Abstract
Purpose of review
HDL is a cardioprotective lipoprotein, at least in part, because of its ability to mediate reverse cholesterol transport (RCT). It is becoming increasingly clear that the antiatherogenic effects of HDL are not only dependent on its concentration in circulating blood but also on its biological ‘quality’. This review summarizes our current understanding of how the biological activities of individual subclasses of HDL particles contribute to overall HDL performance in RCT.
Recent findings
Recent work indicates that apolipoprotein A-I-containing nascent HDL particles are heterogeneous and that such particles exert different effects on the RCT pathway. RCT from macrophages has been examined in detail in mice and the roles of plasma factors (lecithin-cholesterol acyltransferase, cholesterol ester transfer protein, phospholipid transfer protein) and cell factors (ATP-binding cassette transporter A1, ATP-binding cassette transporter G1, scavenger receptor class B type 1) have been evaluated. Manipulation of such factors has consistent effects on RCT and atherosclerosis, but the level of plasma HDL does not reliably predict the degree of RCT. Furthermore, HDL cholesterol or apolipoprotein A-I levels do not necessarily correlate with the magnitude of cholesterol efflux from macrophages; more understanding of the contributions of specific HDL subspecies is required.
Summary
The antiatherogenic quality of HDL is defined by the functionality of HDL subspecies. In the case of RCT, the rate of cholesterol movement through the pathway is critical and the contributions of particular types of HDL particles to this process are becoming better defined.
Keywords: apolipoprotein A-I, atherosclerosis, cholesterol efflux, HDL, reverse cholesterol transport
Introduction
Epidemiological evidence indicates that HDL particles serve an antiatherogenic function in that high levels of HDL cholesterol (HDL-C) are associated with a decreased risk of cardiovascular disease [1]. Consistent with this antiatherogenic function, acute elevation of HDL-C by infusion of synthetic HDL particles can induce regression of atherosclerotic plaque in patients [2]. An important way that HDL achieves these effects is by promoting the reverse cholesterol transport (RCT) pathway, which involves the transport of excess cholesterol from peripheral tissues (including macrophages) back to the liver for eventual excretion from the body [3–5]. The ability of HDL to remove cholesterol and oxysterols from cells such as macrophages in the arterial wall is linked to the anti-inflammatory and immunosuppressive functions of this lipoprotein [6,7••]. These capabilities coupled with the antioxidant, antithrombotic and vasodilatory capabilities of HDL [8•] give rise to the overall cardioprotective behavior of HDL. It is becoming increasingly apparent that these antiatherogenic effects of HDL are not only dependent on its concentration in circulating blood but also on its biological quality [2,4,9]. This review considers the recent progress that is being made in understanding the biological activities of different HDL subspecies and how these activities contribute to the antiatherogenic performance of the mixture of heterogeneous HDL particles that comprise plasma HDL-C and mediate RCT.
HDL heterogeneity
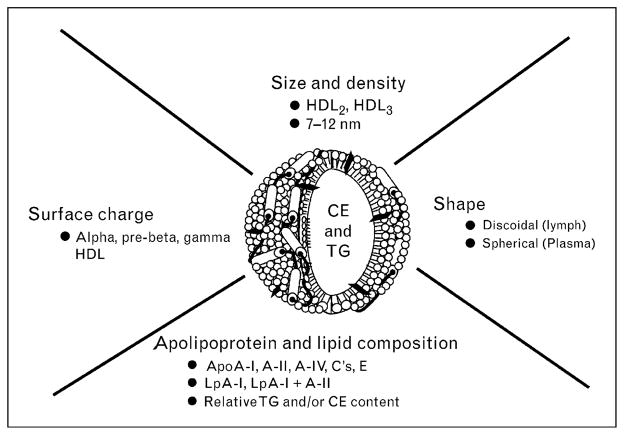
Traditionally, HDL isolated by ultracentrifugation is defined as the lipoprotein with density in the range 1.063–1.21 g/ml. However, HDL constitutes a heterogeneous group of particles differing in density, size, electrophoretic mobility, lipid composition and apolipoprotein content [10] (Fig. 1). Therefore, HDL can be fractionated into discrete subclasses by different techniques according to their physicochemical properties. Human HDL can be separated by ultracentrifugation into two main subfractions on the basis of density, HDL2 (1.063–1.125 g/ml) and HDL3 (1.125–1.21 g/ml), which is relatively protein-rich [11]. HDL2 and HDL3 can be further divided on gradient gel electrophoresis into HDL2b (10.6 nm), HDL2a (9.2 nm), HDL3a (8.4 nm), HDL3b (8.0 nm) and HDL3c (7.6 nm) in decreasing order of particle diameter [12]. In addition, HDL can be separated into two main subpopulations on the basis of electrophoretic mobility: the major subfraction has a relatively high negative surface charge density and is called α-HDL, whereas the other fraction is called preβ-HDL [13,14]. Most of the HDL particles in plasma are α-HDL [15] and preβ-HDL represents only approximately 5% of total apolipoprotein A-I (apoA-I) [3]. Choline-containing phospholipids (phosphatidylcholine, lysolecithin, sphingomyelin) represent 90–95% of the phospholipids in HDL [16]. The calculated lipid composition is 137 phospholipids, 50 free (unesterified) cholesterol, 90 cholesterol ester and 19 triacylglycerol molecules in an HDL2 particle, and 51 phospholipids, 13 free cholesterol, 32 cholesterol ester and nine triacylglycerol molecules in an HDL3 particle [17]. Preβ-HDL contains mainly apoA-I and phospholipids with small amounts of cholesterol [3,18]. Preβ-HDL has been resolved into preβ1, preβ2 and preβ3 HDL particles according to increasing size by two-dimensional gel electrophoresis [14]. HDL can also be separated on the basis of apolipoprotein composition [19] into several subpopulations using immunoaffinity methods [20]: two major apoA-I-containing HDL particles exist, that is, LpA-I+A-II, which includes both apoA-I and apoA-II (molar ratio ~2/1), and LpA-I, which contains only apoA-I. About 65% of the total recovered weight of apoA-I in human plasma is found in LpA-I+A-II and approximately 25% is found in LpA-I. The majority of LpA-I has the same density and size as HDL2, whereas the majority of LpA-I +A-II floats with HDL3 [20]. Table 1 summarizes the main characteristics of the major human HDL subclasses, including preβ-HDL, HDL2 and HDL3.
Figure 1. Factors contributing to the structural heterogeneity of HDL particles.
Different subclasses of the depicted spherical HDL particle can be isolated on the basis of differences in particle diameter and density (lipid/protein ratio). Variations in the surface charge lead to subclasses that can be separated due to differences in electrophoretic mobility. Differences in apolipoprotein composition permit isolation of HDL particles containing either apoA-I alone (LpA-I) or apoA-I along with apoA-II (LpA-I + A-II) by immunoaffinity methods. HDL particles can also be distinguished by their shape: spherical plasma HDL particles contain a neutral lipid core, whereas the discoidal HDL particles that occur in lymph do not. See text for further details. CE, cholesterol ester; TG, triacylglycerol.
Table 1.
