Abstract
Background and methods
In this study, gelatin was blended with polyglycolic acid (PGA) at different ratios (0, 10, 30, and 50 wt%) and electrospun. The morphology and structure of the scaffolds were characterized by scanning electron microscopy, Fourier transform infrared spectroscopy, and differential scanning calorimetry. The mechanical properties were also measured by the tensile test. Furthermore, for biocompatibility assessment, human umbilical vein endothelial cells and human umbilical artery smooth muscle cells were cultured on these scaffolds, and cell attachment and viability were evaluated.
Results
PGA with 10 wt% gelatin enhanced the endothelial cells whilst PGA with 30 wt% gelatin increased smooth muscle cell adhesion, penetration, and viability compared with the other scaffold blends. Additionally, with the increase in gelatin content, the mechanical properties of the scaffolds were improved due to interaction between PGA and gelatin, as revealed by Fourier transform infrared spectroscopy and differential scanning calorimetry.
Conclusion
Incorporation of gelatin improves the biological and mechanical properties of PGA, making promising scaffolds for vascular tissue engineering.
Keywords: polyglycolic acid, gelatin, nanofiber, vascular tissue engineering, biocompatible scaffold
Introduction
The extracellular matrix complex, composed of proteoglycans, collagens, elastin, and various glycoproteins, is central to the maintenance of vessel wall cell integrity and appropriate signal transduction during important biological functions such as adhesion, differentiation, migration, induction of inflammatory responses, and wound healing through the integrin superfamily of receptors.1 Methods influencing cellular functions using electrospun scaffolds remain a challenge because the scaffolds need to mimic some of the components of the natural extracellular matrix, whilst providing appropriate biochemical and mechanical inputs for the cellular environment.2 Previous studies have attempted to develop thromboresistant and long-lasting synthetic fibrous scaffolds for tissue engineering in the field of vascular grafts. Subsequently, due to poor antithrombogenicity and inconsistent material properties,3 clinical outcomes of synthetic vascular grafts have not always proven satisfactory.4 In addition, the bio-compatibility of tissue-engineered scaffolds is of primary concern because this affects cell attachment, proliferation, differentiation, and growth.5
Electrospinning has been used effectively to generate biomimetic nonwoven scaffolds for tissue engineering purposes.6–8 Various synthetic biodegradable polymers have been electrospun into thin fibers for generating fibrous scaffolds, including poly(3-caprolactone),9–11 polyethylene oxide,11 poly(L-lactide-co-3-caprolactone) (PLCL),12 polylactic acid,13 poly(lactideco-glycolide),14,15 and polyglycolic acid (PGA).16 All these scaffolds have been reported to be bio-compatible and to enhance cell functions in vitro. PGA is one of a group of biodegradable aliphatic polyesters currently exploited in a variety of medical applications. The successful clinical use of PGA sutures has demonstrated that PGA-containing polymers can be used safely in soft tissue applications.17 PGA has also been found to be useful in the engineering of many types of tissues18–23 and is also used in vascular tissue engineering.24–27
Gelatin is a polymer derived from partial hydrolysis of native collagen, with uses in the pharmaceutical and medical fields, including as sealants for vascular prostheses,28 carriers for drug delivery,29 and wound dressings.30 Furthermore, it is known that gelatin contains many integrin binding sites for cell adhesion, migration, and differentiation, which are found in natural collagen and other extracellular matrix proteins.
In this study, gelatin was added to PGA and electrospun with different weight ratios of gelatin to develop novel electrospun PGA/gelatin fibrous scaffolds which are noncytotoxic, support cell attachment, maintain viability of the major cellular constituents of the vasculature, namely endothelial and smooth muscle cells, and have suitable biomechanical properties.
Materials and methods
Materials and cells
The PGA and gelatin (type A, porcine skin) were purchased from Sigma-Aldrich (St Louis, MO). For electrospinning, PGA and gelatin were dissolved in 1,1,1,3,3,3-hexafluoro- 2-propanol (Merck, Darmstadt, Germany). Cell culture media, growth factors, and supplements were purchased from Invitrogen (Carlsbad, CA) and disposable tissue culture supplies from Orange Scientific (Brussels, Belgium). Tetrazolium salt (MTT) was purchased from Sigma-Aldrich. Human umbilical vein endothelial cells (HUVECs) were isolated from human umbilical cords according to the method reported in our previous work.1 Human umbilical artery smooth muscle cells (HUASMCs) were obtained from the Pasteur Institute (Tehran, Iran).
Electrospinning
A series of PGA/gelatin solutions with different mixing ratios were prepared by dissolving them in hexafluoroisopropanol at a concentration of 10 wt%. The gelatin contents were 10, 30, and 50 wt%. The PGA/gelatin solutions were delivered by a syringe pump at a constant flow rate of 5 mL/hour. A blunt-ended 18-gauge needle was clamped to the positive electrode of a high-voltage power supply generating 25 kV of electric field, and the negative electrode was connected to an Al foil collector with an air gap distance of 20 cm. The PGA/gelatin solutions were electrospun under the same conditions, producing fibers of varied diameters. All the electrospun PGA/gelatin fibers were vacuum-dried for three days at room temperature and stored in desiccators for subsequent use.
