Abstract
The protease inhibitor saquinavir was administered to 100 human immunodeficiency virus type 1 (HIV-1)-infected patients as a single 600-mg oral dose (hard gelatin capsules) with a standard breakfast, including 200 ml of grapefruit juice, during an open-label trial to assess whether diarrhea and/or wasting syndrome has consequences on its pharmacokinetics. Three groups of patients were enrolled: group 1, asymptomatic patients (n = 30); group 2, AIDS symptomatic patients without body weight loss or diarrhea (n = 37); and group 3, AIDS symptomatic patients with severe body weight loss and/or diarrhea (n = 33). Clinical and biological data (covariates) were collected. A population approach was performed with three blood samples per patient to estimate the mean population pharmacokinetic parameters (clearance [CL]/oral bioavailability [F], V/F, ka, and lag time) and the derived ones (kel, Cmax, Tmax, and area under the curve [AUC]). The relationships between groups, exposure (i.e., estimated individual post hoc AUCs), and covariates were explored by using multiple linear regressions. A significant increase in median AUCs (165, 349, and 705 ng · h · ml−1 for groups 1, 2, and 3, respectively [P < 0.0001]) was observed. The enhancement in saquinavir exposure could be due to the destruction of the transporters in enterocytes and/or to the enlargement of their tight junctions, allowing a paracellular crossing of saquinavir as the illness spreads. Because of grapefruit juice intake by every patient, no implication of CYP3A4 could be assessed. These results strongly suggest that, despite its low intrinsic oral bioavailability, saquinavir can be considered as a relevant treatment for HIV-1-infected patients with diarrhea and/or wasting syndrome. This must be evaluated in a long-term period.
Intestinal troubles (diarrhea and malabsorption) are common symptoms during human immunodeficiency virus (HIV) infection and are responsible for weight loss throughout the course of the illness (32). Several studies have suggested that these intestinal dysfunctions are linked to HIV disease stages (18, 28, 29, 32). Malabsorption, which has nutritional consequences (11), may have great pharmacological effects, particularly from a pharmacokinetic point of view. Moreover, it is usual to consider diarrhea as a factor decreasing drug absorption, but this has not been demonstrated to be fact. Indeed, oral bioavailability (F), i.e., the absorption and first-pass effect-related pharmacokinetic parameter, is expected to be disrupted in such clinical situations. However, there has been no alteration in F reported for some antiretrovirals, such as zidovudine, in AIDS patients with small intestinal disease (SID), i.e., chronic diarrhea with wasting (36). The only change observed in such cases was a delayed absorption (prolonged time to reach the concentration peak [i.e., Tmax]) with respect to the total exposure of the drug: no significant difference in areas under the concentration-time curves (AUCs) from time zero to time 6 h between patients with or without SID (17). Thus, it is possible that the absorption of drugs with relatively high bioavailability, such as zidovudine (15), is not significantly disrupted by chronic diarrhea with wasting compared to that of drugs with low bioavailability at the same stage of disease.
The protease inhibitor (PI) saquinavir (SQV), in a hard gelatin capsule (HGC), has a kinetic profile characterized by a reduced absorption and a high first-pass effect, resulting in the lowest bioavailability (F = 0.04 to 0.06) among the HIV type 1 (HIV-1) PIs available today (3, 27). It has not been pointed out, until now, whether patients with diarrhea and malabsorption could exhibit infratherapeutic SQV concentrations in plasma. Moreover, SQV is known to display a high interindividual variability when administered to HIV-1-infected patients (30). This characteristic, associated with troubled absorption, should be taken into account to either avoid resistance in cases of decreased drug exposure or lower potential side effects in cases of prolonged increased exposure.
New AIDS patients naive to antiretrovirals and with diarrhea and/or wasting syndrome still exist today, particularly in Africa and Asia. Few studies have been published to date that examine the absorption of major HIV oral medications, such as PIs, which exhibit very low oral bioavailability in such clinical situations (5). Given the importance of understanding the effect of diarrhea and/or wasting on SQV oral absorption, we undertook a two-center study (the Lariboisière and Paul Brousse hospitals in Paris and Villejuif, France, respectively) that compares the pharmacokinetic behaviors of SQV in patients with and without diarrhea and/or wasting syndrome. The goal of the present study is to evaluate the consequences of diarrhea and/or wasting syndrome on the pharmacokinetics of SQV in HIV-1-infected patients in order to assess the potential therapeutic relevance of that PI in these patients.
MATERIALS AND METHODS
Study design.
In this open-label trial, which took place from 1996 to 1998, patients enrolled in either of the two centers during the same time period (half of the patients in each center) and received, in addition to their usual antiretroviral treatment (19, 22), SQV-HGC (Produits Roche, Neuilly-sur-Seine, France) as a single oral 600-mg dose immediately after a standard breakfast including 200 ml of grapefruit juice to optimize SQV absorption. All doses were checked to have been taken. To lower drug interactions with SQV, the patients were chosen from an HIV-1-infected population that had never before received any PI. The study was approved by the ethical committee of Paris-Saint-Louis Hospital, Paris, France. A written informed consent was obtained from each patient before starting the trial.
To perform the population pharmacokinetics study, three blood samples (5 ml each) were collected in vials containing lithium heparinate from each patient, chosen randomly among 15 sampling times, i.e., 0, 0.25, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 5.0, 6.0, 8.0, 10.0, and 12.0 h after SQV-HGC administration, according to the following protocol. Each patient had one sample collected randomly among three periods: 0 to 1.5 h, 2 to 4 h, and 5 to 12 h. Hence, each of the 15 points had six determinations in each group. Plasma was separated from blood cells by centrifugation immediately after collection. In accordance with good laboratory practices, a decontamination procedure was set up by heating plasma 30 min at 56°C. After they returned to room temperature, plasma samples were frozen at −20°C until analysis. Concentrations of SQV in plasma were determined by a radioimmunoassay method (Huntington Life Sciences, Ltd., Huntington, Campbridgeshire, England) whose lower limit of quantification was 1 ng/ml (unpublished method).
