Abstract
An extensive series of heterometal-iron-sulfur single cubane-type clusters with core oxidation levels [MFe3S3Q]3+,2+ (M = Mo, W; Q = S, Se) has been prepared by means of a new method of cluster self-assembly. The procedure utilizes the assembly system [(tBu3tach)MVIS3]/FeCl2/Na2Q/NaSR in acetonitrile/THF and affords product clusters in 30–50% yield. The trisulfido precursor acts as a template, binding FeII under reducing conditions and supplying the MS3 unit of the product. The system leads to specific incorporation of a μ3-chalcogenide from an external source (Na2Q) and affords the products [(tBu3tach)MFe3S3QL3]0/1− (L = Cl−, RS−), among which are the first MFe3S3Se clusters prepared. Some 16 clusters have been prepared, 13 of which have been characterized by X-ray structure determinations including the incomplete cubane [(tBu3tach)MoFe2S3Cl2 (μ2-SPh)], a possible trapped intermediate in the assembly process. Comparisons of structural and electronic features of clusters differing only in atom Q at one cubane vertex are provided. In comparative pairs of complexes differing only in Q, placement of one selenide atom in the core increases core volumes by ca. 2% over the Q = S case, sets the order Q = Se > S in Fe-Q bond lengths and Q = S > Se in Fe-Q-Fe bond angles, causes small positive shifts in redox potentials, and has an essentially nil effect on 57Fe isomer shifts. Iron mean oxidation states and charge distributions are assigned to most clusters from isomer shifts. (tBu3tach = 1,3,5-tert-butyl-1,3,5-triazacyclohexane)
Introduction
The large majority of homo- and heterometallic iron-sulfur clusters of biomimetic relevance are synthesized under three protocols:1 (i) self-assembly, consisting of self-organizing reactions between simple mononuclear precursors and ligand reagents, and (ii) fragment condensation in which a preformed di- or polynuclear cluster reacts with a mononuclear species, itself, or another cluster. Certain clusters formed by these methods can undergo (iii) core rearrangement, reorganization of a pre-existing cluster from (i) or (ii) to a different core geometry. Examples include formation of Fe3S(SR)3,2 Fe4S43–5, and MFe3S4 (M = Mo, W)1,6,7 clusters by self-assembly, MFe3S4 (M = V8, Ni9,10), and M2Fe6S8 (M = Fe,11,12 Mo,13–15) clusters by fragment condensation, and M2Fe6S9 clusters (M = Mo,16,17 V18) by core rearrangement. Frequently, clusters employed in fragment condensation are prepared by self-assembly, in which the bridging ligand reagent is metal-bound or free sulfide, hydrosulfide, sulfur, RSSR, or RSSSR and a reductant, or (R3Si)2S. Cluster products obtained in this way have the invariant feature of bridging sulfide core atoms, always of the μ3-S mode when the core structure adopts the prevalent cubane-type geometry. Recent advances in synthesis have afforded new types of clusters with internal (M2Fe6S9) and interstitial (Fe8S7)19–21 μ6-S bridge components.
The foregoing high-nuclearity clusters are directly pertinent to synthetic endeavors directed toward the PN (Fe8S7) and FeMo-cofactor (MoFe7S9X) clusters of nitrogenase which contain internal μ6-S and interstitial μ6-X (X = C, N, or O) bridging elements, respectively.22–24 A largely unsolved problem is the deliberate installation of non-sulfur bridge atoms (preferably X) in clusters of nuclearity four and higher. If the problem is approached with less complex cubane-type species, Fe4(μ3-Se)425–34 and MFe3Se4 (M = Mo, W)35 clusters, prepared by self-assembly, become relevant as a first step in that direction. Lee and coworkers have made significant progress by preparation of two unprecedented types of cubane-type clusters, Fe4(NR)4 by self-assembly,36,37 and the mixed-ligand clusters Fe4Sn(NR)4-n (n = 1–3) by remarkable fragment condensation reactions.38 The latter results are noteworthy because of first successful introduction of nitrogen bridges (as imides) and the isolation of clusters with mixed-atom bridges. Early in the development of iron-sulfur cluster chemistry, it was shown that the clusters [Fe4Q4(SR)4]2−/3− (Q = S, Se) sustain core atom exchange reactions to form [Fe4SnSe4-n(SR)4]2−/3− in solution; mixed bridge atom clusters were detected by 1H NMR but not isolated.26
Recently, we have been experimenting with a new type of cluster assembly reaction based on trisulfido complexes of general formulation [L3MVIS3]0,1−. These are intended to act as templates in cluster self-assembly by providing in the product cluster a desired metal-trisulfido fragment MS3 which supports metal binding when reduced and whose other coordination sites are protected by a tridentate ligand L3. This work utilizes the compounds [(tBu3tach)MVIS3] (M = Mo, W; see Chart for abbreviations) prepared in this laboratory.39 Assembly reaction systems allow specific incorporation of a single selenide atom leading to cores of the type MS3Se that may be isolated in stable clusters. We report the synthesis, structures, and selected properties of such clusters.
Chart 1.
Abbreviations and Designation of Compounds
Experimental Section
Preparation of Compounds
All reactions and manipulations were performed under a pure dinitrogen atmosphere using either Schlenk techniques or an inert atmosphere box. Solvents were passed through an Innovative Technology solvent purification system prior to use. In the preparations that follow, reactants were nearly always used as suspensions in solvents of specified volume, filtrations were through Celite, solvent removal steps were performed in vacuo, and products were washed with ether and dried. Sodium sulfide, sodium selenide, and ferrous chloride were used in anhydrous form from commercial sources. All new compounds were identified by combinations of 1H NMR spectroscopy (in Me2SO-d6), X-ray structure determinations, and elemental analysis (Midwest Microlab, LLC, Indianapolis, IN 46250). Neutral compounds with three thiolate ligands are soluble in acetonitrile, THF and Me2SO. Neutral compounds with one thiolate or no thiolate ligand and cluster salts are soluble in acetonitrile and Me2SO. All compounds are extremely air-sensitive and must be handled accordingly.
[(tBu3tach)MoFe2S3Cl2 (μ2-SPh)]
To a suspension of [(tBu3tach)MoS3]39 (45 mg, 0.10 mmol) in 1 mL of methanol was added a solution of NaSPh (27 mg, 0.20 mmol) in 2 mL of methanol followed by a solution of FeCl2 (25 mg, 0.20 mmol) in 5 mL of methanol. The reaction mixture was stirred for 12 h. The red-brown solution was evaporated to dryness, the solid was extracted with 5 mL of THF, and the extract was filtered. The volume of the filtrate was reduced to ca. 0.5 mL, affording the product as a black crystalline solid (35 mg, 47%) which sometimes contained an impurity of [(tBu3tach)MoFe3S4Cl3]. The compound was identified by an X-ray structure determination.
[(tBu3tach)MoFe3S4Cl3]
To [(tBu3tach)MoS3] (45 mg, 0.10 mmol) in 1 mL of acetonitrile was added NaSPh (13.2 mg, 0.10 mmol) in 2 mL of acetonitrile followed by Na2S (12 mg, 0.15 mmol) in 2 mL of acetonitrile. FeCl2 (38 mg, 0.30 mmol) in 5 mL THF was immediately added to the solution and the reaction mixture was stirred for 36 h. The red-brown solution was evaporated to dryness, the solid was extracted with 5 mL of acetonitrile, and the extract was filtered. The filtrate was evaporated to dryness. The residue was dissolved in 1 mL of acetonitrile, the solution was filtered, and the filtrate was diffused with ether at −35°C for 2 d. The product was obtained as a black crystalline solid (32 mg, 40%), occasionally with the preceding cluster as an impurity. The compound was identified by an X-ray structure determination.