Characteristics of human HDL subclasses
| Property | Preβ-HDL | HDL2 | HDL3 |
|---|---|---|---|
| Molecular weight | Preβ 1: 71 000 | 360 000 | 175 000 |
| Preβ 2: 325 000 | |||
| Electrophoretic mobility | Preβ | α | α |
| Density (g/ml) | 1.210 | 1.063–1.125 | 1.125–1.210 |
| Subpopulations | Preβ 1, Preβ 2, Preβ 3 | HDL2b, HDL2a | HDL3a, HDL3b, HDL3c |
| Diameter (nm) | 8.8–12.9 | 7.2–8.8 | |
| Preβ 1: 5.4–7 | HDL2b: 9.7–12.9 | HDL3a: 8.2–8.8 | |
| Preβ 2: 12–14 | HDL2a: 8.8–9.7 | HDL3b: 7.8–8.2 | |
| HDL3c: 7.2–7.8 |
Remodeling by plasma factors is a critical part of HDL metabolism and underlies the dynamic nature of HDL particles. HDL is a substrate for the plasma factors listed in Table 2 and the lipolytic and lipid transfer activities of these factors cause the various subpopulations of HDL particles that exist in human plasma [10,21] to continually interconvert. As shown in Fig. 2 in section ‘reverse cholesterol transport pathway’ below, the changes in HDL particle shape and size caused by these plasma factors are also central to the participation of HDL in the RCT pathway. The key function of lecithin-cholesterol acyltransferase (LCAT) is to form cholesterol ester and, at a constant apoA-I concentration, larger discoidal HDL particles are the preferred substrate [22]. The remaining enzymes listed in Table 2, hepatic lipase and endothelial lipase, are involved in releasing fatty acids from phospholipids and triacylglycerol. The lipid transfer proteins, cholesterol ester transfer protein (CETP) and phospholipid transfer protein (PLTP) are involved in moving cholesterol ester, triacylglycerol and phospholipid molecules among HDL and other lipoprotein particles [23•,24•]. As shown in Table 2, the interconversion of HDL particles is frequently accompanied by the release of apoA-I molecules into the aqueous phase; rearrangements of HDL particles that are accompanied by a decrease in net surface area lead to desorption of apoA-I molecules and vice-versa. Such cycling of apoA-I molecules between HDL particles and the aqueous phase is critical for HDL metabolism [18,25].
Table 2.
Plasma factors involved in remodeling of HDL
| HDL conversion | Plasma factors |
|---|---|
| Disc → sphere | LCAT |
| Large sphere → small sphere + free apoA-I | CETP and hepatic lipase |
| Large sphere → small sphere | Endothelial lipase |
| Sphere → larger and smaller spheres + free apoA-I | PLTP |
CETP, cholesteryl ester transfer protein; LCAT, lecithin-cholesterol acyltransferase; PLTP, phospholipid transfer protein.
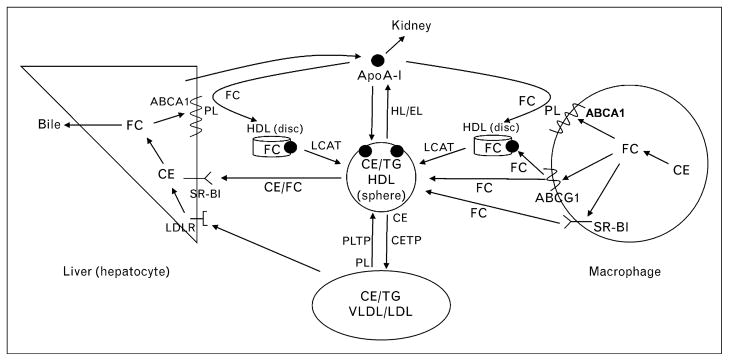
Figure 2. Schematic overview of the major pathways involved in HDL-mediated macrophage cholesterol efflux and reverse cholesterol transport to the liver.
ApoA-I is produced by the liver and acquires free cholesterol and phospholipid from liver and peripheral cells (including macrophages) via the ATP-binding cassette transporter A1 (ABCA1) to form nascent (discoidal) HDL particles. Nonlipidated apoA-I is cleared by the kidney. Free cholesterol efflux from macrophages to HDL particles is also promoted by the ABCG1 transporter and scavenger receptor class B type 1 (SR-BI). As discussed in section ‘HDL heterogeneity’, the free cholesterol in discoidal HDL particles is converted to cholesteryl ester by lecithin-cholesterol acyltransferase (LCAT) activity leading to the formation of mature, spherical HDL particles. Phospholipid transfer protein (PLTP) mediates transfer of phospholipids from VLDL into HDL, thereby providing phospholipids for the LCAT reaction. Mature HDL particles can be remodeled to smaller particles with the release of apoA-I by the actions of hepatic lipase and endothelial lipase, which hydrolyze HDL triglycerides and phospholipids, respectively (Table 2). In humans, but not rodents, HDL cholesterol ester can be transferred to the VLDL/LDL pool by cholesteryl ester transfer protein (CETP) and taken up by endocytosis into hepatocytes via interaction with the LDL receptor (LDLR). HDL cholesterol ester and free cholesterol are also transferred directly to hepatocytes via SR-BI-mediated selective uptake. Cholesterol taken up by the liver can be recycled back into the ABCA1 pathway, secreted into bile as either free cholesterol or bile acids, or assembled into lipoprotein particles that are secreted back into the circulation (not shown). CE, cholesterol ester; FC, free cholesterol; TG, triacylglycerol.
The pool of recycling lipid-free (poor) apoA-I, together with newly synthesized apoA-I secreted by hepatocytes and enterocytes, is involved in the biogenesis of nascent HDL particles by interaction with ATP-binding cassette transporter A1 (ABCA1) [26,27]. Studies over the last several years have shown that multiple sizes of nascent HDL particles (diameters in the range 7–20 nm) are created concurrently when apoA-I is incubated with ABCA1-expressing cells [28–31]. Work in our laboratory has revealed that the predominant HDL species formed are approximately 9 and 12 nm discoidal particles that contain either two or three apoA-I molecules [30]. These particles are also heterogeneous with respect to phospholipid composition and cholesterol content. ApoA-I is the only protein constituent of these nascent HDL particles but, interestingly, if apoM is also expressed by the cells, the particle size increases. ApoM may function intracellularly to transfer lipid onto the HDL particles during or after their formation by ABCA1 [32•]. Depending upon cell type, α-HDL and/or preβ-HDL particles can be formed by the apoA-I/ABCA1 reaction [29]. Besides discoidal particles, preβ1-HDL (or lipid-poor apoA-I) containing a single apoA-I molecule, three to four phospholipid molecules and one to two cholesterol molecules can be formed [33]. Preβ1-HDL is both a product and a substrate in ABCA1-mediated efflux of cellular phospholipids and cholesterol to apoA-I. Lipid-poor apoA-I participates similarly to lipid-free apoA-I in the ABCA1 reaction, but increasing lipidation reduces the ability of apoA-I to react with ABCA1 [31]. Overall, studies of HDL biogenesis indicate that a heterogeneous population of particles is created; subsequent remodeling by plasma factors refines this mixture (which also includes surface remnants created by the action of lipoprotein lipase on triacylglycerol-rich lipoproteins [34]) while preserving the heterogeneity.
The functionality of HDL is impaired in humans with chronic inflammatory diseases, including atherosclerosis [35], and these deleterious effects can involve oxidative modification of apoA-I [36•]. The HDL dysfunction involves reductions in both anti-inflammatory and cholesterol transport properties. Small dense HDL3 particles seem to be particularly sensitive to such oxidative damage and loss of antiatherogenic function [37]. The presence of oxidatively modified HDL particles increases the overall heterogeneity of the HDL pool.
Reverse cholesterol transport pathway
It is clear from the RCT pathway summarized in Fig. 2 that apoA-I is involved in all stages, including the formation of nascent HDL particles, HDL remodeling by LCAT and delivery of HDL cholesterol to the liver via scavenger receptor class B type 1 (SR-BI). ApoA-I exists in several physical states in that it is associated with different HDL subspecies. These various HDL particles interact differently with key proteins in the pathway; these proteins include the cell surface receptors ABCA1, ABCG1 and SR-BI that mediate cholesterol transport between HDL and the cell, as well as LCAT in the plasma that esterifies HDL-associated cholesterol.
The first step in the macrophage RCT process involves the efflux of cholesterol to HDL. The four pathways contributing to this effect are summarized in Table 3 (for reviews, see [3,38–40]). The aqueous diffusion pathway is largely responsible for cholesterol efflux to human serum from mouse peritoneal macrophages containing normal levels of cholesterol; in addition, there are minor contributions from ABCA1 and SR-BI [41]. Cholesterol loading of such cells approximately doubles the fractional cholesterol efflux due to a large increase in the ABCA1 contribution and a smaller increase in the ABCG1 contribution. The situation is different with cholesterol-loaded human macrophages in that ABCG1 activity does not contribute to cholesterol efflux to HDL; as with mouse macrophages, ABCA1 plays a major role in mediating cholesterol efflux [42••].
Table 3.