Scanning electron microscopy
The morphology and pore structure of the electrospun PGA/gelatin blend fibers were observed using a scanning electron microscope. Prior to observation, platinum was coated by ion sputtering for a few seconds. The average diameter, diameter distribution, area of porosity between fibers, and distribution of fiber orientation angles were obtained by analyzing scanning electron microscopy images using a custom code image analysis program.
Fourier transform infrared spectroscopy
Chemical analysis of PGA, gelatin, and the PGA/gelatin nanofibrous scaffolds was performed by attenuated total reflectance-Fourier transform infrared spectroscopy over a range of 4000–400 cm−1. Attenuated total reflectance-Fourier transform infrared spectra of PGA and PGA/gelatin nanofibrous scaffolds were obtained on a Bruker spectrometer system (Vertex 80).
Differential scanning calorimetry
A Perkin-Elmer differential scanning calorimeter was used for studying the thermal behavior of the samples. The temperature was raised from room temperature to 350°C using a linear programmer at a heating rate of 10°C per minute.
Mechanical properties
The mechanical properties of the electrospun PGA/gelatin fiber sheets, approximately 60 mm × 10 mm × 0.1–0.2 mm (width × length × thickness), were measured using a uniaxial testing machine (Santam, STM-20) with a 10 N load cell under a cross-head speed of 1 mm/minute and gauge length of 30 mm (n = 3). From the stress–strain curves, Young’s modulus, tensile strength, and elongation at break were obtained.
Cell culture
PGA/gelatin fibers produced by electrospinning were examined as scaffolds for growth of HUVECs and HUASMCs. Cells were grown in Dulbecco’s modified Eagle’s medium with 4.5 g/L glucose supplemented by 4 mM L-glutamine, 25 units/mL penicillin/streptomycin, and 10% fetal bovine serum under standard culture conditions (37°C, 5% CO2). The electrospun fiber sheets were cut into small circular pieces and rinsed with sterilized phosphate-buffered solution and then transferred to 96-well tissue culture polystyrene (TCPS). Scaffolds were sterilized with 70% ethanol and ultraviolet irradiation, followed by washing with phosphate-buffered solution. Fiber scaffolds were soaked in Dulbecco’s modified Eagle’s medium overnight. The cells were seeded at a density of 1 × 104 cells/well onto the sheets, and cultured with regular replacement of culture medium every two days. Cell adhesion and viability were assayed by a colorimetric MTT assay after days 1, 3, and 5, respectively. The morphology of cell attachment on the scaffolds after one day of cell culture was observed by scanning electron microscopy.
MTT assay
The MTT assay is based on reduction of the yellow tetrazolium salt to purple formazan crystals by dehydrogenase enzymes secreted from the mitochondria of metabolically active cells. The amount of purple formazan crystals formed is proportional to the number of viable cells. First, each sample was incubated at 37°C for four hours with MTT solution 0.5 mg/mL without phenol red. After incubation, the MTT solution was removed. A buffer solution containing dimethylsulfoxide was added into the wells to dissolve the formazan crystals. After 30 minutes of rotary agitation, the solutions were transferred into a cuvette and placed in a spectrophotometer, and absorbance was measured at 570–630 nm.
Scanning electron microscopy
To study the morphology of cell attachment, the cell-cultured fiber sheets were washed with phosphate-buffered solution three times and then fixed with 2.5% glutaraldehyde for three hours, followed by formalin fixation (10%) overnight. After washing three times with distilled water, the fiber sheets were dehydrated through a series of graded ethanol solutions (50%, 60%, 70%, 80%, 90%, and 100%). Completely dried samples were sputter-coated with platinum and observed using scanning electron microscopy.
Statistical analysis
The results are presented as the mean ± standard deviation. Statistical significance was tested using SPSS software (SPSS Inc, Chicago, IL). Values of P < 0.05 were considered to be statistically significant.
Results
Scaffold characterization
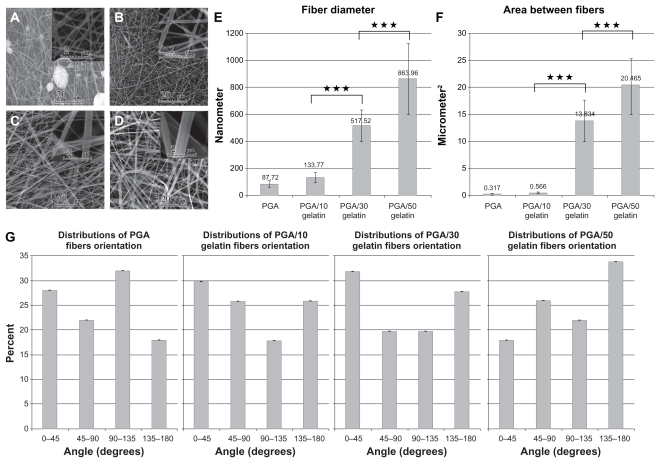
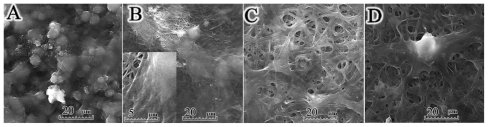
Morphological images of the electrospun PGA/gelatin fibers by scanning electron microscopy are shown in Figure 1. The scanning electron microscopy images of electrospun pure PGA showed a bead-on-string formation (Figure 1A), while PGA with 10 wt% gelatin fibers was a bead-free and homogeneous structure (Figure 1B). As seen in Figure 1C and D, an increase in the concentration of gelatin in the solution was accompanied with an increase in fiber size. Figure 1E shows a quantitative analysis of at least 50 fibers from different blended samples, indicating that the mean fiber diameter increased from 87.72 ± 23.34 nm for pure PGA fibers to 863.96 ± 265.09 nm for PGA/50 gelatin fibers. Statistical analysis revealed that the fiber sizes with different weight ratios of gelatin were significantly different from each other (P < 0.001). As depicted in Figure 1E, the biggest increment in fiber size occurred when the gelatin weight ratio was increased from 10 wt% to 30 wt%. The mean area of porosity between the fibers is displayed in Figure 1F. As seen in Figure 1F, the area between the fibers increased with a higher gelatin concentration similar to fiber diameter. Figure 1G represents the distribution of fiber orientation angles with respect to the horizontal axis, demonstrating that the electrospun nanofiber orientation appeared to be random.
