Abstract
It has been hypothesized that dietary nitrite augments the antimicrobial activity of gastric acid after conversion to nitric oxide and other reactive nitrogen intermediates, thus resulting in increased resistance against gastrointestinal infection. In this study, we showed that the reducing agents ascorbic acid and glutathione reduced the activity of acidified nitrite against Yersinia enterocolitica (P < 0.001). In contrast, iodide and thiocyanate increased the antimicrobial activity (P < 0.001), whereas hydroxyacids (citrate, lactate, and tartarate) had no measurable effects.
Nitrate is a natural component of many foods, particularly vegetables, fruits, and cured meats (1). Dietary nitrate is absorbed in the intestine (23), transported in the blood, actively taken up and concentrated in the salivary glands (20, 21), and then reduced to nitrite by oral nitrate-reducing bacteria (14, 15). Recent in vitro studies showed that the activity of stomach acid against food-borne pathogens might be increased by up to 100-fold by physiological salivary concentrations of nitrite (3). A variety of reactive nitrogen compounds are formed from nitrite in acidic conditions and have been proposed to be the chemical species responsible for killing bacteria in normal human stomach. These compounds include nitrous acid, peroxynitrite, nitrogen dioxide, and, most frequently, nitric oxide (NO) (2, 5, 12).
An increase in NO generation would be expected to augment the antimicrobial activity of acidified nitrite. Ascorbic acid (vitamin C) has been shown to increase the formation of NO from nitrite (17). Other reducing agents, such as glutathione, which are also found in food and gastric secretions, are thought to increase NO production (9). Iodide and thiocyanate are transported competitively into the salivary glands by the same transport mechanism as nitrate (8), and Dykhuisen et al. (6) demonstrated enhanced antibacterial activity of acidified nitrite in vitro when thiocyanate was also added.
Certain hydroxyacids, such as citric acid (13) and lactic acid (11), are frequently added to foods as preservatives and are known to have antimicrobial activities. Therefore, these may augment the activity of the stomach acid. In the study reported here, the effect of selected chemicals found in food and/or salivary and stomach secretions on the antimicrobial activity of hydrochloric acid (HCl) was assessed in vitro in both the presence and absence of nitrite.
An isolate of Yersinia enterocolitica from a patient was used as a test organism and stored at −20°C prior to use in experiments. Microbial colonies grown on nutrient agar plates were transferred to nutrient broth (in 75-ml conical flasks) and regrown on a shaker incubator overnight before being used in assays. Bacterial density in the inoculum suspension was adjusted to give 2 × 107 CFU/ml. Bacterial cells were exposed to acidified nitrite and a range of other compounds in a standard microwell plate assay. First, 50 μl of 2.6% nutrient broth (nitrite levels below 10 μM) was added to each well of a 96-well microwell plate (Quality Plastics, Camlab Ltd., Cambridge, United Kingdom) followed by 30 μl of KCl-HCl buffer. Then 50 μl of the solutions of different compounds, either l-ascorbic acid (Sigma Chemical Co., St. Louis, Mo.), glutathione (Sigma Chemical Co.), potassium thiocyanate (Sigma Chemical Co.), potassium iodide (Sigma Chemical Co.), citric acid (Analar analytical reagent; BDH Chemical Ltd., London, United Kingdom), lactic acid (Analar analytical reagent; BDH Chemical Ltd.), or tartaric acid (Sigma Chemical Co.), was added into the wells. Finally, 50 μl of potassium nitrite (Sigma Chemical Co.) was added at a range of concentrations, followed immediately by transfer of 20 μl of the Y. enterocolitica suspensions to the microwells. The microwell plates were incubated at 37°C, and after being exposed for a specific length of time, 20 μl samples of bacterial suspension (2 × 106 CFU) were transferred to plates containing 180 μl of nutrient broth serving as recovery plates to allow regrowth of viable bacterial cells.
The recovery plates were then placed in an incubator shaker (New Brunswick Scientific Co., Edison, N.J.) at 37°C for 24 h. Regrowth of bacteria in the recovery plates was measured as optical densities at 570 nm by using a microplate reader (MRX Dynatech Medical Products Ltd., West Sussex, England). It was assumed that all the bacteria had been killed during exposure to the different mixtures of chemicals if they failed to regrow on the recovery medium.
