Abstract
S-3578 is a novel beta-lactam with enhanced activity against drug-resistant gram-positive cocci such as methicillin-resistant Staphylococcus aureus (MRSA). We used murine penicillin-resistant Streptococcus pneumoniae lung infection and neutropenic murine systemic MRSA infection models to determine the pharmacokinetic (PK)-pharmacodynamic (PD) parameter that best correlated with efficacy. Pharmacokinetic studies revealed that the maximum concentration in serum/dose values for S-3578 and cefepime in plasma in the lung infection model were 1.21 to 1.54 and 0.97 to 1.29, respectively; those for S-3578 in plasma in the systemic infection model were 0.78 to 1.02. The area under the concentration-time curve (AUC)/dose values for S-3578 and cefepime in plasma in the lung infection model were 0.98 to 1.13 and 0.77 to 1.04, respectively, and those for S-3578 in plasma in the systemic infection model were 1.03 to 1.11. The half-lives of S-3578 and cefepime in plasma in the lung infection model were 0.29 to 0.38 and 0.29 to 0.34, respectively, and those of S-3578 in plasma in the systemic infection model were 0.40 to 0.61. The time above the MIC was the PK-PD parameter that best correlated with efficacy in the murine lung infection model (R2 = 84 and 92% for S-3578 and cefepime in plasma, respectively). There was a twofold increase in the dose of S-3578 in the systemic infection model compared to that in the pneumonia model, yet the AUCs were the same. This may be due to the different MICs for the two pathogens.
In the past two decades, methicillin-resistant Staphylococcus aureus (MRSA), penicillin-resistant Streptococcus pneumoniae (PRSP), vancomycin-resistant enterococci, and extended spectrum β-lactamase-producing bacteria have recently emerged as drug-resistant organisms (7, 12, 14). Among these organisms, MRSA is important, as only a few drugs with activities against MRSA, such as vancomycin and linezolid, are available; and almost all of these drugs have potent activities only against gram-positive cocci, such as MRSA (2, 13). In addition, in patients with polymicrobial infections, Pseudomonas aeruginosa may be isolated in conjunction with MRSA (11). It has been reported that S-3578 has potent in vitro and in vivo activities not only against gram-negative bacteria but also against gram-positive bacteria, including MRSA (6, 16).
The purpose of our study was to measure the pharmacokinetic (PK) and pharmacodynamic (PD) parameters for S-3578 and cefepime and to determine the parameter that best identifies the efficacies of S-3578 and cefepime.
(Part of this work was presented at the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy, 2002.)
MATERIALS AND METHODS
Organisms.
S. pneumoniae strain SR11031 was isolated from the sputum of a patient with a respiratory infection. MRSA strain SR3637 was isolated from the blood of an inpatient. MRSA and S. pneumoniae were cultured overnight in fresh brain heart infusion broth (Difco Laboratoies, Detroit. Mich.) and brain heart infusion broth containing 30% horse serum at 35°C for 5 to 6 h (mid-logarithmic phase), respectively. The organisms were collected by centrifugation, suspended in Dulbecco's phosphate-buffered saline (PBS; Nissui, Tokyo, Japan), and kept at −80°C until use. The viable counts of these two organisms were assayed by plating 0.1 ml of each of the diluents on agar plates.
Antimicrobial agents.
S-3578 and cefepime were obtained from Shionogi & Co. (Osaka, Japan) and Bristol-Myers Squibb K.K. (Tokyo, Japan), respectively; and penicillin G and oxacillin were purchased from the U.S. Pharmacopeia (Rockville, Md.) and Sigma-Aldrich (St. Louis, Mo.), respectively.
Determination of MICs.
The MICs of the drugs for the infecting organisms were determined by the microdilution broth method according to the recommendations of NCCLS (9). Cation-adjusted Mueller-Hinton broth (Difco) and Mueller-Hinton broth supplemented with 5% lysed horse blood, 5 mg of yeast extract (Difco) per ml, and 15 μg of NAD (Sigma-Aldrich) per ml were used as the growth media for MRSA and S. pneumoniae, respectively. S. pneumoniae ATCC 49619 and S. aureus ATCC 25923 were used as standard strains. The assay was done three times.
Experimental models of bronchopneumonia and septicemia.
The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of Toho University School of Medicine.
(i) Bronchopneumonia.
