Abstract
INTRODUCTION
Gastro Intestinal Stromal Tumor (GIST) is the most common mesenchymal tumor of the gastrointestinal tract (GI). GIST that arises primarily outside the GI tract is termed Extragastrointestinal Stromal Tumor (EGIST). To the best of our knowledge, few cases of EGIST in the abdominal wall were reported.
PRESENTATION OF CASE
We present a rare case of EGIST in the abdominal wall of a 57 year-old female patient. The asymptomatic tumor was located in the superior aspect of the left rectus abdominis muscle, measured 5.4 × cm 5.3 × cm 6.9 cm and was well circumscribed. Histological examination showed an epithelioid cell morphology. The mitotic count was 7/50 HPFs. Immunohistochemistry showed diffuse strong CD117 positivity, focal positivity for S100. The tumor was excised and the margins were free of malignancy. The patient was doing well postoperatively and was discharged on STI-571 regimen.
DISCUSSION
Although GIST is the most common mesenchymal tumor of the gastrointestinal tract, a case with EGIST in the abdominal wall is rare. Positive immunohistochemical staining for CD117 is a defining feature of GISTs. A great percentage of EGISTs represent a metastasis from a primary GIST. In our case, the clinical and diagnostic work-up have been proved it to be an EGIST.
CONCLUSION
The existing data on EGIST is insufficient to make a final conclusion regarding the malignant potential and clinicopathological factors of EGISTs that determine patient prognosis. Thus a follow-up for a long period is required. EGISTs should be kept in mind in the differential diagnosis for patients presenting with solid mass of the abdominal wall.
Keywords: GIST, EGIST, Abdominal wall mass, CD117
1. Introduction
Gastrointestinal Stromal Tumors (GISTs) are a subset of GI mesenchymal tumors of varying differentiations. Previously, these tumors were classified as GI leiomyomas, leiomyosarcomas, leioblastomas and schwannomas as a result of their histological findings and apparent origin in the muscularis propria layer of the intestinal wall. However, GISTs now are recognized as a distinct group of mesenchymal tumors due to its immunohistochemical and ultra structural characteristics. GISTs express c-kit protein also known as CD117, which is considered as a highly specific marker that differentiates it from other mesenchymal tumors such as leiomyomas.1
Extragastrointestinal Stromal Tumors (EGISTs) are a rarely reported group of tumors2 that arise outside the GI but histologically resemble their GI counterpart. Approximately 80% are located in the omentum or mesentery, and the remainder develops in the retro peritoneum.3–5 To the best of our knowledge, a few cases were reported with an EGIST in the abdominal wall.6
2. Case report
A 57 year old female with no significant past medical or surgical history presented to the general surgery clinic chiefly complaining of painless abdominal mass which had gradually increased in size over a period of 3 years. Besides the increase in size, she reported no other particular symptoms associated with the mass. The abdominal examination revealed a firm, non-pulsatile, and smooth mass that was fixed in the epigastric region.
As the mass was felt to be not simply subcutaneous, a computerized tomography (CT) scan was obtained, which revealed a well defined, lobulated, heterogeneously enhanced soft tissue mass with flecks of calcification and necrosis originating from the superior aspect of left rectus abdominis muscle, measuring (5.4 × cm 5.3 × cm 6.9 cm). Although the mass was shown to cause local indentation onto the left liver lobe, there was no evidence of invasion or underlying peritoneal infiltration (Fig. 1). Our preoperative differential diagnosis included desmoid tumor and soft tissue sarcoma.
Fig. 1.