[(tBu3tach)MoFe3S4Cl2(SPh)]
To [(tBu3tach)MoS3] (45 mg, 0.10 mmol) in 1 mL of acetonitrile was added NaSPh (27 mg, 0.20 mmol) in 2 mL acetonitrile followed by Na2S (12 mg, 0.15 mmol) in 2 mL of acetonitrile. FeCl2 (38 mg, 0.3 mmol) in 5 mL THF was immediately added and the reaction mixture was stirred for 36 h. The red-brown solution was evaporated to dryness, the residue was extracted with 5 mL of acetonitrile, and the extract was filtered. The filtrate was evaporated to dryness. The residue was dissolved in 1 mL of acetonitrile, filtered, and diffused with ether at −35°C for 2 d to give the product as a black crystalline solid (25 mg, 30%). 1H NMR: δ 14.60 (m-H, 2), 3.33 (CH2, 6), 2.48 (CH3, 27), −2.00 (o-H, 2), −4.18 (p-H, 1).
[(tBu3tach)WFe3S4Cl2 (SPh)]
The preceding method on the same scale but with use of [(tBu3tach)WS3]39 was followed. The product was isolated as a black crystalline solid (28 mg, 30%). 1H NMR: δ 14.23 (m-H, 2), 3.34 (CH2, 6), 2.73 (CH3, 27), −3.60 (o-H, 2), −3.99 (p-H, 1)
[(tBu3tach)MoFe3S3SeCl2(SPh)]
The procedure on the same scale for [(tBu3tach)MoFe3S4Cl2 (SPh)] was followed but with use of Na2Se (19 mg, 0.15 mmol) and no Na2S added. The product was obtained as a black crytalline solid (30 mg, 35%). 1H NMR: δ 14.60 (m-H, 2), 3.33 (CH2, 6), 2.48 (CH3, 27), −1.71 (o-H, 2), −4.27 (p-H, 1).
[(tBu3tach)WFe3S3SeCl2(SPh)]
The preceding method on the same scale for [(tBu3tach)WFe3S4Cl2(SPh)] was employed but with use of Na2Se (19 mg, 0.15 mmol) and no Na2S added. The product was obtained as a black crystalline solid (30 mg, 31%). 1H NMR: δ 14.25 (m-H, 2), 3.34 (CH2, 6), 2.48 (CH3, 27), −3.47 (o-H, 2), −4.11 (p-H, 1).
[(tBu3tach)MoFe3S4(SEt)3]
To [(tBu3tach)MoS3] (45 mg, 0.10 mmol) in 1 mL of acetonitrile was added NaSEt (34 mg, 0.40 mmol) in 2 mL of acetonitrile followed by Na2S (12 mg, 0.15 mmol) in 2 mL acetonitrile. FeCl2 (38 mg, 0.3 mmol) in 5 mL of THF was immediately added and the reaction mixture was stirred for 36 h. The red-brown solution was evaporated to dryness, the solid was extracted with 5 mL of acetonitrile, and the extract was filtered. The filtrate was evaporated to dryness. The residue was dissolved in 1 mL of acetonitrile, filtered, and diffused with ether at −35°C for 2 d, affording the product as a black crystalline solid (45 mg, 54%).1H NMR: δ 9.09 (SCH2 CH3, 9), 3.30 (CH2, 6), 2.06 (CH3, 27), −8.44 (SCH2 CH3, 6).
[(tBu3tach)MoFe3S4(SEt)3](Na)
The preceding procedure on the same scale was used but with an increased amount of NaSEt (42 mg, 0.50 mmol). The compound was isolated as a black crystalline solid (55 mg, 52%). Anal. Calcd. for C21H48Fe3MoN3NaS7: C, 29.55; H, 5.67; N, 4.92. Found: C, 30.03; H, 5.93; N, 4.51. 1H NMR: δ 9.60 (SCH2CH3, 9), 3.30 (CH2, 6), 2.05 (CH3, 27), −9.32 (SCH2CH3, 6).
[(tBu3tach)MoFe3S4(SMe)3][Na(MeCN)]
The preceding method on the same scale was used but with NaSMe (35 mg, 0.50 mmol). The compound was isolated as a black crystalline solid (45 mg, 53%). 1H NMR: δ 3.32 (CH2,6), 2.05 (CH3, 27) −9.67 (SCH3, 9). Anal. Calcd. for C18H42Fe3MoN3NaS7·C2H3N: C, 28.18; H, 5.32; N, 6.57. Found: C, 28.56; H, 5.38; N, 6.34.
[(tBu3tach)MoFe3S3Se(SEt)3][Na(CH3CN)3]
To [(tBu3tach)MoS3] (45 mg, 0.10 mmol) in 1 mL of acetonitrile was added NaSEt (42mg, 0.5 mmol) in 2 mL of acetonitrile followed by Na2Se (19 mg, 0.15 mmol) in 2 mL acetonitrile. FeCl2 (38 mg, 0.3 mmol) in 5 mL THF was immediately added and the reaction mixture was stirred for 36 h. Reaction workup is the same as for the preceding sodium salt, yielding the product as a black crystalline solid (50 mg, 45%). 1H NMR: δ 10.25 (SCH2CH3, 9), 3.30 (CH2, 6), 1.36 (CH3, 27), −8.48 (SCH2CH3, 6).
[(tBu3tach)MoFe3S3Se(SMe)3][Na(Et2O)]
The preceding method on the same scale was used but with NaSMe (35 mg, 0.50 mmol). The compound was isolated as a black crystalline solid (45 mg, 50%). 1H NMR: δ 3.35 (CH2,6), 1.35 (CH3, 27) −8.74 (SCH3, 9). Anal. Calcd. for C18H42Fe3MoN3NaS6Se·C4H10O: C, 26.83; H, 5.29; N, 4.69. Found: C, 26.98; H, 5.09; H, 4.79.
[(tBu3tach)WFe3S3Se(SEt)3]
To [(tBu3tach)WS3] (54 mg, 0.10 mmol) in 1 mL of acetonitrile was added NaSEt (42 mg, 0.50 mmol) in 2 mL of acetonitrile followed by Na2Se (19 mg, 0.15 mmol) in 2 mL of acetonitrile. FeCl2 (38 mg, 0.30 mmol) in 5 mL of THF was immediately added and the reaction mixture was stirred for 36 h. The workup procedure was the same as for [(tBu3tach)MoFe3S4(SEt)3], yielding the product as a black crystalline solid (40 mg, 41%). Anal. Calcd. for C21H48Fe3N3S6SeW: C, 26.13; H, 5.01; N, 4.35. Found: C, 26.02; H, 5.09; N, 4.33. 1H NMR): δ 8.39 (SCH2CH3, 9), 3.29 (CH2, 6), 1.52 (CH3, 27), −7.72 (SCH2CH3, 6).
[(tBu3tach)WFe3S4(SEt)3]
The preceding method was followed on the same scale but with use of Na2S (12 mg, 0.15 mmol) instead of Na2Se, affording the product as black crystals (40 mg, 43%). 1H NMR: δ 8.77 (SCH2CH3, 9), 3.35 (CH2, 6H), 1.91 (CH3, 27), −9.29 (SCH2CH3, 6).
[(tBu3tach)MoFe3S4(SPh)3]
To [(tBu3tach)MoS3] (45 mg, 0.1 mmol) in 1 mL of acetonitrile was added NaSPh (52 mg, 0.4 mmol) in 2 mL acetonitrile followed by Na2S (12 mg, 0.15 mmol) in 2 mL of acetonitrile. FeCl2 (38 mg, 0.3 mmol) in 5 mL of THF was immediately added to the solution and the reaction mixture was stirred for 36 h. The red-brown solution was evaporated to dryness, the solid was extracted with 5 mL of THF, and the extract was filtered. The filtrate was taken to dryness. The residue was dissolved in 1 mL of acetonitrile; the solution was filtered and diffused with ether at −35°C for 2 d, affording the product as a black crystalline solid (33 mg, 31%). Anal. Calcd. for C33H48Fe3MoN3S7: C, 40.66; H, 4.96; N, 4.31. Found: C, 39.55; H, 4.97; N, 4.45. 1H NMR: δ 14.81 (m-H, 6), 3.31 (CH2, 6), 2.48 (CH3, 27), −2.68 (o-H, 6), −4.24 (p-H, 3).
[(tBu3tach)WFe3S4(SPh)3]
The preceding method was followed on the same scale but with use of [(tBu3tach)WS3]. The product was obtained as a black crystalline solid (35 mg, 30%). 1H NMR: δ 14.24 (m-H, 6), 3.31 (CH2, 6), 2.71 (CH3, 27), −3.63 (o-H, 6), −3.99 (p-H, 3).
