Abstract
Mitochondria are affected by low temperature during seedling establishment in maize (Zea mays L.). We evaluated the associated changes in the mitochondrial properties of populations selected for high (C4-H) and low (C4-L) germination levels at 9.5°C. When seedlings of the two populations were grown at 14°C (near the lower growth limit), the mitochondrial inner membranes of C4-H showed a higher percentage of 18-carbon unsaturated fatty acids, a higher fluidity, and a higher activity of cytochrome c oxidase. We found a positive relationship between these properties and the activity of a mitochondrial peroxidase, allowing C4-H to reduce lipid peroxidation relative to C4-L. The specific activity of reconstituted ATP/ADP translocase was positively associated with this peroxidase activity, suggesting that translocase activity is also affected by chilling. The level of oxidative stress and defense mechanisms are differently expressed in tolerant and susceptible populations when seedlings are grown at a temperature near the lower growth limit. Thus, the interaction between membrane lipids and cytochrome c oxidase seems to play a key role in maize chilling tolerance. Furthermore, the divergent-recurrent selection procedure apparently affects the allelic frequencies of genes controlling such an interaction.
Tolerance to low temperatures above 0°C expressed in the first phase of the plant life cycle is an important characteristic for warm-season crops, even when they are grown in temperate regions. In fact, consistent germination and rapid growth in cold soils should increase the stability of performance across environments and should allow early sowing, leading to important agronomic advantages such as flowering prior to the onset of the hottest and driest period of the year.
At the cellular and molecular levels, cold stress affects fatty acid composition, the fluidity of cellular membranes, metabolic rates, and protein turnover (Levitt, 1980; Graham and Patterson, 1982; Miedema, 1982; Blum, 1988; Guy, 1990; Nishida and Murata, 1996). Stewart et al. (1990a, 1990b) studied the influence of various temperatures on germination and seedling establishment of cold-resistant and -susceptible maize (Zea mays L.) genotypes, and concluded that rates of respiration were affected by cold treatment, and that the activity of the respiratory oxidase alternative to COX was stimulated at temperatures near the lower limit of growth.
Mitochondria have been found to be the main cellular compartment affected by chilling treatment, and three chilling-acclimation-responsive nuclear genes have been isolated. One of these proved to be the catalase gene (Prasad et al., 1994a; Anderson et al., 1994, 1995; Prasad, 1997).
Compared with more favorable growth temperatures, chilling temperatures have been reported to lower expression and activity of COX in the mitochondrial inner membranes of a chilling-susceptible genotype of maize (Prasad et al., 1994b). However, the decline in this activity was partially counterbalanced by an increase in the rate of the alternative oxidase (Prasad et al., 1994b). Studies conducted by Rich et al. (1976) and Huq and Palmer (1978) demonstrated that the reduction of complex I and ubiquinone in the respiratory chain (caused by the decrease in terminal oxidase activity) led to an increased production of ROS such as superoxide and H2O2. Therefore, chilling induces oxidative stress, and the ability of maize seedlings to survive depends on their capacity during acclimation to increase the synthesis and the activity of antioxidant enzymes such as superoxide dismutase, catalase, and peroxidases (Prasad et al., 1994a, 1994b, 1995; Zhang et al., 1995; Prasad, 1996, 1997; Hodges et al., 1997a, 1997b). These scavenging mechanisms prevent the accumulation of ROS, and consequently, the irreversible damage to mitochondrial membrane components (Prasad et al., 1995).
In nonacclimated seedlings grown at 4°C, chilling damage was partly due to the oxidation of a large number of protein and lipid molecules caused by ROS produced in mitochondria, and to an inhibition of two protease activities (Prasad, 1996). In acclimated seedlings the early spurt in ROS during acclimation induced expression of antioxidant genes. Consequently, with ROS maintained at steady-state levels, the progression of protease inhibition and the oxidation of proteins and lipids during low-temperature stress were prevented (Prasad, 1996).
In the majority of recent works conducted in maize, the relationships between responses to chilling and other cellular functions were studied by comparing acclimated and nonacclimated seedlings of a susceptible genotype (Anderson et al., 1994, 1995; Prasad et al., 1994a, 1994b, 1995; Prasad, 1996, 1997) or by comparing genotypes of different origins and with varying levels of tolerance to chilling at various growth stages (Stewart et al., 1990a, 1990b; Li et al., 1992; Zhang et al., 1995; Hodges et al., 1997a). However, the chilling tolerance of maize inbreds was not an accurate predictor of that of maize hybrids (Hodges et al., 1997b). The latter proved to be affected by maternal effects associated with germination and early seedling growth (Maryam and Jones, 1983). The level of activities of antioxidant enzymes (catalase, monodehydroascorbate reductase, and ascorbate peroxidase) is a useful screening tool for chilling resistance of maize hybrids (Hodges et al., 1997b).
In previous investigations of few inbred lines and hybrids, the interpretation of the cause-effect relationship between traits related to chilling tolerance could have been biased by specific gene combinations fixed in the materials. For this reason, a suitable approach for obtaining further information on the mechanism of chilling tolerance could be the investigation of materials developed from the same source through divergent selection for chilling tolerance (i.e. for both its high and low expression). These materials, which share a common genetic background, should differ only in the allelic frequencies of the genes controlling the selected trait, thereby allowing a meaningful analysis of their correlated effects on other properties.
Four cycles of divergent-recurrent selection for tolerance to germination at low temperatures were conducted by Landi et al. (1992) in a maize population exposed to a controlled environment. The populations selected downward (for a low level of tolerance) were exceeded by the populations selected upward (for a high level of tolerance) for germination percentage and germination rate at 9.5°C. In contrast, no substantial differences in germination percentage or rate were detected among populations at 25°C (Landi et al., 1992).
In the current study we examined seedlings of the plants developed by this selection procedure at two growth temperatures (favorable, 25°C; and near the lower limit, 14°C) to study the associated changes in mitochondrial properties related to the expression of chilling tolerance. For these purposes we evaluated the fatty acid composition of the inner membranes, membrane fluidity, activities of the terminal respiratory complex COX, activities of antioxidant enzymes within the mitochondria, lipid peroxidation, and the activity of ANT.
When seedlings were grown at 14°C, the population selected for high germination at 9.5°C (H) showed, in comparison with the population selected for low germination (L): (a) a higher content of 18-carbon unsaturated fatty acid species in the mitochondrial inner membrane, (b) a higher membrane fluidity, (c) a higher activity of COX, (d) a higher activity of a mitochondrial peroxidase, (e) a lower level of lipid peroxidation products, and (f) a higher exchange rate of ATP in liposomes reconstituted with purified ANT. Thus, the divergent selection procedure affected allelic frequencies of genes controlling the interactions between membrane lipids and COX activity of mitochondria. Such interactions apparently alleviated the induction of oxidative stress and its effects on ANT activity when seedlings of the tolerant population (C4-H) were grown at a temperature near the lower growth limit.
MATERIALS AND METHODS
Plant Material
The maize (Zea mays L.) populations analyzed in this study were developed through divergent-recurrent selection for tolerance to germination at 9.5°C, conducted in the F2 of the single cross B73 × IABO78. B73 is an inbred line derived from U.S. Corn Belt germplasm that has a dented kernel type and is well-known for its superior agronomic performance. IABO78 is an Italian inbred line with a flint kernel showing a higher ability to germinate under conditions of cold and wet soils compared with B73. The selection procedure has been described in detail by Landi et al. (1992).