Five-week-old male CBA/J mice (Charles River Japan Inc., Atsugi, Japan) were used for the bronchopneumonia model. CBA/J mice are susceptible to pneumonia caused by penicillin-resistant streptococci introduced intranasally (15). The organisms were kept at −80°C prior to use and were thawed just before they were used to infect the mice. They were diluted to a concentration of 4.8 × 107 CFU/ml with PBS. The mice were anesthetized with ketamine-xylazine and then infected by intranasal instillation of 0.07 ml of S. pneumoniae (inoculum size, 3.3 × 106 CFU/mouse).
(ii) Septicemia.
Five-week-old female Jcl:ICR mice (SLC Japan Inc., Shizuoka, Japan) were used for the septicemia model. On days 4 and 1 before infection, cyclophosphamide (150 and 100 mg/kg of body weight, respectively) was injected intraperitoneally to induce neutropenia (16). MRSA strain SR3637 organisms were stored at −80°C and thawed just before they were used to infect the mice. They were diluted to a concentration of 3.7 × 107 CFU/ml with PBS. One milliliter of the bacterial suspension was injected intraperitoneally into the neutropenic mice (inoculum size, 3.7 × 107 CFU/mouse).
Administration of S-3578 or cefepime.
Subcutaneous injection of the drugs commenced 1 day and 2 h after infection with S. pneumoniae and MRSA, respectively. Mice (five animals per group) were treated with S-3578 or cefepime at 2.4 to 480 mg/kg of body weight/day. The dosing regimens were selected by dividing the total daily dosage into one, two, four, or eight doses. The period of treatment was only 1 day. A 0.2-ml portion of saline was injected subcutaneously as a vehicle control.
Evaluation of effects. (i) Viable organisms method.
The animals were killed 24 h after the commencement of treatment, and the infected tissues were dissected and homogenized. The number of viable organisms (number of CFU in the lungs) was determined by agar plating. Evaluation of efficacy was based on the proportional reduction of bacterial counts in the infected tissues of treated animals compared with the counts in the infected tissues of untreated animals at 48 h postinfection. The detection limit of the bacteria was 10 CFU/lungs. The levels of carryover of S-3578 and cefepime in lung homogenates were less than 0.016 and 0.04 μg/ml, respectively.
(ii) Number of surviving animals method.
In an experiment performed simultaneously with the viable organisms method, the number of surviving mice treated with a total daily dosage of 240 mg/kg (seven to eight animals/group) was recorded at day 7 after infection in four groups to which the drugs were given one, two, four, and eight times per day, respectively.
The rate of mortality among mice inoculated intraperitoneally with MRSA was 100% up to 24 h after infection. S-3578 at total daily dosages of 4.8 to 480 mg/kg and cefepime at a total daily dose of 480 mg/kg (divided into doses administered one, two, four, and eight times daily) were administered subcutaneously 1 h after infection. The number of surviving mice in each group was recorded daily up to day 7 after infection.
PK studies.
Studies of the PKs of the drugs in serum and lung tissues after administration of a single dose were performed with mice with bronchopneumonia treated with a subcutaneous dose (0.2 ml/dose) of S-3578 or cefepime (3, 12, 30, or 120 mg/kg of body weight) 1 day postinfection with S. pneumoniae SR11031. Blood samples from three mice in each dosing group were obtained by heart puncture at 5, 15, and 30 min and 1, 2, 3, and 4 h postadministration while the mice were under ketamine-xylazine anesthesia. Just after blood sampling, the lungs were aseptically removed from the mouse and briefly washed with saline in order to minimize contamination with blood. The drug levels in serum and tissues were determined by a bioassay method with Micrococcus luteus ATCC 9341 and Escherichia coli 7437 as the indicator organisms for S-3578 and cefepime, respectively; the indicator organisms were incorporated into the medium (antibiotic medium 2 [Difco] and tryptic soy medium [Eiken], respectively) (16). The lower limits of detection of this assay were 0.016 and 0.04 μg/ml for S-3578 and cefepime, respectively.
Studies of the PKs in serum after the administration of a single dose were performed with mice with septicemia treated with a subcutaneous dose (0.2 ml/dose) of S-3578 (5, 20, 80, or 480 mg/kg of body weight) 1 h postinfection with S. aureus SR3637. Blood samples from three mice in each dosing group were obtained by heart puncture at 5, 15, and 30 min and 1, 2, 3, and 4 h postadministration while the mice were under ketamine-xylazine anesthesia. The coefficient of variation and bias were both lower than 15%.