Patients.
HIV-1-infected men and women over 18 years old, whatever their risk factors for HIV infection, treated or not treated with antiretroviral therapy (if so, no PI was taken), were enrolled in the present study for 21 months. Neither patients with changes in intestinal absorption as a result of intestinal surgery, severe renal failure (creatinine clearance < 20 ml · min−1), or hepatic insufficiency (prothrombin time < 60%) nor patients with signs of clinical or biological pancreatitis or known absorption disorders were included in this clinical trial. After patients were clinically examined, three groups were defined according to the 1993 revised classification of the U.S. Centers for Disease Control and Prevention (CDC): group 1 (n = 30), asymptomatic subjects (CDC-A or -B); group 2 (n = 37), AIDS symptomatic subjects (CDC-C) without wasting syndrome or diarrhea; and group 3 (n = 33), AIDS symptomatic patients (CDC-C) with severe body weight loss (>10% body weight during the past month) and/or chronic diarrhea (more than three daily bowel movements for at least 3 weeks and not related to antiretroviral therapy). All of the drugs taken by each patient were listed in order to check for drug interactions.
Clinical and biological data.
A complete biological profile was defined for each patient, including height and weight to assess the body mass index (BMI), age, total weight of stools per 24 h, viral load, CD4 cell count, creatinine to assess the creatinine clearance (CLCR) according to the Cockroft and Gault formula (8), intestinal permeability and absorption tests, plasma albumin, and alkaline phosphatase.
Intestinal absorption and permeability were assessed by the d-xylose and lactulose-mannitol tests, respectively, in which 5 g of d-xylose (Pharmacie Centrale des Hôpitaux, Nanterre, France) and 5 g of lactulose (Duphalac; Solvay Pharma, Suresnes, France) plus 1 g of mannitol (Pharmacie Centrale des Hôpitaux, Nanterre, France) were given orally to each patient.
The clinical, intestinal function tests and biological parameters are referred to as covariates below.
Population pharmacokinetic analysis.
The blood sampling design was sparse, based on a population pharmacokinetic approach in order to include a large number of subjects in a clinical situation by greatly limiting the number of samples: three blood samples were drawn per patient, according to the recommendations of Bréant et al. (6). Since only three concentration measurements were available for each subject, the kinetics profiles in each group were analyzed separately by using a population approach implemented in the P-PHARM software (InnaPhase Corp., Philadelphia, Pa.). This software allows general nonlinear mixed-effects models to fit experimental data, particularly in sparse individual data situations. P-PHARM uses an EM-like algorithm for computing the maximum-likelihood estimates of the population pharmacokinetic parameters (25).
A one-compartment model with first-order elimination and first-order absorption with a lag time was selected. Since no reference input route was available (intravenous route), the estimation of the absolute bioavailability (F) was not possible. Hence, clearance and volume of distribution of SQV were evaluated as CL/F and V/F, respectively. All pharmacokinetic parameters were assumed to be log-normally distributed. Before the algorithm was applied, initial values of the pharmacokinetic parameters (CL/F, V/F, ka, and Tlag, the so-called independent parameters) were selected from previously reported values (3, 27). The variance model was chosen to display the best fit among constant homoscedastic or heteroscedastic values (1/Y or 1/Y2). The three models were run until convergence. The model retained was the one that gave the lowest maximum-likelihood and Akaike criterion values.
The adequacy of the estimated population models was evaluated by using several standard goodness-of-fit criteria implemented in the P-PHARM software: (i) visual examination of the goodness-of-fit curves (population fitted model and individual fittings); (ii) visual examination of the scatterplot of observed versus predicted concentrations of the drugs in plasma after individual Bayesian fitting; (iii) statistical comparison of observed versus predicted concentrations of the drugs in plasma by a Student paired t test; (iv) comparison of the mean of the normalized residuals to zero by a Student t test; (v) detection of outliers by using the two-sided Student t test; (vi) comparison of the cumulative distribution of the normalized residuals and individual pharmacokinetic parameters to a normal or log-normal distribution, according to the initial hypothesis of this dispersal, by a Kolmogorov-Smirnov test; (vii) visual comparison of the cumulative distribution of the normalized residuals and individual pharmacokinetic parameters to a normal or log-normal distribution; and (viii) checking the decrease of the interindividual variability of the pharmacokinetic parameters, after the covariables were taken into account, compared to the variability before their inclusion in the structural model.
From the estimated mean population pharmacokinetic parameters, the individual (post hoc) parameters were computed (i.e., CL/F, V/F, ka, and Tlag) by the Bayes method implemented in the P-PHARM software. Then, the individual derived parameters, namely, the kel (elimination rate constant) and AUC, were calculated (13). Tmax, the time to reach the maximal concentration, called Cmax, and Cmax were graphically determined from each individual profile. These four last parameters are called the derived parameters (i.e., kel, AUC, Tmax, and Cmax). Since one of the most pertinent parameters to evaluate the total body exposure of a drug is the AUC, this parameter was used as the primary end point for assessing SQV exposure.
Relationships between groups, exposure, and covariates.
