Abstract
Clinical failures of the highly active antiretroviral therapy could result from inefficient intracellular concentrations of antiviral drugs. The determination of drug contents in target cells of each patient would be useful in clinical investigations and trials. The purpose of this work was to quantify the intracellular concentration of ddATP, the active metabolite of dideoxyinosine (ddI), in peripheral blood mononuclear cells (PBMCs) of human immunodeficiency virus (HIV)-infected patients treated with ddI. We have raised antibodies against ddA-citrate, a stable isostere of ddATP selected on the basis of its structural and electronic analogies with ddATP. The anti-ddA-citrate antibodies recognized ddATP and ddA with nanomolar affinities and cross-reacted neither with any of the nucleotide reverse transcriptase inhibitors used in HIV therapy nor with their phosphorylated metabolites. The three phosphorylated metabolites of ddI (ddAMP, ddADP, and ddATP) were purified by anion exchange chromatography and the amount of each metabolite was determined by radioimmunoassay with or without prior phosphatase treatment. The intracellular levels of the three ddI metabolites were measured both in an in vitro model and in PBMCs of HIV-infected patients under ddI treatment. The possibility to measure intracellular levels of ddATP from small blood samples of HIV-infected patients treated with ddI could be exploited to develop individual therapeutic monitoring.
Highly active antiretroviral therapy has been used successfully for treatment of human immunodeficiency virus (HIV) disease. The most common highly active antiretroviral therapy regimens consist of a combination of at least one protease inhibitor and two nucleoside reverse transcriptase inhibitors. Contrary to protease inhibitors, the expression of nucleoside reverse transcriptase inhibitor activity requires intracellular metabolism of the nucleoside precursor into its corresponding 5′-triphosphate nucleotide by the host cell kinases. The active metabolite (nucleoside reverse transcriptase inhibitor-triphosphate) competitively inhibits the HIV reverse transcriptase and acts as a chain terminator of the proviral DNA.
The presence and activity of the intracellular kinases are highly dependent on the type and activation state of the target cell (37). Studies conducted in HIV-infected patients failed to establish a clear relationship between the plasma nucleoside reverse transcriptase inhibitor concentration and the antiviral efficiency of these drugs (3, 4, 18, 39). However, a clinical study showed a significant and linear relationship between the intracellular nucleoside reverse transcriptase inhibitor-triphosphate (zidovudine-triphosphate and lamivudine-triphosphate) concentrations, the percent change in CD4+ cells and the rate of decline of HIV RNA in plasma (17). Thus, intracellular contents of active drugs in target cells seem to give a much better indication of therapeutic efficiency than plasma concentrations of drug precursors.
The intracellular metabolism of ddI leads to three inactive phosphorylated products (ddIMP, ddAMP, and ddADP) and to the active metabolite ddATP (5). The aim of the present study was to develop a sensitive immunoassay suitable for measuring the intracellular concentration of ddATP in human peripheral blood mononuclear cells (PBMCs) from a limited amount of blood of HIV-infected patients treated with ddI. Two different approaches have been considered. In the first (indirect) method, the phosphorylated metabolites were extracted from cells, then purified by anion-exchange chromatography and subjected to phosphatase hydrolysis to generate the ddA epitope before quantification. The second approach involved a direct measurement of the purified ddATP fraction without prior phosphatase treatment. Both ddA and ddATP levels could be measured by means of an antiserum raised against ddA-citrate, a stable isostere of ddATP. We show that both techniques can be used to quantify the intracellular amounts of ddAMP, ddADP, and ddATP after anionic chromatography of cell extracts (PBMC incubated in vitro with ddI). We also present preliminary results on the quantification of these metabolites in PBMCs of HIV-infected patients treated by a polytherapy involving ddI.
MATERIALS AND METHODS
Materials.
Acid phosphatase (EC 3.1.3.2, 500 U per ml), complete Freund's adjuvant, bovine serum albumin, ddATP, and stavudine (d4T) were purchased from Sigma Chemicals; keyhole limpet hemocyanin was from Calbiochem; Na125I (2,150 Ci/mmol) was from New England Nuclear. The culture medium RPMI was from Bio-Whittaker Europe. CPT Vacutainer tubes were purchased from Becton Dickinson (Franklin Lakes, N.J.). ddI was provided by Bristol Myers Squibb and zidovudine and lamivudine by Glaxo Smith Kline. Zidovudine-triphosphate, lamivudine-triphosphate, and stavudine-triphosphate were purchased from Sierra Bioresearch (Tucson, Ariz.). ddA and ddAMP were generous gifts of Gilles Gosselin (Montpellier, France) and Luigi Agrofolio (Orleans, France), respectively.
Molecular modeling.
Geometric optimization of each molecule was performed with the Sybyl molecular modeling package with the tripos 6.0 molecular force field. The partial charges were computed by the Gasteiger Marsilli method. In order to have a reliable conformation, a short molecular dynamics with a annealing method was performed. The Connolly solvent surface access was computed and the surface was colored with the partial charge of the different atoms which are in contact with the surface.
Synthesis of ddA-HS and ddA-citrate.
5′-O-Hemisuccinate-2′,3′-dideoxyadenosine (ddA-HS) was synthesized by coupling ddA (0.42 nmol) to succinic anhydride (0.63 nmol) in the presence of dimethylaminopyridine (0.63 nmol) (21). The product was purified by chromatography on silica gel and characterized by mass spectrometry and 1H and 13C nuclear magnetic resonance.