PK parameters (elimination rate constant, half-life [t1/2], maximum concentration of drug in serum [Cmax], apparent volume of distribution, area under the concentration-time curve [AUC], and time above the MIC [TAM]) were calculated by using a one-compartment model with first-order absorption and first-order elimination by nonlinear least-squares techniques (WinNonlin).
Concentration of blood in lung homogenates.
PBS was perfused through the hearts of healthy mice under deep anesthesia with ketamine-xylazine until the color of the lungs changed to white. Since those lungs then contained minimal blood, they were removed and then dissected into many pieces, which were divided into nine groups (100 mg/group). PBS (400 μl) and heparinized blood (0 to 160 μl) were added to each sample, the mixture was homogenized and then centrifuged, and the supernatant was obtained. The hemoglobin concentration was assayed with a Plasma Hemoglobin kit (Sigma Diagnostics).
Data analysis.
The statistical significance of differences between the numbers of viable organisms in treated mice and untreated mice was examined by the Dunnett test. The Tukey test was performed to determine whether the viable organism counts were different between treatment groups. The statistical significance for all comparisons was set at a P value of <0.05.
The results of these studies were analyzed by using the sigmoid dose-effect model. The model is derived from the Hill equation, E = (Emax × Dn)/(ED50n + Dn), where E is the effect or, in this case, the log change in the number of CFU per lung between treated mice and untreated control mice after the 24-h period of study; Emax is the maximum effect; D is the total dose administered over 24 h; ED50 is the dose required to achieve 50% of Emax; and n is the sigmoidicity factor (8). The indices Emax, ED50, and n were calculated by nonlinear least-squares regression. The correlations between efficacy and each of the three PK or PD parameters (TAM, AUC/MIC, Cmax/MIC) studied were determined by nonlinear least-squares multivariate regression (Statistical Analysis System, version 8.02). The coefficient of determination (R2) was used to estimate the variance that could be due to regression for each of the PK or PD parameters.
RESULTS
MICs of S-3578 and cefepime for the infective organisms.
The MICs of S-3578, cefepime, and penicillin G for S. pneumoniae strain SR11031 as the infecting organism were 1, 1, and 2 μg/ml, respectively. The MICs of S-3578, cefepime, and oxacillin for S. aureus strain SR3637 were 4, >64, and >64 μg/ml, respectively.
Effect of drug administration interval on efficacies of S-3578 and cefepime.
In mice with bronchopneumonia caused by PRSP, the reduction in bacterial numbers in the lungs of mice treated with S-3578 or cefepime for 24 h were dependent upon the total daily dosages and the number of times that the drug was administered per day (Table 1). The counts of viable organisms in mice treated with S-3578 and cefepime four or eight times at total dosages of 8, 24, 80, and 240 mg/kg decreased significantly compared with those in control mice. When the efficacy was compared with the number of times of drug administration per day, the viable organism counts in mice treated with S-3578 four or eight times at total dosages of 24, 80, and 240 mg/kg decreased significantly (P < 0.01) compared with those in mice treated once or twice. At a total dosage of 8 mg/kg, the viable organism counts in groups of mice treated four or eight times decreased significantly compared with those in the group dosed once. In addition, in the case of administration of cefepime four or eight times at total dosages of 24, 80, and 240 mg/kg, the viable organism counts in the lungs decreased significantly compared with those in the lungs of mice treated once or twice, with the exception of those in mice dosed four times at a total dosage of 24 mg/kg. The viable organism counts in the groups treated two or four times at a total dosage of 24 mg/kg were significantly decreased compared with those in the group dosed once.
TABLE 1.
