Abstract
Only a few major studies of chondrosarcoma of the mobile spine have been reported. These studies have shown that spinal chondrosarcomas require complete surgical resection and are notoriously resistant to chemotherapy and radiation. We present 16 cases of chondrosarcoma of the mobile spine diagnosed at a median age of 54.5 (range 20 – 79) years. Diagnosis and treatment studies were based on both CT scans and MRI. Fifteen of our 16 patients had low-grade (grade 1-2) chondrosarcomas. All patients were treated with surgical resection. Fourteen patients had total resection while two patients had subtotal resection. The two patients who had subtotal resection died of their disease. Five of the fourteen patients who had total resection also died. The mean interval to death was 3.6 years. This study confirms that although chondrosarcomas of the spine are low grade, they are dangerous neoplasms. Even with complete resection, they have a high rate of recurrence and metastasis.
INTRODUCTION
Chondrosarcoma is a malignant cartilage-forming bone neoplasm that accounts for 10% of all primary bone tumors.1 Typically low grade, these neoplasms can arise de novo or from a pre-existing cartilage lesion such as an osteochondroma or enchondroma. 1-3 Less than 10% of all chondrosarcomas occur in the spine.1, 2, 4 Most occur in the thoracic spine, and patients typically present in middle age with back pain and/or neurological symptoms.1, 3, 5, 6 Men are affected more often than women.1, 3, 5, 7 Radiologically, these tumors appear as destructive lesions in the spine or as a paraspinal mass with calcification.1
The most successful treatment for spinal chondrosarcoma is complete en bloc resection of the tumor. 1-3,7,8 This often requires spinal reconstruction involving a multidisciplinary team of orthopedic, plastic and neurosurgeons. 5,7, 9 Intralesional removal almost always results in recurrence.7 Chondrosarcomas in general are resistant to chemotherapy and radiation treatment, although postoperative proton-beam therapy, especially for spinal lesions, has shown positive results.1-3, 10 When en bloc resection is not possible, partial removal followed by radiotherapy may provide palliation of pain and improve neurological deficits.1, 11, 12 This paper reports 16 cases of spinal chondrosarcoma.
MATERIALS AND METHODS
These sixteen cases were culled from the IRB-approved database maintained by the senior author. Available data included history and follow-up, radiographic images, and histologic slides.
CASE DESCRIPTIONS
Six patients were female and ten were male (see Table 1). The median age at presentation was 54.5 (range 20 – 79) years. Four cases occurred in the cervical spine alone and seven cases involved only the thoracic spine, while two cases involved the cervicothoracic spine (patients 10 and 13). Three cases involved the lumbar spine.
TABLE 1.
Sixteen Cases of Chondrosarcoma of the Spine
| Patient # | Age/Gender | Location | Pathological Grade | Treatment | Outcome | TimetoFollow-Up (years) |
|---|---|---|---|---|---|---|
| 1 | 76M | T11 | I | R | A | 1.17 |
| 2 | 60F | C2-C4 | I | R | D | 2 |
| 3 | 37F | C4-C5 | I | PR/H+ | D | 4 |
| 4 | 41M | T9-T10 | III | R | A | 7 |
| 5 | 20M | T9-T10 | I | R | A | 1 |
| 6 | 39F | T9-T10 | I | R/H+ | D | 4 |
| 7 | 79M | C5-C6 | II | R | A | 4 |
| 8 | 63F | T8 | II | R | A | 1.25 |
| 9 | 52M | LI | II | R/H+ | A | 3 |
| 10 | 58M | Cervicothoracic | II | R/H+ | A | 3 |
| 11 | 49M | T1-T4 | II | R | D | 7 |
| 12 | 49F | T3-T4 | II | PR | D | 3 |
| 13 | 57M | Cervicothoracic | II | R | D | 3 |
| 14 | 74F | L5 | II | R | D | 3 |
| 15 | 61M | LI | II | R | A | 3 |
| 16 | 46M | C1-C2 | I | R | A | 6.5 |
T = thoracic; C = cervical; L = lumbar; I - grade one; II - grade two; III - grade three; IR = resection; PR = adjuvant proton beam radiation therapy; A = alive; D = deceased. partial resection; H+ =
RADIOGRAPHIC FINDINGS
Radiographic images of thirteen of the sixteen cases were available for study. All thirteen cases had CT scans. Twelve of these thirteen cases also had MRI studies. The lesions were poorly visualized on plain radiographic studies. Lesions were best visualized with a combination of CT and MRI studies.