The effects of growth temperature on mitochondrial properties were studied by comparing the two final populations obtained by the divergent-selection procedure, C4-L and C4-H. The source F2 (the population obtained from the cross B73 × IABO78, hereafter referred to as C0) was also tested to reveal a possible asymmetry of the response to selection. To gain an insight into the genetic variability available in C0 and of the gene distribution between the two parental lines, B73 and IABO78 were tested as well. The five materials (the two parental lines and the three populations) were reproduced in 1995 at Bologna (in northern Italy) to have seeds of the same age and in the same environment. Within each population, approximately 100 pairs of random plants were crossed, and an equal number of seeds was taken from each ear and bulked. For each inbred line 10 plants were selfed, and all seeds obtained were bulked.
Seed Germination and Seedling Growth
Seed germination and seedling growth of the five materials took place in a controlled chamber at 95% RH in darkness. Two continuous temperatures of growth were used: a favorable temperature (25°C) and a cool temperature (14°C). A temperature of 14°C (instead of 9.5°C, as during the selection work) was chosen to obtain seedlings from those seeds that probably would not germinate at 9.5°C. Stewart et al. (1990a, 1990b) also performed their experiments on maize seedlings at 14°C to study the effects of temperature near the lower limit of growth.
Seeds were surface-sterilized for 5 min in 1% (w/v) sodium hypochlorite, rinsed in sterile distilled water, and allowed to imbibe in aerated water overnight at 25°C or 14°C. Seeds were then sown on a layer of hydrophilic cotton in plastic boxes and covered with a sheet of thin, wet paper. Seedlings were grown for 4 d at 25°C or for 21 to 23 d at 14°C, and were harvested at the same growth stage (i.e. when shoots were 4–5 cm tall).
For investigation at both 25°C and 14°C, the five materials were compared in two experiments conducted at different times. In each experiment about 2000 plants were grown for each material. Shoots of all plants were used for the preparation of purified mitochondria. Then, two samples were used for each material (line or population) in each experiment, giving a total of four samples per material across the two experiments.
Purification of Mitochondria
Maize shoot mitochondria were isolated as described by Genchi et al. (1996). Purification of the mitochondria from other contaminants was carried out according to the method of Douce et al. (1972), except that 5 mm Tris-Cl, pH 7.2, was used instead of 10 mm phosphate buffer in all purification steps. Purified mitochondria were suspended at a concentration of 15 to 25 mg protein mL−1 in a medium containing 0.3 m Suc and 5 mm Tris-Cl, pH 7.2.
The purity of mitochondrial preparations was routinely checked by assaying marker enzymes: fumarase for mitochondria, antimycin A-insensitive Cyt c reductase for ER, isocitrate lyase for glyoxysomes, glycolate oxidase for peroxysomes, K+-ATPase for plasma membranes, and lipoxygenase and lipolytic acyl hydrolases for vacuoles. The assay methods have been summarized by Quail (1979) and Neuburger (1985). Etioplast contamination of the mitochondrial membranes was assayed spectrophotometrically by testing for the carotenoid level (Venturoli et al., 1986).
Mitoplasts (mitochondria deprived of the outer membrane) were prepared from the whole, purified mitochondria by hypotonic treatment (10 mm Suc and 5 mm Tris-Cl, pH 7.2) for 15 min (Douce et al., 1973). After centrifugation at 9000g, mitoplasts were suspended in 0.3 m Suc and 5 mm Tris-Cl, pH 7.2, frozen in liquid nitrogen, and stored at −80°C.
Fatty Acid Composition of the Mitochondrial Inner Membranes
Lipids were extracted from mitoplasts by the method of Folch et al. (1957). The preparation of fatty acid methyl esters and the final extraction with n-hexane were carried out according to the method proposed by Kock et al. (1985). The fatty acid methyl esters were analyzed and identified by gas chromatography according to the method of Augustin (1989). The degree of fatty acid unsaturation (Δ/mol) and mean chain length were defined and calculated also according to the method of Augustin (1989).
Fluidity of the Mitochondrial Inner Membranes
The fluidity of the mitochondrial inner membrane was estimated by measuring the steady-state fluorescence polarization of the hydrophobic probe DPH (Shinitzky, 1978). Mitoplasts were incubated for 30 min at room temperature with 7.5 μm DPH to incorporate the probe. The fluorescence polarization of DPH was measured in a spectrofluorometer (model MPF4, Perkin-Elmer) equipped with polarization accessories (excitation and emission wavelengths of 360 and 460 nm, respectively). The degree of polarization (p) was calculated using the following expression:
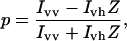 |
where Z = Ihv/Ihh and the first and second subscripts represent the position of the excitation and emission polarizers (vertical and horizontal). The factor Z compensates for slightly unequal horizontal and vertical excitation intensities (Ford and Barber, 1980). We corrected for intrinsic fluorescence and scattering from the membrane suspension by subtracting the values obtained with unlabeled samples.
Although the steady-state polarization cannot discriminate between static and dynamic components (order parameter and viscosity, respectively; Zannoni, 1981), it can be taken as a semiquantitative indication of membrane fluidity (Shinitzky, 1978).
Mitoplasts obtained from shoots of the five materials grown at 25°C or at 14°C were tested for DPH fluorescence polarization at several assay temperatures ranging from 14°C to 33°C.
Enzyme Analyses
COX was determined as described by Prasad et al. (1994b) in a reaction medium containing 90 mm phosphate buffer, pH 7.0, 50 μm Cyt c (previously reduced with 3 mg of sodium hydrosulfite), and 15 μg of mitochondrial protein in a final volume of 1 mL. The activity was determined as the rate of oxidation of the reduced Cyt c measured at A550 (ε550 = 21 mm−1 cm−1). The oxidase content was estimated by measuring spectrophotometrically levels of Cyt a + a3, from ΔA605–630 (ε605–630 = 37.4 mm−1 cm−1) and ΔA605–590 (ε605–590 = 19.3 mm−1 cm−1), as described by van Gelder (1966).
mtPOX was determined by the method of Chance and Maehly (1955) using guaiacol (ε470 = 26.6 mm−1 cm−1) as the electron donor. CPX activity was measured by monitoring the oxidation of reduced Cyt c (ε550 = 21 mm−1 cm−1) according to the method of Verduyn et al. (1988).
Determination of TBARS
One-hundred-fifty-microliter samples of the mitochondrial suspension (containing approximately 3–6 mg of protein) were assayed for TBARS as described by Oteiza and Bechara (1993) with minor modifications. One-hundred-fifty-microliters of 3% (w/v) SDS, 250 μL of 3% (w/v) thiobarbituric acid in 50 mm NaOH, and 250 μL of 25% (v/v) HCl were added sequentially to the mitochondrial suspension, with mixing after each addition. The mixture was heated to 80°C in a water bath for 20 min and cooled on ice. TBARS were extracted with 800 μL of butanol, the specific A532 of the organic phase was measured, and the nonspecific A600 was subtracted. Measurements were expressed as (A532–A600) mg−1 protein.
Purification of ANT Protein from Mitochondria and Reconstitution of Transport Activity in Liposomes
As emphasized by Schünemann et al. (1993), a suitable method to compare the specific activity of carrier proteins from various sources implies their solubilization and chromatographic purification, insertion of protein into liposomes, and functional reconstitution of transport activities (Palmieri et al., 1995).
Purification of the ANT protein, one of the most abundant and physiologically relevant proteins of the mitochondrial inner membrane, was performed as described by Genchi et al. (1996). Only seedlings of C0, C4-H, and C4-L were used, because seeds of the two parental lines produced in the same environment were no longer available.
We assessed the reconstituted transport activities by measuring the amount of substrate transported into proteoliposomes (Genchi et al., 1996). Using the reconstitution method we were able to compare the specific activity of the ANT proteins purified from seedlings of all populations grown at 25°C and 14°C; moreover, we could avoid differences in ANT activity due to changes in the protein level in the inner mitochondrial membrane and/or to differences in the concentration within the mitochondria of the metabolite to be transported. Each analysis of ATP-transport activity was performed at both temperatures used for growing seedlings.
