Abstract
The Staphylococcus lentus plasmid pSCFS2 carries a novel florfenicol-chloramphenicol resistance gene, designated fexA, encoding a protein of 475 amino acids with 14 transmembrane domains. The FexA protein differs from all previously known proteins involved in the efflux of chloramphenicol and florfenicol. Induction of fexA expression by chloramphenicol and florfenicol occurs via translational attenuation.
Florfenicol is a fluorinated derivative of chloramphenicol which was licensed in Germany for the control of bacterial respiratory tract infections in 1995 for cattle and in 2000 for swine. In contrast to chloramphenicol, florfenicol is exclusively used in veterinary medicine (11), and the known chloramphenicol acetyltransferases are unable to inactivate florfenicol (6). While chloramphenicol acetyltransferase genes (cat genes) have been detected in a wide variety of gram-positive bacteria, including staphylococci of human and animal origin (5, 16), only a single gene, cfr, from bovine Staphylococcus sciuri, has been observed to mediate combined resistance to florfenicol and chloramphenicol by a yet-unknown mechanism (13). The cfr gene is located on the 17-kb plasmid pSCFS1, which also confers resistance to macrolide-lincosamide-streptogramin B antibiotics and spectinomycin (12).
In this study, the bovine Staphylococcus lentus isolate no. 8, obtained from the nasal swab of a calf suffering from a respiratory tract infection, was shown to be resistant to chloramphenicol, clindamycin, erythromycin, florfenicol, streptomycin, and tetracycline by agar disk diffusion (7) and was shown to carry six plasmids of ca. 2 to 34 kb. After transformation into protoplasts of Staphylococcus aureus RN4220, a 34-kb plasmid, designated pSCFS2, mediated only resistance to florfenicol and chloramphenicol. MICs for S. aureus RN4220:pSCFS2 were 32 μg of florfenicol/ml and 64 μg of chloramphenicol/ml (Table 1) as determined by the microdilution broth method (7). Preincubation in the presence of either 0.5 μg of chloramphenicol/ml or 0.5 μg of florfenicol/ml led to a fourfold increase of both MICs (Table 1). Since PCR assays and hybridization experiments did not reveal the presence of the cfr gene, cloning experiments using BglII-digested pSCFS2 DNA and BamHI-digested vector pBluescript II SK(+) (Stratagene, Amsterdam, The Netherlands) were performed. After transformation into Escherichia coli JM109, only transformants that carried a 7-kb BglII insert grew on Luria-Bertani agar plates supplemented with 10 μg of florfenicol/ml. They exhibited MICs of 16 μg of florfenicol/ml and 64 μg of chloramphenicol/ml, which could be increased to 64 μg of florfenicol/ml and 128 μg of chloramphenicol/ml, respectively, by preincubation in the presence of either 0.5 μg of chloramphenicol/ml or 0.5 μg of florfenicol/ml (Table 1). These observations suggested that the resistance gene in question is expressed inducibly in gram-positive and gram-negative hosts and that both antimicrobial agents, florfenicol and chloramphenicol, are effective as inducers.
TABLE 1.
MICs of florfenicol and chloramphenicol for the strains used in this studya
| Strain | MIC of florfenicol (μg/ml)
|
MIC of chloramphenicol (μg/ml)
|
||||
|---|---|---|---|---|---|---|
| Noninduced | Ff induced | Cm induced | Noninduced | Ff induced | Cm induced | |
| S. lentus 8 | 32 | 128 | 128 | 64 | 128 | 128 |
| S. aureus RN4220 | 2 | 2 | 2 | 2 | 2 | 2 |
| S. aureus RN4220:pSCFS2 | 32 | 128 | 128 | 64 | 256 | 256 |
| E. coli JM109 | 8 | 8 | 8 | 4 | 4 | 4 |
| E. coli JM109 Bgl30b | 16 | 64 | 64 | 64 | 128 | 128 |
Ff, florfenicol; Cm, chloramphenicol.
E. coli JM109 carrying pBluescript II SK(+) with the 7-kb BglII fragment of pSCFS2 in its BamHI site.
Within the 7-kb BglII fragment, a single EcoRI site was detected. Subclones which carried EcoRI/BglII inserts of 5.2 and 1.8 kb proved to be susceptible to florfenicol and chloramphenicol, suggesting that this EcoRI site is located either within the gene in question or in its regulatory region. Sequence analysis was performed on both strands by primer walking, starting at the EcoRI sites of both subclones and using the M13 universal and reverse primers. The nucleotide sequence of a 1,674-bp fragment of plasmid pSCFS2 was determined. Analysis of this region confirmed the presence of two open reading frames (ORFs), one of them coding for a protein of 475 amino acids (aa) (position 177 to 1604), the other coding for a small peptide of 9 aa (position 118 to 147) preceding the aforementioned ORF.