Effects of S-3578 and cefepime on viable counts in lung tissues of mice infected with S. pneumoniae
| Total daily dose (mg/kg) | No. of times of administration (day) | Viable cells in infected tissues (log CFU/lungs)
|
|
|---|---|---|---|
| S-3578 | Cefepime | ||
| 240 | 1 | 4.33 ± 0.93 | 4.56 ± 0.87 |
| 2 | 3.39 ± 0.73 | 3.93 ± 0.28 | |
| 4 | 1.19 ± 0.32a | 1.90 ± 0.71a | |
| 8 | 1.49 ± 0.63a | 1.10 ± 0.17a | |
| 80 | 1 | 4.20 ± 0.65 | 4.48 ± 0.45 |
| 2 | 3.46 ± 0.29 | 4.05 ± 0.35 | |
| 4 | 2.33 ± 0.57a | 2.87 ± 0.76a | |
| 8 | 1.54 ± 0.49a | 1.94 ± 0.41a | |
| 24 | 1 | 4.82 ± 0.38 | 5.00 ± 0.20 |
| 2 | 4.48 ± 0.45 | 4.21 ± 0.40b | |
| 4 | 2.84 ± 0.71a | 3.70 ± 0.39b | |
| 8 | 1.45 ± 0.48c | 3.13 ± 0.48c | |
| 8.0 | 1 | 5.04 ± 0.42 | 4.90 ± 0.47 |
| 2 | 4.31 ± 0.58 | 4.61 ± 0.22 | |
| 4 | 3.68 ± 0.52b | 4.41 ± 0.32 | |
| 8 | 3.59 ± 0.85b | 4.48 ± 0.84 | |
| 2.4 | 1 | 4.88 ± 0.12 | 5.29 ± 0.52 |
| 2 | 4.98 ± 0.70 | 5.02 ± 0.40 | |
| 4 | 4.51 ± 0.64 | 5.20 ± 0.60 | |
| 8 | 4.76 ± 0.51 | 5.38 ± 0.58 | |
| 0 | 5.34 ± 0.17 | 5.34 ± 0.30 | |
P < 0.01 compared with administration one and two times daily with the same total daily dosage.
P < 0.01 compared with administration once daily.
P < 0.01 compared with administration one, two, and four times daily with the same total daily dosage.
Survival rates of mice with pulmonary S. pneumoniae infections and systemic MRSA infections.
The survival rates of the mice infected with S. pneumoniae and treated with S-3578 two, four, or eight times at a total dosage of 240 mg/kg were 62.5 to 75.0% (Table 2). In mice treated with cefepime under the same conditions, the survival rates were 37.5, 75.0, and 100%, respectively.
TABLE 2.
Mortality rates among mice infected with S. pneumoniae and treated with S-3578 or cefepime
| Drug (dose [mg/kg]) | No. of times of administration | Survival rate (%) |
|---|---|---|
| S-3578 (240) | 1 | 12.5 |
| 2 | 75.0 | |
| 4 | 62.5 | |
| 8 | 75.0 | |
| Cefepime (240) | 1 | 12.5 |
| 2 | 37.5 | |
| 4 | 75.0 | |
| 8 | 100 | |
| Control | 12.5 |
In the systemic MRSA infection model, the survival rates of mice treated with S-3578 four or eight times at a total dosage of 480 mg/kg and eight times at a total dosage of 160 mg/kg were 100% (Table 3). The survival rates of mice treated twice at a total dosage of 160 mg/kg and once or twice at a total dosage of 480 mg/kg were 42.9 to 57.1%. All mice treated with cefepime died, as did all untreated mice.
TABLE 3.
Relationship between survival rates and TAM for S-3578 in murine systemic MRSA infection model
| Total daily dose (mg/kg) | No. of times of administration/day | Survival rate (%) | TAM in plasma (%) |
|---|---|---|---|
| 4.8 | 1 | 0 | 6.7 |
| 2 | 0 | 7.9 | |
| 4 | 0 | 0 | |
| 8 | 0 | 0 | |
| 16 | 1 | 0 | 10.9 |
| 2 | 28.6 | 18.6 | |
| 4 | 0 | 27.2 | |
| 8 | 0 | 25.0 | |
| 48 | 1 | 28.6 | 14.7 |
| 2 | 0 | 24.6 | |
| 4 | 0 | 39.7 | |
| 8 | 14.3 | 58.4 | |
| 160 | 1 | 0 | 18.7 |
| 2 | 42.9 | 32.8 | |
| 4 | 28.6 | 56.1 | |
| 8 | 100 | 93.3 | |
| 480 | 1 | 57.1 | 22.4 |
| 2 | 42.9 | 40.1 | |
| 4 | 100 | 70.8 | |
| 8 | 100 | 100 |
Drug levels in plasma and tissues of infected mice.
Since the percentage of blood in the lung homogenates was ca. 5%, the drug content in peripheral blood had little influence on the drug levels in lung homogenates.