The concentrations of chemicals tested were 0, 125, 250, 500, 750, and 1,000 μM, except for those of thiocyanate, for which concentrations of 0, 25, 50, 125, 250, and 500 μM were used. Each of these concentrations was tested in combination with a range of nitrite concentrations (0, 125, 250, 375, 500, 750, 875, or 1,000 μM). Minimum bactericidal concentrations (MBCs) for exposure times of 10, 15, and 20 min for solutions adjusted to pH 2; 1, 2, 4, and 6 h for solutions adjusted to pH 4 or 6; and 12, 18, and 24 h for solutions adjusted to pH 5 (depending on bacterial survival) were investigated.
Statistical analyses were carried out using the SPSS version 11.0 statistics package (Chicago, Ill.). The effects of different concentrations of the different chemicals (independent variables) on the MBC of nitrite (MBCnitrite) (dependent variable) were analyzed using analysis of variance and regression analysis. Comparison of the effects of different independent variables was done using Student's t test.
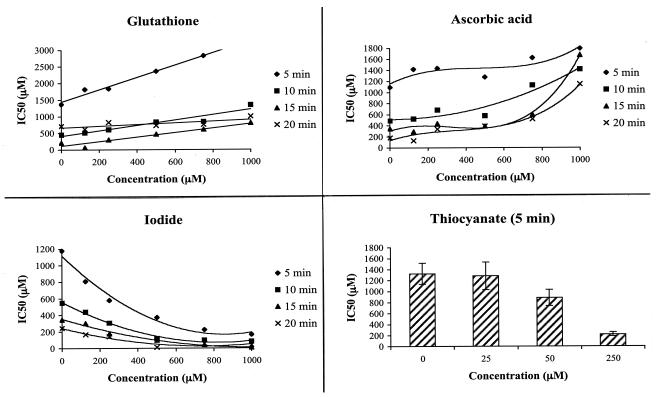
Data were presented in terms of both 50% inhibitory concentrations (IC50), defined as the reduction of bacterial viability to 50% of control (Fig. 1), and the MBCnitrite (≥99.99% of cells killed) (Fig. 2), all in triplicate. IC50s (in μM) were calculated from linear equations of each replicate using percent growth inhibition converted from optical densities using the equation percent growth = (Ax/Ao)100, where Ax is absorbance in the presence of test compounds and Ao is absorbance corresponding to cell solutions without added chemicals (control). Finally, parameters for linear regression equations from curve estimation were developed to compute IC50 values.
FIG. 1.
Time- and concentration-dependent effects of physiologically active compounds on antibacterial effects of acidified nitrite. Y. enterocolitica cells were grown first on nutrient agar for 24 h and then in nutrient broth overnight in a 75-ml conical flask while being shaken (120 rpm) in an incubator shaker at 37°C. The bacterial cells were adjusted to 2 × 107 CFU/ml and plated out in 96-well plates. A concurrent addition of KCl-HCl buffer (pH 2), nitrite, and either glutathione, vitamin C, iodide, or thiocyanate was made into each well. Exposed bacterial cells were rescued by transferring 20 μl from each well to 180 μl of fresh media. Finally, bacterial cell density was determined from optical density. The means are from three independent determinations. Linear curves were first constructed for each replicate using percent growth inhibition as a dependent variable and the dose concentration of nitrite as an independent variable. IC50s (μM) were computed from the regression equations. The antibacterial activity of acidified nitrite was significantly reduced by ascorbic acid and glutathione and was potentiated by thiocyanate and iodide. Bacteria were unable to survive in a concurrent exposure to acidified nitrite and thiocyanate for more than 5 min, and the susceptibility curve for 5 min is shown in the figure. The effects of physiologically active compounds on bacterial growth were time and concentration dependent.
FIG. 2.
MBCnitrite with or without the presence of other physiologically active compounds against Y. enterocolitica at pH 2. Values out of range (i.e., an MBC of >1,000 μM) were included in analysis after assuming an arbitrary value of 1,000 μM, as shown by bars corresponding to high concentrations of vitamin C and glutathione. Error bars show the standard errors of the means, and the different letters indicate significantly different values for the means (P < 0.05, Duncan's multiple range test). Vitamin C and glutathione showed an overall increase in MBCnitrite, whereas iodide and thiocyanate showed reduced MBCs.