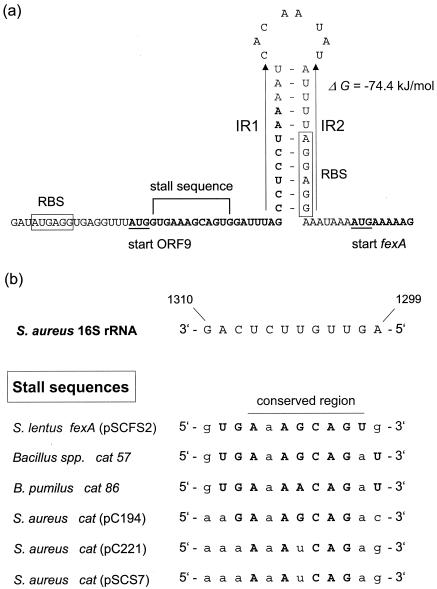
The reading frame for the 475-aa protein was designated fexA (for florfenicol exporter), and the EcoRI site was located within this reading frame at position 1334. Analysis of the fexA upstream region revealed striking homologies to the translational attenuators upstream of chloramphenicol-inducible staphylococcal cat genes (4) and also to that of the chloramphenicol-inducible gene cmlA from Tn1696 (14). In addition to the reading frame for the 9-aa peptide, a pair of inverted repeated sequences (IR1 and IR2) of 11 bp was detected. These inverted repeats might be able to form a stable mRNA stem-loop structure (ΔG = −74.4 kJ/mol) (15) with the fexA-associated ribosome binding site (5′-AGGAGG-3′; position 164 to 169) located within IR2) (Fig. 1a). The sequence of the codons 2 to 5 of the 9-aa peptide (5′-GTGAAAGCAGTG-3′; position 121 to 132) demonstrated significant homology to the ribosome stall sequences previously identified in the regulatory regions of cat57 and cat86 from Bacillus spp. but also to those of staphylococcal cat genes (2, 4, 9, 10) (Fig. 1b). Stalling of a ribosome in the reading frame of the 9-aa peptide might prevent the formation of an mRNA secondary structure between IR1 and IR2 and thus allow translation of the fexA transcript by a second ribosome.
FIG. 1.
(a) Presentation of the fexA regulatory region. The ORF9- and fexA-associated ribosome binding sites are boxed. The start codons of ORF9 and fexA are underlined, and the corresponding coding sequences are displayed in bold letters. The inverted repeated sequences IR1 and IR2 are marked by arrows, and a stable mRNA secondary structure formed by these IR sequences is shown. Calculation of the stability of this stem-loop structure followed the specifications given by Tinoco et al. (15). (b) Comparison of the potential ribosome stall sequence in the fexA regulatory region with those of chloramphenicol-inducible cat genes from Bacillus (4) and Staphylococcus (2, 9, 10). The 16S rRNA sequence was taken from the whole genome sequence of S. aureus N315 (3) (accession no. NC_002745) and is identical for all five rRNA operons detected in this strain. The “G” at position 1300 is a “U,” and the “U” at position 1301 is a “C” in the B. subtilis 16S rRNA (1). Since pairing of U:G and G:U has no negative impact of the stability of the binding (15), such pairing was not considered a mismatch. Matching bases in the stall sequences with regard to the S. aureus 16S rRNA are displayed in bold capital letters, whereas mismatches are displayed as lowercase letters.
Comparisons of the fexA nucleotide sequence using the National Center for Biotechnology Information standard nucleotide BLAST program (http://www.ncbi.nlm.nih.gov/BLAST/) revealed no significant homology to known sequences. The Tmpred program (http://www.ch.embnet.org/software/TMPRED_form.html) predicts that the FexA protein has 14 transmembrane helices. This feature is seen in a wide variety of efflux proteins of the major facilitator superfamily (8), including a cluster of efflux proteins from gram-positive bacteria which confer resistance to antimicrobial agents such as tetracyclines and lincomycin. The deduced FexA amino acid sequence reveals only minor homologies to other proteins deposited in the databases. Closest similarities of 28 or 29% amino acid sequence identity were observed between FexA and a multidrug efflux protein from Lactobacillus plantarum (accession no. NP_84696.1) as well as a metal-tetracycline/H+ antiporter from Bacillus halodurans (NP_242832.1). Comparisons of the amino acid sequences revealed no more than 19% amino acid identity between FexA and known chloramphenicol or florfenicol/chloramphenicol exporter proteins (Fig. 2). Thus, FexA represents a novel type of florfenicol/chloramphenicol efflux system which is distinctly different from those of the FloR subgroup and the CmlA subgroup, both found in clinically important gram-negative bacteria, as well as from the chloramphenicol exporters so far detected in soil and environmental bacteria of the genera Streptomyces, Corynebacterium, and Rhodococcus (Fig. 2).
FIG. 2.
Homology tree of the so far known chloramphenicol exporter proteins. For the different exporter proteins, information on bacterial hosts, database accession numbers, and designations (as given in the database entries) are provided. The tree was constructed using the DNAMAN software (Lynnon Biosoft, Vaudreuil, Quebec, Canada).