Experimental Design and Statistical Analysis
Analysis of variance was conducted separately for the experiments on seedlings grown at 25°C and 14°C, given the large differences in growing conditions. For each growth temperature, the analysis was done separately for each experiment according to a completely randomized design, with two samples per material. Next, a combined analysis of the two experiments was made given the homogeneity of their error variances. The variation among the five materials was partitioned into four orthogonal comparisons as follows: (a) between parental lines, (b) between selected populations C4-L and C4-H, (c) between the mean value of the two selected populations and the source C0, and (d) between the mean value of the two parental lines and the mean value of the three populations. We considered this latter comparison to be residual and therefore irrelevant because it involved both materials that were homozygous (parental lines) and those that were heterozygous, with different allelic frequencies (populations). With respect to the fatty acid composition of the mitochondrial inner membrane, the analysis of variance was carried out only when the mean value of each material was equal to at least 0.1%.
The effects of different temperatures on DPH fluorescence polarization were investigated by regressing the latter on the former variable. Before running regression analysis, we plotted the different temperatures of the assay (expressed in K) as their reciprocal (1/T). We used a Student's t test to make the orthogonal comparisons between regression coefficients.
RESULTS
The purity and identity of the mitochondrial membranes were routinely checked by measuring the activity of the cellular marker enzymes (see Methods). Results of these analyses showed that the tested marker enzymes for other cellular compartments were almost undetectable. On the contrary, fumarase (a mitochondrial marker enzyme) activity was about 24 times higher on a protein basis in purified mitochondrial preparations than in total extract. Etioplasts and glyoxysomes, which have been indicated as the major contaminants of crude mitochondrial fractions (Neuburger, 1985), were also negligible in our preparations: we obtained an average of 1.5 ng mg−1 protein for carotenoids and 0.8 nmol min−1 mg−1 for isocitrate lyase. Thus, we found no significant cross-contaminations by ER, peroxisomes, plasma membranes, enzymes deriving from the vacuole, etioplasts, or glyoxysomes in our mitochondrial fractions purified on Suc gradients.
The analysis of variance pointed out that for most mitochondrial properties the interaction between materials and experiments was not significant (data not shown); therefore, only the mean values of the two experiments on the five materials are presented. The error variance and the coefficient of variation of the traits investigated at 25°C were often lower than the corresponding values at 14°C (data not shown), indicating that the precision level tended to be higher for traits measured on mitochondria isolated from seedlings grown at the favorable temperature.
Fatty Acid Composition of the Mitochondrial Inner Membrane
As a general trend, we found higher contents of saturated fatty acids when seedlings were grown at 25°C, whereas unsaturated fatty acid contents were higher at 14°C (Table I). For most traits, small differences among materials at 25°C were consistent with greater differences observed at 14°C. However, such differences were more often significant at 14°C (despite the tendency for a lower precision level at this temperature than at 25°C), indicating that a greater distinction among materials was achieved when they were grown at a temperature near the lower limit of growth.
Table I.
Fatty acid composition of the mitochondrial inner membrane isolated from shoots of maize seedlings of parental lines and populations grown at 25°C and 14°C
| Material | 12:0 | 12:1 | 14:0 | 16:0 | 16:1 | 18:0 | 18:1[9] | 18:1[11] | 18:2 | 18:3 |
|---|---|---|---|---|---|---|---|---|---|---|
| mass % | ||||||||||
| 25°C | ||||||||||
| Parental lines | ||||||||||
| B73 | 0.3 | +a | 0.5 | 45.4 | 0.4 | 4.0 | 4.4 | + | 42.4 | 2.5 |
| IABO78 | 0.3 | + | 0.5 | 45.1 | 0.4 | 4.0 | 4.5 | + | 42.5 | 2.6 |
| Populations | ||||||||||
| C0 | 0.3 | + | 0.5 | 45.5 | 0.4 | 4.0 | 4.4 | + | 42.3 | 2.6 |
| C4-L | 0.3 | + | 0.5 | 45.4 | 0.4 | 4.2 | 4.6 | + | 41.9 | 2.6 |
| C4-H | 0.3 | + | 0.5 | 44.3 | 0.4 | 3.9 | 4.9 | + | 43.1 | 2.7 |
| Meanb | 0.3 | + | 0.5 | 44.8 | 0.4 | 4.1 | 4.8 | + | 42.5 | 2.7 |
| lsdc | nsd | + | ns | 0.5 | ns | 0.1 | ns | + | 0.4 | ns |
| lsde | ns | + | ns | 0.4 | ns | ns | ns | + | ns | ns |
| 14°C | ||||||||||
| Parental lines | ||||||||||
| B73 | 0.1 | + | 0.3 | 38.5 | 0.5 | 3.2 | 5.4 | 0.3 | 48.3 | 3.3 |
| IABO78 | + | 0.3 | 0.3 | 36.7 | 0.5 | 2.9 | 6.3 | 0.5 | 49.0 | 3.6 |
| Populations | ||||||||||
| C0 | + | + | 0.3 | 36.7 | 0.5 | 3.1 | 5.3 | 0.3 | 50.2 | 3.6 |
| C4-L | 0.1 | + | 0.5 | 39.3 | 0.5 | 5.3 | 4.7 | 0.1 | 47.3 | 2.4 |
| C4-H | + | 0.1 | 0.3 | 32.3 | 0.5 | 2.5 | 7.9 | 0.6 | 49.5 | 6.4 |
| Meanb | + | + | 0.4 | 35.8 | 0.5 | 3.9 | 6.3 | 0.3 | 48.4 | 4.4 |
| lsdc | + | + | 0.1 | 0.9 | ns | 0.4 | 0.3 | 0.1 | 1.0 | 0.5 |
| lsde | + | + | 0.1 | 0.7 | ns | 0.3 | 0.2 | ns | 0.8 | 0.4 |
Results are the mean values of four determinations (two experiments and two samples per experiment).
+, Trace amounts with values < 0.1%; in this case the analysis of variance was not carried out and the lsd was not calculated.
Mean value of the two selected populations, C4-L and C4-H.
lsd (P ≤ 0.05) for comparing parental lines and/or populations.
ns, Not significant.
lsd (P ≤ 0.05) for comparing the source population C0 with the mean of C4-L and C4-H.
Seedlings of the two parental lines did not show any significant difference in mitochondrial fatty acid content when grown at 25°C, but when grown at 14°C, they differed significantly in the levels of 16:0 (palmitic acid), 18:1[9] (oleic acid), and 18:1[11] (vaccenic acid). At 14°C mitochondria from B73 seedlings had a higher content of saturated fatty acids than mitochondria from IABO78 seedlings.
The difference between the two selected populations, C4-L and C4-H, was significant for the content of 16:0, 18:0, and 18:2 in seedlings grown at 25°C and significant for other tested properties except 16:1 content at 14°C. Moreover, at 14°C differences between C4-L and C4-H followed a clear trend that was similar to that observed for differences between the two parental lines. The most relevant changes in the fatty acid composition of C4-H relative to C4-L at 14°C were: a 7.0% decrease in 16:0, a 2.8% decrease in 18:0, a 3.2% increase in 18:1[9], and a 4.0% increase in 18:3. These differences were greater than those observed at 14°C among the parental lines and C0. Indeed, at 14°C and for all fatty acids except 16:1, the two selected populations exhibited transgressive percentages, with C4-L showing values beyond those of the B73 parental line and C4-H showing values beyond those of IABO78. The fatty acid composition of the C0 population at 14°C was generally intermediate between C4-L and C4-H.
Comparison between the mean of the two selected populations and the source C0 was significant for the content of 16:0 at 25°C and for the content of 14:0, 16:0, 18:0, 18:1[9], 18:2, and 18:3 fatty acids at 14°C. The results thus indicate that for these traits the associated responses to selection were asymmetric. The asymmetry of such responses, however, did not follow a clear trend, because C0 was closer to C4-L for the content of some fatty acids, whereas C0 was closer to C4-H for the content of others, irrespective of whether these species were saturated or not.