The isostere of ddATP (5′-O-[3,4-dicarboxy-3-hydroxy]butanoate-2′,3′-dideoxyadenosine, ddA-citrate) was synthesized in six steps, according to the procedure described by Weaver and Gilbert (40). The final product was characterized and identified by 1H nuclear magnetic resonance and mass spectrometry. Nuclear magnetic resonance 1H (CDCl3): δ(ppm): 8.24 (s, 1H, H2); 8.05 (s, 1H, H8); 6.18 (t, 1H, H1′, 3J = 4Hz); 4.46 (m, 1H, H4′); 4.3 (m, 2H, H5′ab); 3.15 and 3.1 (2s, 4H, CH2COO, CH2COOH); 2.6 (m, 2H, H2′ab); 2.2 (m, 2H, H3′ab). Mass spectrometry: C16H18N5O8, m/z (M−H) 408).
Production of antiserum.
The N-hydroxysuccinimide ester of ddA-citrate was coupled to keyhole limpet hemocyanin to give the immunogenic form ddA-citrate-keyhole limpet hemocyanin. This immunogen (500 μg) was emulsified with 1 ml of complete Freund's adjuvant and injected subcutaneously into rabbits (albino strain, Centre d'Élevage des Combes, Ain, France). Booster injections were given every 3 weeks after the priming immunization over a period of 4 months. On the eighth day after each booster injection, blood samples were taken and the antibody titer (i.e., the dilution of antiserum able to bind 50% of the radioactive probe described below) was measured.
Synthesis of the radioactive probe.
The N-hydroxysuccinimide ester of ddA (ddA-HS, 8.93 μmol) was coupled to an ice-cold solution of α-NH2-tyrosyl-lysine-COOH (YK, 9 μmol) dissolved in 700 μl of 50 mM borate-phosphate buffer, pH 8.9. The solution was stirred at 4°C for 4 h. The reaction product (ddA-HS-YK) was purified by HPLC, lyophilized, characterized by mass spectrometry, then radioiodinated by the chloramine T method (20) to give mono-iodo-ddA-HS-125I YK.
Radioimmunoassay.
The ddA-citrate antiserum was used at a dilution of 1/50,000 with ddA-HS-125I YK (5,000 cpm) as tracer. The radioimmunoassay was carried out as described elsewhere (12, 13). Briefly, 100 μl of antibody dilution, 100 μl of ddA-HS-(125I-Y-K) (50,000 cpm/ml), 100 μl of either standard molecules or samples and 200 μl of radioimmunoassay buffer (10 mM Phosphate, 150 mM NaCl, 0.1% bovine serum albumin and 0.01% NaN3 pH 7.4) were incubated at 4°C for 20 h. Then 100 μl of bovine serum, 100 μl of 6% Tween 20 (to decrease the nonspecific background) and 700 μl of polyethylene glycol 6000 (40% in water) were added. The mixture was shaken vigorously and left for 10 min at −20°C. After 25 min of centrifugation (4,200 rpm in a Beckman JS4.2 rotor) at 4°C, the radioactivity of the pellet was determined and the bound/total ratio was calculated. The blank values, i.e., the amount of radioactivity pelleted without antibodies in the presence of 1% Tween 20, were always lower than 6% of the total bound radioactivity.
PBMCs.
PBMCs from healthy volunteers were isolated from blood collected by the Etablissement Français du Sang (EFS, Marseilles, France). Blood samples were diluted (1/3) in RPMI medium then centrifuged on a Ficoll density gradient at 1500 rpm for 30 min. PBMCs were washed three times in sterile PBS (10 mM phosphate,150 mM NaCl, pH 7.4) then suspended in RPMI medium to a density of 5 × 105 cells per ml. Then 2 ml were incubated with or without various concentrations of ddI ranging from 10−4 to 10−7 M, in a CO2 incubator (5% CO2) in Costar dishes for 16 h, then washed three times before extraction.
PBMCs were also isolated from five HIV-infected patients treated with ddI in association with protease inhibitors (highly active antiretroviral therapy treatment); 8 ml of venous blood was placed in CPT Vacutainer tubes. PBMCs were separated from erythrocytes by centrifugation at 1,500 x g for 20 min at room temperature. The mononuclear cell fraction was transferred into a centrifuge tube, the cell number was counted on a KOVA slide and the cell suspension was pelleted by centrifugation. For supernatant ddI concentrations of (4.1 ± 0.7) × 10−5, (5.7 ± 0.3) × 10−6, and (2.7 ± 0.4) × 10−7 M, the cellular ddATP content was 10.6 ± 0.5, 1.06 ± 0.27, and 0.136 ± 0.016 pmol/106 cells, respectively (mean ± SEM, n = 2).
PBMCs from healthy volunteers or HIV-infected patients (from 4 to 10 × 106 cells) were extracted with 0.5 ml of a 70% methanol solution maintained at 4° C for 20 h. The supernatant was dried and stored at −70°C until quantification. Cell extracts were suspended in 1 ml of distilled water and used for quantification of the ddATP and ddA contents with the direct and the indirect methods, respectively, as described below.
Purification and dephosphorylation of the phosphorylated metabolites of ddI.