Pathways for cholesterol efflux from cells to plasma
| Efflux pathway | Energetics | Preferred HDL acceptor | Acceptor binding |
|---|---|---|---|
| Aqueous diffusion | Passive | HDL2 ~ HDL3 (LpA-I ~ LpA-I + A-II) | No |
| SR-BI | Passive | HDL2 > HDL3 | Yes |
| ABCG1 | Active | HDL3 = HDL3 | No |
| ABCA1 | Active | Preβ1-HDL [lipid-free (poor) apoA-I] | Yes |
ABCA1, ATP-binding cassette transporter A1; SR-BI, scavenger receptor class B type 1.
Much has been learned about the molecular mechanisms underlying the four cellular cholesterol efflux pathways listed in Table 3. The aqueous diffusion pathway mediates the bidirectional flux of cholesterol between the cell plasma membrane and HDL in the extracellular medium; the direction of net cholesterol mass transport is determined by the cholesterol concentration gradient as reflected by the free cholesterol/phospholipids ratios in the donor and acceptor particles [38]. The rate-limiting step in efflux from cells is normally the desorption of cholesterol molecules from the cell plasma membrane. The rate of this step is influenced by interactions of free cholesterol molecules with neighboring phospholipid molecules [38,43]; the rate is increased by higher phospholipid unsaturation and decreased by a higher membrane sphingomyelin content [44,45]. The various HDL subclasses are equally effective acceptors in this pathway because the efflux is not affected significantly by alterations in HDL particle size [46]. The diffusion of cholesterol molecules between the cell plasma membrane and HDL particles can be facilitated by SR-BI. Thus, binding of HDL to SR-BI promotes cholesterol efflux [47], apparently by forming a complex [48,49] that contains a hydrophobic channel along which cholesterol molecules can diffuse [50]. Because larger HDL particles bind better to SR-BI [51], such particles are more effective in supporting cholesterol efflux via this receptor [49]. ABCG1 is a member of the ABC family of membrane transporters and is involved in controlling intracellular cholesterol homeostasis [52•]. Expression of ABCG1 enhances efflux of cholesterol to HDL [53] by increasing the pool of plasma membrane free cholesterol and reorganizing it so that it can desorb more readily into the extracellular medium [54•]. Larger HDL2 and smaller HDL3 particles are equally effective acceptors, as expected for the aqueous diffusion pathway. In contrast, the acceptor for cellular cholesterol efflux via the ABCA1 pathway is preβ1-HDL [lipid-free (poor) apoA-I] (Table 3). In this case, there is simultaneous efflux of free cholesterol and phospholipids [55] due to microsolubilization of plasma membrane lipids [56–57] and creation of discoidal nascent HDL particles (c.f. section ‘HDL heterogeneity’). Pools of intracellular cholesterol are also mobilized by the activity of ABCA1 [58,59]. The concentration of active ABCA1 in the cell plasma membrane determines the rate of lipid efflux and nascent HDL particle formation. Strikingly, increased membrane phospholipid unsaturation inhibits ABCA1-mediated cholesterol efflux [60], which, as mentioned above, is opposite to the effects of increased phospholipid unsaturation on efflux via the aqueous diffusion mechanism. Binding of apoA-I to ABCA1 prevents its intracellular degradation and increases the level of transporter in the plasma membrane [61]. Importantly, it has been shown recently that ABCA1 is palmitoylated and that this post-translational modification localizes it to the plasma membrane and regulates its lipid efflux ability [62••]. In addition to the nascent HDL particles, such cholesterol efflux can involve the release of microparticles [30,63••], a fact that is often overlooked in analyzing and interpreting the results of apoA-I-mediated efflux via ABCA1. The discoidal nascent HDL particles created by the apoA-I/ABCA1 reaction are effective acceptors of cellular cholesterol effluxed by the ABCG1 pathway [64,65].
The cholesterol incorporated into nascent HDL particles is transported through the plasma compartment by the various HDL remodeling steps summarized in section ‘HDL heterogeneity’ and Fig. 2. Eventually, the free cholesterol and cholesterol ester in HDL are delivered to the liver. Hepatocytes express SR-BI, which mediates the selective uptake of the HDL cholesterol [21]. This two-step process involves binding of HDL particle to the receptor, followed by diffusion of cholesterol ester and free cholesterol molecules into the cell plasma membrane. Large diameter (10 nm) HDL particles bind better to SR-BI and deliver more cholesterol ester than small (8 nm) HDL particles [49,51]. The presence of apoA-II in an HDL particle modulates both binding to SR-BI and selective uptake of cholesterol [66]. SR-BI protein is situated in both the basolateral and canalicular membranes of hepatocytes [67•]; in the former location, it promotes cholesterol influx and in the latter, it promotes cholesterol efflux. The rate of biliary cholesterol section, a key step in the RCT pathway (Fig. 2), is related to the hepatic expression level of SR-BI [68•].
In-vivo measurement of reverse cholesterol transport
There is great interest in understanding the ability of HDL to mediate RCT from macrophages because this ability underlies the antiatherogenic functions of the lipoprotein. One of the first attempts to examine RCT in intact animals was published in 1999 by Stein et al. [69] who introduced cationized LDL containing labeled cholesterol into the thigh muscle of mice and rats and then followed the disappearance of the labeled cholesterol from this depot. In these studies, the cholesterol was not initially present in macrophages and no attempt was made to examine the individual components of the RCT pathway. In an effort to study RCT as an integrated system, an experimental protocol that measures the flux of labeled cholesterol from macrophages in the peritoneal cavity of mice to the plasma, liver and feces has been developed [70]. In this system murine macrophages, either established cell lines or primary peritoneal or bone marrow, are enriched with radiolabeled cholesterol and injected into recipient mice. At timed intervals, plasma is taken to measure the appearance of labeled cholesterol and at the termination of the study, generally 48 h following injection of the macrophages, the mice are sacrificed and the amount of macrophage-derived labeled cholesterol is measured in the plasma, liver and feces. This protocol, which has been used in a number of studies, has several advantages: it measures the movement of cholesterol out of the macrophages, which is the first step in RCT (Fig. 2), and traces its fate to the last step in RCT, the excretion into the feces; the recipient mice can be genetically manipulated so that the individual steps in RCT can be either enhanced or eliminated so that the contribution of each step can be individually assessed; and the recipient mice can be treated with drugs or diets that may impact on RCT. The basic protocol of introducing macrophages containing labeled cholesterol into the peritoneal cavity of mice has been widely adapted by numerous investigators. A modification of the original protocol has been introduced [71]; this protocol introduces the macrophages subcutaneously into mice and uses two labeled sterols (cholesterol and sitostanol) to permit correction for animal-to-animal variation in biliary cholesterol absorption in the intestine.
Among the numerous studies using the integrated mouse RCT protocol are those that examined the contribution of individual steps in RCT (Fig. 2). The earliest experiment using the macrophage to feces protocol investigated RCT in mice overexpressing human apoA-I [70]. This study validated the experimental protocol and demonstrated greater movement of labeled cholesterol from macrophages to a number of tissues in the mice overexpressing apoA-I. A comparison of RCT in mice expressing either wild-type human apoA-I or apoA-IMilano showed no difference, suggesting that the beneficial effects of apoA-IMilano apparently are not related to more efficient RCT [72•]. Human apoA-II can also maintain effective RCT from macrophages to feces [73].
The role of LCAT in RCT was studied using human apoA-I transgenic mice overexpressing human LCAT [74••]. As both overexpression and reduction in LCAT had only a modest effect on RCT, the results suggest that LCAT may not have a major impact on total macrophage efflux, and RCT may not be as dependent on LCAT as previously thought. This conclusion is consistent with studies demonstrating that in patients with partial or full genetic deficiencies in LCAT, there is no enhanced preclinical atherosclerosis [75]. The studies of LCAT and RCT provide an example of how different HDL particles can differentially mediate both in-vitro cell cholesterol efflux and in-vivo RCT [74••]. Overexpression of LCAT increased HDL-C, but this elevation in HDL-C did not increase RCT from macrophages to feces. Interestingly, the plasma from these mice had a reduced ability to promote cell cholesterol efflux ex vivo via ABCA1. An explanation for this observation is that increased esterification by LCAT resulted in an increase in the production of mature HDL particles and a reduction in lipid-poor preβ-HDL. When homozygous or heterozygous LCAT-null mice were used as macrophage recipients, there was a 45% reduction in labeled cholesterol in the feces of homozygous mice and no difference in RCT between wild-type and heterozygous mice. When the sera from these mice were tested for cholesterol efflux efficiency using J774 cells, total cell efflux was similar to that obtained with wild-type sera, but these sera were more efficient in promoting cellular efflux via the ABCA1 pathway [74••]. This result can be attributed to the greater level of preβ-HDL present in the sera from LCAT-deficient mice. Thus, the level of LCAT activity determines the array of HDL particles present in the plasma, and this in turn influences the efficiency of the various efflux pathways in the removal of cell cholesterol (c.f. Table 3).