MBC was defined as the lowest concentration of nitrite at which resumption of growth was not detected after transfer of 2 × 106 exposed bacterial cells into recovery media. Comparative effects of the tested physiologically active nutrients on the MBCnitrite are presented in Fig. 2. Ascorbic acid increased the MBCnitrite at pH 2. For example, when Y. enterocolitica was exposed for 20 min to exposure media at pH 2, the concentration of nitrite needed to kill bacteria increased (P < 0.001) from about 292 μM in wells without ascorbic acid to >1,000 μM in the presence of 1,000 μM ascorbic acid. Based on results from a further investigation with ascorbic acid, the interaction of nitrite and ascorbic acid was significant at pH 2 and 4 but not at pH 5 (data not shown). The time required to achieve bacterial kill increased to between 2 and 6 h at pH 4 and to between 6 and 24 at pH 5, compared to 20 min at pH 2. Glutathione also reduced the antimicrobial activity of acidified nitrite. For example, the average MBCnitrite increased from 42 μM (without glutathione) to 834 μM (in the presence of 1,000 μM glutathione) at 20 min of exposure time.
The comparative effects of the physiologically active nutrients on the IC50 (with respect to nitrite) are presented in Fig. 1. Most frequently, 500-μM-or-higher concentrations of glutathione significantly reduced the antimicrobial effects of acidified nitrite by significantly increasing the IC50 of nitrite. For example, the IC50 was increased from 450 μM in the absence of glutathione to 1.3 mM in the presence of 1 mM of glutathione after 10 min of exposure. Up to about a fourfold increase in the IC50 of nitrite was determined in 15 min of exposure. Similarly, three- to sixfold increases in the IC50 of nitrite resulted from concurrent exposure of bacteria to combinations of acidified nitrite and ascorbic acid. For instance, the IC50 of nitrite was increased from 178 to 1.15 mM after the addition of 1 mM ascorbic acid.
The concentration-dependent antimicrobial effect of acidified nitrite was dramatically increased by iodide and thiocyanate, thiocyanate having a stronger effect. In the concurrent exposure of bacterial cells to acidified nitrite and thiocyanate, for instance, survival of bacterial cells was limited to only 5 min of exposure and 250 μM of thiocyanate (Fig. 1 and 2). Microbial survival of 10 min or longer was rarely observed with the addition of thiocyanate to acidified nitrite. A synergistic antimicrobial effect of iodide with acidified nitrite was observed. For instance, the IC50 was reduced from 346 to 8 μM after addition of 1 mM iodide (P = 0.001).
The killing of bacteria by acidified nitrite was attributed to the production of NO and other reduced nitrogen compounds, but enhancing the production of NO by the addition of glutathione or ascorbic acid (16, 17, 19, 22) did not result in an increase in antibacterial activity but instead resulted in a significant decrease. This finding contradicts the existing hypothesis that NO is the main chemical species responsible for the increase in antimicrobial activity of acidified nitrite solution. Clearly, further studies are required to precisely identify the chemical species contributing to the increased antimicrobial activity of acidified nitrite.
Ascorbic acid secretion into the stomach is reduced in patients with gastroenteritis (17). Similarly, the ratio of reduced glutathione to oxidized glutathione in patients with gastric carcinoma and ulcer diseases is reduced, indicating an increased glutathione turnover (10) which may show a physiological response designed to augment the antimicrobial activity of the stomach fluid or the host defense system.
Iodide or thiocyanate increased the antimicrobial activity of acidified nitrite. Comparing the means of the MBCnitrite, analysis of variance (Duncan's multiple range test) showed a significant decrease with increasing iodide or thiocyanate concentrations and exposure times (P < 0.001). For example, the average MBCnitrite was reduced from about 1,000 μM (without iodide) to about 250 μM (in the presence of 1,000 μM iodide) and from about 1,000 μM (without thiocyanate) to 0 μM (in the presence of as low as 125 μM thiocyanate) (Fig. 2). Without the addition of nitrite, 50 μM of thiocyanate was sufficient to kill >99.99% of bacterial cells within 15 min. At equimolar concentrations, the increase in antimicrobial activity was significantly higher for thiocyanate than for iodide (P < 0.001).