[(tBu3tach)MoFe3S3Se(SPh)3]
To[(tBu3tach)MoS3] (45 mg, 0.10 mmol) in 1 mL of acetonitrile was added NaSPh (52 mg, 0.40 mmol) in 2 mL of acetonitrile followed by Na2Se (19 mg, 0.15 mmol) in 2 mL of acetonitrile. A suspension of ferrous chloride (38 mg, 0.3 mmol) in 5 mL THF was immediately added to the solution and the reaction mixture was stirred for 36 h. The reaction workup followed that for [(tBu3tach)MoFe3S4(SPh)3], leading to the product as a black solid (35 mg, 33%) which analyzed as an acetonitrile hemisolvate (in conformity with the X-ray structure). Anal. Calcd. for C33H48Fe3MoN3S6Se·0.5 C2H3N: C, 39.19; H, 4.79; N, 4.70. Found: C, 38.90; H, 4.69; N, 4.43. 1H NMR: δ 14.75 (m-H, 6), 3.30 (CH2, 6), 2.22 (CH3, 27), −1.99 (o-H, 6), −4.28 (p-H, 3).
[(tBu3tach)WFe3S3Se(SPh)3]
The preceding procedure was employed but with use of [(tBu3tach)WS3], affording the product as a black solid (35 mg, 31%). Anal. Calcd. for C33H48Fe3N3S6SeW: C, 35.72; H, 4.36; N, 3.79. Found: C, 35.51; H, 4.38; N, 3.77. 1H NMR : δ 14.34 (m-H, 6), 3.31 (CH2, 6), 2.48 (CH3, 27), −3.64 (o-H, 6), −4.15 (p-H, 3).
In the sections following, clusters are numerically designated according to the Chart
X-ray Structure Determinations
The structures of the 13 compounds in Tables 1 and 2 were determined. Diffraction-quality crystals were obtained by ether diffusion into acetonitrile solutions. Crystal mounting and data collections were performed as described40 on a Bruker APEX II CCD single-crystal diffractometer. Data for compound 7b were collected at the Advanced Photon Source. None of the crystals showed significant decay during the data collection procedure. Raw data were integrated and corrected for Lorentz and polarization effects using Bruker APEX II program suite.41 Absorption corrections were performed using SADABS. Space groups were assigned by analysis of symmetry and systematic absences (determined by XPREP) and were further checked by PLATON. Structures were solved by direct methods and refined against all data in the 2θ ranges by full-matrix least-squares on F2 with the SHELXL program suite42 using the OLEX 2 interface.43 Hydrogen atoms at idealized positions were included in final refinements. The OLEX 2 interface was used for structure visualization and drawing ORTEP figures (shown in Figure 3 and 4). Refinement details and explanations (wherever necessary) are included in the individual CIF files. Crystallographic data and final agreement factors are given in Tables 1 and 2.44
Table 1.
| compounds | 2 | 3 | 4a | 4b | 6 | 7a | 8a |
|---|---|---|---|---|---|---|---|
| formula | C21H38Cl2Fe2 MoN3S4. 1.5(CH4O) |
C15H33Cl3Fe3 MoN3S4 |
C21H38Cl2Fe3Mo N3S5 |
C21H38Cl2Fe3 MoN3S4Se |
C21H48Fe3 MoN3S7 |
C21H48Fe3N3 S6SeW |
C18H42Fe3MoN3 NaS7. 0.5(C4H10O) |
| formula weight | 787.42 | 753.56 | 827.28 | 874.17 | 830.6 | 965.39 | 848.57 |
| crystal system | monoclinic | orthorhombic | monoclinic | monoclinic | monoclinic | orthorhombic | monoclinic |
| space group | Cc | Pnma | P21 | P21/n | Pc | Pna21 | P21/n |
| Z | 2 | 4 | 2 | 4 | 2 | 4 | 2 |
| a, Å | 10.314(4) | 18.3532(18) | 14.516(3) | 9.8517(8) | 9.8714(19) | 19.982(3) | 9.8665(14) |
| b, Å | 17.255(7) | 14.7661(14) | 10.013(2) | 19.9821(17) | 10.0395(19) | 16.568(2) | 20.871(3) |
| c, Å | 19.572(9) | 9.5624(9) | 14.518(3) | 17.4884(15) | 19.241(3) | 9.7591(12) | 17.322(2) |
| α, deg | 90 | 90 | 90 | 90.00 | 90 | 90.00 | 90 |
| β, deg | 93.301(9) | 90 | 112.545(3) | 90.024(2) | 120.723(7) | 90.00 | 91.349(3) |
| γ, deg | 90 | 90 | 90 | 90.00 | 90 | 90.00 | 90 |
| V, Å3 | 3477(3) | 2591.5(4) | 1948.9(7) | 3442.7(5) | 1639.2(5) | 3230.8(7) | 3566.0(9) |
| dcalcd, g/cm3 | 1.504 | 1.931 | 1.410 | 1.687 | 1.683 | 1.985 | 1.581 |
| µ, mm−1 | 1.594 | 2.76 | 1.828 | 3.066 | 2.138 | 1.491 | 1.979 |
| max, min peaks, e.Å−3 | 1.709, −1.416 | 3.720, −7.290 | 1.565, −1.106 | 1.173, −1.388 | 1.926, −1.138 | 4.204, −3.746 | 2.794, −2.199 |
| 2θ range, deg | 4.16–51.46 | 4.44–51.48 | 3.38–51.5 | 3.1–51.44 | 4.06–51.4 | 1.86–32.46 | 3.06–51.42 |
| R1c (wR2d) | 0.0638(0.1040) | 0.0687(0.1662) | 0.0787(0.2229) | 0.0801(0.1446) | 0.0973(0.2010) | 0.0659(0.1967) | 0.0565 (0.1549) |
| GOF (F2) | 1.082 | 1.062 | 1.065 | 1.086 | 1.035 | 1.039 | 1.067 |
Mo Kα radiation (λ = 0.71073 Å).
Synchrotron source (λ = 0.41328 Å).
R1=Σ| |F0| − |Fc| | /Σ |F0|.
wR2 = {Σ[w(F02 − Fc2)2]/Σ[w(F02)2]}1/2
Table 2.
Crystallographic Data for Compounds at 100 Ka
| compounds | 8b | 9a | 9b | 10a | 10b | 11a |
|---|---|---|---|---|---|---|
| formula | C18H42Fe3Mo N3NaS6Se. 0.5(C4H10O) |
C27H57Fe3Mo N6NaS7. (C4H10O) |
C27H57Fe3Mo N6NaS6Se. (C4H10O) |
C33H48Fe3Mo N3S7. 2(C2H3N) |
C33H48Fe3Mo N3S6.Se 0.5 (C2H3N) |
C37H54Fe3N5S7W |
| formula weight | 895.46 | 1050.88 | 1097.77 | 1056.83 | 1042.14 | 1144.67 |
| crystal system | monoclinic | monoclinic | monoclinic | monoclinic | triclinic | monoclinic |
| space group | P21/n | P21/n | P21/n | P21/n | P-1 | P21/n |
| Z | 2 | 4 | 4 | 4 | 2 | 4 |
| a, Å | 9.9215(10) | 13.1400(15) | 13.1604(18) | 13.137(3) | 12.296(3) | 13.1609(17) |
| b, Å | 20.895(2) | 18.913(2) | 19.000(3) | 20.952(5) | 18.323(4) | 20.997(3) |
| c, Å | 17.3048(18) | 19.779(2) | 19.816(3) | 16.063(4) | 19.022(4) | 16.036(2) |
| α, deg | 90.00 | 90.00 | 90.00 | 90.00 | 81.471(3) | 90 |
| β, deg | 91.424(2) | 93.348(2) | 93.553(2) | 96.336(4) | 76.904(3) | 96.772(2) |
| γ, deg | 90.00 | 90.00 | 90.00 | 90.00 | 78.096(3) | 90 |
| V, Å3 | 3586.4(6) | 4907.1(10) | 4945.6(12) | 4394.4(19) | 4061.2(14) | 4400.4(10) |
| dcalcd, g/cm3 | 1.658 | 1.423 | 1.474 | 1.597 | 1.704 | 1.728 |
| µ, mm−1 | 2.925 | 1.456 | 2.138 | 1.616 | 2.587 | 3.941 |
| max, min peaks, e.Å−3 | 2.818, −3.046 | 1.504, −1.406 | 1.585, −2.076 | 4.140, −1.396 | 3.149, −1.924 | 3.514, −1.718 |
| 2θ range, deg | 3.06–51.38 | 3.62–51.36 | 3.76–51.4 | 3.20–51.46 | 3.00–51.62 | 3.68–51.38 |
| R1b (wR2c) | 0.0683(0.1942) | 0.0785(0.2070) | 0.0778(0.1927) | 0.0672(0.1573) | 0.0732(0.1930) | 0.0299(0.0803) |
| GOF (F2) | 1.077 | 1.066 | 1.082 | 1.047 | 1.099 | 1.057 |
Mo Kα radiation (λ = 0.71073 Å).