The in-vivo mouse RCT protocol has been used in a limited number of studies designed to probe the role played by two important plasma transfer proteins (c.f. Table 2), CETP [76] and PLTP [77] on RCT. The macrophage to feces pathway was quantitated by injection of cholesterol-labeled and cholesterol-enriched J774 cells into mice stably expressing human CETP in their liver. From this study, which used a number of different genetically manipulated mouse models, it was concluded that liver expression of CETP promotes macrophage RCT and that this effect is dependent on the LDL receptor [76]. Recent studies focusing on the role of PLTP in RCT illustrate how the mouse RCT assay can be used to study the effect of systemic or macrophage-specific expression of a protein [77]. Thus, hepatic overexpression of PLTP in either wild-type or apoA-I transgenic mice impaired RCT, but when wild-type mice were injected with macrophages obtained from PLTP transgenic mice, there was no impact on RCT.
In many of the studies described above, the primary manipulation of the RCT pathway was in components of the pathway that were present in the plasma of the recipient mice. Thus, levels of apoA-I, LCAT, CETP and PLTP varied and the effects of the changes in these proteins on RCT were quantitated. In addition to this approach, the macrophage to feces RCT protocol provides a system that can determine the contribution of various cell-related factors to RCT. One of the best examples of this is a study in which macrophages harvested from mice deficient in either ABCA1, SR-BI or ABCG1 were injected into recipient mice [78]. Using this approach, the relative contributions of the various efflux pathways could be determined, and it was found that elimination of either ABCA1 or ABCG1 reduced RCT and knockdown of both of these proteins produced an additive reduction in RCT. In contrast, RCT in mice receiving macrophages lacking SR-BI was similar to that observed with mice receiving wild-type macrophages [78]. The importance of ABCA1 in promoting RCT has been documented in other investigations using the in-vivo mouse RCT protocol [73,79,80]. Thus, mouse RCT results illustrated the importance of both ABCA1 and ABCG1 in RCT and the lack of a contribution of SR-BI. The relative importance of these proteins in mediating efflux of cholesterol from mouse macrophages in vitro (section ‘reverse cholesterol transport pathway’) is consistent with their contributions to RCT, as estimated from the in-vivo mouse RCT system [78].
In addition to the studies described above, determination of the rate of macrophage RCT in mice has been used to quantitate the effects on RCT of inflammation [81•], dietary manipulations [82] and pharmacologic interventions [83,84]. The numerous genetic interventions that have been investigated using the mouse RCT protocol have resulted in effects on RCT that are largely consistent with the effects of the same interventions on atherosclerosis (Table 4) [85•]. The results in Table 4 show that macrophage RCT mediated by HDL has important implications for the development of atherosclerosis, but the HDL contribution does not correlate well with the HDL-C levels. For instance, the increase in HDL-C due to elimination of SR-BI is associated with decreased RCT and increased atherosclerosis. These results indicate that the rate of macrophage RCT and the quality rather than the quantity of HDL are important in determining the antiatherogenic effects of HDL. It should be noted that though it is generally agreed that ABCA1 plays an important role in preβ1-HDL-mediated RCT from macrophages, this transporter is not involved in the centripetal movement of cholesterol from peripheral parenchymal cells to the liver and intestine, at least not in mice on a low-cholesterol diet [86••].
Table 4.
Relationship between plasma HDL-cholesterol, macrophage reverse cholesterol transport and atherosclerosis in response to genetic manipulation in mice
| Genetic manipulation | Tissue | Effect on plasma HDL-C | Effect on macrophage RCT | Effect on atherosclerosis |
|---|---|---|---|---|
| ApoA-I overexpression | Liver | Increase | Increase | Decrease |
| ApoA-I knockout | Whole body | Decrease | Decrease | Increase |
| SR-BI overexpression | Liver | Decrease | Increase | Decrease |
| SR-BI knockout | Whole body | Increase | Decrease | Increase |
| CETP expression | Liver | Decrease | Increase | Decrease |
| LCAT overexpression | Liver | Increase | Decrease | Increase |
| LCAT + CETP expression | Liver | Variable | Neutral | Neutral |
| LCAT knockout | Whole body | Decrease | Neutral | Variable |
| ABCA1 knockout | Macrophage | No change | Decrease | Increase |
| ABCG1 knockout | Macrophage | No change | Decrease | Decrease |
| ABCA1 + ABCG1 knockout | Macrophage | No change | Decrease | Increase |
| SR-BI knockout | Macrophage | No change | Neutral | Variable |
ABCA1, ATP-binding cassette transporter A1; CETP, cholesteryl ester transfer protein; HDL-C, HDL-cholesterol; LCAT, lecithin-cholesterol acyltransferase; RCT, reverse cholesterol transport; SR-BI, scavenger receptor class B type 1. Table adapted from [85•].
Serum efflux potential
The first step in RCT is cholesterol efflux from macrophages (Fig. 2), and to aid in understanding how these HDL parameters modulate RCT, attention has focused on defining how HDL composition and subclass distribution affect this efflux process. When investigating a limited number of HDL particles that differ in composition and size, comparisons of cellular cholesterol efflux efficiency and efflux pathways can be made by directly isolating and adding the purified particles to the culture media. However, as understanding of the complexity of HDL subfractions has developed, it is very difficult to isolate enough of all of the different particles that are in serum, because of the large number and low concentrations of many of the HDL subfractions. As a consequence of this limitation, determining the efflux efficiency of HDL subfractions has been investigated by exposing cells having different efflux pathways to the total HDL fraction from a number of serum specimens and then establishing the relative efflux efficiencies of each type of HDL particle by determining the correlations between the HDL subfractions and efflux via the different efflux pathways [87]. Using this approach, two HDL subspecies correlated significantly with ABCA1-mediated efflux from macrophages to human serum, namely, small, lipid-poor preβ1-HDL particles and intermediate-sized α2-HDL particles. In contrast to the limited number of HDL particles that were associated with ABCA1 efflux, SR-BI efflux correlated with a large number of HDL subfractions, all of which were particles that contained significant levels of phospholipids [87]. Efflux of cell cholesterol via the ABCG1 pathway exhibited an HDL subfraction efflux pattern generally similar to that of SR-BI [54•].
The efficiency of any serum specimen or HDL preparation in removing cholesterol from cells depends on both the HDL subfraction distribution and the level of expression of the different efflux pathways (Table 3) operational in the specific cell being studied. The selection of cholesterol donor cell is important and macrophages, either established cell lines or primary cells, are particularly useful. Macrophages express, to varying degrees, all of the cholesterol efflux pathways (section ‘reverse cholesterol transport pathway’), but there is great heterogeneity in the macrophage populations in tissues [88], and different pathways are expressed depending upon the origin of the cells [89••] and how the cells were derived [90]. Because of the differences in contribution of different pathways, a prediction of the efflux efficiency of serum or isolated HDL cannot be made based simply on the level of HDL-C or apoA-I. This is exemplified by a recent study of de La Liera-Moya et al. [91••] who measured efflux from cAMP-treated J774 macrophages to the total HDL fraction isolated from a number of normolipemic human serum specimens. In this study, there were a number of serum samples that contained similar concentrations of either HDL-C or apoA-I but promoted significantly different levels of cell cholesterol efflux. Further analysis demonstrated that the differences in efflux efficiency observed with sera having similar HDL-C or apoA-I levels were a reflection of elevated efflux via the ABCA1 pathway, which in turn was associated with differences in the level of preβ1-HDL in the serum. Thus, in this experimental system, total apoA-I was similar, but sera having a greater percentage of the apoA-I as preβ1-HDL enhanced efflux from cells expressing high levels of ABCA1 [91••]. These findings provide a striking demonstration of how HDL quality rather than quantity governs the functionality.