Anion-exchange cartridges were used to separate the three phosphorylated metabolites of ddI as previously described for zidovudine and lamivudine (8, 32, 33). With a ddATP standard solution containing small amounts of ddAMP and ddADP as contaminant, we selected a stepwise elution protocol to obtain a sufficient separation between the three ddI-phosphorylated metabolites. Each collected fraction was checked by anionic HPLC followed by radioimmunoassay detection. Fractions were eluted in the following order: Step 1 (6 ml of 50 mM KCl), ddAMP; step 2 (10 ml of 100 mM KCl), ddADP; step 3 (6 ml of 250 mM KCl), ddATP. The ddATP content of the later fraction was evaluated by the direct method. In the indirect method, each collected fraction was diluted with 1 vol of water, then dephosphorylated by incubation with 2 μl of acid phosphatase (EC 3.1.3.2, 500 U/ml) and 20 μl of 1 M sodium acetate for 30 min at 37°C, before quantification by radioimmunoassay. Control experiments performed on a ddATP standard solution showed that the ddA molecule was generated from ddATP by the phosphatase treatment with a recovery yield of 98 ± 2%. The KCl solutions (from 50 mM to 250 mM) did not modified the antigen-antibody interaction.
RESULTS
Choice of the citrate chain to mimic the triphosphate group of ddATP.
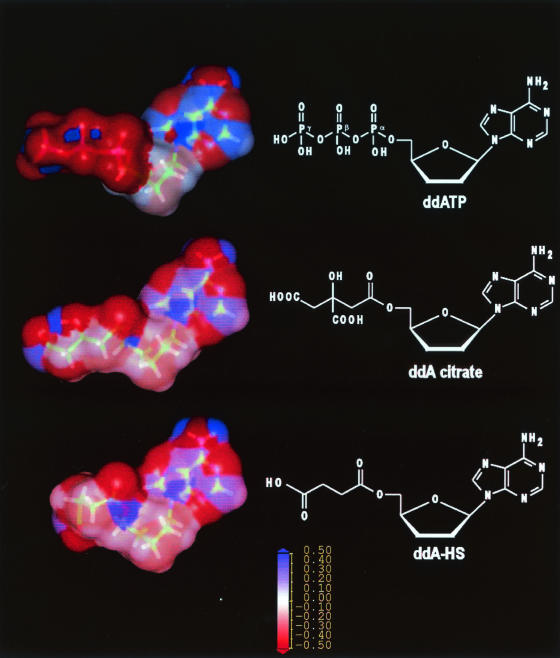
The development of antibodies with directly polyphosphate nucleoside molecules as hapten is a difficult task because the hapten phosphodiester bonds are rapidly hydrolyzed by endogenous phosphatases. To overcome this difficulty, we chose to raise antibodies against a stable isostere molecule mimicking the ddATP structure (French patent BF 02/02782). Based upon Weaver's design for HIV reverse transcriptase inhibitors (40), we selected the citrate chain to mimic the triphosphate part of the ddATP molecule. Figure 1 presents a comparison between the molecular modeling of ddATP, the ddA-citrate isostere, and ddA-HS. When a superposition of the molecules was performed starting from the 2′,3′-dideoxyadenosine moiety, surface analysis confirmed that the citrate linker mimics the triphosphate group. The hydroxyl function and one of the carboxyl functions of the citrate moiety are located in a position similar to that occupied by the β phosphate group in the triphosphate. The negative charges of the citrate and phosphate groups can establish similar electrostatic interactions with the antibody binding site.
FIG. 1.
Structural and electronic analogies between ddATP, ddA-citrate, and ddA-HS.
Characteristics of the anti-ddA-citrate antibodies.
The antibody titers of the two antisera obtained with ddA-citrate-keyhole limpet hemocyanin as immunogen were 1:6,000 and 1:260,000, respectively. Only the last one (CLS-3) was fully characterized. The antibody affinity (IC50) towards ddATP was found to be 1.1 ± 0.18 10−9 M (mean ± standard deviation, n = 28). The minimal detectable value of the radioimmunoassay test, corresponding to the amount of ddATP able to inhibit 20% of the antigen-antibody binding, was found to be 17 ± 4 fmol (mean ± standard deviation, n = 22). The standard curve was linear from 0.1 to 300 nM (100 fmol/ml to 300 pmol/ml). As shown in Table 1, the anti-isostere antibodies exhibited the following order of decreasing affinity: ddA-HS > ddA > ddATP > ddAMP > ddI. These antibodies recognize neither the other nucleoside inhibitors of reverse transcriptase used in HIV therapy (i.e., zidovudine, d4T, and lamivudine) nor their biological metabolites (i.e., zidovudine-triphosphate, d4T-triphosphate, and lamivudine-triphosphate).
TABLE 1.