The mean values of the unsaturation degree, the chain length, and other properties of mitochondrial fatty acids of the five materials grown at 25°C were in most cases lower than the corresponding mean values at 14°C (Table II).
Table II.
Characterization of fatty acid composition of the mitochondrial inner membrane isolated from shoots of maize seedlings of parental lines and populations grown at 25°C and 14°C
| Material | Unsaturation Degreea | Mean Chain Lengthb | Monounsaturated Species | Unsaturated Species | Unsaturated 18-Carbon Species | 18:1/18:0 | Unsat C18/Sat C18c |
|---|---|---|---|---|---|---|---|
| Δ/mol | Carbon atom no. | mass % | |||||
| 25°C | |||||||
| Parental lines | |||||||
| B73 | 0.970 | 17.04 | 4.9 | 49.8 | 49.3 | 1.1 | 12.3 |
| IABO78 | 0.980 | 17.05 | 5.1 | 50.2 | 49.6 | 1.1 | 12.5 |
| Populations | |||||||
| C0 | 0.970 | 17.04 | 4.9 | 49.8 | 49.3 | 1.1 | 12.3 |
| C4-L | 0.968 | 17.05 | 5.1 | 49.6 | 49.2 | 1.1 | 11.6 |
| C4-H | 0.990 | 17.07 | 5.3 | 51.0 | 50.7 | 1.2 | 12.8 |
| Meand | 0.979 | 17.06 | 5.2 | 50.3 | 49.9 | 1.2 | 12.2 |
| lsde | 0.011 | 0.02 | ns | 0.6 | 0.6 | 0.1 | 0.5 |
| lsdf | nsg | ns | ns | 0.5 | 0.5 | ns | ns |
| 14°C | |||||||
| Parental lines | |||||||
| B73 | 1.125 | 17.20 | 6.2 | 57.8 | 57.3 | 1.8 | 17.8 |
| IABO78 | 1.163 | 17.23 | 7.6 | 60.1 | 59.3 | 2.3 | 20.5 |
| Populations | |||||||
| C0 | 1.173 | 17.24 | 6.1 | 59.9 | 59.4 | 1.8 | 19.3 |
| C4-L | 1.068 | 17.18 | 5.1 | 54.9 | 54.4 | 0.9 | 10.4 |
| C4-H | 1.273 | 17.33 | 9.1 | 65.0 | 64.4 | 3.4 | 25.6 |
| Meand | 1.170 | 17.25 | 7.1 | 59.9 | 59.4 | 2.1 | 18.0 |
| lsde | 0.020 | 0.02 | 0.3 | 1.3 | 1.3 | 0.2 | 1.2 |
| lsdf | ns | ns | 0.3 | ns | ns | 0.2 | 1.0 |
Results are the mean values of four determinations (two experiments and two samples per experiment).
Calculated as follows: Δmol = (1[% monoenes]/100) + (2[% dienes][/100) + (3[% trienes]/100).
Calculated as follows: ∑ (percentage of the fatty acid × its carbon atom number)/100.
Unsat C18/Sat C18, Ratio of unsaturated 18-carbon species and saturated 18-carbon species.
Mean of the two selected populations C4-L and C4-H.
lsd (P ≤ 0.05) for comparing parental lines and/or populations.
lsd (P ≤ 0.05) for comparing the source population C0 with the mean C4-L and C4-H.
ns, Not significant.
Compared with B73, IABO78 had higher values for all properties at both temperatures (with the exception of the ratio between 18:1 and 18:0 at 25°C); however, such higher values were always not significant at 25°C and were always significant at 14°C. Likewise, C4-H exceeded C4-L for all mitochondrial properties at both temperatures, and these differences were significant in almost all instances. At 14°C the overall shift from saturated to unsaturated fatty acids in C4-H relative to C4-L was about 10%, including minor fatty acid components. This shift was due almost completely to the increase in 18-carbon unsaturated fatty acids. Thus, at 14°C, C4-H relative to C4-L showed an increase of 3.8 times in the ratio between 18:1 and 18:0 fatty acid content and an increase of 2.5 times in the ratio of total 18-carbon unsaturated:18-carbon saturated fatty acids.
Differences between the means of the two selected populations and C0 were not significant for the unsaturation degree and for the chain length at either 25°C or at 14°C, indicating that the associated responses to divergent selection were symmetric. However, these symmetric responses were achieved by balancing the asymmetric responses observed for some components. In fact, the comparison between C0 and the mean of C4-L and C4-H was significant for the sum of unsaturated species, the sum of unsaturated 18-carbon fatty acid species at 25°C, the content of monounsaturated species, and the ratios of 18:1 to 18:0 and 18-carbon unsaturated to 18-carbon saturated fatty acids at 14°C.
DPH Fluorescence Polarization
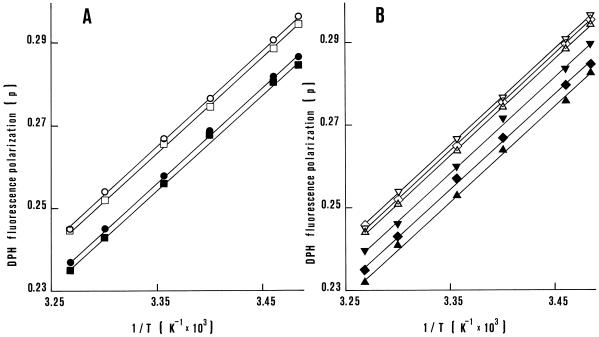
DPH fluorescence polarizations of the mitochondrial inner membrane obtained from seedlings of the five materials grown at 25°C and at 14°C were markedly affected by the assay temperature (Fig. 1). A stringent consistency was found between the observed data and the ones predicted by the regression lines, with the determination coefficients (r2) ranging from 99.84% for IABO78 and C4-H seedlings grown at 14°C to 99.98% for C4-H at 25°C. Moreover, these results showed that no transition phase of the mitochondrial inner membranes occurred within the range of temperatures investigated. No significant differences were found among the regression coefficients of the five groups grown at 25°C or at 14°C, nor between the regression coefficients at the two temperatures averaged over the five genetic materials. The common slope for all of the regression lines was 0.234 ± (1 × 10−3 p)K.
Figure 1.
Effect of the assay temperature on DPH fluorescence polarization of the mitochondrial inner membrane. A, Mitochondria isolated from shoots of maize seedlings of parental lines B73 (○ and •) and IABO78 (□ and ▪) grown at 25°C (○ and □) and at 14°C (• and ▪). B, Mitochondria isolated from shoots of seedlings of populations C0 (⋄ and ♦), C4-L (▿ and ▾), and C4-H (▵ and ▴) grown at 25°C (⋄, ▿, and ▵) and at 14°C (♦, ▾, and ▴). Data are the means of four determinations (two experiments and two samples per experiment). The assay temperatures were transformed as 1/T × 103. Errors were homogeneous; the se of each mean value was 0.2 × 10−3 p.
Given the lack of significant differences among the regression coefficients, the DPH fluorescence polarization of each material grown at 25°C and at 14°C could be also examined as the mean value of the six assayed temperatures. Such mean values for plants grown at 25°C were 0.272 (B73), 0.270 (IABO78), 0.271 (C0), 0.272 (C4-L), and 0.270 p (C4-H). When seedlings were grown at 14°C, the mean values were 0.263 (B73), 0.261 (IABO78), 0.261 (C0), 0.265 (C4-L), and 0.258 p (C4-H). The lsd at P ≤ 0.05 for comparing parental lines and/or populations was 1 × 10−3 p at both 25°C and 14°C. Mean values were higher for mitochondria of seedlings grown at 25°C, whereas differences among mean values were larger for mitochondria of plants grown at 14°C. At both temperatures B73 was significantly higher than IABO78, and the same was true for C4-L in comparison with C4-H. Moreover, at 14°C the two selected populations showed transgressive mean values, with C4-L being beyond B73 and C4-H beyond IABO78.