Figure 1.
Scanning electron microscopic morphology of electrospun PGA/gelatin nanofibers (2000× and 20,000× magnification). (A) Pure PGA, (B) PGA/10 wt% gelatin, (C) PGA/30 wt% gelatin, and (D) PGA/50 wt% gelatin. (E) Mean diameter (± standard deviation) variations of electrospun PGA/gelatin fibers, (F) mean area (± standard deviation) between fibers, and (G) distribution of fiber orientation angles (n = 50).
Notes: ***P < 0.001, significantly different from the previous group.
Abbreviation: PGA, polyglycolic acid.
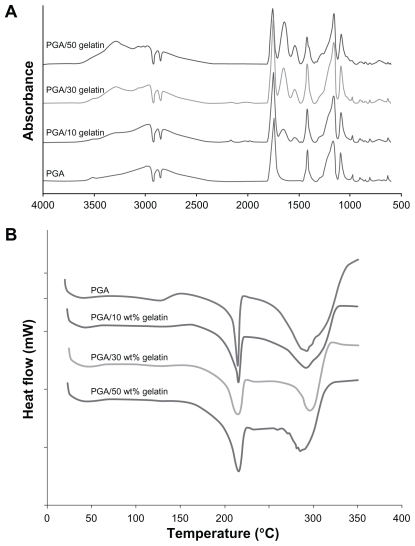
Fourier transform infrared spectroscopy
The infrared absorbance spectra for the scaffolds are shown in Figure 2A. The typical characteristic features observed in the infrared spectra of all scaffolds are reported here. Pure PGA was typically around 2960 cm−1 (γ–CH), 2882 cm−1, 2820 cm−1 (–CH2), 1417 cm−1 (δ–CH), 1163 cm−1, and 1090 cm−1 (γ–C–O), and the group of bands in the region of 1000–800 cm−1 was possibly due to a mix of vibrational modes of [–C–C–]n repeat units and –CH twist. The strong band around 1744 cm−1 is characteristic of [C=O] groups. In the PGA/gelatin scaffolds, the bond groups of PGA are presented; in addition, the 3290 cm−1 region is donated by N–H bond-stretching mode of hydrogen-bonded amide A groups. Common bands of protein appeared at approximately 1642 cm−1 (amide I) and 1539 cm−1 (amide II), corresponding to the stretching vibrations of C=O bond, and coupling of the bending of the N–H bond and stretching of the C–N bonds, respectively.
Figure 2.
(A) Attenuated total reflectance-Fourier transform infrared spectra of PGA/gelatin scaffolds and (B) differential scanning calorimetric curves of PGA/gelatin scaffolds.
Abbreviation: PGA, polyglycolic acid.
Differential scanning calorimetry
The differential scanning calorimetry results of the samples are displayed in Figure 2B. As shown in this figure, the first endothermic peaks are at about 210°C–220°C, representing the melting point of PGA. When comparing the PGA curve and the PGA/gelatin curves, in the presence of gelatin, the first peak becomes broader and shifts slightly to a higher temperature.
