC. (A) CD47-coated fluorescent bead binding to CHO cells expressing empty vector (i.e., “CHO”), SIRPα1, or SIRPαBIT. The 12C4 and 1.23A mAbs (but not isotype IgG1 control mAb) block the binding of CD47 beads to either both SIRPα1- and SIRPαBIT-expressing CHO cells (12C4) or only to SIRPα1-expressing CHO cells. The proportion (in %) of cells binding CD47 beads is indicated in the upper right of each panel. Shown is one representative experiment out of three. (B) Enhancing effect of 12C4 mAb on ADCC toward trastuzumab-opsonized SKBR-3 cells using neutrophils from (n = 12) individuals in four independent experiments. (C) Enhancing effect of 1.23A mAb on ADCC toward trastuzumab-opsonized SKBR-3 cells using neutrophils from (n = 9) individuals with different SIRPα genotypes (α1/α1 or αBIT/αBIT homozygotes or α1/αBIT heterozygotes) in three independent experiments. P values of statistically significant differences, as determined by Student's t test, are indicated. n.s., nonsignificant.
Discussion
In the present study, we have investigated the role of CD47–SIRPα interactions in the context of antibody therapy against cancer. In general, our results provide evidence that CD47–SIRPα interactions, and the resultant intracellular signals generated via SIRPα in myeloid cells, suppress antibody-mediated destruction of tumor cells.
To study the role of SIRPα in vivo, we used mutant mice lacking the SIRPα cytoplasmic tail to investigate whether inhibitory signaling via SIRPα could regulate the antibody-mediated elimination of syngeneic B16F10 melanoma cells in immunocompetent mice. Our results demonstrate that SIRPα signaling does indeed limit the capacity of cancer therapeutic antibodies to eliminate tumor cells in vivo. The effects could not be attributed to direct effects of SIRPα on tumor homing or outgrowth, as identical tumor development was shown in the absence of therapeutic antibody. This provides evidence for a role of SIRPα in antibody-mediated tumor cell destruction in vivo.
The role of CD47–SIRPα interactions in a human context was investigated with an in vitro ADCC method using trastuzumab-opsonized Her2/Neu-positive SKBR-3 breast cancer cells as target cells and neutrophils as effector cells. In this assay, the addition of F(ab′)2 fragments of the antibody B6H12, which is known to block CD47–SIRPα interactions (30), substantially enhanced trastuzumab-mediated cancer cell killing, supporting the idea that CD47–SIRPα interactions negatively control ADCC. Of note, the interference with CD47–SIRPα interactions in the absence of trastuzumab did not enhance ADCC. The latter is in apparent contrast with the results of Chao et al., who did show significant effects of anti-CD47 antibody alone on tumor cell phagocytosis in vitro and in vivo. However, Chao et al. used intact B6H12 anti-CD47 antibody in the vast majority of their experiments, including all of their in vivo experiments. We now demonstrate that this intact anti-CD47 antibody causes direct ADCC in neutrophils (Fig. S1), and similar observations have also been made for monocytes/macrophages, thereby indicating, in retrospect, that the results of Chao et al. did not really justify the conclusion that the effects were due to the interference with CD47–SIRPα interactions. On the contrary, our findings, which are based on both antibody-blocking experiments performed with anti-CD47 F(ab′)2 fragments as well as CD47 knockdowns in breast cancer cells, do indeed exclude alternative explanations and thereby provide direct evidence for a regulatory role of CD47–SIRPα interactions in antibody-dependent cancer cell destruction.