Conclusion
The antiatherogenic quality of HDL is not simply defined by the plasma HDL-C level but rather by the functionality of HDL subspecies. In the case of RCT, the rate of cholesterol movement through the pathway is critical [85•] and the contributions of particular types of HDL particles to this are becoming better defined, especially in terms of interactions with cell-surface cholesterol transporters. The ability of HDL to transport cholesterol provides additional indirect cardioprotective benefits; for instance, ABCA1-mediated efflux of cholesterol from arterial macrophages to apoA-I suppresses inflammation [92•,93•]. The fact that intimal arterial smooth muscle foam cells have impaired ABCA1 activity [94••] further underscores the critical role played by this transporter in preventing atherosclerosis. The tissue-specific contributions of ABCA1 to atherosclerosis susceptibility remain controversial; hepatic ABCA1 clearly plays an antiatherogenic role but, depending upon the particular animal model, expression of ABCA1 in macrophages may or may not be antiatherogenic [95••]. The successful development of HDL-based therapies will benefit from the increasing knowledge of the functionalities of HDL subspecies, as well as a means for reliably measuring RCT in humans [96•].
Acknowledgments
The authors’ work described here was supported by National Institutes of Health grant PPG HL 22633.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 271–272).
- 1.Castelli WP. Epidemiology of coronary heart disease: the Framingham study. Am J Med. 1984;76:4–12. doi: 10.1016/0002-9343(84)90952-5. [DOI] [PubMed] [Google Scholar]
- 2.Nicholls SJ, Nissen SE. New targets of high-density lipoprotein therapy. Curr Opin Lipidol. 2007;18:421–426. doi: 10.1097/MOL.0b013e32821f603b. [DOI] [PubMed] [Google Scholar]
- 3.Fielding CJ, Fielding PE. Molecular physiology of reverse cholesterol transport. J Lipid Res. 1995;36:211–228. [PubMed] [Google Scholar]
- 4.von Eckardstein A, Nofer J-R, Assmann G. High density lipoproteins and apolipoproteins. Role of cholesterol efflux and reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 2001;21:13–27. doi: 10.1161/01.atv.21.1.13. [DOI] [PubMed] [Google Scholar]
- 5.Cuchel M, Rader DJ. Macrophage reverse cholesterol transport. Key to the regression of atherosclerosis? Circulation. 2006;113:2548–2555. doi: 10.1161/CIRCULATIONAHA.104.475715. [DOI] [PubMed] [Google Scholar]
- 6.Tall AR. Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins. J Internal Med. 2008;263:256–273. doi: 10.1111/j.1365-2796.2007.01898.x. [DOI] [PubMed] [Google Scholar]
- 7••.Yvan-Charvet L, Wang N, Tall AR. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler Thromb Vasc Biol. 2010;30:139–143. doi: 10.1161/ATVBAHA.108.179283. This paper summarizes the evidence showing that the sterol efflux activities of ABCA1 and ABCG1 modulate macrophage expression of inflammatory cytokines and chemokines. Thus, the roles of HDL and ABC transporters in cholesterol efflux and RCT are mechanistically linked to anti-inflammatory and immunosuppressive functions of HDL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8•.Tabet F, Rye K-A. High-density lipoproteins, inflammation and oxidative stress. Clin Sci. 2009;116:87–98. doi: 10.1042/CS20080106. A comprehensive review of the origins of HDL and its anti-inflammatory and antioxidant properties. [DOI] [PubMed] [Google Scholar]
- 9.Movva R, Rader DJ. Laboratory assessment of HDL heterogeneity and function. Clin Chem. 2008;54:788–800. doi: 10.1373/clinchem.2007.101923. [DOI] [PubMed] [Google Scholar]
- 10.Lund-Katz S, Liu L, Thuahnai ST, Phillips MC. High density lipoprotein structure. Front Biosci. 2003;8:d1044–d1054. doi: 10.2741/1077. [DOI] [PubMed] [Google Scholar]
- 11.Havel RJ, Eder HA, Bragdon JH. The distribution and chemical composition of untracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955;34:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nichols AV, Krauss RM, Musliner TA. Nondenaturing polyacrylamide gradient gel electrophoresis. Methods Enzymol. 1986;128:417–431. doi: 10.1016/0076-6879(86)28084-2. [DOI] [PubMed] [Google Scholar]
- 13.Kunitake ST, La Sala KJ, Kane JP. Apolipoprotein A-I-containing lipoproteins with prebeta electrophoretic mobility. J Lipid Res. 1985;26:549–555. [PubMed] [Google Scholar]
- 14.Castro GR, Fielding CJ. Early incorporation of cell-derived cholesterol into prebeta-migrating high-density lipoprotein. Biochemistry. 1988;27:25–29. doi: 10.1021/bi00401a005. [DOI] [PubMed] [Google Scholar]
- 15.Skipski VP. Lipid composition of lipoproteins in normal and diseased states. In: Nelson GJ, editor. Blood lipids and lipoproteins: quantitation composition, and metabolism. New York: Wiley-Interscience; 1972. pp. 471–583. [Google Scholar]
- 16.Jonas A, Phillips MC. Lipoprotein structure. In: Vance DE, Vance JE, editors. Biochemistry of lipids, lipoproteins and membranes. Elsevier; 2008. pp. 485–506. [Google Scholar]
- 17.Shen BW, Scanu AM, Kezdy FJ. Structure of human serum lipoproteins inferred from compositional analysis. Proc Natl Acad Sci U S A. 1977;74:837–841. doi: 10.1073/pnas.74.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rye KA, Barter PJ. Formation and metabolism of prebeta-migrating, lipid-poor apolipoprotein A-I. Arterioscler Thromb Vasc Biol. 2004;24:421–428. doi: 10.1161/01.ATV.0000104029.74961.f5. [DOI] [PubMed] [Google Scholar]
- 19.Scanu AM, Edelstein C. HDL: bridging past and present with a look at the future. FASEB J. 2008;22:4044–4054. doi: 10.1096/fj.08-117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheung MC, Albers JJ. Characterization of lipoprotein particles isolated by immunoaffinity chromatography. J Biol Chem. 1984;259:12201–12209. [PubMed] [Google Scholar]
- 21.Zannis VI, Chroni A, Krieger M. Role of apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL. J Mol Med. 2006;84:276–294. doi: 10.1007/s00109-005-0030-4. [DOI] [PubMed] [Google Scholar]
- 22.Cavigiolio G, Shao B, Geier EG, et al. The interplay between size, morphology, stability, and functionality of high-density lipoprotein subclasses. Biochemistry. 2008;47:4770–4779. doi: 10.1021/bi7023354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.Masson D, Jiang XC, Lagrost L, Tall AR. The role of plasma lipid transfer proteins in lipoprotein metabolism and atherogenesis. J Lipid Res. 2009;50:S201–S206. doi: 10.1194/jlr.R800061-JLR200. The central role of CETP and PLTP in regulating HDL levels is reviewed, and the potential therapeutic benefits of CETP inhibition are discussed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24•.Rye KA, Bursill CA, Lambert G, et al. The metabolism and antiatherogenic properties of HDL. J Lipid Res. 2009;50:S195–S200. doi: 10.1194/jlr.R800034-JLR200. This review focuses on the consequences of HDL heterogeneity and points out the need to assess HDL subpopulation distribution and functionality when considering interventions that raise HDL levels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pownall HJ, Ehnholm C. The unique role apolipoprotein A-I in HDL remodeling and metabolism. Curr Opin Lipidol. 2006;17:209–213. doi: 10.1097/01.mol.0000226110.66942.e8. [DOI] [PubMed] [Google Scholar]
- 26.Lee JY, Parks JS. ATP-binding cassette transporter AI and its role in HDL formation. Curr Opin Lipidol. 2005;16:19–25. doi: 10.1097/00041433-200502000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Yokoyama S. Assembly of high-density lipoprotein. Arterioscler Thromb Vasc Biol. 2006;26:20–27. doi: 10.1161/01.ATV.0000195789.39418.e8. [DOI] [PubMed] [Google Scholar]