R1=Σ | |F0| − |Fc| | /Σ |F0|.
wR2 = {Σ[w(F02 − Fc2)2]/Σ[w(F02)2]}1/2
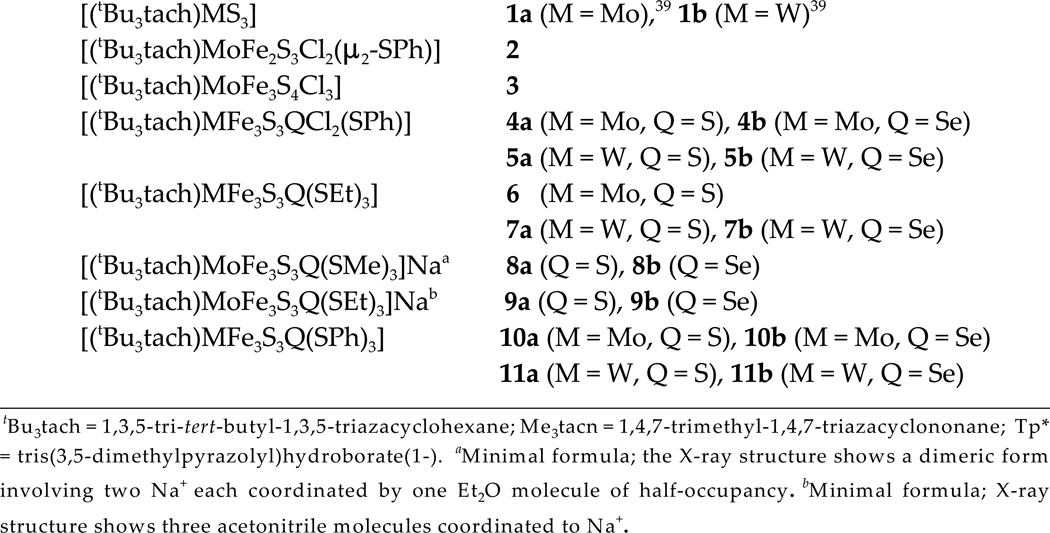
Figure 3.
Structures of single cubanes (2, 3, 7b, 9a, 9b and 11a) shown with 50% probability ellipsoids and partial atom labeling schemes. Hydrogen atoms are omitted for clarity.
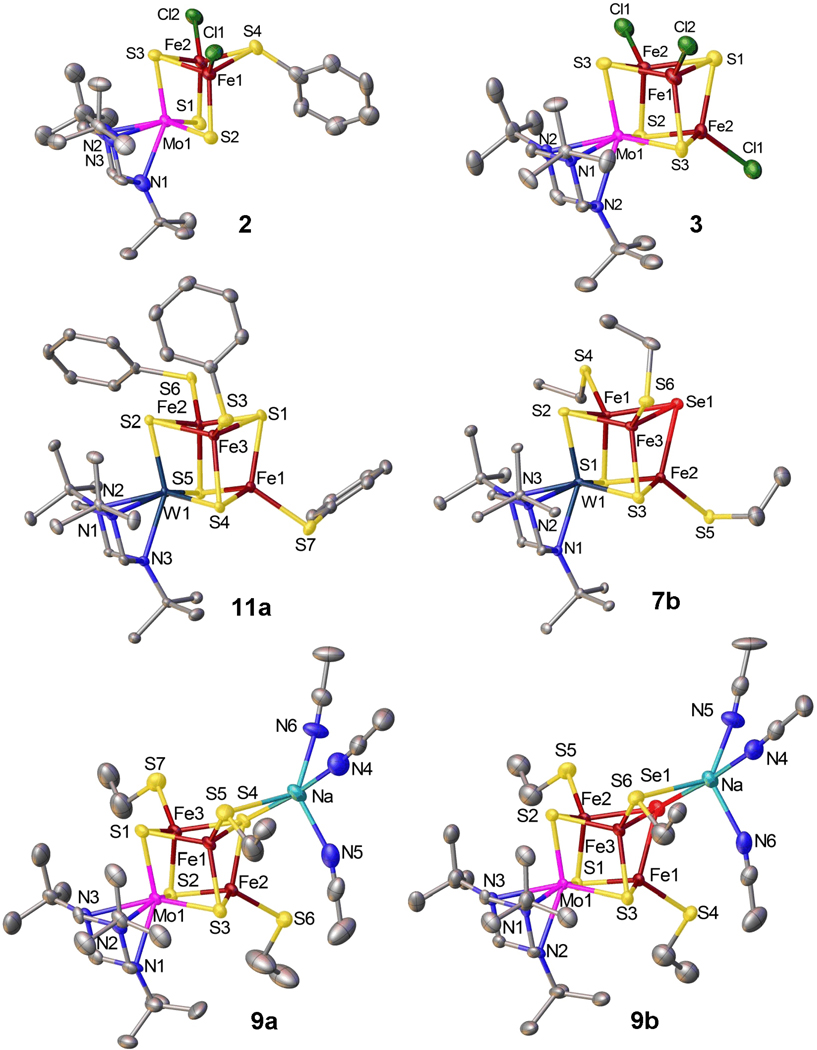
Figure 4.
Structures of single cubanes (4a, 4b, 8a, 8b, 10a and 10b) shown with 50% probability ellipsoids and partial atom labeling schemes. Hydrogen atoms are omitted for clarity. 8a and 8b exist in dimeric forms involving two sodium atoms each coordinated with diethylether molecule of one half occupancy.
Other Physical Measurements
1H NMR spectra were obtained with a Varian M400 spectrometer in Me2SO-d6 solutions. Absorption spectra were measured on a Varian Cary 50 Bio spectrophotometer. 57Fe Mössbauer spectra were measured with a constant acceleration spectrometer. Data were analyzed with Igor Pro 6 software (Wavemetrics, Portland, OR); isomer shifts are referenced to iron metal at room temperature. Cyclic voltammetry measurements were made with a BioAnalytical Systems Epsilon potentiostat/galvanostat in acetonitrile solutions at 100 mV/s using a glassy carbon working electrode, 0.1 M (Bu4N)(PF6) supporting electrolyte, and an SCE reference electrode.