Although the above clearly supported a role for CD47–SIRPα interactions in antibody-dependent tumor cell elimination, it was considered important to confirm these results with antagonistic antibodies against SIRPα. Moreover, because of its much more limited tissue distribution compared with CD47, SIRPα appears to be the preferred target for potential future therapeutic intervention. Because antagonistic antibodies of the appropriate specificity were unavailable, we attempted to generate new reagents. Two antagonistic antibodies were identified and characterized that reacted with one or both of the two major [and apparently equally functional (32)] polymorphic SIRPα variants, SIRPα1 and SIRPαBIT, found in the Caucasian population, and both were shown to be able to enhance trastuzumab-mediated ADCC in breast cancer cells. Notably, the inability of the SIRPα1-specific antibody to enhance antibody-dependent tumor cell elimination when effector cells from heterozygote SIRPα1/SIRPαBIT individuals were used suggests that inhibitory signals from both alleles are required to provide substantial control over antibody-mediated cytotoxicity. It will be of interest to test the in vivo efficacy of our antibodies in appropriately humanized mouse xenograft tumor models.
Clearly, an interesting and clinically highly relevant question is whether CD47–SIRPα interactions play a regulatory role in the context of antibody therapy in human cancer patients, and whether antagonists targeting the CD47–SIRPα interaction, such as the antibodies against SIRPα described herein, can be used to enhance the clinical efficacy of trastuzumab. Although the present study does not provide direct evidence for this, our findings do suggest a preliminary link between CD47 expression on breast cancer cells and clinical trastuzumab responsiveness in breast cancer. In particular, pathologically complete responders were found to have significantly lower CD47 mRNA levels compared with trastuzumab-treated patients lacking a pathologically complete response.
It should be emphasized that CD47–SIRPα interactions may not form the only mechanism by which tumor cells can evade phagocyte-mediated immune destruction. In fact, recent studies have shown that the interaction between the self CD200 molecule, expressed on tumor cells and many other cell types, and the nonconventional (i.e., ITIM-lacking) inhibitory CD200 receptor (CD200R) on myeloid cells may also limit the immune-mediated elimination of leukemic cells such as B-CLL (33–35). However, this can apparently occur in the absence of therapeutic antibodies, and may also be mediated by a different effector mechanism involving cytotoxic T cells. The observation that different nonredundant mechanisms may actually underlie the regulatory effects of the CD47–SIRPα and CD200–CD200R interactions may actually generate opportunities for simultaneous targeting of these pathways to increase therapeutic benefit.
Collectively, our results provide direct evidence for a homeostatic regulatory role of CD47–SIRPα interactions in the context of antibody-mediated destruction of tumor cells by myeloid cells. Together with the findings of Chao et al. (27), this provides a strong rational basis for combining therapeutic antibodies against cancer cells with antagonists of the CD47–SIRPα interaction, such as the mAb against SIRPα described here. This is anticipated to enhance the clinical efficacy of cancer-targeting therapeutic antibodies and/or reduce the need for chemotherapy or other nonspecific treatment regimens.
Methods
Mice and B16 Melanoma Model.
C57BL/6 mice with a targeted deletion of the SIRPα cytoplasmic region have been described previously (21). These mice, originally generated onto the 129/Sv background and backcrossed onto C57BL/6 mice for 10 generations, were bred and maintained under specific pathogen-free conditions, together with wild-type C57BL/6 mice from the same genetic background, and used between 8 and 12 wk of age. Age-matched wild-type and SIRPα-mutant mice were injected i.v. with 1.5 × 105 B16F10 tumor cells in 100 μL of HBSS on day 0. Mice were injected i.p. with a suboptimal dose of 10 μg of TA99 antibody (or PBS as control) on days 0, 2, and 4. At day 21 the mice were killed. Their lungs were excised and scored for the number of metastases and tumor load as described (28).
Antibodies, cell lines, culture conditions, procedures for the production of monoclonal antibodies, the CD47 bead binding assay, and flow cytometry are described in SI Methods.
CD47 mRNA Expression in Breast Cancer.