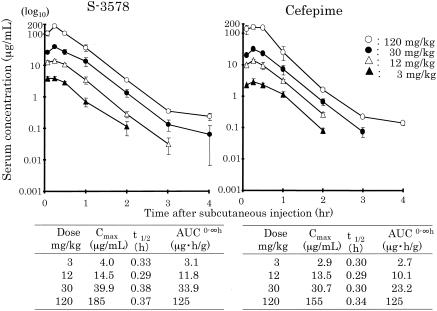
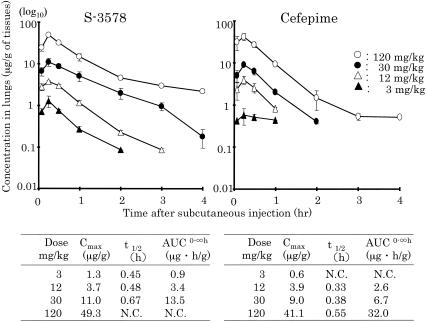
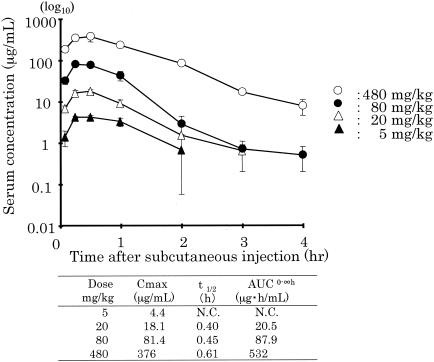
The levels of S-3578 and cefepime in the plasma and lungs of mice with bronchopneumonia caused by S. pneumoniae along with the calculated values of the PK parameters are presented in Fig. 1 and 2, respectively. The values of the PK parameter Cmax/dose for plasma and lung homogenates were 1.21 to 1.54 and 0.31 to 0.43, respectively, for S-3578 and 0.97 to 1.13 and 0.20 to 0.34, respectively, for cefepime. The values of the PK parameter AUC/dose for plasma and lung homogenates were 0.98 to 1.13 and 0.28 to 0.45, respectively, for S-3758 and 0.77 to 1.04 and 0.22 to 0.26, respectively, for cefepime. The t1/2 values of S-3578 in plasma and lung homogenates were 0.29 to 0.38 and 0.45 to 0.67, respectively, and those of cefepime were 0.29 to 0.34 and 0.33 to 0.55, respectively. In the model of murine systemic MRSA infection, the values of the PK parameters for S-3578 in plasma were 0.78 to 1.02 for Cmax/dose, 1.03 to 1.11 for AUC/dose, and 0.40 to 0.61 for t1/2 (Fig. 3). t1/2 values in the lung homogenates of mice in the lung infection model and the plasma of mice in the systemic infection model tended to be longer than those in the corresponding plasma of mice in the lung infection model.
FIG. 1.
Plasma S-3578 or cefepime concentration after administration of single doses of 3, 12, 30, and 120 mg/kg to mice infected with S. pneumoniae strain SR11031. Each symbol represents the mean levels in the plasma of three mice. AUC0-∞, AUC from time zero to infinity.
FIG. 2.
S-3578 and cefepime concentrations in lung homogenates after administration of single doses of 3, 12, 30, and 120 mg/kg to mice infected with S. pneumoniae strain SR11031. Each symbol represents the mean level in the lung homogenates of three mice. AUC0-∞, AUC from time zero to infinity; N.C., not calculable.
FIG. 3.
Plasma S-3578 concentrations after administration of single doses of 5, 20, 80, and 480 mg/kg to mice infected with S. aureus strain SR3637. Each symbol represents the mean levels in the plasma of three mice. AUC0-∞, AUC from time zero to infinity; N.C., not calculable.
PK parameters.