Specificity of the anti-ddA-citrate antibodiesa
| Analogue | IC50 (M) |
|---|---|
| ddA-citrate | 5 ± 1.5 × 10−11 |
| ddA-HS | 1 ± 0.06 × 10−10 |
| ddA-HS-(Y-K) | 1.1 ± 0.14 × 10−10 |
| ddA | 4 ± 0.6 × 10−10 (n = 18) |
| ddATP | 1.1 ± 0.18 × 10−9 (n = 28) |
| ddA-MP | 3.4 ± 0.8 × 10−9 |
| ddI | 2 ± 0.3 × 10−8 |
| dA | 3 ± 0.4 × 10−7 |
| Adenosine | 2.4 ± 0.2 × 10−6 |
| A-TP | 3.5 ± 0.6 × 10−5 |
| A-DP | >10−4 |
| A-MP | >10−4 |
| AZT, AZT-TP | >10−4 |
| 3TC, 3TC-TP | >10−4 |
| d4T, d4T-TP | >10−4 |
| ddC | >10−4 |
IC50 is the concentration of analogue that inhibits 50% of the binding of ddA-HS- ([125I]YK) to the CLS-3 antibody. Values are the mean ± SEM of 3 to 28 experiments performed in duplicate. TP, triphosphate.
ddATP content of control PBMCs incubated with ddI.
In order to validate both the anion exchange purification step and the CLS-3 radio-immunoassay, PBMCs (2 × 106 cells) from healthy donors were incubated with ddI concentrations ranging from 10−5 to 10−7 M for 16 h (23, 27) and the immunoreactive material present in the supernatant and cell extracts was analyzed independently. The supernatant immunoreactivity was eluted in the 1 to 5 ml (void volume) fraction, corresponding to the elution volume of the ddI standard molecule. The methanol-extracted immunoreactive material eluted at the elution volume of a ddATP standard (between 16 and 18 ml) was also quantified directly, i.e., without phosphatase treatment. The intracellular ddATP level was found to increase with the ddI concentration. The presence of a ddI immunoreactivity was barely detectable in intracellular extracts (data not shown).
In control experiments, cell extracts of PBMCs (4 × 106 cells) not incubated with ddI were submitted to anion exchange chromatography and the eluted fractions were analyzed by radioimmunoassay before and after phosphatase digestion. Whatever the method (direct or indirect) used, we never detected any ddATP or ddA immunoreactivities, indicating that endogenous nucleotides (i.e., ATP) and deoxynucleotides (i.e., dATP) did not interfere in our immunoassay (data not shown). Moreover, cellular proteins recovered in the PBMC pellet did not inhibited the antigen-antibody binding (data not shown).
Detection of ddAMP and ddADP in PBMCs incubated with ddI.
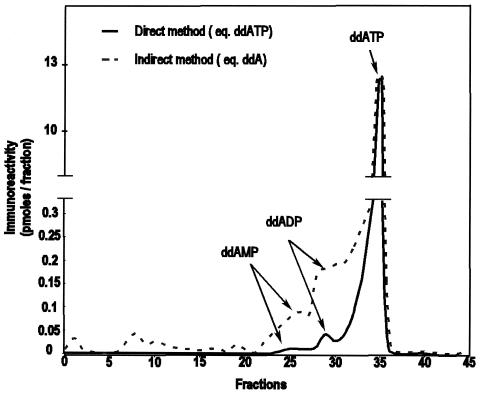
In the course of the purification of PBMC extracts on the anion-exchange cartridge, two minor immunoreactive peaks eluting at fractions 24 to 26 and 27 to 30 were also present in addition to ddATP (Fig. 2). Although this material was initially detected without prior phosphatase treatment, its immunoreactivity was greatly increased after phosphatase hydrolysis (Fig. 2). The increasing factors were 6.6 and 4.7 for fractions 24 to 26 and 27 to 30, respectively. Thus, both fractions corresponded to phosphorylated derivatives of ddA eluted at lower ionic strength (and therefore less negatively charged) than ddATP itself. An aliquot of synthetic ddAMP eluted at fractions 24 to 26. Taken together, these observations identify fractions 24 to 26 as ddAMP. Fractions 27 to 30 contain a phosphorylated metabolite of ddA eluted at an intermediate ionic strenght between ddAMP and ddATP and therefore correspond very probably to ddADP. The low amount of material and the presence of KCl in fractions 27 to 30 prevented us from definitely identifying this product by mass spectrometry.
FIG. 2.
Immunoreactivity of ddI-treated PBMC extracts after anion exchange chromatography by the indirect and direct methods.
Detection of the ddI phosphorylated metabolites in blood samples of patients.
Blood samples (8 ml) of HIV-infected patients receiving daily doses of ddI were used to quantify the intracellular levels of ddAMP, ddADP, and ddATP by the chromatography-radioimmunoassay technique described above. The ddI plasma content was also determined (Table 2). Significant levels of ddATP were detected in three patients. For patient 4, the ddATP immunoreactivity was barely detectable. For all the HIV-infected patients, immunoreactive material was found in the ddAMP fraction, representing around 25 to 32% of the total immunoreactivity. A main immunoreactive material, representing 50 to 67% of the total immunoreactivity, was found in the fraction attributed to ddADP. As already observed in vitro, the immunoreactive material measured in these two later fractions by the direct method were greatly increased after phosphatase hydrolysis with increasing factors close to 6. By contrast, we did not see any significant increase of the immunoreactive material after phosphatase treatment of the 41 to 44 fractions, corresponding of the elution volume of ddATP. For patient 1, we found three minor but significant immunoreactive peaks at elution volumes of 2.5 to 3, 7.5 to 8.5, and 10 to 11 ml, respectively. These weak peaks were only detected after phosphatase hydrolysis of the fractions and might correspond to other phosphorylated metabolites of ddI. The 2.5- to 3-ml fractions might be intracellular ddI.