These results are consistent with most of the results seen in Tables I and II. In particular, highly significant and negative linear relationships were found at 14°C between the mean values of the five materials for DPH fluorescence polarization versus the percentage of the unsaturated species of fatty acids (r = −0.995) and versus the ratio between unsaturated and saturated 18-carbon species (r = −0.972). These findings therefore indicate that most of the variation observed for DPH fluorescence was accounted for by these linear relationships. The DPH fluorescence of C0 was intermediate between that of C4-L and that of C4-H at 25°C or at 14°C, revealing that the associated response to selection was symmetric.
COX and mtPOX Activities and Levels of TBARS
For COX activity mean values of the materials grown at 25°C were higher than the corresponding mean values at 14°C, whereas the reverse was found for the other three traits (higher mean values were detected at 14°C than at 25°C; Table III). Differences among materials grown at 25°C reached the significance level for COX and CPX activities, whereas at 14°C differences were significant for all of the traits except CPX.
Table III.
Activities of COX, mtPOX, and CPX, and the degree of lipid peroxidation (TBARS) of the mitochondria isolated from shoots of maize seedlings of parental lines and populations grown at 25°C and 14°C
| Material | COX | mtPOX | CPX | TBARS |
|---|---|---|---|---|
| μmol Cyt c min−1 mg−1 protein | μmol guaiacol min−1 mg−1 protein | μmol Cyt c min−1 mg−1 protein | (A532–A600) mg−1 protein | |
| 25°C | ||||
| Parental lines | ||||
| B73 | 1.81 | 0.21 | 0.13 | 0.13 |
| IABO78 | 1.87 | 0.23 | 0.15 | 0.13 |
| Populations | ||||
| C0 | 1.86 | 0.23 | 0.14 | 0.14 |
| C4-L | 1.82 | 0.22 | 0.13 | 0.12 |
| C4-H | 1.88 | 0.24 | 0.14 | 0.14 |
| Meana | 1.85 | 0.23 | 0.14 | 0.13 |
| lsdb | 0.02 | nsc | 0.02 | ns |
| lsdd | ns | ns | ns | ns |
| 14°C | ||||
| Parental lines | ||||
| B73 | 1.23 | 0.36 | 0.35 | 0.29 |
| IABO78 | 1.33 | 0.40 | 0.36 | 0.25 |
| Populations | ||||
| C0 | 1.27 | 0.38 | 0.35 | 0.26 |
| C4-L | 0.80 | 0.27 | 0.34 | 0.46 |
| C4-H | 1.41 | 0.51 | 0.35 | 0.22 |
| Meana | 1.11 | 0.39 | 0.34 | 0.34 |
| lsdb | 0.03 | 0.02 | ns | 0.02 |
| lsdd | 0.02 | ns | ns | 0.02 |
Results are the averages of four determinations (two experiments and two samples per experiment).
Mean value of the two selected populations, C4-L and C4-H.
lsd (P ≤ 0.05) for comparing parental lines and/or populations.
ns, Not significant.
lsd (P ≤ 0.05) for comparing the source population C0 with the mean of C4-L and C4-H.
When grown at 14°C, IABO78 exceeded B73, as did C4-H compared with C4-L for COX and mtPOX, whereas the opposite findings were noted for TBARS accumulation. In particular, the negative relationship between the former two traits and the latter was not significant for mtPOX versus TBARS, but it was highly significant (r = −0.998) for COX versus TBARS. Moreover, for the three traits for which significant differences among materials were found, the two selected populations exhibited transgressive mean values: C4-H and C4-L were beyond IABO78 and B73, respectively. The associated response to selection was symmetric for mtPOX and asymmetric for both COX and TBARS (the mean value of the two selected populations being significantly higher than that of the source).
As noted for DPH fluorescence polarization, COX, mtPOX, and TBARS levels at 14°C showed consistency with the results seen in Tables I and II. In particular, the ratio between unsaturated and saturated 18-carbon species (Table II) was correlated positively with COX (r = 0.960; P ≤ 0.01) and mtPOX (r = 0.983; P ≤ 0.01) and negatively with TBARS (r = −0.950; P ≤ 0.05).
Because the COX activity decreased markedly (especially for C4-L) at 14°C, we also investigated whether such changes were dependent on the different COX level in the inner membrane. Results not shown in Table III indicated that Cyt a + a3 content was very similar for the five materials grown at 14°C (ranging from 0.87 to 0.93 nmol Cyt a + a3 mg−1 mitochondrial protein). Therefore, differences in COX activities among the investigated materials at 14°C should not be ascribed to variations in COX content but, rather, to COX specific activity.
Rate of ATP Transport Activity across Liposome Membranes Reconstituted with Purified ANT Protein
Mean values of the reconstituted ANT specific activities (Table IV) were higher when seedlings were grown at 25°C than at 14°C, irrespective of the assay temperature (25°C or 14°C). In all instances the rate of reconstituted ATP transport obtained with ANT protein purified from the C4-H population was significantly higher than that obtained from C4-L, whereas the rate of C0 was intermediate. At both assay temperatures, differences between the two selected populations were quite limited when seedlings were grown at 25°C and much more marked when seedlings were grown at 14°C.
Table IV.
Rate of ATP transport in proteoliposomes reconstituted with ANT protein purified from mitochondrial membranes isolated from maize seedlings of populations grown at 25°C and at 14°C
| Population | ATP
Transport
|
|
|---|---|---|
| 25°C | 14°C | |
| nmol 10 min−1 mg−1 protein | ||
| Seedlings grown at 25°C | ||
| C0 | 9109 | 8481 |
| C4-L | 9033 | 8327 |
| C4-H | 9239 | 8604 |
| Meana | 9136 | 8466 |
| lsdb | 84 | 149 |
| lsdc | nsd | ns |
| Seedlings grown at 14°C | ||
| C0 | 7624 | 6648 |
| C4-L | 6824 | 5571 |
| C4-H | 8920 | 7730 |
| Meana | 7872 | 6651 |
| lsdb | 615 | 228 |
| lsdc | ns | ns |
Proteoliposomes were loaded with 20 mm ATP. Transport was initiated by adding 0.1 mm [3H]ATP. Results are the averages of four determinations (two experiments and two samples per experiment).
Mean value of the two selected populations C4-L and C4-H.
lsd (P ≤ 0.05) for comparing populations.
lsd (P ≤ 0.05) for comparing the source population C0 with the mean of C4-L and C4-H.
ns, Not significant.
DISCUSSION
Effect of Temperature
The two growth temperatures utilized in this study (25°C versus 14°C, constant) led to different effects on the physiological properties of the mitochondria of the investigated materials. In particular, mitochondria isolated from seedlings grown at the temperature near the lower limit of growth (14°C) showed, compared with mitochondria isolated from seedlings grown at the more favorable temperature (25°C): (a) a higher unsaturation degree, (b) higher values of DPH polarization over a wide range of assayed temperatures, (c) a lower activity of the inner membrane complex COX, (d) higher activities of the enzymes mtPOX and CPX, (e) a higher presence of TBARS, and (f) a lower rate of reconstituted ATP transport.
Stewart et al. (1990a) grew seedlings of the B73 inbred line at 14°C and at 30°C, and found that at the lower temperature mitochondria showed a higher capacity of alternative oxidase activity, which resulted in elevated respiration rates. The authors' hypothesis was that a higher respiration rate prevents the accumulation of a toxic metabolite, and that the alternative pathway functions in that respiration. A subsequent study by Stewart et al. (1990b) conducted on genotypes showing different levels of chilling tolerance indicated that the ability of maize seedlings to grow at temperatures near the lower limit is influenced by a number of genetic determinants and not only by the alternative oxidase activity.