- 28.Liu L, Bortnick AE, Nickel M, et al. Effects of apolipoprotein A-I on ATP-binding cassette transporter A1-mediated efflux of macrophage phospholipid and cholesterol. J Biol Chem. 2003;278:42976–42984. doi: 10.1074/jbc.M308420200. [DOI] [PubMed] [Google Scholar]
- 29.Krimbou L, Hassan HH, Blain S, et al. Biogenesis and speciation of nascent apoA-I-containing particles in various cell lines. J Lipid Res. 2005;46:1668–1677. doi: 10.1194/jlr.M500038-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Duong PT, Collins HL, Nickel M, et al. Characterization of nascent HDL particles and microparticles formed by ABCA1-mediated efflux of cellular lipids to apoA-I. J Lipid Res. 2006;47:832–843. doi: 10.1194/jlr.M500531-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Mulya A, Lee JY, Gebre AK, et al. Minimal lipidation of prebeta HDL by ABCA1 results in reduced ability to interact with ABCA1. Arterioscler Thromb Vasc Biol. 2007;27:1828–1836. doi: 10.1161/ATVBAHA.107.142455. [DOI] [PubMed] [Google Scholar]
- 32•.Mulya A, Seo J, Brown AL, et al. Apolipoprotein M expression increases the size of nascent prebeta HDL formed by ATP binding cassette transporter A1 (ABCA1) J Lipid Res. 2010;51:514–524. doi: 10.1194/jlr.M002162. This study shows that the size of nascent preβ-HDL formed by apoA-I/ABCA1 interaction is increased by the coexpression of apoM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duong PT, Weibel GL, Lund-Katz S, et al. Characterization and properties of pre beta-HDL particles formed by ABCA1-mediated cellular lipid efflux to apoA-I. J Lipid Res. 2008;49:1006–1014. doi: 10.1194/jlr.M700506-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisenberg S. High density lipoprotein metabolism. J Lipid Res. 1984;25:1017–1058. [PubMed] [Google Scholar]
- 35.Navab M, Reddy ST, Van Lenten BJ, et al. The role of dysfunctional HDL in atherosclerosis. J Lipid Res. 2009;50:S145–S149. doi: 10.1194/jlr.R800036-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.Smith JD. Dysfunctional HDL as a diagnostic and therapeutic target. Arterioscler Thromb Vasc Biol. 2010;30:151–155. doi: 10.1161/ATVBAHA.108.179226. Myeloperoxidase modification of apoA-I impairs its function as a cholesterol acceptor via ABCA1 and this review summarizes the molecular changes in apoA-I that make oxidized apoA-I dysfunctional. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kontush A, Chapman MJ. Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol Rev. 2006;58:342–374. doi: 10.1124/pr.58.3.1. [DOI] [PubMed] [Google Scholar]
- 38.Phillips MC, Johnson WJ, Rothblat GH. Mechanisms and consequences of cellular cholesterol exchange and transfer. Biochim Biophys Acta. 1987;906:223–276. doi: 10.1016/0304-4157(87)90013-x. [DOI] [PubMed] [Google Scholar]
- 39.Johnson WJ, Mahlberg FH, Rothblat GH, Phillips MC. Cholesterol transport between cells and high density lipoproteins. Biochim Biophys Acta. 1991;1085:273–298. doi: 10.1016/0005-2760(91)90132-2. [DOI] [PubMed] [Google Scholar]
- 40.Yancey PG, Bortnick AE, Kellner-Weibel G, et al. Importance of different pathways of cellular cholesterol efflux. Arterioscler Thromb Vasc Biol. 2003;23:712–719. doi: 10.1161/01.ATV.0000057572.97137.DD. [DOI] [PubMed] [Google Scholar]
- 41.Adorni MP, Zimetti F, Billheimer JT, et al. The roles of different pathways in the release of cholesterol from macrophages. J Lipid Res. 2007;48:2453–2462. doi: 10.1194/jlr.M700274-JLR200. [DOI] [PubMed] [Google Scholar]
- 42••.Larrede S, Quinn CM, Jessup W, et al. Stimulation of cholesterol efflux by LXR agonists in cholesterol-loaded human macrophages is ABCA1-dependent but ABCG1-independent. Arterioscler Thromb Vasc Biol. 2009;29:1930–1936. doi: 10.1161/ATVBAHA.109.194548. A comprehensive study demonstrating that cholesterol efflux to HDL from THP-1 human macrophages is mediated by ABCA1 and that ABCG1 is not essential for cholesterol efflux in these cells. In addition, by using anti-Cla-1 (SR-BI) blocking antibody, it was shown that Cla-1 made a significant contribution to cholesterol efflux from cholesterol-loaded human macrophages. [DOI] [PubMed] [Google Scholar]
- 43.Lange Y, Steck TL. Cholesterol homeostasis and the escape tendency (activity) of plasma membrane cholesterol. Prog Lipid Res. 2008;47:319–332. doi: 10.1016/j.plipres.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lund-Katz S, Laboda HM, McLean LR, Phillips MC. Influence of molecular packing and phospholipid type on rates of cholesterol exchange. Biochemistry. 1988;27:3416–3423. doi: 10.1021/bi00409a044. [DOI] [PubMed] [Google Scholar]
- 45.Gold JC, Phillips MC. Effects of membrane lipid composition on the kinetics of cholesterol exchange between lipoproteins and different species of red blood cells. Biochim Biophys Acta. 1990;1027:85–92. doi: 10.1016/0005-2736(90)90052-p. [DOI] [PubMed] [Google Scholar]
- 46.Davidson WS, Rodrigueza WV, Lund-Katz S, et al. Effects of acceptor particle size on the efflux of cellular free cholesterol. J Biol Chem. 1995;270:17106–17113. doi: 10.1074/jbc.270.29.17106. [DOI] [PubMed] [Google Scholar]
- 47.Ji Y, Jian B, Wang N, et al. Scavenger receptor BI promotes high density lipoprotein-mediated cellular cholesterol efflux. J Biol Chem. 1997;272:20982–20985. doi: 10.1074/jbc.272.34.20982. [DOI] [PubMed] [Google Scholar]
- 48.Liu B, Krieger M. Highly purified scavenger receptor class B, type I reconstituted into phosphatidylcholine/cholesterol liposomes mediates high affinity high density lipoprotein binding and selective lipid uptake. J Biol Chem. 2002;277:34125–34135. doi: 10.1074/jbc.M204265200. [DOI] [PubMed] [Google Scholar]
- 49.Thuahnai ST, Lund-Katz S, Dhanasekaran P, et al. SR-BI-mediated cholesteryl ester selective uptake and efflux of unesterified cholesterol: influence of HDL size and structure. J Biol Chem. 2004;279:12448–12455. doi: 10.1074/jbc.M311718200. [DOI] [PubMed] [Google Scholar]
- 50.Rodrigueza WV, Thuahnai ST, Temel RE, et al. Mechanism of scavenger receptor class B type I-mediated selective uptake of cholesteryl esters from high density lipoprotein to adrenal cells. J Biol Chem. 1999;274:20344–20350. doi: 10.1074/jbc.274.29.20344. [DOI] [PubMed] [Google Scholar]
- 51.de Beer MC, Durbin DM, Cai L, et al. Apolipoprotein A-I conformation markedly influences HDL interaction with scavenger receptor BI. J Lipid Res. 2001;42:309–313. [PubMed] [Google Scholar]
- 52•.Tarr PT, Tarling EJ, Bojanic DD, et al. Emerging new paradigms for ABCG transporters. Biochim Biophys Acta. 2009;1791:584–593. doi: 10.1016/j.bbalip.2009.01.007. A comprehensive review of the ABCG family of transporters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang N, Lan D, Chen W, et al. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc Natl Acad Sci U S A. 2004;101:9774–9779. doi: 10.1073/pnas.0403506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54•.Sankaranarayanan S, Oram JF, Asztalos BF, et al. Effects of acceptor composition and mechanism of ABCG1-mediated cellular free cholesterol efflux. J Lipid Res. 2009;50:275–284. doi: 10.1194/jlr.M800362-JLR200. Using baby hamster kidney (BHK) cells transfected with human ABCG1, the relative efficiencies of individual HDL subfractions and other acceptors for promoting cholesterol efflux were established. HDL composition and acceptor particle size were the major determinants of efflux efficiency. ABCG1 expression did not enhance cholesterol influx from HDL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gillotte KL, Davidson WS, Lund-Katz S, et al. Removal of cellular cholesterol by prebeta-HDL involves plasma membrane microsolubilization. J Lipid Res. 1998;39:1918–1928. [PubMed] [Google Scholar]