TABLE 2.
ddA-phosphorylated metabolite content of PBMCs from HIV-infected patients under ddI treatmenta
| Patient no. | Treatment | PBMC count (106 cells/ml) | Plasma ddI (μM) | ddAMP (fmol/106 cells) | ddADP (fmol/106 cells) | ddATP (fmol/106 cells) | ddATP (fmol/106 cells) |
|---|---|---|---|---|---|---|---|
| 1 | ddI, AZT, 3TC | 3.6 ± 0.2 | 4.3 ± 1.1 | 267 ± 85 | 596 ± 119 | 174 ± 49 | 104 ± 44 |
| 2 | ddI, SQV, RTV, d4T | 7.8 ± 0.5 | 1.95 ± 0.3 | 122 ± 22 | 311 ± 24 | 26 ± 5 | 18.5 ± 6.1 |
| 3 | ddI, SQV, RTV, d4T | 10.8 ± 0.7 | 2.95 ± 0.8 | 139 ± 27 | 252 ± 34 | 130 ± 7 | 94 ± 23 |
| 4 | ddI, abacavir, d4T | 3.9 ± 0.5 | 6.10 ± 1.9 | 282 ± 124 | 577 ± 100 | 6 ± 1.7 | ND |
The cell content in ddA-phosphorylated metabolites was determined in four HIV-infected patients under ddI treatment after PBMC extraction, Sep-Pak purification, and phosphatase hydrolysis with the anti-ddA-citrate antibodies and expressed as ddA equivalent per 106 cells. The ddATP content was also measured without phosphatase treatment and expressed as ddA-TP equivalent per 106 cells. Cells were from 4 ml of blood. ND, undetectable. AZT, zidoviudine; SQV, saquinavir; RTV, ritonavir; 3TC, lamivudine; d4T, ? Results are means ± SEM of at least 2 experiments performed in duplicate.
DISCUSSION
Quantification of the active triphosphate metabolites of nucleoside reverse transcriptase inhibitors used in anti-HIV therapy would be useful for clinical investigations. The major difficulty is the small amount of intracellular triphosphate metabolite present in patients (a few fmol per 106 cells). Thus, sensitive methodologies have been developed to detect such small levels from limited amounts of blood. Several methods including a purification step by HPLC or Sep-Pak Cartridge coupled mainly with UV detection were used for quantification of zidovudine, d4T and lamivudine phosphorylated metabolites (17, 29, 32, 33, 36). Recently, liquid chromatography-tandem mass spectrometry and capillary electrophoresis-tandem mass spectrometry techniques were also described for the same nucleoside reverse transcriptase inhibitors (i.e., zidovudine, lamivudine, and d4T) (11, 22, 26, 28, 30, 34) and for ddATP (6). Another possible approach involved the development of sensitive and specific antibodies. Most of the immunoassays described for nucleoside reverse transcriptase inhibitors and ddNTPs were developed against zidovudine, d4T, and lamivudine. Generally, the methods used were indirect, involving antibodies raised against the parent drug and detection of the phosphorylated metabolites after chromatography and phosphatase digestion to generate the epitope recognized by the antibodies (2, 16, 29, 32).
The intracellular ddATP levels were found to be the lowest among various ddNTPs (23, 27). Thus, most of the technologies previously described were not sensitive enough to detect intracellular ddATP in HIV-infected patients. To our knowledge, no sensitive immunoassay has been described for ddA and ddATP. The production of antibodies with directly the nucleoside triphosphate as hapten is a doubtful approach. During the immunogen synthesis step or during the priming of immune system, the phosphodiester bonds can be hydrolyzed either chemically or by endogenous phosphatases and phosphodiesterases (37). However such a direct approach has been used for the development of zidovudine-monophosphate and zidovudine-triphosphate antibodies (2, 19). Our own assays to obtain sensitive antibodies directly against the ddATP hapten were unsuccessful. The hapten instability can be overcome with analogs of ddNTPs in which the triphosphate link is replaced by a stable isosteric group. Numerous works have reported the synthesis of enzymatically stable analogs of ddNTPs used as potential antiviral agents in the HIV reverse transcriptase field. Most of the bio-isosteres of ddNTP involved the substitution of the P-O-P bonds either by P-NH-P (35) or by P-CH2-P bonds (15, 25, 38). With the P-CH2-P bond strategy, an isostere of zidovudine was synthesized and used as hapten to produce antibodies (9, 10). However until now, no quantification of intracellular endogenous zidovudine-triphosphate with these antibodies has been reported.
Recently, Weaver et al. demonstrated that the citrate link was a good mimic of the triphosphate moiety for developing phosphate-modified analogs of d4T (40, 41). In order to obtain antibodies recognizing the ddATP molecule, we chose to use the citrate link to synthesize a stable isostere of ddATP. The anti-isostere antibodies raised against the ddA-citrate hapten exhibited a high antibody-titer (1:260,000) and a good affinity towards ddATP (IC50 = 1.1 ± 0.18 ×10−9 M). Our antibody specificity studies show that the citrate group is a good mimic of the triphosphate moiety towards the B-cell receptors.