The following covariates were studied: gender, age, BMI, mean weight of stools per 24 h, plasma albumin, CLCR, xylose, lactulose/mannitol (L/M) ratio, alkaline phosphatase levels, and CD4 cell count. The variables that were not normally distributed, i.e., xylose, L/M ratio, alkaline phosphatase levels, and CD4 cell count, were log transformed. Changes in AUC between groups and relationships between AUC and several biological and clinical parameters, such as intestinal absorption, were both studied. Initially, comparisons between groups of the covariates and the logarithm of all of the individual pharmacokinetic parameters were performed by using univariate analysis of variance (ANOVA) for quantitative variables and a χ2 test for gender. When the results of the ANOVA were significant, the pairwise comparisons among the three groups were performed by using a Tukey correction. AUCs were log transformed by using decimal logarithm. Then, univariate relationships between the log(AUC) and the covariates were tested by using a Pearson correlation coefficient for quantitative variables and a Student t test for gender. A Bonferroni correction was performed to account for multiple testing all of the univariate analyses. A P value of <10−3 was consequently considered statistically significant.
Lastly, a multiple linear regression was performed to assess the association between covariates and log(AUC). Two models were built. The first model included all covariates; then, a step-by-step deletion was performed, beginning with the covariates with the higher P value. The second model included only the variable “group.” A step-by-step backward selection method with a “P = 0.05 criterion” was used. Goodness of fit was assessed according to scatter plots and quantile-quantile plots of the residuals.
RESULTS
Patients.
One hundred patients participated in the present study (19 females and 81 males). Thirty, thirty-seven, and thirty-three patients were enrolled in groups 1, 2, and 3, respectively, as described above. The female/male ratios were 6/24 in group 1, 7/30 in group 2, and 6/27 in group 3. The antiretroviral treatments used were zidovudine, didanosine, zalcitabine, lamivudine, and stavudine. No drug known to affect SQV metabolism was reported except in 12 patients: 2 patients with rifabutin in group 1; 3 patients with rifabutin and 1 patient with rifampin in group 2; and 5 patients with rifabutin and 1 patient with rifampin in group 3. No antidiarrheal agents were noted. Twenty patients were naive to antiretrovirals (11, 8, and 1 patients in groups 1, 2, and 3, respectively). The main demographic, clinical, and biological characteristics are listed in Table 1. Gender, viral load, and age were not found to differ significantly among the three groups. As expected, BMI, CD4 cell count, CLCR, and albumin significantly decreased from group 1 to group 3. The decrease in BMI is related to an incremental decrease in total body weight from group 1 to group 3 since the height of patients was statistically the same in the three groups (result not shown in Table 1).
TABLE 1.
Mean biological values, intestinal absorption, and permeability test values of the HIV-1-infected patients included in the present study
| Parameter or test | Mean value (SD)
|
Pa | ||
|---|---|---|---|---|
| Group 1 (n = 30) | Group 2 (n = 37) | Group 3 (n = 33) | ||
| Parameter | ||||
| Body mass index (kg · m−2) | 24.2 (5.2) | 21.9 (2.2) | 18.7 (2.8) | <0.0001* |
| Age (yr) | 40 (10) | 40 (9) | 39 (9) | NS |
| Viral load (expressed as log10) | 4.44 (1.05) | 4.85 (1.04) | 4.92 (0.87) | NS |
| CD4 count (cells · mm−3) | 248 (244) | 118 (120) | 53 (77) | <0.0001A |
| CLCR (ml · min−1) | 102 (26) | 97 (25) | 80 (19) | <0.001B |
| Plasma albumin level (g · liter−1) | 42 (4) | 38 (7) | 35 (8) | <0.001C |
| Alkaline phosphatase level (IU · liter−1) | 79 (27) | 122 (94) | 220 (239) | <0.001B |
| Intestinal absorption and permeability tests | ||||
| d-Xylose (g excreted in urine) | 1.42 (0.58) | 0.91 (0.45) | 0.66 (0.44) | <0.001D |
| L/M ratio | 0.042 (0.036) | 0.055 (0.071) | 0.106 (0.092) | <0.01E |
| Mean wt of stools (g/24 h) | 195 (80) | 147 (98) | 248 (219) | <0.05F |
*, all pairwise comparisons were significant. Superscripts: A, group 3 versus groups 1 and 2; B, group 3 versus groups 1 and 2; C, group 1 versus group 3; D, group 1 versus groups 2 and 3; E, group 3 versus groups 1 and 2; F, group 3 versus groups 1 and 2. NS, not significant.
It should be noted, as already described by Pernet et al. (29), that intestinal absorption and permeability were abnormal in every patient of our study, regardless the stage of illness, especially for those in group 3. These observations are quite consistent with findings published earlier (7, 14, 23). Chronic diarrhea and/or severe body weight loss, as defined above, permitted us to identify three subgroups in group 3: patients with chronic diarrhea (n = 18; 54.5%), patients with severe body weight loss (n = 26; 78.8%), and patients with diarrhea and severe body weight loss (n = 14; 42.4%).
A significant decrease in d-xylose elimination (group 1 compared to groups 2 and 3) and a significant increase in L/M ratio (group 3 compared to groups 1 and 2) were found. d-Xylose excretion was decreased in AIDS patients (group 3) with chronic diarrhea and severe body weight loss compared to those with severe body weight loss alone. However, L/M ratio did not significantly differ among patients in these three subgroups. Moreover, the mean weight of stools per 24 h was significantly higher in group 3 than in groups 1 and 2.
Population pharmacokinetic analysis.