- 56.Vedhachalam C, Duong PT, Nickel M, et al. Mechanism of ATP-binding cassette transporter A1-mediated cellular lipid efflux to apolipoprotein A-I and formation of high density lipoprotein particles. J Biol Chem. 2007;282:25123–25130. doi: 10.1074/jbc.M704590200. [DOI] [PubMed] [Google Scholar]
- 57.Gillotte KL, Zaiou M, Lund-Katz S, et al. Apolipoprotein-mediated plasma membrane microsolubilization. J Biol Chem. 1999;274:2021–2028. doi: 10.1074/jbc.274.4.2021. [DOI] [PubMed] [Google Scholar]
- 58.Marcel YL, Ouimet M, Wang MD. Regulation of cholesterol efflux from macrophages. Curr Opin Lipidol. 2008;19:455–461. doi: 10.1097/MOL.0b013e32830f4a1d. [DOI] [PubMed] [Google Scholar]
- 59.Boadu E, Bilbey NJ, Francis GA. Cellular cholesterol substrate pools for adenosine-triphosphate cassette transporter A1-dependent high-density lipoprotein formation. Curr Opin Lipidol. 2008;19:270–276. doi: 10.1097/MOL.0b013e3282feea99. [DOI] [PubMed] [Google Scholar]
- 60.Sun Y, Hao M, Luo Y, et al. Stearoyl-CoA desaturase inhibits ATP-binding cassette transporter A1-mediated cholesterol efflux and modulates membrane domain structure. J Biol Chem. 2003;278:5813–5820. doi: 10.1074/jbc.M208687200. [DOI] [PubMed] [Google Scholar]
- 61.Lu R, Arakawa R, Ito-Osumi C, et al. ApoA-I facilitates ABCA1 recycle/accumulation to cell surface by inhibiting its intracellular degradation and increases HDL generation. Arterioscler Thromb Vasc Biol. 2008;28:1820–1824. doi: 10.1161/ATVBAHA.108.169482. [DOI] [PubMed] [Google Scholar]
- 62••.Singaraja RR, Kang MH, Vaid K, et al. Palmitoylation of ATP-binding cassette transporter A1 is essential for its trafficking and function. Circ Res. 2009;105:138–147. doi: 10.1161/CIRCRESAHA.108.193011. ABCA1 is located in different membrane environments and uses lipids from various sources to lipidate apoA-I and create nascent HDL particles. The regulation of ABCA1 targetting to membranes has been poorly understood. This study shows that ABCA1 is palmitoylated at four cysteine sites, which localizes the transporter at the plasma membrane. [DOI] [PubMed] [Google Scholar]
- 63••.Nandi S, Ma L, Denis M, et al. ABCA1-mediated cholesterol efflux generates microparticles in addition to HDL through processes governed by membrane rigidity. J Lipid Res. 2009;50:456–466. doi: 10.1194/jlr.M800345-JLR200. This study demonstrates that, in addition to generating nascent HDL, the interaction of apoA-I with cells expressing ABCA1 results in the production of cholesterol-containing, apoA-I-free microparticles. A significant part of the cholesterol released when apoA-I interacts with ABCA1 can be in the microparticle fraction. These results demonstrate that all of the cholesterol release by the apoA-I/ABCA1 interaction should not be considered as nascent HDL. [DOI] [PubMed] [Google Scholar]
- 64.Gelissen IC, Harris M, Rye KA, et al. ABCA1 and ABCG1 synergize to mediate cholesterol export to apoA-I. Arterioscler Thromb Vasc Biol. 2006;26:534–540. doi: 10.1161/01.ATV.0000200082.58536.e1. [DOI] [PubMed] [Google Scholar]
- 65.Vaughan AM, Oram JF. ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. J Lipid Res. 2006;47:2433–2443. doi: 10.1194/jlr.M600218-JLR200. [DOI] [PubMed] [Google Scholar]
- 66.de Beer MC, Durbin DM, Cai L, et al. Apolipoprotein A-II modulates the binding and selective lipid uptake of reconstituted high density lipoprotein by scavenger receptor BI. J Biol Chem. 2001;276:15832–15839. doi: 10.1074/jbc.M100228200. [DOI] [PubMed] [Google Scholar]
- 67•.Wiersma H, Gatti A, Nijstad N, et al. Scavenger receptor class B type I mediates biliary cholesterol secretion independent of ATP-binding cassette transporter g5/g8 in mice. Hepatology. 2009;50:1263–1272. doi: 10.1002/hep.23112. This study shows that hepatic expression of SR-BI in mice increases biliary cholesterol secretion by promoting efflux from the canalicular membrane. [DOI] [PubMed] [Google Scholar]
- 68•.Wiersma H, Gatti A, Nijstad N, et al. Hepatic SR-BI, not endothelial lipase, expression determines biliary cholesterol secretion in mice. J Lipid Res. 2009;50:1571–1580. doi: 10.1194/jlr.M800434-JLR200. A companion study to Ref. [67•] showing that the biliary cholesterol secretion rate is related to hepatic expression of SR-BI and not the ABCG5/G8 transporters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stein O, Dabach Y, Hollander G, et al. High levels of human apolipoprotein A-I and high density lipoproteins in transgenic mice do not enhance efflux of cholesterol from a depot of injected lipoproteins. Relevance to regression of atherosclerosis? Atherosclerosis. 1999;144:367–374. doi: 10.1016/s0021-9150(99)00006-4. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Y, Zanotti I, Reilly M, et al. Overexpression of apoA-I promotes reverse cholesterol transport of cholesterol from macrophages to feces. Circulation. 2003;108:661–663. doi: 10.1161/01.CIR.0000086981.09834.E0. [DOI] [PubMed] [Google Scholar]
- 71.Sehayek E, Hazen SL. Cholesterol absorption from the intestine is a major determinant of reverse cholesterol transport from peripheral tissue macrophages. Arterioscler Thromb Vasc Biol. 2008;28:1296–1297. doi: 10.1161/ATVBAHA.108.165803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72•.Alexander ET, Weibel GL, Joshi MR, et al. Macrophage reverse cholesterol transport in mice expressing ApoA-I Milano. Arterioscler Thromb Vasc Biol. 2009;29:1496–1501. doi: 10.1161/ATVBAHA.109.191379. This study compares the abilities of human wild-type apoA-I and human apoA-I Milano to promote macrophage RCT when the apoproteins are expressed in apoA-I-null mice. It was demonstrated that both wild-type and Milano apoA-I are equally efficient at promoting macrophage RCT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rotllan N, Ribas V, Calpe-Berdiel L, et al. Overexpression of human apolipoprotein A-II in transgenic mice does not impair macrophage-specific reverse cholesterol transport in vivo. Arterioscler Thromb Vasc Biol. 2005;25:e128–e132. doi: 10.1161/01.ATV.0000175760.28378.80. [DOI] [PubMed] [Google Scholar]
- 74••.Tanigawa H, Billheimer JT, Tohyama J, et al. Lecithin: cholesterol acyltransferase expression has minimal effects on macrophage reverse cholesterol transport in vivo. Circulation. 2009;120:160–169. doi: 10.1161/CIRCULATIONAHA.108.825109. In this study, the macrophage to feces in-vivo RCT protocol was used to determine the importance of LCAT in RCT. Human LCAT overexpression in human apoA-I transgenic mice increased plasma HDL levels but did not increase macrophage RCT. RCT in LCAT−/− and LCAT+/− mice were compared to that in wild-type mice. LCAT-null mice had only a 50% reduction in RCT and LCAT+/− mice had normal RCT despite a significant reduction in HDL. These data suggest that macrophage RCT may not be as dependent on LCAT activity as previously believed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Calabresi L, Favari E, Moleri E, et al. Functional LCAT is not required for macrophage cholesterol efflux to human serum. Atherosclerosis. 2009;204:141–146. doi: 10.1016/j.atherosclerosis.2008.08.038. [DOI] [PubMed] [Google Scholar]
- 76.Tanigawa H, Billheimer JT, Tohyama J, et al. Expression of cholesteryl ester transfer protein in mice promotes macrophage reverse cholesterol transport. Circulation. 2007;116:1267–1273. doi: 10.1161/CIRCULATIONAHA.107.704254. [DOI] [PubMed] [Google Scholar]