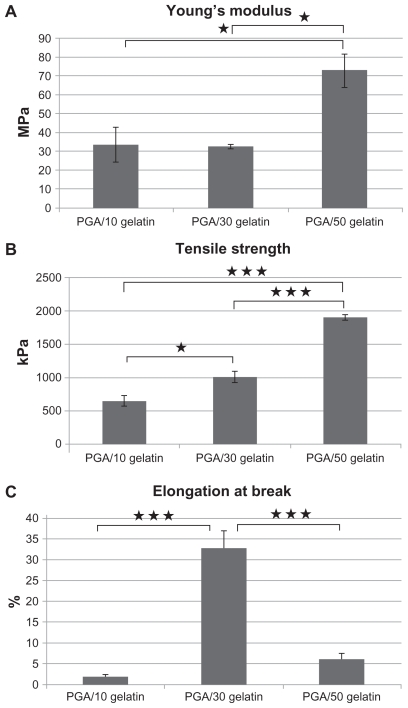
Mechanical properties
The tensile properties of electrospun PGA/gelatin fiber sheets were characterized. Young’s modulus, tensile strength, and elongation at break were calculated from stress–strain curves, as shown in Figure 3. The pure PGA fiber sheet showed very rigid and brittle characteristics, so no further studies were done. The PGA with 10 wt% gelatin sheets showed a tensile strength of 650 kPa. The PGA with 30 wt% gelatin sheet showed very soft and flexible characteristics, with a Young’s modulus of 32 MPa and a high elongation at break of 32%. PGA/gelatin 50 wt% showed an enhanced tensile strength of 1907 kPa, which was increased about three-fold when compared with that of PGA with 10 wt% gelatin. It also showed a high Young’s modulus of 72 MPa and an elongation break of 6%. It was observed that the addition of gelatin significantly increased tensile strength and the Young’s modulus of the structures.
Figure 3.
(A) Young’s modulus of PGA/gelatin scaffolds, mean ± standard deviation (n = 3), (B) tensile strength of PGA/gelatin scaffolds, mean ± standard deviation (n = 3), and (C) elongation at break of PGA/gelatin scaffolds, mean ± standard deviation (n = 3).
Notes: *P < 0.05; ***P < 0.001, significantly different compared with the previous group.
Abbreviation: PGA, polyglycolic acid.
Cell study
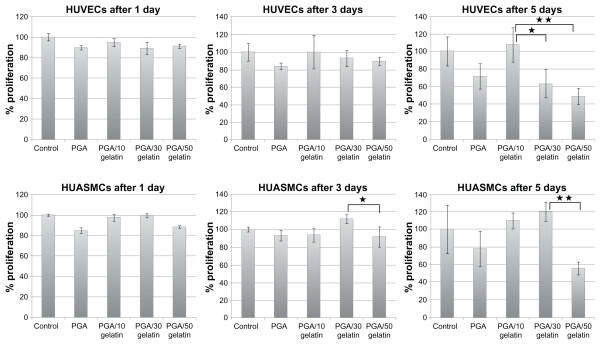
HUVECs and HUASMCs were seeded at a density of 10,000 cells/well in triplicate for each experiment. The cells were allowed to proliferate for up to five days. Over the five-day course, relative viable cell numbers were determined continuously on days 1, 3, and 5 using the MTT assay. Figure 4 shows the number of viable cells on electrospun PGA/gelatin fiber and control (TCPS). HUVECs and HUASMCs cells proliferated similarly on all substrates at day 1 of culture period, albeit with some slight differences. On day 1, the number of viable cells on the fibrous substrates was slightly lower than on the controls (P > 0.05). Conversely, on days 3 and 5, there were more endothelial cells on the PGA with 10 wt% gelatin (P < 0.05) substrates. Moreover, there was a high number of smooth muscle cells on the PGA with 30 wt% gelatin substrate, indicating a higher degree of cell viability (P < 0.05).
Figure 4.
Cell viability (MTT results) on PGA/gelatin scaffolds after days 1, 3, and 5. Mean ± standard deviation (n = 3).
Notes: *P < 0.05; **P < 0.01.
Abbreviation: PGA, polyglycolic acid.
The enhanced cell adhesion on PGA/gelatin fibers was also confirmed by the scanning electron microscopic images (Figures 5 and 6). The endothelial cell density on the PGA with 10 wt% gelatin increased significantly and enough to cover the electrospun sheets confluently. It also had high cell adhesion and penetration into the fibers (Figure 5B), whereas adherent cells on substrates above this surface displayed flattened morphologies. Figure 5D shows a spheroid cell with some filapodial extensions onto the scaffold due to high concentrations of gelatin. This kind of structure may reduce cell spreading, migration, and viability compared with the cell culture achieved using the lower concentration of gelatin. As shown in Figure 6C, smooth muscle cells can attach to the PGA/gelatin fibers, and the larger space between fibers aids high cell penetration into the PGA with 30 wt% gelatin. The scanning electron microscopy results confirm the data obtained by MTT assay.
Figure 5.
Scanning electron micrographs of human umbilical vein endothelial cells cultured on PGA/gelatin scaffolds (2000× magnification). (A) Pure PGA, (B) PGA/10 wt% gelatin, (C) PGA/30 wt% gelatin, and (D) PGA/50 wt% gelatin.
Abbreviation: PGA, polyglycolic acid.
Figure 6.
Scanning electron micrographs of human umbilical artery smooth muscle cells cultured on PGA/gelatin scaffolds (2000× magnification). (A) Pure PGA, (B) PGA/10 wt% gelatin, (C) PGA/30 wt% gelatin, and (D) PGA/50 wt% gelatin.
Abbreviation: PGA, polyglycolic acid.
Discussion
Vascular tissue engineering has emerged as one of the most promising approaches for the development of ideal substitutes for native tissue with similar properties. The design of these scaffolds must meet several physical and chemical criteria of the normal vascular system, such as being biocompatible and biodegradable, and with mechanical properties close to that of native blood vessels.