The detection of the florfenicol-chloramphenicol resistance gene fexA in staphylococci is to the best of our knowledge the first report of a gene coding for a florfenicol efflux protein in staphylococci. The location of this gene on a plasmid and the observation that it is functionally active even in E. coli suggest a potential transfer of fexA-mediated resistance between members of different bacterial species and genera.
Nucleotide sequence accession number.
The sequence of the fexA gene has been deposited with the EMBL database under accession number AJ549214.
Acknowledgments
This study was supported by grants of the Deutsche Forschungsgemeinschaft (SCHW 382/6-1 and SCHW 382/6-2).
We thank Stefan Hörmansdorfer for providing S. lentus isolate 8 and Vera Nöding for excellent technical assistance.
REFERENCES
- 1.Green, C. J., G. C. Stewart, M. A. Hillis, B. S. Vold, and K. F. Bott. 1985. Nucleotide sequence of the Bacillus subtilis ribosomal RNA operon rrnB. Gene 37:261-266. [DOI] [PubMed] [Google Scholar]
- 2.Horinouchi, S., and B. Weisblum. 1982. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J. Bacteriol. 150:815-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuroda, M., T. Ohta, I. Uchiyama, T. Baba, H. Yuzawa, I. Kobayashi, L. Cui, A. Oguchi, K. Aoki, Y. Nagai, J. Lian, T. Ito, M. Kanamori, H. Matsumaru, A. Maruyama, H. Murakami, A. Hosoyama, Y. Mizutani-Ui, N. K. Takahashi, T. Sawano, R. Inoue, C. Kaito, K. Sekimizu, H. Hirakawa, S. Kuhara, S. Goto, J. Yabuzaki, M. Kanehisa, A. Yamashita, K. Oshima, K. Furuya, C. Yoshino, T. Shiba, M. Hattori, N. Ogasawara, H. Hayashi, and K. Hiramatsu. 2001. Whole genome sequencing of methicillin-resistant Staphylococcus aureus. Lancet 357:1225-1240. [DOI] [PubMed] [Google Scholar]
- 4.Lovett, P. S. 1990. Translational attenuation as the regulator of inducible cat genes. J. Bacteriol. 172:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyon, B. R., and R. Skurray. 1987. Antimicrobial resistance of Staphylococcus aureus: genetic basis. Microbiol. Rev. 51:88-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray, I. A., and W. V. Shaw. 1997. O-Acetyltransferases for chloramphenicol and other natural products. Antimicrob. Agents Chemother. 41:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals. Approved standard. NCCLS document M31-A2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 8.Paulsen, I. T., M. H. Brown, and R. A. Skurray. 1996. Proton-dependent multidrug efflux systems. Microbiol. Rev. 60:575-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Projan, S. J., J. Kornblum, S. L. Moghazeh, I. Edelman, M. L. Gennaro, and R. P. Novick. 1985. Comparative sequence and functional analysis of pT181 and pC221, cognate plasmid replicons from Staphylococcus aureus. Mol. Gen. Genet. 199:452-464. [DOI] [PubMed] [Google Scholar]
- 10.Schwarz, S., and M. Cardoso. 1991. Nucleotide sequence and phylogeny of a chloramphenicol acetyltransferase encoded by the plasmid pSCS7 from Staphylococcus aureus. Antimicrob. Agents Chemother. 35:1551-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwarz, S., and E. Chaslus-Dancla. 2001. Use of antimicrobials in veterinary medicine and mechanisms of resistance. Vet. Res. 32:201-225. [DOI] [PubMed] [Google Scholar]
- 12.Schwarz, S., C. Kehrenberg, and K. K. Ojo. 2002. Staphylococcus sciuri gene, erm(33), encoding inducible resistance to macrolides, lincosamides, and streptogramin B antibiotics is a product of recombination between erm(C) and erm(A). Antimicrob. Agents Chemother. 46:3621-3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwarz, S., C. Werckenthin, and C. Kehrenberg. 2000. Identification of a plasmid-borne chloramphenicol-florfenicol resistance gene in Staphylococcus sciuri. Antimicrob. Agents Chemother. 44:2530-2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stokes, H. W., and R. M. Hall. 1991. Sequence analysis of the inducible chloramphenicol resistance determinant in the Tn1696 integron suggests regulation by translational attenuation. Plasmid 26:10-19. [DOI] [PubMed] [Google Scholar]
- 15.Tinoco, I., P. Borer, B. Dengler, M. Levine, O. Uhlenbeck, D. Crothers, and J. Gralla. 1973. Improved estimation of secondary structure in ribonucleic acid. Nat. New Biol. 246:171-172. [DOI] [PubMed] [Google Scholar]
- 16.Werckenthin, C., M. Cardoso, J.-L. Martel, and S. Schwarz. 2001. Antimicrobial resistance in staphylococci from animals with particular reference to bovine Staphylococcus aureus, porcine Staphylococcus hyicus and canine Staphylococcus intermedius. Vet. Res. 32:341-362. [DOI] [PubMed] [Google Scholar]