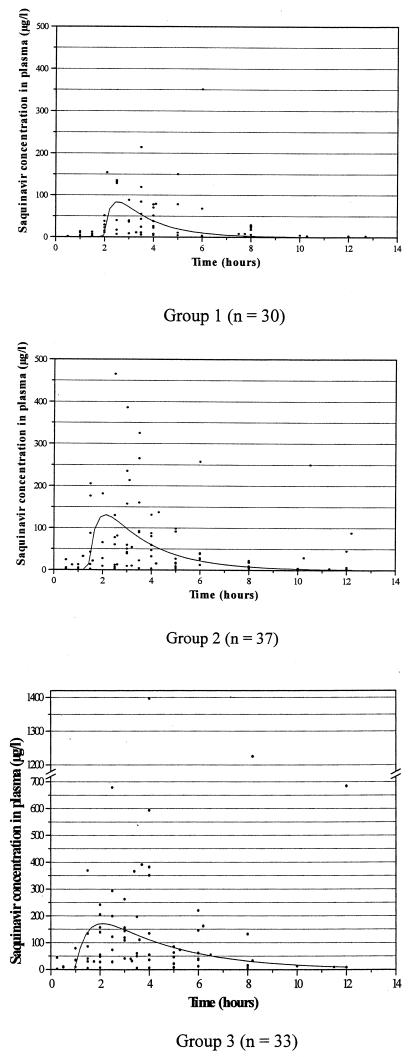
The one-compartment model adequately fit each group with satisfactory goodness-of-fit plots. The best fits were obtained by using a homoscedastic variance model for the three groups. The individual concentrations (solid black circles) and the corresponding mean estimated concentration profiles are displayed in Fig. 1 for each group. Population parameter mean values, interindividual variability, and residual errors are given in Table 2. Also, the mean population values of the derived parameters are given (namely, kel and AUC). The kel and AUC were calculated by using CL/V and dose/(CL/F), respectively. Cmax and Tmax were graphically determined. It should be noted that because the derived population mean parameters were calculated from estimated mean population parameters (CL/F, V/F, ka, and Tlag), no direct estimates of interpatient variability of these parameters were available. It is of particular interest that during the evolution of illness (from group 1 to group 3), the total body clearance of SQV expressed as CL/F decreased (Table 2), whereas Cmax and AUC increased (Table 2 and Fig. 1). The high interindividual variability in SQV kinetics (30) previously evoked is illustrated in Fig. 1.
FIG. 1.
Individual observed concentrations (indicated by solid black circles [three per patient]) and mean population profile curves after the administration of 600 mg of SQV-HGC as a single oral dose after a standard breakfast that included 200 ml of grapefruit juice.
TABLE 2.
Population mean estimated pharmacokinetic parameters, median (25th to 75th percentiles) post hoc individual values, and derived parameters for each groupa
| Parameter | Group 1 (n = 30)
|
Group 2 (n = 37)
|
Group 3 (n = 33)
|
Pb | |||
|---|---|---|---|---|---|---|---|
| Population mean value (CV %) | Median post hoc individual value (IQR) | Population mean value (CV %) | Median post hoc individual value (IQR) | Population mean value (CV %) | Median post hoc individual value (IQR) | ||
| Experimental parameters | |||||||
| CL/F (ml · h−1) | 6,820 (196.6) | 3,691 (1,620-6,685) | 1,643 (181.7) | 1,718 (831-3,333) | 769 (151.3) | 851 (472-1,755) | <0.0001* |
| V/F (liter) | 837 (76.8) | 800 (771-832) | 534 (172.0) | 506 (483-570) | 403 (96.0) | 394 (361-402) | <0.0001* |
| ka (h−1) | 0.658 (28.5) | 0.622 (0.595-0.680) | 0.510 (56.0) | 0.507 (0.452-0.575) | 0.309 (60.8) | 0.307 (0.253-0.343) | <0.0001* |
| Tlag (h) | 1.94 (7.4) | 1.95 (1.86-2.00) | 1.41 (4.5) | 1.41 (1.41-1.41) | 0.97 (1.5) | 0.98 (0.95-0.99) | <0.0001* |
| SDREc (ng · ml−1) | 5.2 | 18.9 | 34.5 | ||||
| Derived parameters | |||||||
| kel (h−1)d | 8.15 | 5.62 (1.69-9.42) | 3.08 | 0.58 (0.18-1.29) | 1.91 | 2.35 (1.30-4.48) | <0.0001* |
| Cmax (ng · ml−1)e | 83 | 85 (43-128) | 131 | 116 (75-183) | 170 | 148 (85-226) | 0.0041A |
| Tmax (h)e | 2.5 | 2.45 (2.21-3.20) | 2.1 | 1.90 (1.90-2.40) | 2.1 | 1.90 (1.66-2.37) | 0.0335B |
| AUCd (ng · h · ml−1) | 88 | 165 (90-370) | 365 | 349 (179-767) | 780 | 705 (342-1,271) | <0.0001* |
CV %, percent coefficient of variation as calculated by P-PHARM; IQR, interquartile range (25th to 75th percentiles are given in parentheses).
*, all pairwise comparisons were significant. Superscripts: A, group 1 versus group 3; B, group 1 versus group 3. P values were estimated from the logarithm of the post hoc individual values.
SDRE, standard deviation of residual error.
Derived parameters calculated from CL/F, V/F, and dose.
Observed values on curves.
Relationships between groups, exposure, and covariates.