In the electrospinning process, there are a number of parameters affecting fiber morphology and fiber diameter, including polymer concentration/viscosity, voltage applied, needle diameter, and the delivery rate of polymer solution.6 Pure PGA solutions of high concentrations have been found to be less viscous than PGA/gelatin solutions. Low-viscosity solutions are more prone to produce beads, and bead formation reduces as viscosity is increased by addition of gelatin. Kim et al investigated the electrospinning of polyurethane and gelatin, and reported that addition of gelatin also increased fiber diameter.31 Gu et al also showed that when the gelatin concentration was increased to 10%, uniform fibers were obtained, suggesting a change from beads-on-string structures to smooth fibers.32 Therefore, the addition of gelatin to PGA has a significant effect on the spinnability of a polymer solution and the fiber morphology, resulting in bead-free nanofibers (Figure 1).
Normally, in the Fourier transform infrared spectra of gelatin, a free N–H stretching vibration occurs in the range of 3400–3440 cm−1. It has been shown that when the N–H group of a peptide is involved in a hydrogen bond, the position is shifted to lower frequencies.33 At the amide A region, a lower amplitude, as well as a lower wave number, was found in the PGA/gelatin structure, compared with gelatin, indicating that the N–H group of shorter peptide fragments in PGA/gelatin was involved in hydrogen bonding. Gelatin might also form new covalent intermolecular cross-links during electrospinning. The amide A also tends to join with the –CH2 stretch peak when carboxylic acid groups exist in a dimeric intermolecular interaction.34
In the current work, the appearance of an amide group in the Fourier transform infrared spectra of PGA/gelatin nanofibrous scaffolds indicates that the PGA chains were chemically bonded to the gelatin side chains, leading to introduction of functional groups, such as –NH2 and –COOH, on the surface of the PGA/gelatin scaffolds. Moreover, the combination of PGA and gelatin with a ratio of 30 wt% gave rise to a strong and flexible structure, which confirms appropriate interaction between PGA and gelatin.
As shown in the differential scanning calorimetry results (Figure 2B), the area under the first peak in the differential scanning calorimetry curves was determined as the enthalpy of the melting of samples (ΔHm).35 The broadening of the melting peak in the samples containing gelatin shows that these samples consume more energy for melting than pure PGA. Therefore, the presence of gelatin molecules in the scaffolds stabilizes the PGA molecules, based on molecular interaction between gelatin and PGA. These results are indicative of interactions between PGA and gelatin, thus confirming the Fourier transform infrared spectra results.
The mechanical properties of the blended fibrous scaffolds that are produced from natural extracellular matrix proteins, namely collagen and other byproducts, are not sufficient for tissue-engineering applications due to their low stability, but nanofibrous membranes made of a polymeric blend system have the advantage of suitable mechanical strength of both natural and synthetic polymers.31 In this study, it was found that PGA nanofibers showed inferior mechanical properties with beads present. Nanofibers with beads have a lower Young’s modulus, tensile strength, and elongation at break. This is due to stress concentrations induced by the beads as the nanofibers are stretched. One study showed that addition of a natural polymer like collagen or gelatin to a synthetic polymer such as PLCL decreased the strength of the polymer,36 whereas Zhang et al showed that Young’s modulus of electrospun poly(3-caprolactone) fiber increased with the addition of gelatin, but the tensile strength decreased to some extent.37 Wang et al tested the mechanical properties of a normal human saphenous vein and showed a Young’s modulus of around 15 MPa and a tensile strength of around 1000 kPa for a natural vessel.24 Our results demonstrate that electrospun PGA with 10 wt% and 30 wt% gelatin scaffolds are mechanically suitable for vascular tissue engineering, with tensile strength values approximating natural vein values and a higher Young’s modulus of 30 MPa, which is about twice as high and, therefore, stiffer than natural veins.
Matsuda et al studied the cell adhesion and proliferation of HUVECs on 0.3 μm, 1.1 μm, and 7 μm PLCL fibers. The dense surface of the small fibers (0.3 μm, 1.1 μm) provided good cell adhesion, spreading, and proliferation, while on the large diameter (7 μm) fibers, cells were sparsely distributed with less proliferation.12 Similar to our observations, they also reported that addition of collagen (5 wt% or 10 wt%) enhanced cell adhesion/proliferation, but the effects were diminished for fibers with a higher collagen content.38
It is well known that basement membranes and their interaction with endothelial cells play a major role in vascular development. Endothelial cells can produce a variety of basement membrane components. Fibronectin and interstitial collagens seem to promote migration and proliferation, whereas basement membrane collagen and laminin stimulate attachment and differentiation.39 Reactivity of the synthetic peptide analogs of adhesive proteins with regard to the interaction of human endothelial cells with the extracellular matrix is also well described in the literature.40 As an example, endothelial cell attachment on uncoated vascular prostheses is very weak, but the vascular graft surface with cell adhesion promoting Arg-Gly-Asp (RGD)-containing synthetic peptides significantly improves this important step in endothelial cell seeding of vascular grafts.4,41 Gelatin also has many integrin binding sites (such as RGD) for cell adhesion, migration, and differentiation, which are found in natural collagen and other extracellular matrix proteins. As expected, blending of PGA and gelatin improves cell attachment and proliferation compared with cell growth on pure PGA nanofibrous scaffolds.