Results and Discussion
Synthesis and Scope
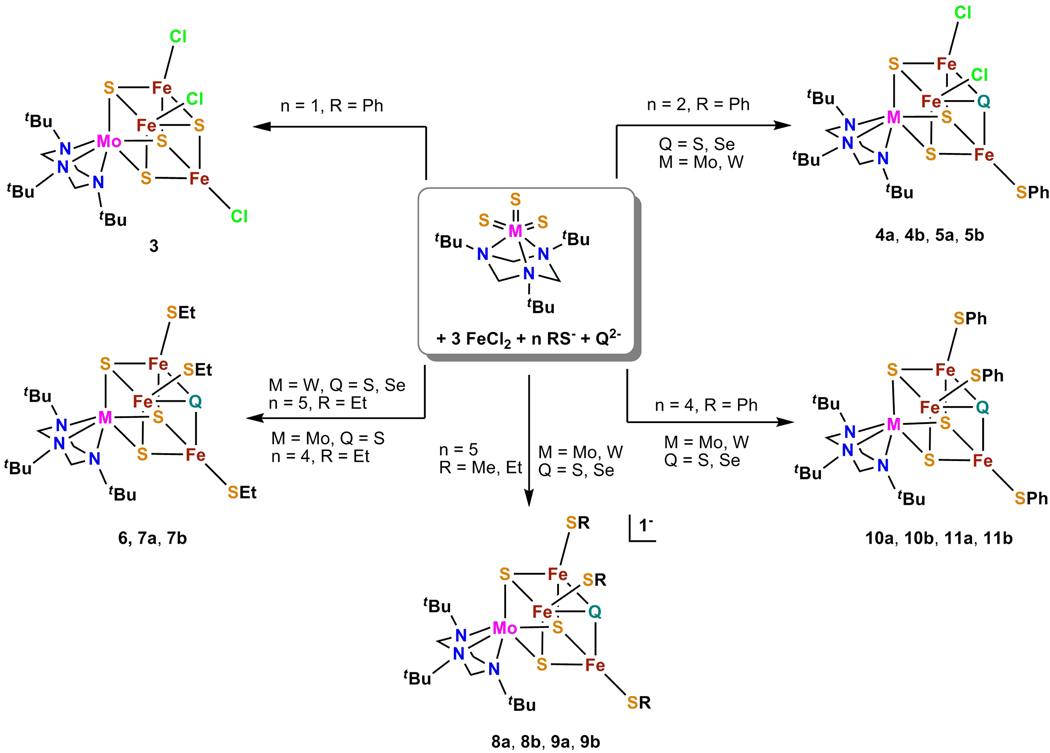
In the cluster assembly systems of Figure 1, the trisulfido complexes [(tBu3tach)MVIS3] (M = Mo, W) are utilized as scaffolds in which an intact MS3 group is converted to the plausible heterometal unit [MFe3(μ3-S)3] and the tridentate tBu3tach ligand blocks reaction at three M sites. This unit is capable of incorporating a fourth chalcogenide atom to complete the cubane-type core [MFe3S3Q]3+,2+ (Q = S, Se). Preparation of 1a and 1b involves sulfide transfer to [(tButach)MVIO3] by Lawesson's reagent ((p-MeOC6H4PS2)2).39 During reinvestigation of the isolation of 1a, we obtained a molybdenum derivative of this reagent, [(tBu3tach)MoO(μ2-S)2(S)P(p-C6H4OMe)], whose X-ray structure suggests that it may be an intermediate in the reaction. Because no Mo or W complex with Lawesson's reagent has been obtained previously, we include the structure of this compound.44
Figure 1.
Schematic depiction of the synthesis of (Mo/W)-Fe-(S/Se) clusters.
The cluster assembly systems in Figure 1 consist of 1a or 1b, FeCl2, Na2Q, and NaSR in THF/acetonitrile. The quantity of thiolate controls the number of iron-bound ligands and core redox state as reflected by cluster charge, leading to isolation of the neutral clusters 3, 4ab, 6, 10ab, and 11ab with 3+ cores and monanionic clusters 8ab and 9ab with 2+ cores. With 1b only the neutral clusters 5ab, 7ab, and 11ab have been isolated. Neutral clusters are formed under the generalized stoichiometry of reaction 1 (n = 1, 2, 4) and monoanionic clusters by reaction 2 (Q = S, Se for both) in which additonal thiolate acts as a reductant. Under the conditions that afford reduced molybdenum clusters 9ab, neutral tungsten clusters 7ab are isolated. This result is apparently another manifestation of the greater difficulty in reducing the tungsten member of an isoligated isostructural Mo/W pair of complexes.45
| (1) |
| (2) |
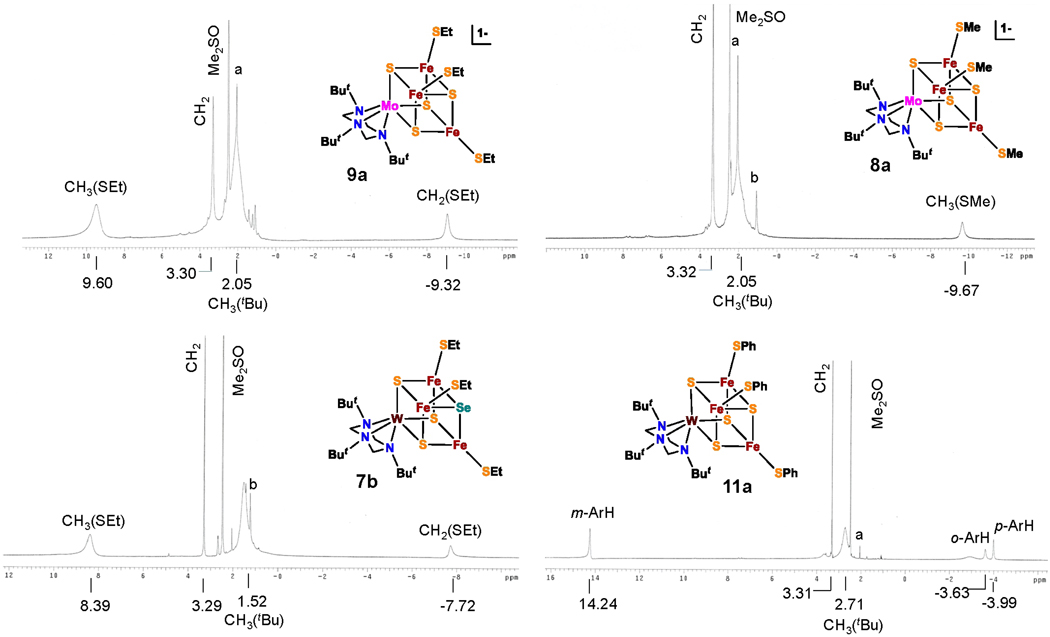
The cubane clusters exhibit isotropically shifted 1H NMR spectra as a consequence of paramagnetism, presumably similar to that of the [MoFe3S4]3+ (S = 3/246) and [MoFe3S4]2+ (S = 246,47) oxidation states determined for other clusters. These spectra, four of which (7b, 8a, 9a, 11a) encompassing both oxidation states, are included in Figure 2, are useful in cluster identification. The alternating signs of the o-H (+10.9 ppm), m-H (−7.0 ppm) and p-H (+11.1 ppm) isotropic shifts of benzenethiolate cluster 11a indicate that these shifts are dominantly contact in origin.48 The same behavior is exhibited by 4ab, 5ab, 10ab, and 11b. The spectrum of methanethiolate cluster 8a (−9.67 ppm) leads the assignment of the CH2 (−9.32 ppm)) and CH3 (9.60ppm) signals of ethanethiolate cluster 9a and also 6, 7ab, and 9b. While these spectra could have been assigned on the basis of relative intensities, the oppositely signed isotropic shifts of the SEt group (CH2, −8.57 ppm; CH3, +11.52 ppm) are unexpected. Nearly always, contact shifted proton resonances of n-alkyl groups attenuate along the chain with the same sign in the absence of dipolar contributions to the shifts, a behavior observed for other [MoFe3S4]3+ clusters.49–51 The first examples of 1H NMR spectra of [MoFe3S4]2+ clusters with alkylthiolate ligands are reported here.
Figure 2.
1H NMR spectra of representative clusters in Me2SO-d6. Signal assignments are indicated. Peaks denoted as a and b are CH3 CN and (CH3 CH2)2O respectively.
Also prepared in this work, by means of reaction 3, is the incomplete cubane 2, so designated because of the vacancy of an iron atom in one vertex of a cubane. The cluster, shown in Figure 3, may be considered as a trapped intermediate in the cluster assembly
| (3) |
process. Once the μ2-SPh bridge is formed, cubane formation does not proceed and 2 is isolated as a stable solid. On occasion, cubane 3 is found as an impurity in the preparation of 2 and conversely. The X-ray structure of one crystal contained both 2 and 3.44 Cluster 3 has also been isolated in low yield by reaction 1 with no externally added Na2S, indicating that initial complex 1a is susceptible to sulfide extraction. This event may lead to undesirable side reactions and may contribute to the observed moderate yields (ca. 30–50%) of the cubane clusters. However, all compounds were isolated by essentially the same procedure as crystalline solids that meet analytical and/or 1H NMR standards of purity, and are readily accessible.