We analyzed CD47 mRNA expression in 353 invasive breast carcinomas and 11 normal breast samples profiled (36) using whole-genome Affymetrix oligonucleotide microarrays (Gene Expression Omnibus accession no. GSE21653). Only two of the probe sets representing CD47, 211075_s_at and 213857_s_at, mapped exclusively to constitutively transcribed CD47 exons according to NetAffx, RefSeq, and the University of California Santa Cruz Genome Browser (27). Their expression strongly correlated (Spearman correlation, 0.87). We retained that with the highest variance (211075_s_at). Before analysis, the CD47 expression level for each tumor was centered by the average expression level of the normal breast samples. We analyzed the correlation between CD47 expression and patients’ age (≤/>50 y), pathological tumor size (≤/>2 cm), axillary lymph node status (negative/positive) and grading (I/II/III), immunohistochemisty estrogen and progesterone receptor status (negative/positive; positivity threshold 10% of tumor cells), and molecular subtypes (luminal A/luminal B/basal/Her2/Neu+/normal-like), defined as described (37). We also analyzed a public (http://caarraydb.nci.nih.gov/ccarray) expression data set of Her2/Neu-positive breast cancers treated with primary trastuzumab plus vinorelbine weekly for 12 wk followed by surgery (29). Pathological complete response was defined as the absence of invasive cancer in the breast and axillary lymph nodes at the time of surgery.
ADCC Assay.
Neutrophils were isolated by density centrifugation from heparinized blood obtained from healthy volunteers using isotonic Percoll (Pharmacia) followed by red cell lysis with hypotonic ammonium chloride solution. Cells were cultured in complete RPMI medium in the presence of 10 ng/mL clinical grade G-CSF (Neupogen; Amgen) and 50 ng/mL recombinant human IFN-γ (PeproTech) at a concentration of 5 × 106 cells/mL for 16–20 h. Monocytes were isolated from the peripheral blood mononuclear cells fraction by magnetic cell sorting using anti-CD14-coated beads according to the manufacturer's instructions (Miltenyi Biotec) or by counterflow elutriation. Washed tumor cells (5–8 × 106 cells) were collected and labeled with 100 μCі 51Cr (PerkinElmer) in 1 mL for 90 min at 37 °C. The cells were preincubated with anti-CD47 and/or the therapeutic antibodies, as indicated, and washed again. The target cells (5 × 103 per well) and effector cells were cocultured in 96-well U-bottom tissue culture plates in complete medium in an E:T ratio of 50:1, unless indicated otherwise, for 4 h at 37 °C in 5% CO2 in RPMI with 10% FCS medium. Aliquots of supernatant were harvested and analyzed for radioactivity in a gamma counter. The percent relative cytotoxicity was determined as [(experimental cpm − spontaneous cpm)/(total cpm − spontaneous cpm)] × 100%. All conditions were measured in triplicate.
Statistical Analysis.
Statistical differences were determined using ANOVA or Student's t test as indicated.
Supplementary Material
Acknowledgments
We thank Dr. L. A. Aarden and Dr. S. Rodenhuis for useful discussions and supplying anti-CD3, rituximab, and trastuzumab antibodies; and Dr. D. Roos and Dr. R. van Lier for their advice on the manuscript. Miap301 and B6H12 antibodies were generously provided by Dr. E. Brown (University of California at San Francisco). Dr. Peter Steenbakkers is acknowledged for his help in generating anti-SIRPα mAb. Paul Verkuijlen and Peter Kooyman are gratefully acknowledged for their technical support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1106550108/-/DCSupplemental.