Both central and surface chondrosarcomas were represented in this study. There were seven central sarcomas and six surface lesions. Of the central chondrosarcomas, three involved the vertebral body (Figure 1) and four involved the pedicle (Figure 2). Lesions in the pedicle usually had extraosseous extensions. Often, the extraosseous extensions were best visualized with an MRI scan (Figure 3). All but one of the CT scans showed rings and stipples characteristic of cartilage matrix (Figure 4). Lesions on MRI were lobulated and bright on T2-weighted images. One patient (patient 16) had synovial chondromatosis of a cervical vertebra with secondary chondrosarcoma (Figure 5)
Figure 1.
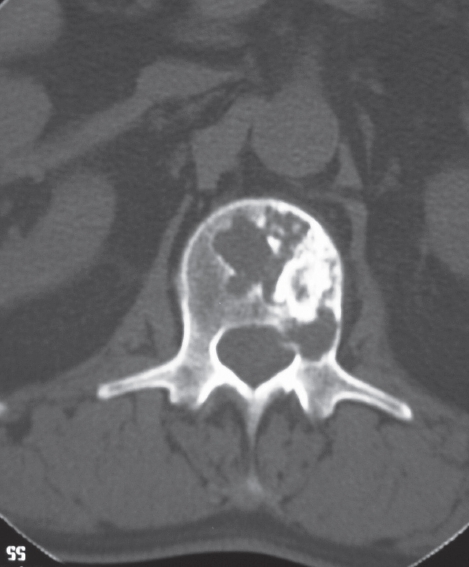
Axial CT scan of the L1 vertebra of patient #15. There is a poorly defined lytic lesion mixed with nodular radiodensities characteristic of cartilage.
Figure 2.
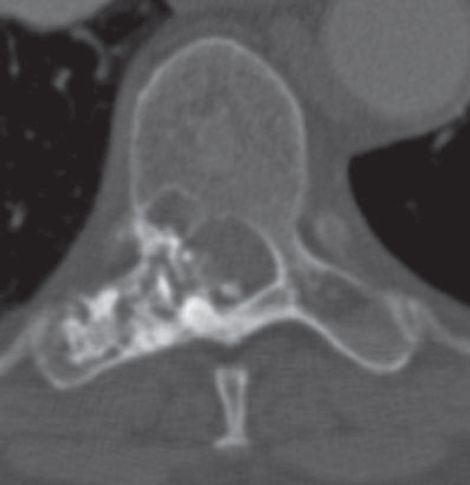
Axial CT scan of the T8 vertebra of patient #8 showing a poorly defined lytic lesion in the pedicle with associated ring-like radiodensities.
Figure 3.

A) Axial CT scan of the C8 of patient #10. There is a poorly defined lytic lesion with cortical destruction. B) AT2-weighted sagittal MRI of patient #10 showing the anterior extraosseous extension of the lobulated cartilage mass which was not visible on CT scan.
Figure 4.
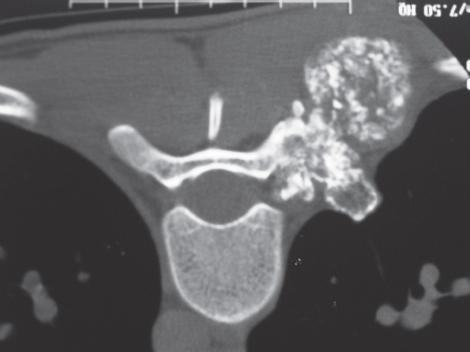
Axial CT scan of the T9 vertebra of patient #5. There is significant extraosseous spread from the pedicle. There are abundant rings and stippled calcification characteristic of cartilage.
Figure 5.
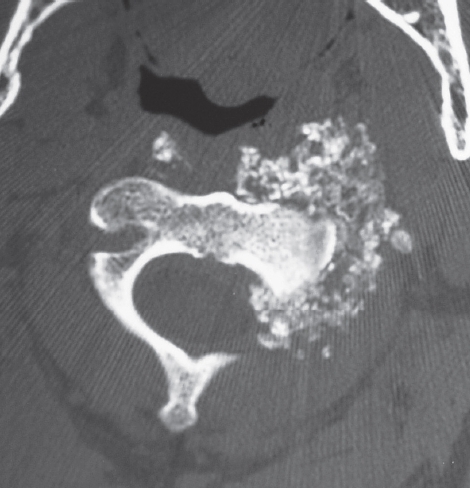
Axial CT scan of the C3 vertebra of patient #16. There is extensive synovial chondromatosis with destruction of the vertebral body secondary to a chondrosarcomatous transformation.