- 77.Samyn H, Moerland M, van Gent T, et al. Elevation of systemic PLTP, but not macrophage-PLTP, impairs macrophage reverse cholesterol transport in transgenic mice. Atherosclerosis. 2009;204:429–434. doi: 10.1016/j.atherosclerosis.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 78.Wang X, Collins HL, Ranalletta M, et al. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J Clin Invest. 2007;117:2216–2224. doi: 10.1172/JCI32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Calpe-Berdiel L, Rotllan N, Palomer X, et al. Direct evidence in vivo of impaired macrophage-specific reverse cholesterol transport in ATP-binding cassette transporter A1-deficient mice. Biochim Biophys Acta. 2005;1738:6–9. doi: 10.1016/j.bbalip.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 80.Wang MD, Franklin V, Marcel YL. In vivo reverse cholesterol transport from macrophages lacking ABCA1 expression is impaired. Arterioscler Thromb Vasc Biol. 2007;27:1837–1842. doi: 10.1161/ATVBAHA.107.146068. [DOI] [PubMed] [Google Scholar]
- 81•.McGillicuddy FC, de la Llera Moya M, Hinkle CC, et al. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009;119:1135–1145. doi: 10.1161/CIRCULATIONAHA.108.810721. The results from this study provide in-vivo evidence that inflammation impairs RCT at multiple steps in the RCT pathway, particularly cholesterol flux through liver, bile and feces. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nishimoto T, Pellizzon MA, Aihara M, et al. Fish oil promotes macrophage reverse cholesterol transport in mice. Arterioscler Thromb Vasc Biol. 2009;29:1502–1508. doi: 10.1161/ATVBAHA.109.187252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Naik SU, Wang X, da Silva JR, et al. Pharmacological activation of liver X receptors promotes reverse cholesterol transport in vivo. Circulation. 2006;113:90–97. doi: 10.1161/CIRCULATIONAHA.105.560177. [DOI] [PubMed] [Google Scholar]
- 84.Tohyama J, Billheimer JT, Fuki IV, et al. Effects of nevirapine and efavirenz on HDL cholesterol levels and reverse cholesterol transport in mice. Atherosclerosis. 2009;204:418–423. doi: 10.1016/j.atherosclerosis.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85•.Rader DJ, Alexander ET, Weibel GL, et al. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res. 2009;50(Suppl):S189–S194. doi: 10.1194/jlr.R800088-JLR200. This review discusses the individual steps in macrophage to feces RCT. Serum factors and macrophage transporters are discussed in terms of the flux of cholesterol from macrophages through the plasma, liver and into the feces. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86••.Xie C, Turley SD, Dietschy JM. ABCA1 plays no role in the centripetal movement of cholesterol from peripheral tissues to the liver and intestine in the mouse. J Lipid Res. 2009;50:1316–1329. doi: 10.1194/jlr.M900024-JLR200. In this study, the absolute rates of cholesterol flux each day from the individual peripheral tissues to the blood as well as the rates of fecal sterol excretion from the whole mouse, in the presence and absence of ABCA1 function, were measured systematically. The results show that though ABCA1 plays a critical role in preserving apoA-I and in removing cholesterol from macrophages, it seems to play no role in controlling the major flux of sterol from the peripheral organs to the liver. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Asztalos B, de la Llera-Moya M, Dallal GE, et al. Differential effects of HDL subpopulations on cellular ABCA1- and SR-BI-mediated cholesterol efflux. J Lipid Res. 2005;46:2246–2253. doi: 10.1194/jlr.M500187-JLR200. [DOI] [PubMed] [Google Scholar]
- 88.Waldo SW, Li Y, Buono C, et al. Heterogeneity of human macrophages in culture and in atherosclerotic plaques. Am J Pathol. 2008;172:1112–1126. doi: 10.2353/ajpath.2008.070513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89••.Johnson JL, Newby AC. Macrophage heterogeneity in atherosclerotic plaques. Curr Opin Lipidol. 2009;20:370–378. doi: 10.1097/MOL.0b013e3283309848. This review article discusses the importance of macrophage heterogeneity in atherosclerotic plaques and how monocyte and macrophage diversity is important in atherosclerosis. Such differences between either primary macrophages or established macrophage cell lines can explain, in part, the conflicting data that are sometimes observed among experiments that probe the expression of different macrophage cholesterol transporters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Turchyn LR, Baginski TJ, Renkiewicz RR, et al. Phenotypic and functional analysis of murine resident and induced peritoneal macrophages. Comp Med. 2007;57:574–580. [PubMed] [Google Scholar]
- 91••.de La Llera-Moya M, Drazul-Schrader D, Asztalos BF, et al. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar HDL-C to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol. 2010 doi: 10.1161/ATVBAHA.109.199158. (in press). In this study that measured efflux from J774 macrophage to the total HDL fraction from a number of human sera, it was established that there were numerous instances of serum samples that had similar HDL-C or apoA-I, yet produced significant differences in cholesterol efflux. By probing the contribution of cholesterol transporters, it was determined that the differences were associated with the ABCA1 pathway, which in turn was linked to the level of preβ-HDL in the serum. These results demonstrate that efflux efficiency is a function of HDL subfraction distribution and not simply HDL-C or apoA-I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92•.Tang C, Liu Y, Kessler PS, et al. The macrophage cholesterol exporter ABCA1 functions as an anti-inflammatory receptor. J Biol Chem. 2009;284:32336–32343. doi: 10.1074/jbc.M109.047472. This paper demonstrates that the apoA-I/ABCA1 pathways in macrophages can activate signaling molecules that promote an anti-inflammatory response; this links ABCA1-mediated cholesterol efflux and suppressed inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93•.Tang C, Oram JF. The cell cholesterol exporter ABCA1 as a protector from cardiovascular disease and diabetes. Biochim Biophys Acta. 2009;1791:563–572. doi: 10.1016/j.bbalip.2009.03.011. This interesting review focuses on the pathophysiology of ABCA1, especially with regard to its activity in pancreatic β-cells and the consequences for diabetes. [DOI] [PubMed] [Google Scholar]
- 94••.Choi HY, Rahmani M, Wong BW, et al. ATP-binding cassette transporter A1 expression and apolipoprotein A-I binding are impaired in intima-type arterial smooth muscle cells. Circulation. 2009;119:3223–3231. doi: 10.1161/CIRCULATIONAHA.108.841130. The expression and activity of ABCA1 in human intimal and medial smooth muscle cells and in the same cells in atherosclerotic lesions were examined. The results show that reduced ABCA1 activity contributes to smooth muscle foam cell formation in the intima. [DOI] [PubMed] [Google Scholar]
- 95••.Brunham LR, Singaraja RR, Duong M, et al. Tissue-specific roles of ABCA1 influence susceptibility to atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:548–554. doi: 10.1161/ATVBAHA.108.182303. This study investigated in which tissues ABCA1 exerts its effect on atherosclerosis using mice with tissue-specific inactivation of ABCA1. In the mouse models used, and contrary to prior findings, a lack of macrophage ABCA1 does not influence atherogenesis. However, physiological hepatic ABCA1 expression provides ather-oprotection. [DOI] [PubMed] [Google Scholar]
- 96•.Duffy D, Rader DJ. Update on strategies to increase HDL quantity and function. Nat Rev Cardiol. 2009;6:455–463. doi: 10.1038/nrcardio.2009.94. This review considers new therapeutic strategies that have the potential to increase plasma levels of HDL-C and/or improve HDL function. [DOI] [PubMed] [Google Scholar]