Cluster Structures
The cubane formulation of clusters has been verified by X-ray structure determinations of 12 compounds. Structures (except 6) are presented in Figures 3 and 4. Bond distances and angles involving variable atom Q = S, Se are collected in Table 3 together with cluster volumes calculated from atom coordinates. Other bond distances and angles are unexceptional and are available elsewhere.44 We make several brief observations. (i) Mo-S and W-S distances in the entire set of clusters occur in the range 2.29–2.35 Å, and are substantially longer that the MoVI-S distance of 2.173(6) Å in 1a,39 a behavior consistent with reduction to the MIII/IV level in reactions 1–3. (ii) N-M-N angles are nearly invariant at 59.5–61.3° and correspond closely to those in other M0,IV,VI complexes (58–61°) with the same 3 ligand.39,52,53 These angles, dictated by the 6-membered ring of tBu3tach, suggest chelate ring strain larger than that in the less trigonally distorted, generally robust complexes of the type [MIII–VI(Me3tacn)L3]z in which the ligand is a 9-membered ring with N-M-N angles in the range 73–79° (M = Mo, W).39,54–57 Ring detachment owing to strain may occur during synthesis but the binding is sufficient to allow serviceable yields. (iv) Structurally comparable pairs of clusters 4ab, 8ab, 9ab, and 10ab differ only in atom Q and afford the bond length and angle orders Fe-Se > Fe-S and Fe-S-Fe > Fe-Se-Fe for individual and mean values. Although the standard deviation of mean bond lengths is large, the differences between comparable pairs is 0.10–0.13 Å; likewise, the difference between angles is 1.6–3.4°.
Table 3.
Selected Bond Distances, Angles and Core Volumes.
| compoundsa | Fe-Q bond distancesb (Å) | Fe-Q-Fe anglesb (°) | ||
|---|---|---|---|---|
| Q = S | Q = Se | Q = S | Q = Se | |
| 3 | 2.267(3), 2.299(3)c, 2.299(3)c | ----- | 72.75(9)c, 72.75(9)c, 71.3(1) | ----- |
| 4a | 2.234(3), 2.255(3), 2.264(4) | ----- | 71.4(1), 71.4(1), 71.62(10) | ----- |
| 4b | ----- | 2.339(2), 2.382(2), 2.418(3) | ----- | 68.25(7), 70.60(7), 70.77(7) |
| 6 | 2.249(7), 2.235(6), 2.322(7) | ----- | 73.8(2), 71.7(2), 71.0(2) | ----- |
| 7b | ----- | 2.349(3), 2.413(3), 2.424(3) | ----- | 67.43(8), 69.81 (8), 70.46(8) |
| 8a | 2.264(2), 2.303(2), 2.331(2) | ----- | 69.12(5), 71.70(5), 73.11(6) | ----- |
| 8b | ----- | 2.388(2), 2.389(2), 2.412(2) | ----- | 66.90(4), 68.88(5), 70.12(5) |
| 9a | 2.270(3), 2.328(2), 2.336(3) | ----- | 69.98(8), 71.99(8), 72.55(8) | ----- |
| 9b | ----- | 2.367(2), 2.425(2), 2.434(2) | ----- | 67.05(5), 69.40(5), 69.90(5) |
| 10a | 2.236(2), 2.287(3), 2.315(3) | ----- | 70.99(8), 73.21(8), 73.75(8) | ----- |
| 10b | ----- | 2.326(2), 2.400(2), 2.410 (2) | ----- | 67.64(5), 70.03(5), 70.06 (5) |
| 11a | 2.220(1), 2.292(1), 2.306(1) | ----- | 70.97(3), 73.24(3), 74.11(3) | ----- |
| core volumes (Å3) | ||||
| MFe3 | Q4 | MFe3Q4 | ||
| 9a | 2.34 | 5.72 | 9.46 | |
| 9b | 2.36 | 5.94 | 9.64 | |
| 10a | 2.33 | 5.57 | 9.35 | |
| 10b | 2.33 | 5.83 | 9.49 | |
M = Mo except 7b and 11a (M = W);
See Figure 1 for position of Q in clusters;
Equal by symmetry element.
Oxidation States and Charge Distribution
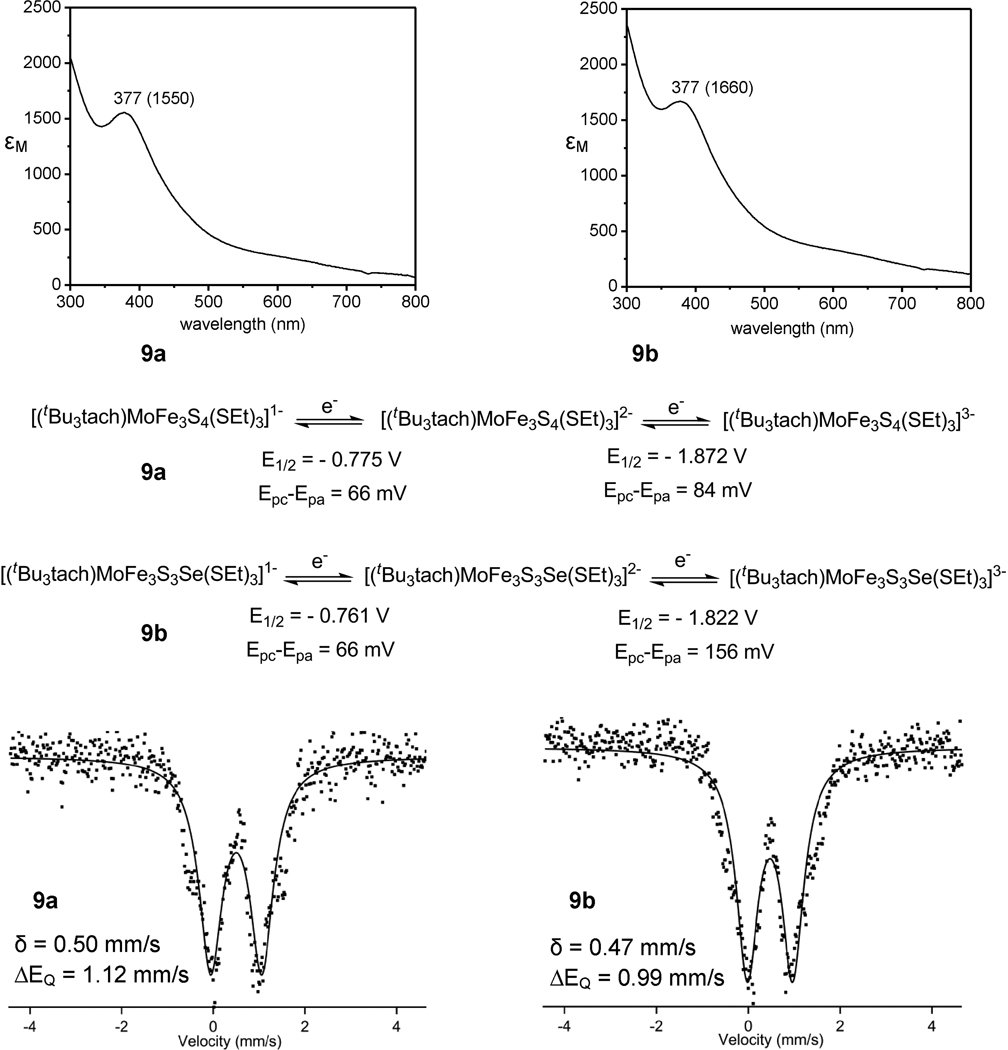
A property of interest in heterometal iron-sulfur clusters is charge distribution as approximated by metal oxidation states. The procedure in this laboratory is to determine iron (mean) oxidation states by 57Fe isomer shifts and infer that of the heterometal by difference. Mössbauer spectra of eight clusters were measured at 100 K; isomer shifts (δ) and quadrupole splittings are listed in Table 4. All spectra consist of a single quadrupole doublet, indicating delocalized electronic structures of these mixed valence clusters. Iron oxidation states s were calculated from the empirical relation δ = 1.43 – 0.40s which applies to tetrahedral FeS4 sites at or near 77 K.4 The values s = 2.33+ and 2.67+ correspond to the formal designations M3+Fe2+2Fe3+ and M3+Fe2+Fe3+2, respectively. Reduced clusters 9a (2.32+) and 9b (2.40+) belong to the first designation. Oxidized clusters 8a and 10a (both 2.67+), as practically all [MFe3S4]3+ clusters examined in this laboratory, belong to the second. The empirical relationship does not strictly apply to 4ab because of chloride ligation. Values for 7b and 10b are intermediate, and no conclusions can be drawn.