HISTOLOGICAL FINDINGS
Histologic study was available on all 16 cases. In all but one case, the diagnosis was made on the CT-guided needle biopsies. One case required an open biopsy. The histologic findings of hyaline cartilage were interpreted in the light of the radiographic images. After resection of the lesion, the diagnosis of chondrosarcoma was confirmed in all cases.
Histologic grading of the chondrosarcomas ranged from grade 1 to grade 3. However, there was only one grade 3 chondrosarcoma. There were six grade 1 chondrosarcomas (Figure 6) and nine grade-2 chondrosarcomas.
Figure 6.
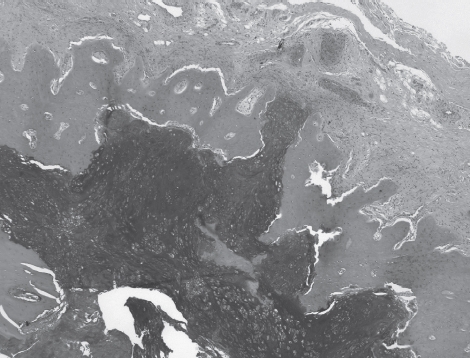
Medium-power photomicrograph of a grade-2 chondrosarcoma of patient #8. The cartilage shows penetration of the thinned cortex.
TREATMENT
All cases were treated with surgical resection. Fourteen patients had total resection of their tumors. Ten of these cases were treated with one procedure, while two cases required a two-stage procedure (patients 1 and 16) and two cases required a three-stage procedure (patients 2 and 9).
Two patients had subtotal excision of their tumors. Patient 12 underwent resection of the tumor down to the right pedicle with residual tumor noted at the close of the operation. Another patient was found to have residual tumor with postoperative magnetic resonance imaging, despite attempted en bloc resection (patient 3).
No patient underwent neoadjuvant or adjuvant chemotherapy or traditional radiation therapy. Four patients received postoperative proton beam radiation (patients 3,6,9 and 10). Two of these patients were deceased within four years while one is alive at seven years with no evidence of disease and the fourth has been lost to follow-up.
FOLLOW-UP AND OUTCOME
Follow-up was available for all sixteen patients with a mean time of follow-up of 3.5 (range 1-7) years.
Of the 16 patients, seven (43.8%) had died of their disease due to pulmonary metastasis. Both patients who had only subtotal resection and five of the 14 who had total en bloc resection died. The interval to death ranged from two to seven years (mean 3.6 years). Four of the patients who died had a local recurrence before pulmonary metastasis. The interval to local recurrence ranged from 1.3 to four years. No surviving patient has serious neurologic deficit.
Seven patients developed pulmonary metastases; one of these patients also had metastases to the chest wall and ribs (patient 11). Five of these seven originally had total resection of their tumors while the other two were the only patients who had subtotal tumor resection (patients 3 and 12). Therefore five of 14, or 35.7% of patients with total tumor resection later had metastases, while 100% of patients with subtotal tumor resection suffered metastases. The interval from surgical treatment to metastatic disease was available for two of the seven patients. For the patient who had subtotal tumor resection (patient 3) the interval to metastases was four years, while the patient who underwent total tumor resection had metastases within two years (patient 11).
DISCUSSION
Three other similar large series of spinal chondrosarcoma have been reported. Shives et al. described 20 patients from Mayo Clinic while York et al. described 21 patients cared for at the University of Texas M.D. Anderson Cancer Center13, 14 Boriani et al. reviewed 22 cases of chondrosarcoma of the mobile spine.7 Lloret et al. described a smaller series of five patients.6 Other studies include smaller series and case reports.2,4,6,8,11
Our study confirms some clinical observations by these previous authors. Similar to the Mayo Clinic series, our series shows that men are most commonly affected. However, an equal gender distribution was observed at M.D. Anderson Cancer Center. The median age of our patients was 54.5 (range 20 – 79) years, similar to that observed in series at all sites and consistent with the literature.1, 3-6, 6,13, 14 Also, the increased involvement of the thoracic spine compared to the cervical and lumbar spine seen in our patients is similar to previous studies.6, 13, 14
Chondrosarcoma of the spine may present diagnostic difficulties. Differential diagnosis includes three lesions that can be ruled out based on radiographic and histologic findings. The first, synovial chondromatosis of the facet joint, may masquerade as an aggressive cartilage lesion. Our case #16 showed synovial chondromatosis, but there was a secondary chondrosarcoma that invaded the bone. Second, chondroblastic osteosarcoma must be ruled out This can only be excluded by examination of multiple sections after resection of the tumor. The presence of neoplastic osteoid among the cartilage is diagnostic of osteosarcoma. This diagnosis is extremely difficult to make based on a small-needle biopsy sample. Finally, the diagnosis of chordoma must be excluded. Although most chordomas are either in the skull base or the sacrum, ten percent occur in the mobile spine and may be mistaken for chondrosarcoma.15 Histologically, both lesions have abundant extracellular matrix and both are positive with an S-100 immunostain. Chordoma may be distinguished by the presence of keratin-positive cells, a feature not present in chondrosarcoma. Therefore, keratin stains should be performed on all suspected chondrosarcomas of the spine to rule out chordoma.