Cell penetration is highly important for obtaining cell-cell contacts and also the outcome of engineered tissue. In two-dimensional systems, contact inhibition in a monolayer culture caused a decrease in cell proliferation when cultured on TCPS as control. In three-dimensional scaffold systems, cells can penetrate into the scaffold structure, but due to the incorporation of high concentrations of gelatin, cell penetration may be hampered even in the presence of adhesion molecules, due to the physical obstruction posed by the dense matrix.42 Mann et al showed that a low concentration of cell adhesion ligand (RGD) aids cell migration, while a higher concentration impedes cell migration, and cells growing on the surfaces with a greater RGD produce less matrix.43,44 Neff et al demonstrated that an intermediate RGD concentration provides maximum proliferation of fibroblasts, indicating that excessive peptide levels on the surface causes a decrease in proliferation.45 Other studies have shown that maximal migration of cells on surfaces coated with matrix proteins, such as RGD, collagen and fibronectin, occurs at an intermediate level of cell-substrate adhesiveness.46–49 These results reflect the fact that cells require cell adhesion ligands for migration, but they also demonstrate that there may be an optimal ligand concentration for migration and proliferation, and indicate that cell viability decreases in scaffolds with a high concentration of gelatin after five days. Therefore, it can be concluded that only a certain amount of gelatin (10 wt% for endothelial cells and 30 wt% for smooth muscle cells) in the PGA/gelatin-blended fiber scaffolds can improve cell viability significantly, and show better vascular cell compatibility than PGA fiber scaffolds and controls.
Conclusion
In this study, gelatin was added to PGA and electrospun with different weight ratios. The results demonstrate that the properties of nanofibrous scaffolds were strongly influenced by the concentration of gelatin in the scaffolds. Given the suitable mechanical properties of these PGA/gelatin scaffolds and favorable biocompatibility with vascular cells, it is suggested that tubular scaffolds with an inner layer of PGA/10 wt% gelatin and an outer layer of PGA/30 wt% gelatin are promising scaffolds for vascular tissue engineering and regeneration in vivo.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Shahgasempour S, Woodroffe SB, Sullivan-Tailyour G, Garnett HM. Alteration in the expression of endothelial cell integrin receptors α5β1 and α2β1 and α6β1 after in vitro infection with a clinical isolate of human cytomegalovirus. Arch Virol. 1997;142:125–138. doi: 10.1007/s007050050063. [DOI] [PubMed] [Google Scholar]
- 2.Nisbet DR, Forsythe JS, Shen W, Finkelstein DI, Horne MK. Review paper: A review of the cellular response on elecrospun nanofibers for tissue engineering. J Biomed Appl. 2009;24:7–29. doi: 10.1177/0885328208099086. [DOI] [PubMed] [Google Scholar]
- 3.Nerem RM, Ensley AE. The tissue engineering of blood vessels and the heart. Am J Transplant. 2004;4:36–42. doi: 10.1111/j.1600-6135.2004.0343.x. [DOI] [PubMed] [Google Scholar]
- 4.Meinhart JG, Deutsch M, Fischlein T, Howanietz Froschl A, Zilla P. Clinical autologous in vitro endothelialization of 153 infrainguinal ePTFE grafts. Ann Thorac Surg. 2001;71:327–331. doi: 10.1016/s0003-4975(01)02555-3. [DOI] [PubMed] [Google Scholar]
- 5.Williams DF. On the mechanisms of biocompatibility. Biomaterials. 2008;29:2941–2953. doi: 10.1016/j.biomaterials.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 6.Nukavarapu SP, Kumbar SG, Merrell JG, Laurencin CT. Electrospun polymeric nanofiber scaffolds for tissue regeneration. In: Laurencin CT, Nair LS, editors. Nanotechnology and Tissue Engineering: The Scaffold. Boca Raton, FL: CRC Press; 2008. [Google Scholar]
- 7.Vasita R, Katti DS. Nanofibers and their applications in tissue engineering. Int J Nanomedicine. 2006;1:15–30. doi: 10.2147/nano.2006.1.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang YZ, Su B, Venugopal J, Ramakrishna S, Lim CT. Biomimetic and bioactive nanofibrous scaffolds from electrospun composite nanofibers. Int J Nanomedicine. 2007;2:623–638. [PMC free article] [PubMed] [Google Scholar]