PBMCs collected from healthy subjects were used as an in vitro model to validate our methodology. With this model, we demonstrated that anion-exchange chromatography on a Sep-Pak cartridge efficiently separated the three phosphorylated metabolites of ddI. The very low level (if any) of intracellular ddI is eluted in the void volume and cannot interfere with the phosphorylated metabolites. The cross-reactivity of the anti-ddA-citrate antibodies towards ddI (IC50 = 2 × 10−8 M) offers the possibility to quantify the plasma ddI content in HIV-infected patients under ddI treatment. The sensitivity of our test towards ddI is much higher than that of HPLC methods described by Ray et al. and Knupp et al. (i.e., (5 × 10−7 M and 1 × 10−5 M, respectively) (24, 31) but quite similar to that of the ddI radioimmunoassay described by DeRemer et al. (14).
The sensitivity of our test is comparable to the liquid chromatography MS/MS analytical method recently described, involving the detection of ddATP by an indirect method with a limit of ddA quantification of 0.1 ng/ml (4 x 10−10 M) (1, 11). It is important to note that the ddATP content found in our PBMC model (picomole) is much higher than that reported in the literature (femtomole) (18, 23, 27). Such discrepancies are most likely attributable to the differences between the cellular models and the detection methods of ddATP contents (mainly HPLC/UV detection and radiolabeled experiments studies) used in these previous studies. Our human PBMC model involves mainly lymphocytes and probably better reflects the physiological situation compared to studies performed in cell lines like human monocytoid U937 cells, human lymphoid MOLT 4 cells and human fibroblastoid H1080 cells.
Application to a limited number of human PBMC samples collected from ddI-treated HIV-infected patients, show that our methodology can be used for quantifying the active ddATP metabolite from a reasonable amount of blood sample (4 to 8 ml). It is worth noting that the intracellular ddATP contents detected in the present work are similar those found in HIV-infected patients with an HPLC/tandem Mass Spectrometry method (6, 7). The quantification of ddATP is a crucial point in the follow-up of HIV-infected patients, in order to adapt the nucleoside reverse transcriptase inhibitor regimen to each individual. Thus, if our preliminary results were confirmed, treatment of patient 4 with ddI would be questionable since this patient is unable to transform ddI into its active form ddATP. The immunological approach described here offers the opportunity to detect and quantify not only ddATP, but also the two other phosphorylated metabolites of ddI after purification and phosphatase hydrolysis. Taken together these measurements should constitute an indication of the intracellular metabolism status of HIV-infected patient and should help to better understand therapeutic failures.
The immunological method involving the use of an isostere of the triphosphate group to produce antibodies against phosphorylated molecules appears to be a powerful and general approach to detect the phosphorylated metabolites of nucleoside reverse transcriptase inhibitors used in HIV therapy. We are currently trying to extend this technique to other anti-HIV nucleosides.
Acknowledgments
This work was supported by research grants from the Centre National de la Recherche Scientifique and the Agence Nationale de Recherche contre la Sida. C.L.S. was supported by the Région Provence-Alpe-Côte d’Azur and the Fondation “Ensemble contre le Sida.”
We thank P. Vierling for fruitful discussions and T. Tran and F. Akeb for technical advice.
Footnotes
This work is dedicated to the memory of Elisa Cupo (1919-2003).
REFERENCES
- 1.Agrofoglio, L. A., X. Cahours, T. T. Tran, H. Dessans, C. Kieda, and P. Morin. 2001. Analysis of anti-HIV nucleoside inhibitors by capillary electrophoresis-electrospray ionization mass spectrometry. Nucleosides Nucleotides Nucleic Acids 20:375-381. [DOI] [PubMed] [Google Scholar]
- 2.Akeb, F., C. Creminon, J. Grassi, R. Guedj, and D. Duval. 2001. The production and the evaluation for enzyme immunoassay of AZTTP. Nucleosides Nucleotides Nucleic Acids 20:243-250. [DOI] [PubMed] [Google Scholar]
- 3.Back, D., J., G. Gatti, C. Fletcher, R. Garaffo, R. Haubrich, R. Hoetelmans, M. Kurowski, A. Luber, C. Merry, and C. F. Perno. 2002. Therapeutic drug monitoring in HIV infection current status and future directions. AIDS 16:S5-S37. [DOI] [PubMed] [Google Scholar]