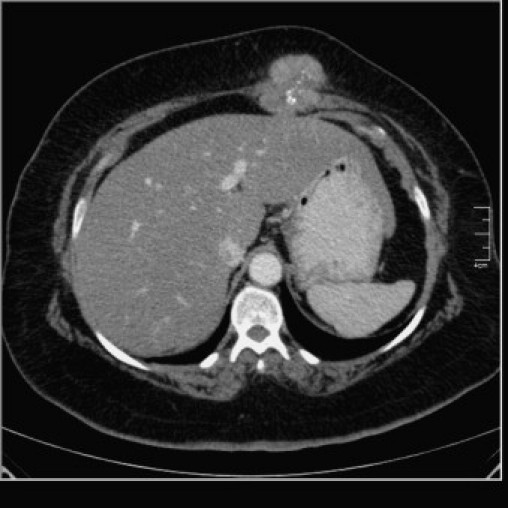
Axial enhanced CT scan of abdomen showing heterogeneously enhancing lobulated soft tissue mass with flecks of calcification and necrosis originating from the superior aspect of left rectus abdominis muscle and bulging posteriorly, causing indentation on left liver lobe.
Routine blood test results were unremarkable. Chest X-ray showed no lung parenchyma or bone lesion, CT scan of the abdomen showed no evidence of metastasis, liver lesion or presence of other masses. The esophagogastroduodenoscopy (EGD) was performed postoperatively and showed that esophagus, stomach, pylorus, duodenal bulb, and the duodenum to the third portion were normal. Fine Needle Aspiration (FNA) was of low yield in the pre operative diagnosis. Excision was done through a 4 cm incision placed over the mass and revealed a large, white tan, rubbery mass not attached to intraperitoneal structures (Fig. 2). The margins were sent for frozen section and came back as negative for malignancy and recovery was uneventful.
Fig. 2.
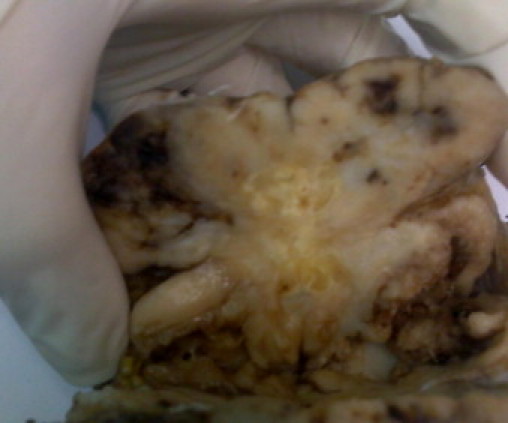
Gross specimen showing lobulated soft tissue mass containing flecks of calcification.
Histopathological examination of the specimen showed a well circumscribed lobulated mass with surrounding fibrous capsule and pushing borders made up of proliferation of epitheliod cells. The mass showed perivascular cohesion with loss of cohesiveness away from the blood vessels. There were cells shaped round to polygonal with variably eosinophilic and clear cytoplasm and vesicular chromatin and some prominent nucleoli. Average mitotic count was that of 7 mitoses per 50 high power fields (HPFs).
Immunohistochemical analysis showed diffusely strong CD117 positivity for both cytoplasmic and membranous components (Fig. 3) and focal positivity for S100, while it was completely negative for Vimentin (which might be negative in 5% of cases with GISTs), cytokeratin, CD34 (which can be observed in 25% of cases with GISTs), neuron specific enolase (NSE), CD99, desmin, and myogenine, so the histopathological diagnosis was that of epithelioid GIST. Molecular genetic analysis for c-KIT was not available at our institution with limited resources.
Fig. 3.
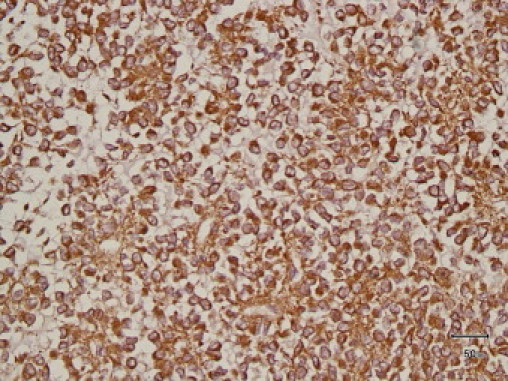
Immunohistochemical analysis showed diffuse strong CD117 positivity for both cytoplasmic and membranous components.