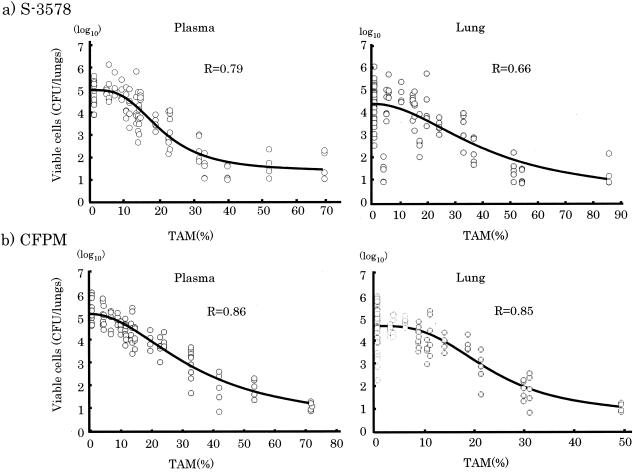
The relationship between the numbers of viable cells in lung tissues as an effective indicator and AUC/MIC, Cmax/MIC and TAM in plasma and lung tissue was calculated. The correlation coefficients between efficacy and AUC/MIC and between efficacy and Cmax/MIC for S-3578 were 0.45 and 0.03, respectively, for plasma and 0.45 and 0.03, respectively, for lung tissue. For cefepime the correlation coefficients were 0.56 and 0.01, respectively, for plasma and 0.56 and 0.01, respectively, for lung tissue. On the other hand, the correlation coefficients between efficacy and TAM for S-3578 were 0.79 for plasma and 0.66 for lung tissue, and those for cefepime were 0.86 for plasma and 0.85 for lung tissue (Fig. 4).
FIG. 4.
Relationship between TAM for S. pneumoniae SR11031 and log10 numbers of CFU per lungs for S-3578 or cefepime (CFPM) after 24 h of therapy.
The survival rate among mice with systemic MRSA infection (Table 3) was 100% when the TAM in the plasma was ≥71%, but it was less than 60% when the TAM in plasma was <60%. The correlation coefficients for the relationship between survival rate and AUC/MIC, Cmax/MIC, and TAM in our systemic infection model were 0.78, 0.36, and 0.82, respectively.
DISCUSSION
In the present study, PRSP (S-3578 MIC, 1 mg/liter) and MRSA (S-3578 MIC,4 mg/liter) were used because the MICs for the organisms corresponded to the MIC at which 50% of isolates are inhibited (MIC50) and the MIC90s for clinical isolates reported by Fujimura et al. (6). Since the novel drug S-3578 is a parenterally administered drug, it can be used for the treatment of severe infections such as bacteremia or septicemia. Thus, in the present study, we used S. pneumoniae lung infection as a local infection and systemic MRSA infection as a severe infection to analyze the PK and PD parameters for S-3578. Two of the most important parameters that affect the efficacy of an antimicrobial agent are the MIC of the therapeutic agent for the infecting pathogen and the magnitude of exposure of the organism to the drug.
The antibacterial effects of β-lactams are time dependent. Once drug concentrations exceed a critical value (usually two to four times the MIC), the rate of bacterial killing is maximized; that is, further increases in drug concentration do not result in proportional increases in the rate or extent of bacterial killing (1, 5, 10). Craig and Ebert (4) reported that the binding of antimicrobials to serum proteins could reduce antimicrobial activity and that binding levels of 80% or more had the potential to significantly reduce free drug levels and affect therapeutic efficacy in patients. The level of serum protein binding of S-3578 is 22% in mice (16) and therefore does not affect the therapeutic efficacy of the drug. The present study showed that TAM is the PK-PD parameter that best predicts the efficacy of the novel drug S-3578 against gram-positive pathogens and that for S-3578 the correlation coefficient between efficacy and TAM in mouse plasma is higher than that in lung homogenates. Our findings also confirm that the antibacterial effect of cefepime reflects the duration that the drug concentration remains above the MIC of the drug for the pathogen. In this study, a TAM of S-3578 in plasma of 22% was required to decrease viable organism counts in infected tissues by ≥2 log10. In the case of cefepime, a TAM in plasma of 32% was required to decrease the viable organism counts to the same level as that achieved with S-3578. Craig (3) reported that the TAMs required for a static effect after 24 h of therapy with cefotaxime, ceftriaxone, ceftazidime, and cefpirome were 36 to 41% in the S. pneumoniae infection model. These values were similar to those for cefepime but were slightly higher than those for S-3578 obtained in the present study. When the TAMs of S-3578 and cefepime were more than 22 and 32%, respectively, the survival rates were 62.5% or greater. This result was associated with a ≥2-log10 decrease in viable organism counts.