Table 4.
Mössbauer Parameters (100 K) for Selected Clusters.
| compounds | δ (mm/s)a | ΔEQ (mm/s) |
|---|---|---|
| 4a | 0.42 | 0.88 |
| 4b | 0.45 | 0.84 |
| 7b | 0.44 | 0.88 |
| 8a | 0.36 | 0.76 |
| 9a | 0.50 | 1.12 |
| 9b | 0.47 | 0.99 |
| 10a | 0.36 | 0.76 |
| 10b | 0.41 | 0.83 |
Referenced with Fe metal at room temperature.
Sulfur vs. Selenium
The difference in ionic radii between Se2− and S2− is 1.91 – 1.84 = 0.07 Å and between covalent radii is 1.17 – 1.04 = 0.13 Å.58 These differences are reflected in the preceding bond distance and angle orders, which are characteristic of all comparative pairs of S/Se compounds of any type in which only atom Q is varied. A further indication of these effects is seen in the core volumes of two comparative pairs, 9ab and 10ab. While values of the MFe3 tetrahedra are practically invariant, the entire core MFe3Q4 volume increases by 1.9% (9ab) and 1.5% (10ab). The effect is small and expectedly smaller than replacing 4S with 4Se, as in the pair [Fe4Q4(SPh)4]2− where the core volumes are 9.55 Å3 (Q = S)59 and 10.54 Å3 (Q = Se),25 a 10.4% increase. Other properties are summarized in Figure 5 for the comparative pair 9ab as an example. The energy of the charge transfer band near 380 nm is unchanged and band intensites are only slightly affected. The clusters shown two chemically reversible redox steps in the −0.7 to −2.0 V range with the potentials of the selenium-containg cluster being more positive25 by 10 and 50 mV. Mössbauer spectra are nearly the same and isomer shifts are within experimental uncertainty of ca. ±0.02 mm/s.
Figure 5.
Absorption spectra (in acetonitrile), redox couples (in acetonitrile) and Mössbauer spectra (100 K) of complexes 9a and 9b.
Summary
This report describes the synthesis, characterization, and comparative study of isostructural Mo/W-Fe-S single cubane clusters that differ by only one vertex atom Q = S or Se. The following are the principal results and findings of this investigation.
A new self-assembly system has been developed for heterometal-iron-sulfur single cubane clusters that allows selective incorporation of sulfide or selenide at one vertex of the structure. The method utilizes as templates the compounds [(tBu3tach)MVIS3] (M = Mo, W), which furnish three of the four chalcogenide atoms in the product cubane core [MFe3S3Q] as the MS3 fragment.
An extensive series of clusters with the cores [MFe3S3Q]3+,2+ has been prepared by assembly reactions 1 and 2 (30–50% purified yields) and characterized by X-ray structure determinations. The presence of Q = Se retains the cubane structure with small changes in bond distances and angles that are intrinsic to the structural behavior of selenium vs. sulfur.
A possible trapped intermediate [(tBu3tach)MoFe2S3Cl2(μ2-SPh)] in the assembly process has been isolated and shown to have an incomplete cubane structure with one vertex iron atom missing.
Based on the comparative properties of [(tBu3tach)MoFe3S3Q(SEt)3]1−, the Q = Se atom produces an ca. 2% increase in core volume, slightly less negative redox potentials (less easily oxidized), and virtually no change in 57Fe isomer shift or UV-visible absorption spectrum. Isomer shifts indicate that both clusters correspond to the M3+Fe2+2Fe3+ oxidation state formalism. These findings are consistent with the properties of the pair [Fe4Q4(SPh)4]2− for which, however, the effects of selenide become more pronounced because of complete chalcogenide substitution.25
The existence of the cluster [Fe4S3(NtBu)Cl4]2–38 makes evident the ability of weak-field cubane sterechemistry to incorporate a much smaller non-sulfur atom (Fe-N 1.95 Å) in addition to sulfide itself (mean Fe-S 2.31(4) in 9a) and the larger selenide (mean Fe-Se 2.41(4) in 9b). This raises the possibility that anions containing oxygen or nitrogen may be incorporated as μ3 bridges using the methods employed in this work. This investigation is underway. Thereafter, it remains to be learned if non-sulfur bridge atoms can be introduced in larger structures and manipulated into an interstitial position by core rearrangement.
Supplementary Material
Acknowledgments
This research was supported by NIH Grant GM-28856. We thank Dr. Shao-Liang Zheng for his help with X-ray data collection and structure determinations. We thank Prof. S. C. Lee for volume calculations and Dr. Yu-Sheng Chen at ChemMat CARS, Advanced Photon Source, Argonne National Laboratory, for his assistance with single crystal data for compound 7b. ChemMat CARS Sector 15 is principally supported by the National Science Foundation/Department of Energy under Grant No. NSF/CHE-0822838. Use of the Advanced Photon Source was supported by the Department of Energy, Office of Science, Office Basic Energy Sciences under Contract No. DE-AC02-06CH11357.