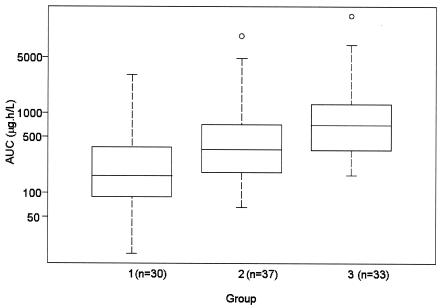
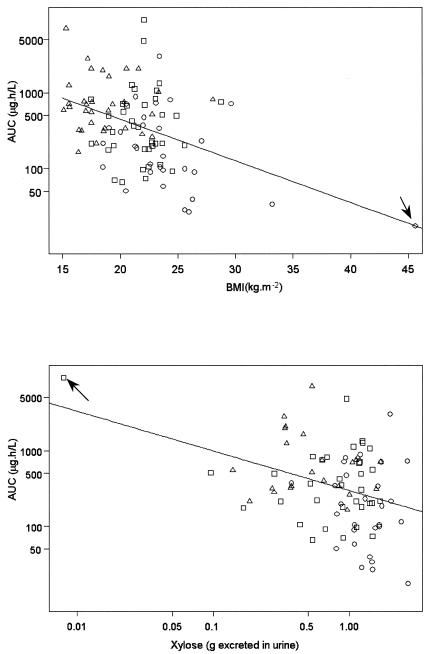
The median AUC values (interquartile range, 25th to 75th percentiles) for each group were estimated from the individual post hoc estimated AUCs: these were 165 (range, 90 to 370), 349 (range, 179 to 767), and 705 (range, 342 to 1,271) ng · h · ml−1 for groups 1, 2, and 3, respectively. The log(AUC) was found to increase significantly from group 1 to group 3 (P < 0.0001; Fig. 2). In the univariate analysis, the log(AUC) was found to be significantly correlated to BMI (r = −0.42, P < 0.0001) and log(xylose) (r = −0.36, P = 0.0007). Each of these variables had a negative effect on the log(AUC) (Fig. 3). The correlations remained statistically significant for BMI (r = −0.35 and P = 0.0004) and xylose (r = −0.24 and P = 0.0271) when, for each analysis, one outlier with very high BMI or very low xylose (see arrows in Fig. 3) was removed from the analysis. No significant relationship was found between log(AUC) and sex, age, CLCR, mean weight of stools per 24 h, and log(alkaline phosphatase) by using the Bonferroni correction.
FIG. 2.
Boxplots of individual estimated AUC in each group. The boxes represent the 25th and 75th percentiles, with the median (solid lines) shown within the boxes. The error bars represent 1.5 times the interquartile range, and the circles indicate extreme values.
FIG. 3.
Correlations between AUC and BMI and log(xylose) for patients from group 1 (○), patients from group 2 (□), and patients from group 3 (▵). Arrows indicate outliers. The correlations remain statistically significant for BMI (r = −0.35 and P = 0.0004) and log(xylose) (r = −0.24 and P = 0.0271) when the outliers are removed from the analysis.
When all covariates, except “group,” entered the descending procedure of the multivariate analysis, BMI and log(xylose) remained in the final model. This strongly suggests that AUCs were increased from groups 1 to 3 because of two independent effects: decrease in BMI and decrease in xylose, i.e., decrease in intestinal absorption. When the variable “group” was added to the model with all covariates, only BMI remained in the model. In that case, groups 2 and 3 had higher values for log(AUC) and BMI had a negative effect on SQV exposure. Goodness-of-fit plots were satisfying for both multivariate models.
DISCUSSION
Homogeneity in the three groups with regard to demographic parameters has been found, whereas the patients differed markedly with respect to several biological covariates (Table 1). The association of high viral load (means of 4.44, 4.85, and 4.92 log copies/ml for groups 1, 2 and 3, respectively) with low CD4 cell counts (means of 248, 118, and 53 cells/mm3 for groups 1, 2, and 3, respectively) reflects relatively advanced stages of the illness in every group (mainly in groups 2 and 3). The decreases in BMI and CD4 cell counts, in addition to the damaged hepatic function (revealed by the decrease in plasma albumin and the increase in alkaline phosphatase), are clearly a sign of the evolution of the illness from group 1 to group 3.
The population approach we used, limiting the blood sample schedule, was chosen for the comfort of the patients. The population analyses were performed separately for the three groups of patients, allowing us to adequately estimate the population parameters for each of them. The large interpatient variability of the observed concentrations, although a single identical dose of SQV was given (Fig. 1), was previously described by Regazzi et al. (30). A great scatter in the observations was especially noted around Cmax for groups 2 and 3.
The pharmacokinetics of SQV is often described as a two-compartment model (3). The overall parameters in such a model can be estimated only when enough experimental data are available, especially in the terminal elimination phase. In the present study, the sample schedule tends to better characterize the absorption phase up to Tmax than the elimination phase. Moreover, since some patients prematurely abandoned the study, the later points (i.e., 10 and 12 h after dosing) were not often available, preventing the algorithm from correctly fitting the elimination phase. Therefore, a one-compartment model with first-order absorption with a lag-time and first-order elimination was selected. Possible misspecifications on the estimated parameters with respect to the final elimination phase may have been made as a result. To avoid this main limitation on the pharmacokinetic parameter evaluation, another sample schedule could have been proposed with more sampling times during the elimination phase. For example, two samples per phase (i.e., absorption, distribution, and elimination) could have been judiciously chosen as we recommended for another study already published (26). Furthermore, an optimal population design approach using the Fisher information matrix-based method could have been implemented to define the best sampling schedule to consider (24, 31).
The present study mainly displayed a significant increase in SQV exposure in HIV-1-infected patients when disease worsens. This is brought to the fore by a steady increase in AUCs, observed from group 1 (asymptomatic HIV-1-infected patients) to group 3 (AIDS symptomatic patients with severe body weight loss and/or diarrhea) (Fig. 2 and Table 2). The multivariate analysis of the individual post hoc AUC estimates showed that this increase was mainly correlated to two independent effects: (i) a decrease in intestinal absorption and (ii) an increase of the dose of SQV expressed in milligrams per kilogram (because of the decrease in total body weight).