Surgical resection is the recommended treatment for chondrosarcoma of the spine; these tumors are notoriously resistant to chemotherapy or radiation therapy All patients in our series underwent surgical treatment, either gross total resection or subtotal resection, similar to those in all other studies.6, 13, 14
Our study confirms the poor prognosis of spinal chondrosarcoma reported by other investigators. The Mayo Clinic authors observed a five-year survival of 55% with a median time to death of six years, while York et al. observed five-year and ten-year survivals of 64% and 40% with a median survival of six years after surgery.14
Our study strongly suggests that surgical margins are an important prognostic factor when predicting disease progression and recurrence. Both of our patients who had subtotal resection died of pulmonary metastases. Our nine surviving patients all had total resection with tumor-free margins. York et al found a significant increase in disease-free interval after gross resection of the tumor versus subtotal excision. Local recurrence was noted in one of five patients who had a total resection with an average time to recurrence of 5.4 years, while nine of 13 patients with subtotal tumor excisions had disease recurrence at an average of 3.7 years.14 Furthermore, Shives et al. described survival to be significantly related to the surgical margin obtained - in all eleven patients who underwent intralesional excision, disease progression was observed. Half of the six patients with contaminated marginal excision had local disease recurrence, and both patients who had gross total resections had no evidence of disease at five years.13 Boriani et al. observed three recurrences in 12 patients treated with en bloc excision while all ten out of ten patients treated with intralesional excision experienced recurrence.7
Post-operative radiation therapy may be tried when complete resection of the tumor is not possible. However, radiation therapy provided no survival benefit for the 21 patients in the MD Anderson Cancer Center series.14 Similarly, Boriani et al. found that conventional radiation therapy did not affect outcomes. Three of their eleven patients who had intralesional tumor excision underwent post-operative radiation; two patients had disease recurrence while one patient progressed.7 None of our patients received conventional radiation therapy, however, four patients received postoperative proton-beam radiation therapy, and two of these patients died. Our sample size is not large enough to make conclusions regarding the success of proton-beam radiation therapy.
Our study confirms that chondrosarcomas of the spine are aggressive and many are lethal. This is despite the finding that most lesions are low to intermediate grade (grade 1 or grade 2). Whereas in the long bones, only 10 to 15% of grade 1 and 2 chondrosarcomas metastasized, this figure is much higher in spinal chondrosarcomas.16 This is most likely due to the incomplete resection of the tumor that leads to local recurrence and ultimately to pulmonary metastasis. Therefore, early and wide en bloc excision is the treatment of choice for chondrosarcomas of the spine. Tumor-free margins should be obtained. In order to obtain tumor-free margins, MRI studies should be performed on all patients since this can map the extraosseous extension of the tumor, a feature common to spinal chondrosarcoma.
The poor prognosis is unexpected given that most chondrosarcomas of the spine are low grade. In long-bone chondrosarcomas, a low-grade lesion is an indicator of a better prognosis. In the spine, where incomplete resection often occurs, chondrosarcomas of any histologic grade are not responsive to adjuvant measures such as chemotherapy or radiation. Thus, the prognosis of spinal chondrosarcomas, even for low-grade lesions, is poor if total resection is not achieved. In this location and for these lesions, therefore, wide en bloc excision with tumor-free margins is the only possible curative procedure.