- 9.Zong X, Bien H, Chung C, et al. Electrospun fine-textured scaffolds for heart tissue constructs. Biomaterials. 2005;26:5330–5338. doi: 10.1016/j.biomaterials.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 10.Bakhshandeh H, Soleimani M, Hosseini SS, et al. Poly (∑caprolactone) nanofibrous ring surrounding a polyvinyl alcohol hydrogel for the development of a biocompatible two-part artificial cornea. Int J Nanomedicine. 2011;6:1509–1515. doi: 10.2147/IJN.S19011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neves NM, Campos R, Pedro A, Cunha J, Macedo F, Reis RL. Patterning of polymer nanofiber meshes by electrospinning for biomedical applications. Int J Nanomedicine. 2007;2:433–448. [PMC free article] [PubMed] [Google Scholar]
- 12.Kwon IK, Kidoaki S, Matsuda T. Electrospun nano- to microfiber fabrics made of biodegradable copolyesters: structural characteristics, mechanical properties and cell adhesion potential. Biomaterials. 2005;26:3929–3939. doi: 10.1016/j.biomaterials.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Zeng J, Chen X, Xu X, et al. Ultrafine fibers electrospun from biodegradable polymers. J Appl Polym Sci. 2003;59:1085–1092. [Google Scholar]
- 14.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60:613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 15.Seil JT, Webster TJ. Spray deposition of live cells throughout the Electrospinning process produces nanofibrous three-dimensional tissue scaffolds. Int J Nanomedicine. 2011;6:1095–1099. doi: 10.2147/IJN.S18803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boland ED, Wnek GE, Simpson DG, Pawlowski KJ, Bowlin GL. Tailoring tissue engineering scaffolds by employing electrostatic processing techniques: a study of poly(glycolic acid) J Macromol Sci. 2001;A38:1231–1243. [Google Scholar]
- 17.Perrin DE, English JP. Polyglycolide and polylactide. In: Domb AJ, Kost J, Wiseman DM, editors. Handbook of Biodegradable Polymers. Boca Raton, FL: CRC Press; 1997. [Google Scholar]
- 18.Xu L, Cao D, Liu W, Zhou G, Zhang WJ, Cao Y. In vivo engineering of a functional tendon sheath in a hen model. Biomaterials. 2010;31:3894–3902. doi: 10.1016/j.biomaterials.2010.01.106. [DOI] [PubMed] [Google Scholar]
- 19.Cui L, Wu Y, Cen L, et al. Repair of articular cartilage defect in non-weight bearing areas using adipose derived stem cells loaded polyglycolic acid mesh. Biomaterials. 2009;30:2683–2693. doi: 10.1016/j.biomaterials.2009.01.045. [DOI] [PubMed] [Google Scholar]
- 20.Abbushi A, Endres M, Cabraja M, et al. Regeneration of intervertebral disc tissue by resorbable cell-free polyglycolic acid-based implants in a rabbit model of disc degeneration. Spine. 2008;33:1527–1532. doi: 10.1097/BRS.0b013e3181788760. [DOI] [PubMed] [Google Scholar]
- 21.Kim WS, Kim HK. Tissue engineered vascularized bone formation using in vivo implanted osteoblast-polyglycolic acid scaffold. J Korean Med Sci. 2005;20:479–482. doi: 10.3346/jkms.2005.20.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kojima K, Bonassar LJ, Roy AK, Mizuno H, Cortiella J, Vacanti CA. A composite tissue-engineered trachea using sheep nasal chondrocyte and epithelial cells. FASEB J. 2003;17:823–828. doi: 10.1096/fj.02-0462com. [DOI] [PubMed] [Google Scholar]
- 23.Cooper ML, Hansbrough JF, Spielvogel RL, Cohen R, Baxtel RL, Naughtm G. In vivo optimization of a living dermal substitute employing cultured human fibroblasts on a biodegradable polyglycolic acid or polyglactin mesh. Biomaterials. 1991;12:243–248. doi: 10.1016/0142-9612(91)90207-q. [DOI] [PubMed] [Google Scholar]
- 24.Wang C, Cen L, Yin S, et al. A small diameter elastic blood vessel wall prepared under pulsatile conditions from polyglycolic acid mesh and smooth muscle cells differentiated from adipose-derived stem cells. Biomaterials. 2010;31:621–630. doi: 10.1016/j.biomaterials.2009.09.086. [DOI] [PubMed] [Google Scholar]
- 25.Xu ZC, Zhang WJ, Li H, et al. Engineering of an elastic large muscular vessel wall with pulsatile stimulation in bioreactor. Biomaterials. 2008;29:1464–1472. doi: 10.1016/j.biomaterials.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 26.Iwasaki K, Kojima K, Kodama S, et al. Bioengineered three-layered robust and elastic artery using hemodynamically-equivalent pulsatile bioreactor. Circulation. 2008;30:52–57. doi: 10.1161/CIRCULATIONAHA.107.757369. [DOI] [PubMed] [Google Scholar]
- 27.Rashid ST, Salacinski HJ, Hamilton G, Seifalian AM. The use of animal models in developing the discipline of cardiovascular tissue engineering: a review. Biomaterials. 2004;25:1627–1637. doi: 10.1016/s0142-9612(03)00522-2. [DOI] [PubMed] [Google Scholar]
- 28.Gui R, Marceau D, Rao TJ, et al. In vitro and in vivo characterization of an impervious polyester arterial prosthesis: the Gelseal triaxial graft. Biomaterials. 1987;8:433–441. doi: 10.1016/0142-9612(87)90079-2. [DOI] [PubMed] [Google Scholar]