Genetic Aspects of the Associated Responses to Selection
The present study on chilling tolerance used maize populations obtained by a divergent-recurrent selection procedure for tolerance to germination at low temperature. Results indicated that the associated changes for several physiological properties of the mitochondria were obtained. The size of such associated changes was for most properties reduced or even negligible at 25°C, but much greater at 14°C. At the lower temperature of growth, the two selected populations showed mean values beyond those of the two parental lines. This transgression was always consistent in that C4-H exceeded IABO78 (the parental line showing greater chilling tolerance), as did C4-L compared with B73. We can account for these transgressions by assuming that the traits in question are controlled by more than one gene and that the favorable alleles are to some extent dispersed in the two parents. On the other hand, the hypothesis that the less-tolerant parental line, B73, is homozygous for favorable alleles at some loci is not surprising, because in field trials (Mock and McNeill, 1979) and laboratory studies (Stewart et al., 1990a, 1990b) the line demonstrated a high level of chilling tolerance.
Changes associated with recurrent selection can be ascribed to random genetic drift, linkage, and/or pleiotropy (Falconer, 1981). For each cycle of the recurrent selection, 15 families were selected upward and 15 downward (Landi et al., 1992), so that some genetic drift may have occurred due to the reduction of the effective population size. Changes caused by genetic drift are expected to be erratic, however. But the observed changes followed consistent trends, as revealed by the comparisons involving the three populations and the two parental lines. Also, linkage could have contributed to the associated changes because the source was an F2 population (i.e. a population with a high level of linkage disequilibrium). However, C4-H and C4-L were developed after four cycles of recurrent selection, a procedure in which materials are selected and then intermated before starting the subsequent selection cycle. Because intermating allows recombination among linked loci, we can assume that the linkage contribution was limited mainly in the first selection cycles and for those genes tightly linked. In all likelihood, and given both the consistency and the size of the associated changes, pleiotropy should have played a basic role in the achievement of such changes. This would imply that the recurrent selection for a high or low level of chilling tolerance at germination changed the allelic frequencies at genes that, either directly or indirectly, also affect mitochondrial characteristics of seedlings grown at a temperature near the lower limit (14°C).
For several traits C0 was intermediate between the two selected populations (symmetry of response), indicating that the associated changes were consistent (similar in absolute value) in both upward and downward directions. Also, in the recurrent selection for tolerance to germination at 9.5°C conducted by Landi et al. (1992), the direct response (i.e. for the trait under selection) was symmetric. According to Falconer (1981), and given the material used as a source population (an F2 population with equal allelic frequencies at segregating genes), the prevalence of symmetric responses could be due to the fact that selection acted on genes showing important additive effects.
Involvement of Fatty Acid Composition and Membrane Fluidity
The selected populations differed markedly in the fatty acid composition of mitochondrial inner membrane and DPH fluorescence polarization when grown at 14°C. Compared with C4-L, C4-H showed a higher concentration of 18-carbon unsaturated fatty acids and, therefore, a higher ratio between unsaturated and saturated 18-carbon fatty acids. It is worth noting that 18-carbon fatty acids represented the most abundant component of unsaturated fatty acids. Moreover, C4-H showed lower values of DPH polarization and consequently higher fluidity in the whole range of temperatures used in the assay. The term “fluidity” (the reciprocal of viscosity) is used loosely to describe the extent of disorder and molecular motion within a lipid bilayer (Cossins, 1994). This single term includes all of the very different dynamic characteristics of a lipid bilayer, such as lateral diffusion of molecules, molecular wobbling, and chain flexing (Murata and Los, 1997). A role of fluidity in plant cell membranes in the perception of low temperatures and the involvement of a membrane component in the subsequent signal transduction was also proposed by Murata and Los (1997).
Prasad (1996) suggested a possible involvement of the content of the unsaturated fatty acids in chilling tolerance of maize seedlings. Our data emphasize a role in chilling tolerance for the ratio of the level of 18-carbon unsaturated fatty acids to the level of 18-carbon saturated fatty acids, which is linked to changes in the fluidity of the membrane.
Involvement of COX Activity
When seedlings were grown at 14°C, the activity of COX was higher in C4-H than in C4-L. Moreover, this property was positively associated with the ratio between unsaturated and saturated 18-carbon species. The higher level of COX activity is probably due to higher mitochondrial membrane fluidity of C4-H compared with C4-L, at least in some specific microdomains. Trivedi et al. (1986) investigated the nature of the interactions between COX and fatty acid composition in phospholipids of the mitochondrial membranes in a double-fatty-acid mutant of Saccharomyces cerevisiae that was auxotrophic for both unsaturated and saturated fatty acids. The activity and level of COX changed when the unsaturated and saturated fatty acid content was altered by varying the fatty acid supplements in the growth medium. In particular, mitochondria whose membranes were characterized by enhanced levels of 18:1 and depressed levels of 18:0 fatty acids had the highest heme Cyt a + a3 content and also showed higher COX specific activity, whereas those membranes that contained 18:2 and increased 16:0 levels had the lowest heme Cyt a + a3 level and decreased activity of COX.
Prasad et al. (1994a, 1994b, 1995) and Prasad (1996, 1997) studied chilling tolerance of dark-grown seedlings of the susceptible G50 maize inbred line subjected or not to acclimation at 14°C and then chilled to 5°C. They observed a decline of the synthesis and activity of COX after acclimation and after chilling treatment. Therefore, our data on the COX activity of C4-H and C4-L are consistent with data concerning the mechanism of acclimation to chilling conditions studied by Prasad and coworkers. In addition we can hypothesize a relationship between fatty acid unsaturation in the mitochondrial membrane and COX activity, as Trivedi et al. (1986) found in yeast.
Effects on Peroxidase Activities and on the Accumulation of TBARS in Mitochondria
The exposure of seedlings to a temperature near the lower limit of growth (14°C) led to a higher activity of mtPOX in the C4-H than in the C4-L population. The fact that the antioxidant effect of this enzyme might also occur in whole seedlings was confirmed by the finding that the TBARS accumulation proved to be lower in C4-H than in C4-L. The accumulation of TBARS measures the level of short- and long-chain aldehydes in the membrane. This assay is considered by several authors (Oteiza and Bekara, 1993; Zhang et al., 1995; Prasad, 1996) as a general indication of lipid peroxidation, even if it cannot be taken as a strictly quantitative assay. The physiological need to maintain membrane fluidity at low temperatures is generally associated with increased lipid unsaturation (Guy, 1990; Nishida and Murata, 1996). The increased level of unsaturated fatty acid that we observed when maize seedlings were grown at 14°C provided a higher substrate concentration for peroxidation, a necessary price to be paid for the maintenance of membrane function. The cells of C4-H, however, counteracted this challenge by enhancing their antioxidant properties, thus keeping the peroxidation damage as low as possible. It is plausible that the cells of C4-H synthesized unsaturated fatty acids at a higher rate than the rate of their chemical modification.
To demonstrate that oxidative stress might be responsible for lipid peroxidation, seedlings of C4-H grown at 14°C were treated with 0.1 mm H2O2 for 4 h in the dark, and mitochondria were then isolated and purified. Treatment with H2O2 caused a small but significant increase in TBARS accumulation (about 8% on the average; results not shown), confirming that this assay method measures the effects of lipid peroxidation.
Our results on the mtPOX activities of C4-H and C4-L are consistent with the hypothesis of Prasad et al. (1994b) and Zhang et al. (1995), suggesting that tolerance to chilling temperature requires the scavenging of H2O2 (the enhanced production of which is related to lowered COX synthesis and activity) by mtPOX. As a consequence, the level of lipid peroxidation in the mitochondrial membranes of the tolerant population (C4-H) is lowered.