Both iodide and thiocyanate are concentrated in salivary glands at up to 30 and 20 times, respectively, the concentration found in plasma (4, 8). In comparison, nitrate is concentrated to levels 10 times that found in plasma. Previous studies have demonstrated that iodide, thiocyanate, and nitrate uptake into the salivary glands is facilitated by the same active transport mechanism. This finding and the additive effect of the compounds in enhancing bacterial killing may represent a common physiological role of the three compounds in host defense.
Hydroxyacids (citric acid, lactic acid, and tartaric acid) had no effect on the bactericidal activity of acidified nitrite at concentration ranges between 0 and 1 mM. Though similar works done on the bactericidal activity of these compounds is scarce, Ruzickova (18) reported that up to 0.2% of citric acid inhibited the growth of Salmonella enteritidis in a defined medium at acidic conditions. Similarly, 3% lactic acid showed high bactericidal activity against Y. enterocolitica and other bacterial species when applied to pork fat (12). Clearly, the concentrations used in these studies is higher than the physiological concentrations tested in this study. These hydroxyacids are, therefore, unlikely to affect the stomach disinfecting system.
The findings of this study are in agreement with the findings of Dykhuizen et al. (6), who tested a panel of enteric bacterial pathogens. Helicobacter pylori is one of the important enteric bacteria that is also susceptible to physiological toxicity of acidified nitrite (7). The susceptibility of bacterial pathogens to metabolites of dietary nitrate is more complex in vivo because of the reactivity of nitrogen intermediates in the presence of other physiological substances. Thus, this in vitro study provided relevant evidence for further studies to validate of the health benefit of nitrate when it is ingested with fruits and vegetables, which also contain both ascorbic acid and glutathione.
The overall results of this study demonstrate that a wide range of dietary compounds or salivary and gastric secretions, including nitrite, ascorbic acid, glutathione, iodide, and thiocyanate, affect antimicrobial activity in the stomach and the nonimmunological host defense mechanism.
REFERENCES
- 1.Anonymous. 1981. The health effects of nitrate, nitrite and N-nitroso compounds. Part 1 of a 2-part study by the committee on nitrite and alternative curing agent in food. National Academy Press, Washington, D.C.
- 2.Beckman, J. S., J. Chen, H. Ischiropoulos, and J. P. Crow. 1994. Oxidative chemistry of peroxynitrite. Meth. Enzymol. 223:229-240. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin, N., F. O'Driscol, H. Dougall, C. Duncan, L. Smith, M. Golden, and H. Mckenzie. 1994. Stomach NO synthesis. Nature 368:502. [DOI] [PubMed] [Google Scholar]
- 4.Das, D., P. K. De, and R. K. Banerjee. 1995. Thiocyanate, a plausible physiological electron donor of gastric peroxidase. Biochem. J. 305:59-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dizdaroglu, M. 1993. Chemistry of free radical damage to DNA and nucleoproteins, p. 19-39. In B. Halliwell and O. I. Aruoma (ed.), DNA and free radicals. Prentice Hall, Inc., Englewood Cliffs, N.J.