Footnotes
Supporting Information Available. X-ray crystallographic files in CIF form for all compounds in Tables 1 and 2, depiction of additional cluster structures, and additional 1H NMR and Mössbauer spectra. This material is available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- 1.Lee SC, Holm RH. Chem. Rev. 2004;104:1135–1157. doi: 10.1021/cr0206216. [DOI] [PubMed] [Google Scholar]
- 2.Pryadun R, Holm RH. Inorg. Chem. 2008;47:3366–3370. doi: 10.1021/ic7023742. [DOI] [PubMed] [Google Scholar]
- 3.Holm RH. Iron-Sulfur Clusters. In: Que L Jr, Tolman WA, editors. Bio-coordination Chemistry. Oxford: Elsevier; 2004. pp. 61–90. [Google Scholar]
- 4.Rao PV, Holm RH. Chem. Rev. 2004;104:527–559. doi: 10.1021/cr020615+. [DOI] [PubMed] [Google Scholar]
- 5.Sharp CR, Duncan JS, Lee SC. Inorg. Chem. 2010;49:6697–6705. doi: 10.1021/ic100742c. [DOI] [PubMed] [Google Scholar]
- 6.Holm RH, Simhon ED. In: Molybdenum Enzymes. Spiro TG, editor. New York: Wiley; 1985. pp. 1–87. [Google Scholar]
- 7.Fomitchev DV, McLauchlan CC, Holm RH. Inorg. Chem. 2002;41:958–966. doi: 10.1021/ic011106d. [DOI] [PubMed] [Google Scholar]
- 8.Kovacs JA, Holm RH. Inorg. Chem. 1987;26:702–711. [Google Scholar]
- 9.Ciurli S, Ross PK, Scott MJ, Yu S-B, Holm RH. J. Am. Chem. Soc. 1992;114:5415–5423. [Google Scholar]
- 10.Panda R, Berlinguette CP, Zhang Y, Holm RH. J. Am. Chem. Soc. 2005;127:11092–11101. doi: 10.1021/ja052381s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou H-C, Holm RH. Inorg. Chem. 2003;42:11–21. doi: 10.1021/ic020464t. [DOI] [PubMed] [Google Scholar]
- 12.Deng L, Holm RH. J. Am. Chem. Soc. 2008;130:9878–9886. doi: 10.1021/ja802111w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demadis KD, Campana CF, Coucouvanis D. J. Am. Chem. Soc. 1995;117:7832–7833. [Google Scholar]
- 14.Berlinguette CP, Miyaji T, Zhang Y, Holm RH. Inorg. Chem. 2006;45:1997–2007. doi: 10.1021/ic051770k. [DOI] [PubMed] [Google Scholar]
- 15.Scott TA, Holm RH. Inorg. Chem. 2008;47:3426–3432. doi: 10.1021/ic702372f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Holm RH. J. Am. Chem. Soc. 2003;125:3910–3920. doi: 10.1021/ja0214633. [DOI] [PubMed] [Google Scholar]
- 17.Berlinguette CP, Holm RH. J. Am. Chem. Soc. 2006;128:11993–12000. doi: 10.1021/ja063604x. [DOI] [PubMed] [Google Scholar]
- 18.Zuo J-L, Zhou H-C, Holm RH. Inorg. Chem. 2003:4624–4631. doi: 10.1021/ic0301369. [DOI] [PubMed] [Google Scholar]
- 19.Ohki Y, Ikagawa Y, Tatsumi K. J. Am. Chem. Soc. 2007;129:10457–10465. doi: 10.1021/ja072256b. [DOI] [PubMed] [Google Scholar]
- 20.Ohki Y, Imada M, Murata A, Sunada Y, Ohta S, Honda M, Sasamori T, Katada M, Tatsumi K. J. Am. Chem. Soc. 2009;131:13168–13178. doi: 10.1021/ja9055036. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto T, Ohki Y, Tatsumi K. Inorg. Chem. 2010;49:6102–6109. doi: 10.1021/ic100692v. [DOI] [PubMed] [Google Scholar]
- 22.Peters JW, Stowell MHB, Soltis SM, Finnegan MG, Johnson MK, Rees DC. Biochemistry. 1997;36:1181–1187. doi: 10.1021/bi9626665. [DOI] [PubMed] [Google Scholar]
- 23.Mayer SM, Lawson DM, Gormal CA, Roe SM, Smith BE. J. Mol. Biol. 1999;292:871–891. doi: 10.1006/jmbi.1999.3107. [DOI] [PubMed] [Google Scholar]
- 24.Einsle O, Tezcan FA, Andrade SLA, Schmid B, Yoshida M, Howard JB, Rees DC. Science. 2002;297:1696–1700. doi: 10.1126/science.1073877. [DOI] [PubMed] [Google Scholar]
- 25.Bobrik MA, Laskowski EJ, Johnson RW, Gillum WO, Berg JM, Hodgson KO, Holm RH. Inorg. Chem. 1978;17:1402–1410. [Google Scholar]
- 26.Reynolds JG, Holm RH. Inorg. Chem. 1981;20:1873–1878. [Google Scholar]
- 27.Rutchik S, Kim S, Walters MA. Inorg. Chem. 1988;27:1513–1515. [Google Scholar]
- 28.Carney MJ, Papaefthymiou GC, Whitener MA, Spartalian K, Frankel RB, Holm RH. Inorg. Chem. 1988;27:346–352. doi: 10.1021/ja00226a025. [DOI] [PubMed] [Google Scholar]
- 29.Stack TDP, Weigel JA, Holm RH. Inorg. Chem. 1990;29:3745–3760. [Google Scholar]
- 30.Yu S-B, Papaefthymiou GC, Holm RH. Inorg. Chem. 1991;30:3476–3485. [Google Scholar]
- 31.Zhou C, Holm RH. Inorg. Chem. 1997;36:4066–4077. [Google Scholar]
- 32.Kornienko A, Huebner L, Freedman D, Emge TJ, Brennan JG. Inorg. Chem. 2003;43:8476–8480. doi: 10.1021/ic030204r. [DOI] [PubMed] [Google Scholar]
- 33.Kern A, Näther C, Studt F, Tuczek F. Inorg. Chem. 2004;43:5003–5010. doi: 10.1021/ic030347d. [DOI] [PubMed] [Google Scholar]
- 34.Hauptmann R, Schneider J, Chen C-N, Henkel G. Acta Crystallogr. 1999;C55:192–194. [Google Scholar]
- 35.Greaney MA, Coyle CL, Pilato RS, Stiefel EI. Inorg. Chim. Acta. 1991;189:81–96. [Google Scholar]
- 36.Verma AK, Lee SC. J. Am. Chem. Soc. 1999;121:10838–10839. [Google Scholar]
- 37.Zdilla MJ, Verma AK, Lee SC. Inorg. Chem. 2011;50:1551–1562. doi: 10.1021/ic1021627. [DOI] [PubMed] [Google Scholar]
- 38.Chen X-D, Duncan JS, Verma AK, Lee SC. J. Am. Chem. Soc. 2010;132:15884–15886. doi: 10.1021/ja106478k. [DOI] [PubMed] [Google Scholar]
- 39.Partyka DV, Staples RJ, Holm RH. Inorg. Chem. 2003;42:7877–7886. doi: 10.1021/ic030185l. [DOI] [PubMed] [Google Scholar]
- 40.Groysman S, Holm RH. Inorg. Chem. 2007;46:4090–4102. doi: 10.1021/ic062441a. [DOI] [PubMed] [Google Scholar]
- 41.APEX II, v. Madison, WI: Bruker Analytical X-ray Systems, Inc.; 2009. [Google Scholar]
- 42.Sheldrick GM. Acta Crystallogr. 2009;A64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 43.Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H. J. Appl. Cryst. 2009;42:339–341. doi: 10.1107/S0021889811041161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.See paragraph at the end of the article concerning supporting information available.
- 45.Holm RH, Solomon EI, Majumdar A, Tenderholt A. Coord. Chem. Rev. 2011;255:993–1015. [Google Scholar]
- 46.Mascharak PK, Papaefthymiou GC, Armstrong WH, Foner S, Frankel RB, Holm RH. Inorg. Chem. 1983;22:2851–2858. [Google Scholar]
- 47.Xi B, Holm RH. Inorg. Chem. 2011;50:6280–6288. doi: 10.1021/ic200641k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holm RH, Phillips WD, Averill BA, Mayerle JJ, Herskovitz T. J. Am. Chem. Soc. 1974;96:2109–2117. doi: 10.1021/ja00814a020. [DOI] [PubMed] [Google Scholar]
- 49.Wolff TE, Berg JM, Holm RH. Inorg. Chem. 1981;20:174–180. [Google Scholar]
- 50.Armstrong WH, Mascharak PK, Holm RH. J. Am. Chem. Soc. 1982;104:4373–4383. [Google Scholar]
- 51.Zhang Y-P, Bashkin JK, Holm RH. Inorg. Chem. 1987;36:694–702. [Google Scholar]
- 52.Armanasco NL, Baker MV, North MR, Skelton BW, White AH. J. Chem. Soc., Dalton Trans. 1998:1145–1149. [Google Scholar]
- 53.Baker MV, North MR, Skelton BW, White AH. Inorg. Chem. 1999;38:4515–4521. doi: 10.1021/ic990031z. [DOI] [PubMed] [Google Scholar]
- 54.Backes-Dahmann G, Herrmann W, Wieghardt K, Weiss J. Inorg. Chem. 1985;24:485–491. [Google Scholar]
- 55.Bürger KS, Haselhorst G, Stötzel S, Weyhermüller T, Wieghardt K, Nuber B. J. Chem. Soc., Dalton Trans. 1993:1987–1997. [Google Scholar]
- 56.Shores MP, Sokol JJ, Long JR. J. Am. Chem. Soc. 2002;124:2279–2292. doi: 10.1021/ja011645h. [DOI] [PubMed] [Google Scholar]
- 57.Wieghardt K, Backes-Dahmann G, Nuber B, Weiss J. Angew. Chem. Int. Ed. 1985;24:777–778. [Google Scholar]
- 58.Emsley J. The Elements. 3rd ed. Oxford: Oxford University Press; 1998. pp. 188–198. [Google Scholar]
- 59.Que L, Jr, Bobrik MA, Ibers JA, Holm RH. J. Am. Chem. Soc. 1974;96:4168–4178. doi: 10.1021/ja00820a018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.