- 29.Tabata Y, Hijikata S, Ikada Y. Enhanced vascularization and tissue granulation by basic fibroblast growth factor impregnated in gelatin hydrogels. J Control Release. 1994;31:189–199. [Google Scholar]
- 30.Choi YS, Hong SR, Lee YM, Song KW, Park MH, Nam YS. Study on gelatin-containing artificial skin: 1. Preparation and characteristics of novel gelatin alginate sponge. Biomaterials. 1999;20:409–417. doi: 10.1016/s0142-9612(98)00180-x. [DOI] [PubMed] [Google Scholar]
- 31.Kim SE, Heo DN, Lee JB, et al. Electrospun gelatin/polyurethane blended nanofibers for wound healing. Biomed Mater. 2009;4:44–106. doi: 10.1088/1748-6041/4/4/044106. [DOI] [PubMed] [Google Scholar]
- 32.Gu SY, Wang ZM, Ren J, Zhang CY. Electrospinning of gelatin and gelatin/poly(l-lactide) blend and its characteristics for wound dressing. Mater Sci Eng C Mater Biol Appl. 2009;29:1822–1828. [Google Scholar]
- 33.Doyle BB, Blout ER, Bendit EG. Infrared spectroscopy of collagen and collagen like polypeptides. Biopolymers. 1975;14:937–957. doi: 10.1002/bip.1975.360140505. [DOI] [PubMed] [Google Scholar]
- 34.Kemp W. Organic Spectroscopy. 2nd ed. Hampshire, UK: Macmillan Education Ltd; 1987. [Google Scholar]
- 35.Pungor E. A Practical Guide to Instrumental Analysis. Boca Raton, FL: CRC Press; 1995. [Google Scholar]
- 36.Lee J, Tae G, Kim YH, Park IS, Kim SH, Kim SH. The effect of gelatin incorporation into electrospun poly(L-lactide-co-3-caprolactone) fibers on mechanical properties and cytocompatibility. Biomaterials. 2008;29:1872–1879. doi: 10.1016/j.biomaterials.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Ouyang H, Lim CT, Ramakrishna S, Huang ZM. Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. J Biomed Mater Res B. 2005;72:156–165. doi: 10.1002/jbm.b.30128. [DOI] [PubMed] [Google Scholar]
- 38.Kwon IK, Matsuda T. Co-electrospun nanofiber fabrics of poly (L-lactideco-3-caprolactone) with type I collagen or heparin. Biomacromolecules. 2005;6:2096–2105. doi: 10.1021/bm050086u. [DOI] [PubMed] [Google Scholar]
- 39.Olsen BR. Matrix molecules and their ligands. In: Lanza RP, Langer R, Chick WL, editors. Principles of Tissue Engineering. Georgetown, TX: RG Landes; 1997. [Google Scholar]
- 40.Chen CS, Hawiger J. Reactivity of synthetic peptide analogs of adhesive proteins in regard to the interaction of human endothelial cells with extracellular matrix. Blood. 1991;77:2200–2206. [PubMed] [Google Scholar]
- 41.Walluscheck KP, Steinhoff G, Kelm S, Haverich A. Improved endothelial cell attachment on ePTFE vascular grafts pretreated with synthetic RGD-containing peptides. Eur J Vasc Endovasc Surg. 1996;12:321–330. doi: 10.1016/s1078-5884(96)80251-6. [DOI] [PubMed] [Google Scholar]
- 42.Peyton SR, Raub CB, Keschrumrus VP, Putnam AJ. The use of poly(ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cells. Biomaterials. 2006;27:4881–4893. doi: 10.1016/j.biomaterials.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Mann BK, West JL. Cell adhesion peptides alter smooth muscle adhesion, proliferation, migration and matrix protein synthesis on modified surfaces and in polymer scaffolds. J Biomed Mater Res. 2002;60:86–93. doi: 10.1002/jbm.10042. [DOI] [PubMed] [Google Scholar]
- 44.Mann BK, Tsai AT, Scott-Burden T, West JL. Modification of surfaces with cell adhesion peptides alters extracellular matrix deposition. Biomaterials. 1999;20:2281–2286. doi: 10.1016/s0142-9612(99)00158-1. [DOI] [PubMed] [Google Scholar]
- 45.Neff JA, Tresco PA, Caldwell KD. Surface modification for controlled studies of cell-ligand interactions. Biomaterials. 1999;20:2377–2393. doi: 10.1016/s0142-9612(99)00166-0. [DOI] [PubMed] [Google Scholar]
- 46.Palecek SP, Loftus JC, Ginsberg MH, Luffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- 47.Burgess BT, Myles JL, Dickinson RB. Quantitative analysis of adhesion-mediated cell migration in three-dimensional gels of RGD-grafted collagen. Ann Biomed Eng. 2000;28:110–118. doi: 10.1114/1.259. [DOI] [PubMed] [Google Scholar]
- 48.DiMilla PA, Stone JA, Quinn JA, Albelda SM, Lauffenburger DA. Maximal migration of human smooth muscle cells on fibronectin and type IV collagen occurs at an intermediate attachment strength. J Cell Biol. 1993;122:729–737. doi: 10.1083/jcb.122.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olbrich KC, Andersen TT, Blumenstock FA, Bizios R. Surfaces modified with covalently-immobilized adhesive peptides affect fibroblast population motility. Biomaterials. 1996;17:759–764. doi: 10.1016/0142-9612(96)81412-8. [DOI] [PubMed] [Google Scholar]