- 6.Dykhuizen, R. S., R. Frazer, C. Duncan, C. C. Smith, M. Golden, N. Benjamin, and C. Leifert. 1996. Antimicrobial effect of acidified nitrite on gut pathogens: importance of dietary nitrate in host defense. Antimicrob. Agents Chemother. 46:1422-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dykhuizen R. S., A. Fraser, H. McKenzie, M. Golden, C. Leifert, and N. Benjamin. 1998. Helicobacter pylori is killed by nitrite under acidic conditions. Gut 42:334-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards, D. A. W., M. D. Camb, K. Fletcher, B. A. Camb, and E. N. Rowlands. 1954. Antagonism between perchlorate, iodide, thiocyanate, and nitrate for secretion in human saliva: analogy with the iodide trap of the thyroid. Lancet i:498-499. [DOI] [PubMed] [Google Scholar]
- 9.Flagg, E. W., R. J. Coates, J. W. Eley, D. P. Jones, E. W. Gunter, T. E. Byers, G. S. Block, and R. S. Greenberg. 1994. Dietary glutathione intake in humans and the relationship between intake and plasma total glutathione level. Nutr. Cancer 21:33-46. [DOI] [PubMed] [Google Scholar]
- 10.Giorgi G., L. Micheli, G. Segre, and A. Pecchi. 1989. Glutathione (GSH) in human stomach mucosa. Riv. Eur. Sci. Med. Farmacol. 112:163. (Abstract.) [PubMed] [Google Scholar]
- 11.Greer, G. G., and B. D. Dilts. 1995. Lactic acid inhibition of the growth of spoilage bacteria and cold tolerant pathogens on pork. Int. J. Food Microbiol. 25:141-151. [DOI] [PubMed] [Google Scholar]
- 12.Groote, M. A. D., and F. C. Fang. 1995. NO inhibitors: antimicrobial properties of nitric oxide. Clin. Infect. Dis. 21:S162-S165. [DOI] [PubMed] [Google Scholar]
- 13.Gundidza, M., and N. Gaza. 1993. Antimicrobial activity of Dalbergia melanoxylon extracts. J. Ethnopharmacol. 40:127-130. [DOI] [PubMed] [Google Scholar]
- 14.Hong, Li., C. Duncan, J. Townend, K. Killham, L. M. Smith, P. Johnston, R. Dykhuizen, D. Kelly, M. Golden, N. Benjamin, and C. Leifert. 1997. Nitrate-reducing bacteria on rat tongues. Appl. Environ. Microbiol. 63:924-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishiwata H, P. Boriboon, Y. Nakamura, M. Harada, A. Tanimura, and M. Ishididate. 1975. Changes of nitrite and nitrate concentration in incubated human saliva. J. Food Hyg. Soc. Jpn. 16:93-98. [Google Scholar]
- 16.Mirvish, S. S., L. Wallcave, M. Eagen, and P. Shubik. 1972. Ascorbate-nitrite reaction: possible means of blocking the formation of carcinogenic N-nitroso compounds. Science 177:65-68. [DOI] [PubMed] [Google Scholar]
- 17.Reed, P. I., P. L. R. Smith, H. Haines, F. R. House, and C. L. Walters. 1981. Gastric juice N-nitrosamines in health and gastroduodenal disease. Lancet ii:550-552. [DOI] [PubMed] [Google Scholar]
- 18.Ruzickova, V. 1996. The effect of acidification on the virulent strains of Salmonella enteriditis in a defined medium. Vet. Med. (Prague) 41:25. (In Czech.) (Abstract.) [PubMed]
- 19.Sobala, G. M., C. J. Schorah, M. Sanderson, M. F. Dixon, D. S. Tompkins, P. Godwin, and A. T. R. Axon. 1989. Ascorbic acid in the human stomach. Gastroenterology 97:357-363. [DOI] [PubMed] [Google Scholar]
- 20.Stephany, R. W., and P. L. Schuller. 1980. Daily dietary intakes of nitrate, nitrite and volatile N-nitrosamines in the Netherlands using the duplicate portion sampling technique. Oncology 37:203-210. [DOI] [PubMed] [Google Scholar]
- 21.Tannenbaum, S. R., M. Weisman, and D. Fett. 1976. The effect of nitrate intake on human concentration in human saliva. Food Cosmet. Toxicol. 14:549-552. [DOI] [PubMed] [Google Scholar]
- 22.Tannenbaum, S. R., J. S. Wishnok, and C. D. Leaf. 1991. Inhibition of nitrosamine formation by ascorbic acid. Am. J. Clin. Nutr. 53:247S-250S. [DOI] [PubMed] [Google Scholar]
- 23.Witter, J. P., E. Balish, and S. J. Gatley. 1979. Distribution of nitrogen 13 from labeled nitrate and nitrite in germ-free and conventional-flora rats. Appl. Environ. Microbiol. 38:870-878. [DOI] [PMC free article] [PubMed] [Google Scholar]