Involvement of ANT Activity
Seedlings of the selected population C4-H grown at 14°C showed a greater specific activity of reconstituted ATP transport than those of C4-L when the assay was performed at both 25°C and 14°C. The lesser effect of chilling temperatures on ANT activity of C4-H may be due to the protection of the enhanced mtPOX activity, which lowered peroxidation of unsaturated fatty acids.
Metabolite transfer by carrier protein in the inner membrane of animal mitochondria proved to be strongly dependent on fatty acid changes in specific phospholipid molecules tightly bound with carrier molecules (cardiolipin) (Hoch, 1992; Paradies et al., 1992, 1994; Petit et al., 1994; Brustovetsky and Klingenberg, 1996). The induction of lipid peroxidation in rat heart mitochondria preferentially affected ANT protein (Zwizininsky and Schmid, 1992). The peroxidative modification was found to be a small increase in its apparent molecular mass, up to 1.2 kD (Girón-Calle et al., 1994), which occurred under relatively mild peroxidative conditions similar to those observed in the present study in maize.
The active center of maize ANT has not yet been characterized; we know only that the purified maize ANT1 protein can transport ATP, ADP, and also GTP, GDP, and deoxy-ATP (Genchi et al., 1996). Thus, its active site is similar but not identical to the ANT protein of rat heart mitochondria. Further investigations into the peroxidative modification of specific phospholipids connected with the active center of maize ANT protein might clarify the mechanism by which the decrease in ANT activity is related to chilling sensitivity in maize seedlings.
CONCLUSIONS
The application of divergent-recurrent selection procedures to the maize population herein investigated using germination percentage at 9.5°C as the selective trait affected allelic frequencies of genes controlling mitochondrial functions connected with chilling tolerance. The final two selected populations showed transgressive segregation in that they were beyond the parental boundaries, indicating that such mitochondrial functions are quantitatively inherited. These populations allowed us to investigate the cause-effect relationship between functions related to chilling tolerance. In particular, our data demonstrated that the content of 18:1 and 18:3 unsaturated fatty acids in the inner mitochondrial membrane can control the level of activity of COX at temperatures near the lower limit of growth. Moreover, the transport of ATP by the transmembrane ANT protein was positively associated with 18-carbon unsaturated fatty acid level and with mtPOX activity, suggesting that ANT activity is also affected by chilling.
The information that this study provides can be useful for breeding purposes; some of the mitochondrial properties of seedlings grown at 14°C (such as the 18:1[9] and the 18:1[11] contents or the level of fluidity of the inner membrane) could help identify suitable parental lines for the development of chilling-tolerant plants.
ACKNOWLEDGMENTS
We are grateful to Prof. B.A. Melandri (University of Bologna) and to Prof. J.A. Olson (Iowa State University, Ames) for critical reading of the manuscript.
Abbreviations:
- ANT
adenine nucleotide translocator
- COX
Cyt c oxidase
- CPX
Cyt c peroxidase
- DPH
1,6-diphenyl-1,3,5-hexatriene
- mtPOX
mitochondrial guaiacol peroxidase
- ROS
reactive oxygen species
- TBARS
thiobarbituric acid-reactive species
Footnotes
This research was supported by the Italian Ministry of University and Scientific Research (National Research Unit: Biology of Differentiation and Development of Plants), by the National Research Council of Italy, and by the University of Bologna.
LITERATURE CITED
- Anderson MC, Prasad TK, Martin BA, Stewart CS. Differential gene expression in chilling-acclimated maize seedlings and evidence for the involvement of abscisic acid in chilling tolerance. Plant Physiol. 1994;105:331–339. doi: 10.1104/pp.105.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MC, Prasad TK, Stewart CS. Changes in isozyme profiles of catalase, peroxidase, and glutatione reductase during acclimation to chilling in mesocotyls of maize seedlings. Plant Physiol. 1995;109:1247–1257. doi: 10.1104/pp.109.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin OPH. Differentiation between yeast species, and strains within a species, by cellular fatty acid analysis. 2. Saccharomyces cerevisiae. S Afr J Enol Vitic. 1989;10:8–15. [Google Scholar]
- Blum A (1988) Cold resistance. In Plant Breeding for Stress Environments. CRC Press, Boca Raton, FL, pp 99–127
- Brustovetsky N, Klingenberg M. Mitochondrial ADP/ATP carrier can be reversibly converted into a large channel by Ca2+ Biochemistry. 1996;35:8483–8488. doi: 10.1021/bi960833v. [DOI] [PubMed] [Google Scholar]
- Chance B, Maehly AC. Assay of catalase and peroxidases. Methods Enzymol. 1955;2:764–775. [Google Scholar]
- Cossins AR (1994) Homeoviscous adaptation of biological membranes and its functional significance. In AR Cossins, ed, Temperature Adaptation of Biological Membranes. Portland Press, London, pp 63–76
- Douce R, Christensen EL, Bonner WD., Jr Preparation of intact plant mitochondria. Biochim Biophys Acta. 1972;275:148–160. doi: 10.1016/0005-2728(72)90035-7. [DOI] [PubMed] [Google Scholar]
- Douce R, Mannella CA, Bonner WD., Jr The external NADH dehydrogenases of plant mitochondria. Biochim Biophys Acta. 1973;292:105–116. doi: 10.1016/0005-2728(73)90255-7. [DOI] [PubMed] [Google Scholar]
- Falconer DS (1981) Correlated characters. In Introduction to Quantitative Genetics. Longman Inc., New York, pp 190–194, 286–290
- Folch J, Lees M, Sloane-Stanley GH. A simple method for isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Ford RC, Barber J. The use of diphenylhexatriene to monitor the fluidity of the thylakoid membrane. Photobiochem Photobiophys. 1980;1:263–270. [Google Scholar]
- Genchi G, Ponzone C, Bisaccia F, De Santis A, Stefanizzi L, Palmieri F. Isolation and characterization of reconstitutively active ATP/ADP carrier from maize mitochondria. Plant Physiol. 1996;112:845–851. doi: 10.1104/pp.112.2.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girón-Calle J, Zwizinski CW, Schmid HHO. Peroxidative damage to cardiac mitochondria. II. Immunological analysis of modified adenine nucleotide translocase. Arch Biochem Biophys. 1994;315:1–7. doi: 10.1006/abbi.1994.1463. [DOI] [PubMed] [Google Scholar]
- Graham D, Patterson BD. Responses of plants to low, nonfreezing temperatures: proteins, metabolism, and acclimation. Annu Rev Plant Physiol. 1982;33:347–372. [Google Scholar]
- Guy CL. Cold acclimation and freezing stress tolerance: role of protein metabolism. Annu Rev Plant Physiol Plant Mol Biol. 1990;41:187–223. [Google Scholar]
- Hoch FL. Cardiolipins and biomembrane functions. Biochim Biophys Acta. 1992;1113:71–133. doi: 10.1016/0304-4157(92)90035-9. [DOI] [PubMed] [Google Scholar]
- Hodges DM, Andrews CJ, Johnson DA, Hamilton RI. Antioxidant enzyme responses to chilling stress in differentially sensitive inbred maize lines. J Exp Bot. 1997a;48:1105–1113. [Google Scholar]
- Hodges DM, Andrews CJ, Jonhson DA, Hamilton RI. Antioxidant enzyme and compound responses to chilling stress and their combining abilities in differentially sensitive maize hybrids. Crop Sci. 1997b;37:857–863. [Google Scholar]