The d-xylose test assesses intestinal absorption. d-Xylose is a monosaccharide absorbed unchanged by the duodenum and the jejunum due to a facilitated transport after oral administration. It is poorly metabolized. A decrease in its urinary excretion signals a decrease in the mucosal functional area of the jejunum. In HIV-1-infected patients, small-bowel histological modifications such as villous atrophy, which leads to a decreased d-xylose absorption, are already well known (9, 18, 21). In the present study, this test displayed a significant incremental urinary d-xylose decrease as the illness spreads from group 1 to 3. This argues for possible disabled absorption transporters whose consequence should be a decrease in SQV absorption. Furthermore, it is now well known that peptides and peptidomimetics, such as the HIV PIs, are substrates of P-glycoprotein and/or CYP3A4 (2, 4, 12, 20, 33, 34). P-glycoprotein, an ATP-dependent drug efflux pump, typically associated with multidrug resistance in cancer chemotherapy, has been shown to be present in the enterocyte brush border and to closely interact with the major phase I drug-metabolizing enzyme in humans, CYP3A4 (35), further limiting the bioavailability of many drugs. In addition to its hepatic biotransformation (see below), it has been shown that SQV undergoes another metabolism in the enterocyte that potentially contributes to its high first-pass metabolism (12). Thus, it may be that in normal situations (without intestinal disease) P-glycoprotein, together with CYP3A4, acts as a concerted barrier to SQV absorption, which is responsible for its fairly low bioavailability. As the HIV infection spreads, i.e., as wasting syndrome develops and diarrhea appears, the histological integrity of the villus is challenged. Hence, the destruction, at least in part, of the efflux, together with the metabolism mechanisms, permits larger drug quantities to be passively absorbed by the body, causing an increase in its AUCs. However, in our study, all patients used grapefruit juice, which acts as a blocker of CYP3A4 (10, 22). Indeed, when the present study started (at the end of the 1990s), it was usual practice to coadminister grapefruit juice in order to increase SQV absorption (22) because of the high cost of that treatment when prescribed in doses high enough to be effective. Therefore, the design of this protocol corresponds to the routinely prescribed method at that time. As a consequence, the mechanism involving P-glycoprotein should be considered the only one in our study that could explain the increase in AUCs from group 1 to group 3. Furthermore, since grapefruit juice was given to every patient at the same dose, it allowed comparison between the groups. Finally, some of our patients were treated with rifabutin and/or rifampin. Both substances strongly induce the CYP3A4 PI metabolism, thus decreasing their concentrations. The many patients with rifabutin and/or rifampin in group 1 could have explained the lower disposition of SQV observed in this group compared to that of group 3 (AUC1 < AUC3, P < 0.0001). However, most patients receiving rifabutin and/or rifampin were in group 3 (2 of 30 in group 1 [6.7%], 4 of 37 in group 2 [10.8%], and 6 of 33 in group 3 [18.2%]). However, an increase in SQV disposition from group 1 to group 3 was observed despite these drug interactions. Hence, the presence of rifabutin and/or rifampin in some of our patients does not invalidate our overall findings.
The lactulose-mannitol test assesses intestinal permeability. In this test, the two sugars are simultaneously given orally, and their urinary recovery is determined. The intestinal permeability measures the ability of compounds to passively cross the intestinal mucosa through paracellular tight junction areas (16). The urinary recovery of the two sugars is expressed as the L/M ratio, which is known to be a reproducible measure of the mucosal integrity of the small intestine. Many studies in the literature utilized that test. Some of these studies demonstrated that children with small-intestine mucosal damage, determined by jejunal biopsy, had a significantly higher L/M urinary excretion ratio than control groups (1). In these situations, the sugar permeability test has shown good correlation with jejunal biopsy results. The enhanced L/M ratio detected in our study as the sickness worsened (Table 1) probably reveals an SQV-increased intestinal permeability from group 1 to group 3 that could, at least in part, explain and argue for the increased SQV disposition. Although the L/M ratio significantly increased from group 1 to group 3 (P < 0.01), the multivariate analysis did not correlate it with log(AUC). A higher number of patients in every group could probably have revealed such a correlation. Nevertheless, the increase in L/M ratio from group 1 to group 3 was probably related to an enlargement of the tight junctions, allowing a passive paracellular crossing of SQV. This latter mechanism could take over from the transporter-mediated absorption that takes place in normal situations (patients in group 1 without digestive disease).
The total body clearance of SQV (expressed as CL/F = dose/AUC) decreased as the illness spread (Table 2). This effect can be explained by a decrease in the dose of SQV, the increase in AUC being a consequence of the decrease in CL/F. If one expresses the SQV dosing in milligrams per kilogram, the doses are 8.6 ± 1.5, 9.3 ± 1.5, and 11.1 ± 1.8 mg/kg for groups 1, 2, and 3, respectively. The mean dose of SQV was 1.3-fold greater in group 3 than in group 1. This is related to an incremental decrease in the total body weights of our patients from group 1 to group 3, their heights being not significantly different in the three groups (Table 1, decrease in BMI). Thus, the increase in SQV dosing, expressed in milligrams per kilogram, should not be the only explanation for the CL/F decrease, whose median post hoc individual value was 4.3 times greater in group 3 than in group 1. The changes observed in CL/F could then be related to a decrease in CL and/or an increase in F. As already stated above, CL could not be evaluated because the intravenous data were not available. If bioavailability was implicated (increase in F), this could be due to an increase in the “absorption” (from a pharmacokinetic point of view) or to a decrease in the first-pass metabolism effect. Even if the liver function was disrupted (decrease in plasma albumin and increase in alkaline phosphatase), the ANOVA did not show any significant relationship between plasma albumin or alkaline phosphatase and log(AUC). Moreover, since grapefruit juice was given to all patients, the argument involving CYP3A4 in the enterocytes cannot be put forward to explain the possible increase in F. This increase in SQV bioavailability could then be explained, at least in part, by the increase of the intestinal permeability observed in the present study.