References
- 1.Glennie MJ, van de Winkel JG. Renaissance of cancer therapeutic antibodies. Drug Discov Today. 2003;8:503–510. doi: 10.1016/s1359-6446(03)02714-4. [DOI] [PubMed] [Google Scholar]
- 2.Oldham RK, Dillman RO. Monoclonal antibodies in cancer therapy: 25 years of progress. J Clin Oncol. 2008;26:1774–1777. doi: 10.1200/JCO.2007.15.7438. [DOI] [PubMed] [Google Scholar]
- 3.Strome SE, Sausville EA, Mann D. A mechanistic perspective of monoclonal antibodies in cancer therapy beyond target-related effects. Oncologist. 2007;12:1084–1095. doi: 10.1634/theoncologist.12-9-1084. [DOI] [PubMed] [Google Scholar]
- 4.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 5.Barok M, et al. Trastuzumab causes antibody-dependent cellular cytotoxicity-mediated growth inhibition of submacroscopic JIMT-1 breast cancer xenografts despite intrinsic drug resistance. Mol Cancer Ther. 2007;6:2065–2072. doi: 10.1158/1535-7163.MCT-06-0766. [DOI] [PubMed] [Google Scholar]
- 6.Spiridon CI, Guinn S, Vitetta ES. A comparison of the in vitro and in vivo activities of IgG and F(ab′)2 fragments of a mixture of three monoclonal anti-Her-2 antibodies. Clin Cancer Res. 2004;10:3542–3551. doi: 10.1158/1078-0432.CCR-03-0549. [DOI] [PubMed] [Google Scholar]
- 7.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 8.van Spriel AB, van Ojik HH, Bakker A, Jansen MJ, van de Winkel JG. Mac-1 (CD11b/CD18) is crucial for effective Fc receptor-mediated immunity to melanoma. Blood. 2003;101:253–258. doi: 10.1182/blood.V101.1.253. [DOI] [PubMed] [Google Scholar]
- 9.Musolino A, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26:1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 10.Fujioka Y, et al. A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol Cell Biol. 1996;16:6887–6899. doi: 10.1128/mcb.16.12.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kharitonenkov A, et al. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature. 1997;386:181–186. doi: 10.1038/386181a0. [DOI] [PubMed] [Google Scholar]
- 12.Adams S, et al. Signal-regulatory protein is selectively expressed by myeloid and neuronal cells. J Immunol. 1998;161:1853–1859. [PubMed] [Google Scholar]
- 13.van Beek EM, Cochrane F, Barclay AN, van den Berg TK. Signal regulatory proteins in the immune system. J Immunol. 2005;175:7781–7787. doi: 10.4049/jimmunol.175.12.7781. [DOI] [PubMed] [Google Scholar]
- 14.Barclay AN, Brown MH. The SIRP family of receptors and immune regulation. Nat Rev Immunol. 2006;6:457–464. doi: 10.1038/nri1859. [DOI] [PubMed] [Google Scholar]
- 15.Jiang P, Lagenaur CF, Narayanan V. Integrin-associated protein is a ligand for the P84 neural adhesion molecule. J Biol Chem. 1999;274:559–562. doi: 10.1074/jbc.274.2.559. [DOI] [PubMed] [Google Scholar]
- 16.Seiffert M, et al. Signal-regulatory protein α (SIRPα) but not SIRPβ is involved in T-cell activation, binds to CD47 with high affinity, and is expressed on immature CD34(+)CD38(−) hematopoietic cells. Blood. 2001;97:2741–2749. doi: 10.1182/blood.v97.9.2741. [DOI] [PubMed] [Google Scholar]
- 17.Vernon-Wilson EF, et al. CD47 is a ligand for rat macrophage membrane signal regulatory protein SIRP (OX41) and human SIRPα 1. Eur J Immunol. 2000;30:2130–2137. doi: 10.1002/1521-4141(2000)30:8<2130::AID-IMMU2130>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 18.Timms JF, et al. Identification of major binding proteins and substrates for the SH2-containing protein tyrosine phosphatase SHP-1 in macrophages. Mol Cell Biol. 1998;18:3838–3850. doi: 10.1128/mcb.18.7.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oldenborg PA, et al. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 20.Olsson M, Bruhns P, Frazier WA, Ravetch JV, Oldenborg PA. Platelet homeostasis is regulated by platelet expression of CD47 under normal conditions and in passive immune thrombocytopenia. Blood. 2005;105:3577–3582. doi: 10.1182/blood-2004-08-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamao T, et al. Negative regulation of platelet clearance and of the macrophage phagocytic response by the transmembrane glycoprotein SHPS-1. J Biol Chem. 2002;277:39833–39839. doi: 10.1074/jbc.M203287200. [DOI] [PubMed] [Google Scholar]