However, the TAM calculated by Craig (3) was for the dose required to achieve stasis, but the TAM used in the present study was for the dose needed to obtain a 2-log reduction. In addition, there were differences between the two studies in terms of the models, the infective organisms, and the endpoints used. In the near future, it will be necessary to conduct more detailed studies to obtain a better understanding of this phenomenon.
REFERENCES
- 1.Ambrose, P. G., R. C. Owens, Jr., M. J. Garvey, and R. N. Jones. 2002. Pharmacodynamic considerations in the treatment of moderate to severe pseudomonal infections with cefepime. J. Antimicrob. Chemother. 49:445-453. [DOI] [PubMed] [Google Scholar]
- 2.Betriu, C., M. Redondo, A. Boloix, M. Gomez, E. Culebras, and J. J. Picazo. 2001. Comparative activity of linezolid and other new agents against methicillin-resistant Staphylococcus aureus and teicoplanin-intermediate coagulase-negative staphylococci. J. Antimicrob. Chemother. 48:911-913. [DOI] [PubMed] [Google Scholar]
- 3.Craig, W. A. 1995. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn. Microbiol. Infect. Dis. 22:89-96. [DOI] [PubMed] [Google Scholar]
- 4.Craig, W. A., and S. C. Ebert. 1989. Protein binding and its significance in antibacterial therapy. Infect. Dis. Clin. N. Am. 3:407-414. [PubMed] [Google Scholar]
- 5.Eagle, H., R. Fleischman, and M. Levy. 1953. Continuous vs. discontinuous therapy with penicillin. N. Engl. J. Med. 238:481-488. [DOI] [PubMed] [Google Scholar]
- 6.Fujimura, T., Y. Yamano, I. Yoshida, J. Shimada, and S. Kuwahara. 2003. In vitro activity of S-3578, a new broad-spectrum cephalosporin active against methicillin-resistant staphylococci. Antimicrob. Agents Chemother. 47:923-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gniadkowski, M. 2001. Evolution and epidemiology of extended-spectrum beta-lactamses (ESBLs) and ESBL-producing microorganisms. Clin. Microbiol. Infect. 7:597-608. [DOI] [PubMed] [Google Scholar]
- 8.Keggett, J. E., S. Ebert, B. Fantin, and W. A. Craig. 1990. Comparative dose-effect relations at several dosing intervals for beta-lactam, aminoglycoside and quinolone antibiotics against gram-negative bacilli in murine thigh-infection and pneumonitis models. Scand. J. Infect. Dis. 74:179-184. [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, approved standard, 5th ed. M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 10.Nishida, M., T. Murakawa, and T. Kaminura. 1978. Bactericidal activity of cephalosporins in an in vitro model of simulating serum levels. Antimicrob. Agents Chemother. 14:6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimizu, K., M. Orizu, H. Kanno, S. Kitamura, T. Konishi, K. Soma, H. Nishitani, Y. Noguchi, S. Hasegawa, H. Hasegawa, and K. Wada. 1996. Clinical studies on vancomycin in the treatment of MRSA infection. Jpn. J. Antibiot. 49:782-799. (In Japanese.) [PubMed] [Google Scholar]
- 12.Snydman, D. R., N. V. Jacobus, L. A. McDermott, J. R. Lonks, and J. M. Boyce. 2000. Comparative in vitro activities of daptomycin and vancomycin against resistant gram-positive pathogens. Antimcirob. Agents Chemother. 44:3447-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevens, D. L., D. Herr, H. Lampiris, J. L. Hunt, D. H. Batts, and B. Hafkin. 2002. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin. Infect. Dis. 34:1481-1490. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi, Y., N. Masuda, M. Otsuki, M. Miki, and T. Nishino. 1997. In vitro activity of HSR-903, a new quinolone. Antimicrob. Agents Chemother. 41:1326-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tateda, K., K. Takashima, H. Miyazaki, T. Matsumoto, T. Hatori, and K. Yamaguchi. 1996. Noncompromised penicillin-resistant pneumococcal pneumoniae CBA/J mouse model and comparative efficacies of antibiotics in this model. Antimicrob. Agents Chemother. 40:1520-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuji, A., M. Takema, H. Miwa, J. Shimada, and S. Kuwahara. 2003. In vivo antibacterial activity of S-3578, a new broad-spectrum cephalosporin: methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa experimental infection models. Antimicrob. Agents Chemother. 47:2507-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]