The present study demonstrated that SQV, despite its low intrinsic oral bioavailability, remains a relevant treatment for HIV-1-infected patients with diarrhea and/or wasting syndrome, mainly in developing countries where these digestive troubles still persist. The results support a major role of the efflux protein, P-glycoprotein, together with the integrity of the gut barrier, in determining the level of the bioavailability of SQV. The role of the metabolism enzyme CYP3A4 in the enterocyte, although reduced because of the coadministration of grapefruit juice to our patients, is not to be neglected. Further investigations are needed to determine the clinical efficacy of SQV for HIV-1-infected patients with diarrhea and/or wasting syndrome over a long-term period.
Acknowledgments
We thank Produits Roche and the Agence Française du Médicament for financial support.
We also thank to Huntington Life Sciences Ltd., Huntington, Campbridgeshire, England, for SQV assays; Bertrand Diquet for reviewing the manuscript; the patients for their kind contribution in that trial; and Martine Mallet, Maguy Parrinello, Laurence Bonhomme-Faivre, and the nurses of the Departments of Infectious Diseases of Lariboisière and Paul Brousse Hospitals, who actively participated in this study.
REFERENCES
- 1.Akinbami, F. O., G. A. Brown, and A. S. McNeish. 1989. Intestinal permeability as a measure of small mucosal integrity: correlation with jejunal biopsy. Afr. J. Med. Med. Sci. 18:187-192. [PubMed] [Google Scholar]
- 2.Alsenz, J., H. Steffen, and R. Alex. 1998. Active apical secretory efflux of the HIV protease inhibitors saquinavir and ritonavir in Caco-2 cell monolayers. Pharm. Res. 15:423-428. [DOI] [PubMed] [Google Scholar]
- 3.Barry, M., S. Gibbons, D. Back, and F. Mulcahy. 1997. Protease inhibitors in patients with AIDS disease: clinically important pharmacokinetic considerations. Clin. Pharmacokinet. 32:194-209. [DOI] [PubMed] [Google Scholar]
- 4.Benet, L. Z., T. Izumi, Y. Zhang, J. A. Silverman, and V. J. Wacher. 1999. Intestinal MDR transport proteins and P-450 enzymes as barriers to oral drug delivery. J. Control Release 62:25-31. [DOI] [PubMed] [Google Scholar]
- 5.Bobin, S., D. Bouhour, S. Durupt, A. Boibieux, V. Girault, and D. Peyramond. 1998. Importance of antiproteases in the treatment of microsporidia and/or cryptosporidia infections in HIV-seropositive patients. Pathol. Biol. 46:418-419. [PubMed] [Google Scholar]
- 6.Bréant, V., B. Charpiat, J. M. Sab, P. Maire, and R. W. Jelliffe. 1996. How many patients and blood levels are necessary for population pharmacokinetic analysis? A study of a one-compartment model applied to cyclosporine. Eur. J. Clin. Pharmacol. 51:283-288. [DOI] [PubMed] [Google Scholar]
- 7.Carlson, S. J., C. Webster, and R. M. Craig. 1997. Urinary recovery of lactulose compared to d-xylose absorption kinetics in HIV patients with diarrhea and weight loss. Dig. Dis. Sci. 42:2599-2602. [DOI] [PubMed] [Google Scholar]
- 8.Cockcroft, D. W., and M. H. Gault. 1976. Predictive creatinine clearance from serum creatinine. Nephron 15:545-553. [DOI] [PubMed] [Google Scholar]
- 9.Cummins, A. G., J. T. Labrooy, D. P. Stanley, M. Rowland, and D. J. C. Shearman. 1990. Quantitative histological study of enteropathy associated with HIV infection. Gut 31:317-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eagling, V. A., L. Profit, and D. J. Back. 1999. Inhibition of the CYP3A4-mediated metabolism and P-glycoprotein-mediated transport of the HIV-1 protease inhibitor saquinavir by grapefruit juice components. Br. J. Clin. Pharmacol. 48:543-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehrenpreis, E. D., S. J. Carlson, H. L. Boorstein, and R. M. Craig. 1994. Malabsorption and deficiency of vitamin B12 in HIV-infected patients with chronic diarrhea. Dig. Dis. Sci. 39:2159-2162. [DOI] [PubMed] [Google Scholar]
- 12.Fitzsimmons, M. E., and J. M. Collins. 1997. Selective biotransformation of the human immunodeficiency virus protease inhibitor saquinavir by small-intestinal cytochrome P4503A4: potential contribution to high first-pass metabolism. Drug Metab. Dispos. 25:256-266. [PubMed] [Google Scholar]
- 13.Gibaldi, M., and D. Perrier. 1982. One-compartment model, p. 1-43. In M. Dekker (ed.), Pharmacokinetics. Marcel Dekker, Inc., New York, N.Y.