- 4.Back, D., J., S. Khoo, H., S. Gibbons, E., and C. Merry. 2000. The role of therapeutic drug monitoring in treatment of HIV infection. Br. J. Clin. Pharmacol. 51:301-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Back, D. J., S. Ormesher, J. F. Tjia, and R. Macleod. 1992. Metabolism of 2′,3′-dideoxyinosine (ddI) in human blood. Br. J. Clin. Pharmacol. 33:319-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becher, F., A. Pruvost, J. Gale, P. Couerbe, C. Goujard, V. Boutet, E. Ezan, J. Grassi, and H. Benech. 2003. A strategy for liquid chromatography/tandem mass spectometric assays of intracellular drugs: application to the validation of the triphosphorylated anabolite of antiretrovirals in peripheral blood mononuclear cells. J. Mass Spectrosc. 38:879-890. [DOI] [PubMed] [Google Scholar]
- 7.Becher, F., A. Pruvost, C. Goujard, C. Guerreiro, J. F. Delfraissy, J. Grassi, and H. Benech. 2002. Improved method for the simultaneous determination of d4T, lamivudine and ddl intracellular phosphorylated anabolites in human peripheral-blood mononuclear cells with high-performance liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 16:555-565. [DOI] [PubMed] [Google Scholar]
- 8.Brody, S. R., and F. T. Aweeka. 1997. Pharmacokinetics of intracellular zidovudine and its phosphorylated anabolites in the absence and presence of stavudine with an in vitro human peripheral blood mononuclear cell (PBMC) model. Int. J. Antimicrob. Agents 9:131-135. [DOI] [PubMed] [Google Scholar]
- 9.Brossette, T., M. C. Nevers, C. Creminon, C. Mioskowski, J. Grassi, and L. Lebeau. 1999. Synthesis of analogues of 5′-mono-, 5′-di-, and 5′-triphosphate-zidovudine for the development of specific enzyme immunoassay for monitoring of intracellular levels of zidovudine-MP, zidovudine-DP, and zidovudine-triphosphate. Nucleosides Nucleotides.. 18:939-940. [DOI] [PubMed] [Google Scholar]
- 10.Brossette, T., A. Valleix, L. Goujon, C. Creminon, J. Grassi, C. Mioskowski, and L. Lebeau. 1999. Synthesis of a non-hydrolysable zidovudine-triphosphate analogue designed for the production of anti-zidovudine-triphosphate antibodies. Tetrahedron Lett. 40:3391-3394. [Google Scholar]
- 11.Cahours, X., T. T. Tran, N. Mesplet, C. Kieda, P. Morin, and L. A. Agrofoglio. 2001. Analysis of intracellular didanosine triphosphate at sub-ppb level with LC-MS/MS. J. Pharm. Biomed. Anal. 26:819-827. [DOI] [PubMed] [Google Scholar]
- 12.Cucumel, K., I. Garreau, J. Mery, D. Moinier, A. Mansour, H. Akil, and A. Cupo. 1996. Production and characterization of site-directed antibodies against dermorphin and dermorphin-related peptides. Peptides 17:973-982. [DOI] [PubMed] [Google Scholar]
- 13.Cupo, A., and T. Jarry. 1985. Detection of methionine-enkephalin at the 10−16 mole levels. J. Neuroimmunol. 8:57-67. [DOI] [PubMed] [Google Scholar]
- 14.DeRemer, M., R. D'Ambrosio, and G. D. Morse. 1996. Didanosine measurement by radioimmunoassay. Antimicrob. Agents Chemother. 40:1331-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dyatkina, N., E. Shirokova, F. Theil, S. M. Roberts, and A. Krayevsky. 1996. Modified triphosphates of carbocyclic nucleoside analogues: synthesis, stability towards alkaline phosphatase and substrat properties for some DNA polymerases. Bioorg. Med. Chem. Lett. 6:2639-2642. [Google Scholar]
- 16.Ferrua, B., T. T. Tran, J. F. Quaranta, J. Kubar, C. Roptin, R. Condom, J. Durant, and R. Guedj. 1994. Measurement of the anti-HIV agent 2′,3′-didehydro-2′,3′-dideoxythymidine (D4T) by competitive ELISA. J. Immunol. Methods 176:103-110. [DOI] [PubMed] [Google Scholar]
- 17.Fletcher, C. V., S. P. Kawle, T. N. Kakuda, P. L. Anderson, D. Weller, L. R. Bushman, R. C. Brundage, and R. P. Remmel. 2000. Zidovudine triphosphate and lamivudine triphosphate concentration-response relationships in HIV-infected persons. AIDS 14:2137-2144. [DOI] [PubMed] [Google Scholar]
- 18.Gao, W. Y., R. Agbaria, J. S. Driscoll, and H. Mitsuya. 1994. Divergent anti-human immunodeficiency virus activity and anabolic phosphorylation of 2′,3′-dideoxynucleoside analogs in resting and activated human cells. J. Biol. Chem. 269:12633-12638. [PubMed] [Google Scholar]
- 19.Goujon, L., T. Brossette, N. Dereudre-Bosquet, C. Creminon, P. Clayette, D. Dormont, C. Mioskowski, L. Lebeau, and J. Grassi. 1998. Monitoring of intracellular levels of 5′-monophosphate-zidovudine with an enzyme immunoassay. J. Immunol. Methods 218:19-30. [DOI] [PubMed] [Google Scholar]
- 20.Hunter, W. M., and F. C. Greenwood. 1962. Preparation of iodine-131-labelled growth hormone of high specific activity. Nature 194:495-496. [DOI] [PubMed] [Google Scholar]
- 21.Kaul, S., B. Stouffer, V. Mummaneni, N. Turabi, S. Mantha, P. Jayatilak, and R. Barbhaiya. 1996. Specific radioimmunoassays for the measurement of stavudine in human plasma and urine. J. Pharm. Biomed. Anal. 15:165-174. [DOI] [PubMed] [Google Scholar]
- 22.Kenney, K. B., S. A. Wring, R. M. Carr, G. N. Wells, and J. A. Dunn. 2000. Simultaneous determination of zidovudine and lamivudine in human serum with HPLC with tandem mass spectrometry. J. Pharm. Biomed. Anal. 22:967-983. [DOI] [PubMed] [Google Scholar]