- 22.Ishikawa-Sekigami T, et al. SHPS-1 promotes the survival of circulating erythrocytes through inhibition of phagocytosis by splenic macrophages. Blood. 2006;107:341–348. doi: 10.1182/blood-2005-05-1896. [DOI] [PubMed] [Google Scholar]
- 23.Okazawa H, et al. Negative regulation of phagocytosis in macrophages by the CD47-SHPS-1 system. J Immunol. 2005;174:2004–2011. doi: 10.4049/jimmunol.174.4.2004. [DOI] [PubMed] [Google Scholar]
- 24.Oldenborg PA, Gresham HD, Lindberg FP. CD47-signal regulatory protein α (SIRPα) regulates Fcγ and complement receptor-mediated phagocytosis. J Exp Med. 2001;193:855–862. doi: 10.1084/jem.193.7.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takenaka K, et al. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol. 2007;8:1313–1323. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- 26.van den Berg TK, van der Schoot CE. Innate immune ‘self’ recognition: A role for CD47–SIRPα interactions in hematopoietic stem cell transplantation. Trends Immunol. 2008;29:203–206. doi: 10.1016/j.it.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Chao MP, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hara I, Takechi Y, Houghton AN. Implicating a role for immune recognition of self in tumor rejection: Passive immunization against the brown locus protein. J Exp Med. 1995;182:1609–1614. doi: 10.1084/jem.182.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris LN, et al. Predictors of resistance to preoperative trastuzumab and vinorelbine for HER2-positive early breast cancer. Clin Cancer Res. 2007;13:1198–1207. doi: 10.1158/1078-0432.CCR-06-1304. [DOI] [PubMed] [Google Scholar]
- 30.Latour S, et al. Bidirectional negative regulation of human T and dendritic cells by CD47 and its cognate receptor signal-regulator protein-α: Down-regulation of IL-12 responsiveness and inhibition of dendritic cell activation. J Immunol. 2001;167:2547–2554. doi: 10.4049/jimmunol.167.5.2547. [DOI] [PubMed] [Google Scholar]
- 31.Chao MP, et al. Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia. Cancer Res. 2011;71:1374–1384. doi: 10.1158/0008-5472.CAN-10-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hatherley D, Graham SC, Harlos K, Stuart DI, Barclay AN. Structure of signal-regulatory protein α: A link to antigen receptor evolution. J Biol Chem. 2009;284:26613–26619. doi: 10.1074/jbc.M109.017566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McWhirter JR, et al. Antibodies selected from combinatorial libraries block a tumor antigen that plays a key role in immunomodulation. Proc Natl Acad Sci USA. 2006;103:1041–1046. doi: 10.1073/pnas.0510081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kretz-Rommel A, et al. CD200 expression on tumor cells suppresses antitumor immunity: New approaches to cancer immunotherapy. J Immunol. 2007;178:5595–5605. doi: 10.4049/jimmunol.178.9.5595. [DOI] [PubMed] [Google Scholar]
- 35.Kretz-Rommel A, et al. Blockade of CD200 in the presence or absence of antibody effector function: Implications for anti-CD200 therapy. J Immunol. 2008;180:699–705. doi: 10.4049/jimmunol.180.2.699. [DOI] [PubMed] [Google Scholar]
- 36.Bertucci F, et al. Gene expression profiling shows medullary breast cancer is a subgroup of basal breast cancers. Cancer Res. 2006;66:4636–4644. doi: 10.1158/0008-5472.CAN-06-0031. [DOI] [PubMed] [Google Scholar]
- 37.Sabatier R, et al. A gene expression signature identifies two prognostic subgroups of basal breast cancer. Breast Cancer Res Treat. 2011;126:407–420. doi: 10.1007/s10549-010-0897-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.