- 14.Gillin, J. S., M. Shike, N. Alcock, C. Urmacher, S. Krown, R. C. Kurtz, C. J. Lightdale, and S. J. Winawer. 1985. Malabsorption and mucosal abnormalities of the small intestine in the acquired immunodeficiency syndrome. Ann. Intern. Med. 102:619-622. [DOI] [PubMed] [Google Scholar]
- 15.Hoetelmans, R. M., D. M. Burger, P. L. Meenhorst, and J. H. Beijnen. 1996. Pharmacokinetic individualisation of zidovudine therapy: current state of pharmacokinetic-pharmacodynamic relationship. Clin. Pharmacokinet. 30:314-327. [DOI] [PubMed] [Google Scholar]
- 16.Hollander, D. 1992. The intestinal permeability barrier: a hypothesis as to its regulation and involvement in Crohn's disease. Scand. J. Gastroenterol. 27:721-726. [DOI] [PubMed] [Google Scholar]
- 17.Kapembwa, M. S., S. C. Fleming, M. Orr, C. Wells, M. Bland, D. Back, and G. E. Griffin. 1996. Impaired absorption in patients with AIDS-related small intestinal disease. AIDS 10:1509-1514. [DOI] [PubMed] [Google Scholar]
- 18.Keating, J., I. Bjarnason, S. Somasundaram, A. Macpherson, N. Francis, A. B. Price, D. Sharpstone, J. Smithson, I. S. Menzies, and B. G. Gazzard. 1995. Intestinal absorption capacity, intestinal permeability, and jejunal histology in HIV and their relation to diarrhea. Gut 37:623-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kenyon, C. J., F. Brown, G. R. McClelland, and I. R. Wilding. 1998. The use of pharmacoscintigraphy to elucidate food effects observed with a novel protease inhibitor (saquinavir). Pharm. Res. 15:417-422. [DOI] [PubMed] [Google Scholar]
- 20.Kim, A. E., J. M. Dintaman, D. S. Waddel, and J. A. Silverman. 1998. Saquinavir, an HIV protease inhibitor, is transported by P-glycoprotein. J. Pharmacol. Exp. Ther. 286:1439-1445. [PubMed] [Google Scholar]
- 21.Kotler, D. P., H. P. Gaetz, M. Lange, E. B. Klein, and P. R. Holt. 1984. Enteropathy associated with acquired immunodeficiency syndrome. Ann. Intern. Med. 101:421-428. [DOI] [PubMed] [Google Scholar]
- 22.Kupferschmidt, H. H., K. E. Fattinger, H. R. Ha, F. Follath, and S. Krahenbuhl. 1998. Grapefruit juice enhances the bioavailability of the HIV protease inhibitor saquinavir in man. Br. J. Clin. Pharmacol. 45:355-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim, S. G., I. S. Menzies, C. A. Lee, M. A. Johnson, and R. E. Pounder. 1993. Intestinal permeability and function in patients infected with human immunodeficiency virus: a comparison with celiac disease. Scand. J. Gastroenterol. 28:573-580. [DOI] [PubMed] [Google Scholar]
- 24.Mentré, F., C. Dubruc, and J. P. Thénot. 2001. Population pharmacokinetic analysis of mizolastine solution in children with optimization of the experimental design. J. Pharmacokinet. Pharmacodyn. 28:299-319. [DOI] [PubMed] [Google Scholar]
- 25.Mentré, F., and R. Gomeni. 1995. A two-step iterative algorithm for estimation in nonlinear mixed-effect models with an evaluation in population pharmacokinetics. J. Biopharm. Stat. 5:141-158. [DOI] [PubMed] [Google Scholar]
- 26.Mouly, S., G. Aymard, J. P. Tillement, C. Caulin, J. F. Bergmann, and S. Urien. 2001. Increased oral ganciclovir bioavailability in HIV-infected patients with chronic diarrhoea and wasting syndrome: a population pharmacokinetic study. Br. J. Clin. Pharmacol. 51:557-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noble, S., and D. Faulds. 1996. Saquinavir: a review of its pharmacology and clinical potential in the management of HIV infection. Drugs 52:93-112. [DOI] [PubMed] [Google Scholar]
- 28.Oktedalen, O., V. Skar, E. Dahl, and A. Serk-Anssen. 1998. Changes in small intestinal structure and function in HIV-infected patients with chronic diarrhoeas. Scand. J. Infect. Dis. 30:459-463. [DOI] [PubMed] [Google Scholar]
- 29.Pernet, P., D. Vittecoq, A. Kodjo, M. H. Randrianarisolo, L. Dumitrescu, H. Blondon, J. F. Bergmann, J. Giboudeau, and C. Aussel. 1999. Intestinal absorption and permeability in human immunodeficiency virus-infected patients. Scand. J. Gastroenterol. 4:29-34. [DOI] [PubMed] [Google Scholar]
- 30.Regazzi, M. B., P. Villani, R. Maserati, L. Cocchi, R. Giacchino, D. Burroni, and M. Rettani. 1999. Pharmacokinetic variability and strategy for therapeutic drug monitoring of saquinavir (SQN) in HIV-1-infected individuals. Br. J. Clin. Pharmacol. 47:379-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Retout, S., S. Duffull, and F. Mentré. 2001. Development and implementation of the population Fisher information matrix for evaluation of population pharmacokinetic designs. Comp. Methods Programs Biomed. 65:141-151. [DOI] [PubMed] [Google Scholar]
- 32.Stockmann, M., H. Schmitz, M. Fromm, W. Schmidt, G. Pauli, P. Scholz, E. O. Riecken, and J. D. Schulzke. 2000. Mechanisms of epithelial barrier impairment in HIV infection. Ann. N. Y. Acad. Sci. 915:293-303. [DOI] [PubMed] [Google Scholar]
- 33.Wacher, V. J., J. A. Silverman, Y. Zhang, and L. Z. Benet. 1998. Role of P-glycoprotein and cytochrome P450 3A in limiting oral absorption of peptides and peptidomimetics. J. Pharm. Sci. 87:1322-1330. [DOI] [PubMed] [Google Scholar]
- 34.Washington, C. B., G. E. Duran, M. C. Man, B. I. Sikic, and T. F. Blaschke. 1998. Interaction of anti-HIV protease inhibitors with the multidrug transporter P-glycoprotein (P-gp) in human cultured cells. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 19:203-209. [DOI] [PubMed] [Google Scholar]
- 35.Watkins, P. B. 1997. The barrier function of CYP3A4 and P-glycoprotein in the small bowel. Adv. Drug Deliv. Rev. 27:161-170. [DOI] [PubMed] [Google Scholar]
- 36.Zorza, G., L. Beaugerie, A. M. Taburet, Y. Le Quintrec, and E. Singlas. 1993. Absorption of zidovudine in patients with diarrhoea. Eur. J. Clin. Pharmacol. 44:501-503. [DOI] [PubMed] [Google Scholar]