- Huq S, Palmer JM. Superoxide and hydrogen peroxide production in cyanide resistant Arum maculatum mitochondria. Plant Sci Lett. 1978;11:351–358. [Google Scholar]
- Kock JLF, Botes PJ, Erasmus SC, Lategan PM. A rapid method to differentiate between four species of the Endomycetaceae. J Gen Microbiol. 1985;131:3393–3396. [Google Scholar]
- Landi P, Frascaroli E, Lovato A. Divergent full-sib recurrent selection for germination at low temperature in a maize population. Euphytica. 1992;64:21–29. [Google Scholar]
- Levitt J (1980) Chilling injury and resistance. In TT Kozlowsky, ed, Chilling, Freezing, and High Temperature Stresses: Responses of Plant to Environmental Stresses, Vol 1. Academic Press, New York, pp 23–64
- Li J, Zhang J, Cui S, Wei J. The relationship among membrane lipids, membrane linked enzymes, and maize cold resistance. Acta Agric Sin (in Chinese with English abstract) 1992;7:50–53. [Google Scholar]
- Maryam B, Jones DA. The genetics of maize (Zea mays L.) growing at low temperatures. I. Germination of inbred lines and their F1s. Euphytica. 1983;32:535–542. [Google Scholar]
- Miedema P (1982) The effect of low temperature on Zea mays. In NC Brady, ed, Advances in Agronomy. Academic Press, New York, pp 93–128
- Mock JJ, McNeill MJ. Cold tolerance of maize inbred lines adapted to various latitudes in North America. Crop Sci. 1979;19:239–242. [Google Scholar]
- Murata N, Los DA. Membrane fluidity and temperature perception. Plant Physiol. 1997;115:875–879. doi: 10.1104/pp.115.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuburger M. Preparation of plant mitochondria. In: Douce R, Day DA, editors. Encyclopedia of Plant Physiology. New Series, Vol 18. New York: Springer-Verlag; 1985. pp. 1–24. [Google Scholar]
- Nishida I, Murata N. Chilling sensitivity in plants and cyanobacteria: the crucial contribution of membrane lipids. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:541–568. doi: 10.1146/annurev.arplant.47.1.541. [DOI] [PubMed] [Google Scholar]
- Oteiza PI, Bechara EJH. 5-Aminolevulinic acid induces lipid peroxidation in cardiolipin-rich liposomes. Arch Biochem Biophys. 1993;305:282–287. doi: 10.1006/abbi.1993.1424. [DOI] [PubMed] [Google Scholar]
- Palmieri F, Indiveri C, Bisaccia F, Iacobazzi V. Mitochondrial metabolite carrier proteins: purification, reconstitution and transport studies. Methods Enzymol. 1995;260:349–369. doi: 10.1016/0076-6879(95)60150-3. [DOI] [PubMed] [Google Scholar]
- Paradies G, Ruggiero FM, Gadaleta MN, Quagliariello E. The effect of aging and acetyl-l-carnitine on the activity of the phosphate carrier and on phospholipid composition in rat heart mitochondria. Biochim Biophys Acta. 1992;1103:324–326. doi: 10.1016/0005-2736(92)90103-s. [DOI] [PubMed] [Google Scholar]
- Paradies G, Ruggiero FM, Petrosillo G, Gadaleta MN, Quagliariello E. Effect of aging and acetyl-l-carnitine on the activity of cytochrome oxidase and adenine nucleotide translocase in rat heart mitochondria. FEBS Lett. 1994;350:213–215. doi: 10.1016/0014-5793(94)00763-2. [DOI] [PubMed] [Google Scholar]
- Petit JM, Huet O, Gallet PF, Maftah A, Ratinaud MH, Julien R. Direct analysis and significance of cardiolipin transverse distribution in mitochondrial inner membranes. Eur J Biochem. 1994;220:871–879. doi: 10.1111/j.1432-1033.1994.tb18690.x. [DOI] [PubMed] [Google Scholar]
- Prasad TK. Mechanism of chilling-induced oxidative stress injury and tolerance in developing maize seedlings: changes in antioxidant system, oxidation of proteins and lipids, and protease activities. Plant J. 1996;10:1017–1026. [Google Scholar]
- Prasad TK. Role of catalase in inducing chilling tolerance in pre-emergent maize seedlings. Plant Physiol. 1997;114:1369–1376. doi: 10.1104/pp.114.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad TK, Anderson MD, Martin BA, Stewart CR. Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell. 1994a;6:65–74. doi: 10.1105/tpc.6.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad TK, Anderson MD, Stewart CR. Acclimation, hydrogen peroxide, and abscisic acid protect mitochondria against irreversible chilling injury in maize seedlings. Plant Physiol. 1994b;105:619–627. doi: 10.1104/pp.105.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad TK, Anderson MD, Stewart CR. Localization and characterization of peroxidases in the mitochondria of chilling-acclimated maize seedlings. Plant Physiol. 1995;108:1597–1605. doi: 10.1104/pp.108.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail PH. Plant cell fractionation. Annu Rev Plant Physiol. 1979;30:425–484. [Google Scholar]
- Rich PR, Boveris A, Bonner WD, Moore AL. Hydrogen peroxide generation by the alternate oxidase of higher plants. Biochem Biophys Res Commun. 1976;71:695–703. doi: 10.1016/0006-291x(76)90887-1. [DOI] [PubMed] [Google Scholar]
- Schünemann D, Borchert S, Flügge UI, Heldt HW. ADP/ATP translocator from pea root plastids. Comparison with translocators from spinach chloroplasts and pea leaf mitochondria. Plant Physiol. 1993;103:131–137. doi: 10.1104/pp.103.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinitzky M, Barenholz Y. Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim Biophys Acta. 1978;515:367–394. doi: 10.1016/0304-4157(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Stewart CF, Martin BA, Reding L, Cerwick S. Respiration and alternative oxidase in corn seedling tissues during germination at different temperatures. Plant Physiol. 1990a;92:755–760. doi: 10.1104/pp.92.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CF, Martin BA, Reding L, Cerwick S. Seedling growth, mitochondrial characteristics, and alternative respiratory capacity of corn genotypes differing in cold tolerance. Plant Physiol. 1990b;92:761–766. doi: 10.1104/pp.92.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi A, Fantin DJ, Tustanoff ER. Role of phospholipid fatty acids on the kinetics of high and low affinity sites of cytochrome c oxidase. Biochem Cell Biol. 1986;64:1195–1210. doi: 10.1139/o86-157. [DOI] [PubMed] [Google Scholar]
- van Gelder BF. On cytochrome c oxidase. I. The extinction coefficient of cytochrome a and cytochrome a3. Biochim Biophys Acta. 1966;118:36–46. doi: 10.1016/s0926-6593(66)80142-x. [DOI] [PubMed] [Google Scholar]
- Venturoli G, Fernández-Velasco JG, Crofts AR, Melandri BA. Demonstration of a collisional interaction of ubiquinol with the ubiquinol-cytochrome c2 oxidoreductase complex in chromatophores from Rhodobacter sphaeroides. Biochim Biophys Acta. 1986;851:340–352. doi: 10.1016/0005-2728(86)90070-8. [DOI] [PubMed] [Google Scholar]
- Verduyn C, Giuseppin MLF, Scheffers WA, van Dijiken JP. Hydrogen peroxide metabolism in yeasts. Appl Environ Microbiol. 1988;54:2086–2090. doi: 10.1128/aem.54.8.2086-2090.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannoni C. A theory of fluorescence depolarization in membranes. Mol Physiol. 1981;42:1303–1320. [Google Scholar]
- Zhang J, Cui S, Li J, Wei J, Kirkham MB. Protoplasmic factors, antioxidant responses, and chilling resistance in maize. Plant Physiol Biochem. 1995;33:567–575. [Google Scholar]
- Zwizinski CW, Schmid HHO. Peroxidative damage to cardiac mitochondria: identification and purification of modified adenine nucleotide translocase. Arch Biochem Biophys. 1992;294:178–183. doi: 10.1016/0003-9861(92)90154-o. [DOI] [PubMed] [Google Scholar]