REFERENCES
- 1.Sundaresan N, Rosen G, Boriani S. Primary Malignant Tumors of the Spine. Orthop Clin North Am. 2009;40(1):21–36. doi: 10.1016/j.ocl.2008.10.004. January 1. [DOI] [PubMed] [Google Scholar]
- 2.Tessitore E, Burkhardt K, Payer M. Primary Clear-Cell Chondrosarcoma of the Cervical Spine. J Neurosurg Spine. 2006;4(5):424. doi: 10.3171/spi.2006.4.5.424. May 1. [DOI] [PubMed] [Google Scholar]
- 3.Knoeller SM, Uhl M, Gahr N, Adler CP, Herget GW. Differential diagnosis of primary malignant bone tumors in the spine and sacrum. The radiological and clinical spectrum. Neoplasma. 2008;55(1):16–22. January 1. [PubMed] [Google Scholar]
- 4.Panelos J, Voulgaris S, Michos E, Doukas M, Ch-aralabopoulos K, Batistatou A. Chondrosarcoma of the spine: A rare case with unusual presentation. Diagn Pathol. 2006;30(1):39. doi: 10.1186/1746-1596-1-39. October 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergh P, Gunterberg B, Meis-Kindblom JM, Kindblom LG. Prognostic Factors and Outcome of Pelvic, Sacral, and Spinal Chondrosarcomas. Cancer. 2001;91(7):1201–12. doi: 10.1002/1097-0142(20010401)91:7<1201::aid-cncr1120>3.0.co;2-w. April 1. [DOI] [PubMed] [Google Scholar]
- 6.Lloret I, Server A, Bjerkehagen B. Primary Spinal Chondrosarcoma: Radiologic Findings with Pathologic Correlation. Acta Radiol. 2006;47(1):77–84. doi: 10.1080/02841850500406852. February 1. [DOI] [PubMed] [Google Scholar]
- 7.Boriani S, De Lure F, et al. Chondrosarcoma of the mobile spine. Spine. 2000;25(7):804–12. doi: 10.1097/00007632-200004010-00008. April 1. [DOI] [PubMed] [Google Scholar]
- 8.Li YH, Yao XH. Primary intradural mesenchymal chondrosarcoma of the spine in a child. Pediatr Radiol. 2007;37(11):1155–8. doi: 10.1007/s00247-007-0576-0. November 1. [DOI] [PubMed] [Google Scholar]
- 9.Rawlins JM, Batchelor AG, Liddington MI, Towns G. Tumor Excision and Reconstruction of the Upper Cervical Spine: A Multidisciplinary Approach. Plast Reconstr Surg. 2004;114(6):1534–8. doi: 10.1097/01.prs.0000138239.12968.31. November 1. [DOI] [PubMed] [Google Scholar]
- 10.Foweraker KL, Buron KE, et al. High-dose Radiotherapy in the Management of Chordoma and Chondrosarcoma of the Skull Base and Cervical Spine; Part 1 - Clinical Outcomes. Clin Oncol. 2007;19(7):509–16. doi: 10.1016/j.clon.2007.04.004. September 1. [DOI] [PubMed] [Google Scholar]
- 11.Prevedello DM, Cordeiro JG, Koerbel A, Ditzel LF, Araujo JC. Management of Primary Spinal Chondrosarcoma. Arq Neuropsiquiatr. 2004;62(3B):875–8. doi: 10.1590/s0004-282x2004000500026. September 1. [DOI] [PubMed] [Google Scholar]
- 12.Rao G, Suki D, et al. Surgical management of primary and metastatic sarcoma of the mobile spine. J Neurosurg Spine. 2008;9(2):120–8. doi: 10.3171/SPI/2008/9/8/120. August 1. [DOI] [PubMed] [Google Scholar]
- 13.Shives T, McLeod R, Unni K. Chondrosarcoma of the Spine. J Bone Joint Surg Am. 1989;71(8):1158–65. September 1. [PubMed] [Google Scholar]
- 14.York JE, Berk RH, et al. Chondrosarcoma of the spine: 1954 to 1997. J Neurosurg. 1999;90(1 Suppl):73–8. doi: 10.3171/spi.1999.90.1.0073. January 1. [DOI] [PubMed] [Google Scholar]
- 15.Smith J, Ludwig RL, Marcove RC. Sacrococcygeal chordoma. A clinicoradiological study of 60 patients. Skeletal Radiol. 1987;16:37–44. doi: 10.1007/BF00349926. [DOI] [PubMed] [Google Scholar]
- 16.Rizzo M, Ghert MA, Harrelson JM, Scully SP. Chondrosarcoma of bone: analysis of 108 cases and evaluation for predictors of outcome. Clin Orthop Relat Res. 2001;(391):224–33. October 1. [PubMed] [Google Scholar]