- 23.Kewn, S., P. G. Hoggard, J. S. Henry-Mowatt, G. J. Veal, S. D. Sales, M. G. Barry, and D. J. Back. 1999. Intracellular activation of 2′,3′-dideoxyinosine and drug interactions in vitro. AIDS Res. Hum. Retroviruses 15:793-802. [DOI] [PubMed] [Google Scholar]
- 24.Knupp, C. A., F. A. Stancato, E. A. Papp, and R. H. Barbhaiya. 1990. Quantitation of didanosine in human plasma and urine by high-performance liquid chromatography. J. Chromatogr. 533:282-290. [DOI] [PubMed] [Google Scholar]
- 25.Labataille, P., H. Pélicano, G. Maury, J.-L. Imbach, and G. Gosselin. 1995. Synthesis and HIV-1 reverse transcriptase inhibition properties of two new methylenephosphonate analogues of 3′-azido-3′-deoxythymidine-5′-triphosphate. Bioorg. Med. Chem. Lett. 5:2315-2320. [Google Scholar]
- 26.Moore, J. D., G. Valette, A. Darque, X. J. Zhou, and J. P. Sommadossi. 2000. Simultaneous quantitation of the 5′-triphosphate metabolites of zidovudine, lamivudine, and stavudine in peripheral mononuclear blood cells of HIV infected patients by high-performance liquid chromatography tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 11:1134-1143. [DOI] [PubMed] [Google Scholar]
- 27.Mukherji, E., J. L. Au, and L. E. Mathes. 1994. Differential antiviral activities and intracellular metabolism of 3′-azido-3′-deoxythymidine and 2′,3′-dideoxyinosine in human cells. Antimicrob. Agents Chemother. 38:1573-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pereira, A. S., K. B. Kenney, M. S. Cohen, J. E. Hall, J. J. Eron, R. R. Tidwell, and J. A. Dunn. 2000. Simultaneous determination of lamivudine and zidovudine concentrations in human seminal plasma with high-performance liquid chromatography and tandem mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 742:173-183. [DOI] [PubMed] [Google Scholar]
- 29.Peter, K., J. P. Lalezari, and J. G. Gambertoglio. 1996. Quantification of zidovudine and individual zidovudine phosphates in peripheral blood mononuclear cells by a combined isocratic high performance liquid chromatography radioimmunoassay method. J. Pharm. Biomed.Anal. 14:491-499. [DOI] [PubMed] [Google Scholar]
- 30.Pruvost, A., F. Becher, P. Bardouille, C. Guerrero, C. Creminon, J. F. Delfraissy, C. Goujard, J. Grassi, and H. Benech. 2001. Direct determination of phosphorylated intracellular anabolites of stavudine (d4T) by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 15:1401-1408. [DOI] [PubMed] [Google Scholar]
- 31.Ray, G., and E. Murrill. 1987. Determination of 2′,3′-dideoxyinosine in plasma by high performance liquid chromatography. Analytical Lett. 20:1815-1838. [Google Scholar]
- 32.Robbins, B. L., T. T. Tran, F. H. Pinkerton, Jr., F. Akeb, R. Guedj, J. Grassi, D. Lancaster, and A. Fridland. 1998. Development of a new cartridge radioimmunoassay for determination of intracellular levels of lamivudine triphosphate in the peripheral blood mononuclear cells of human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 42:2656-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robbins, B. L., B. H. Waibel, and A. Fridland. 1996. Quantitation of intracellular zidovudine phosphates by use of combined cartridge-radioimmunoassay methodology. Antimicrob. Agents Chemother. 40:2651-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez, J. F., J. L. Rodriguez, J. Santana, H. Garcia, and O. Rosario. 2000. Simultaneous quantitation of intracellular zidovudine and lamivudine triphosphates in human immunodeficiency virus-infected individuals. Antimicrob. Agents Chemother. 44:3097-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saady, M., A. Valleix, L. Lebeau, and C. Mioskowski. 1995. First synthesis of fully deprotected diiminotriphosphoric acid and derivatives designed for the synthesis of “PNPNP” nucleotides and dinucleotides. J. Org. Chem. 60:3685-3691. [Google Scholar]
- 36.Sarasa, M., N. Riba, L. Zamora, and X. Carné. 2000. Determination of stavudine in human plasma and urine by high-performance liquid chromatography with reduced sample volume. J. Chrom. B. 746:183-189. [DOI] [PubMed] [Google Scholar]
- 37.Sommadossi, J. P. 1993. Nucleoside analogs: similarities and differences. Clin. Infect. Dis. 16 Suppl. 1:S7-S15. [DOI] [PubMed] [Google Scholar]
- 38.Trowbridge, D.,. B., and G. L. Kenyon. 1970. Adenosine 5′-Bis(dihydrophosphinylmethyl)-phosphinate, the α, β:β, γ-bismethylene analog of adenosine 5′-triphosphate. J. Am. Chem. Soc. 92:2181-2182. [DOI] [PubMed] [Google Scholar]
- 39.Van Heeswijk, R. P. 2002. Critical issues in therapeutic drug monitoring of antiretroviral drugs. Ther. Drug Monit. 24:323-331. [DOI] [PubMed] [Google Scholar]
- 40.Weaver, R., and I. H. Gilbert. 1997. The design and synthesis of nucleoside triphosphate isosteres as potential inhibitors of reverse transcriptase. Tetrahedron 53:5537-5562. [Google Scholar]
- 41.Weaver, R., I. H. Gilbert, N. Mahmood, and J. Balzarini. 1996. Isosteres of nucleoside triphosphates. Bioorg. Med. Chem. Lett. 6:2405-2410. [Google Scholar]