Post operatively, the patient was doing well and discharged on STI-571 regimen. After 5 months of follow-up, no recurrence or metastasis was detected by clinical or radiological exams.
3. Discussion
Gastrointestinal Stromal Tumors (GISTs) are the most common mesenchymal tumors of the GI tract,7 many of which are identified in the fifth or sixth decades.8 They can occur at any site along the GI tract but mainly occur in the stomach or small intestine, with a smaller percentage in other locations.9 The pathogenesis is a gain of function mutations in the c-KIT gene which results in over expression of the KIT receptor also called CD117 or stem cell factor receptor. The counterpart that arises outside the gastrointestinal tract was termed as Extragastrointestinal Stromal Tumor (EGIST) by Reith et al.10 The majority of EGISTs (14 cases) that were re-classified by Agaimy and Wünsch was found to be either GISTs with extensive extramural growth resulting in loss of contact to the external muscle coat of the gut (8/14) or as a metastasis from an inoperable GIST (2/14) or from a previously resected deceptively benign tumor (1/14). Thus, a great percentage of EGISTs appeared to be due to metastasis from a primary GIST.11 In our case, the diagnostic workups, including CT scans and endoscopies proved that the mass was likely to be a genuine EGIST. In addition, other findings suggestive of EGIST were the features of immunohistochemical staining, and the large size of the asymptomatic tumor.
EGISTs were also reported in pleura, omentum, mesentry, pancreas and abdominal wall. Thalheimer et al. reported an EGIST of abdominal wall in a 40-year-old patient with 24 cm-sized tumor with low mitotic index (1/50 HPFs), in which tumor cells showed positivity for CD117, CD99, CD34, Vimentin, and actin.12
The diagnosis of GIST was established based on the morphology and immunophenotyping. Histologically, there are three types of GISTs: spindle celled (70%), epithelioid (20%) and mixed.13 The tumor in our case showed epithelioid type histology and was immunopositive for c-KIT. Approximately 95% of GISTs carry an activating somatic mutation of CD117.11 CD117 is the product of proto-onco-gene c-KIT, a tyrosine kinase transmembrane receptor located on chromosome 4 (4q11-q12).15 Positive immunohistochemical staining for CD117 is a defining feature of GISTs14; however there are GISTs that have a mutation of PDGFRA instead of c-kit and therefore they do not show the characteristic CD117 positive immunostaining. C-kit positive tumors are most responsive to the treatment with c-kit selective tyrosine kinase inhibitor, STI-571; some reports support a therapeutic trial of STI-571 for all GIST patients regardless of CD117 expression.14
Other neoplasms, in which CD117 expression is detected, such as melanoma and others,16 can be differentiated by its characteristic morphology with immunohistochemical analysis. This case has negative smooth muscle and neuronal markers with total negativity for CD34 and Vimentin, resulting in exclusion of most of these differential diagnoses. However, with a simple immunohistochemical method optimized for clinical use, desmoid can be regarded as a c-Kit-negative tumor.17 In our case, desmoid tumor diagnosis was excluded due to the epithelioid morphology and the pushing nature of borders as well as the pattern of CD117 immunostaining as it cytoplasmic and membranous, while desmoid tumors express cytoplasmic positivity only. Also desmoid tumor has CD117 positivity either focal or diffuse typically expressed more weakly than in GIST,18 in addition desmoid tumors are characterized by a spatially homogeneous proliferation of wavy spindle cells without atypia, associated with collagen deposition (often of the keloidal type), and an infiltrative border rather than pushing borders as in our case.19
The National Institute of Health (NIH) developed a new classification system for risk of malignant behavior in 2001, which ranged from very low risk to high risk and was based on tumor size and mitotic count.16 In general, tumors larger than 5 cm, with more than 5 mitoses per 50 HPFs are considered to be high risk. In our case, the tumor size was (5.4 × cm 5.3 × cm 6.9 cm), with mitotic count of 7 per 50 HPFs, so it was considered a high risk tumor.
4. Conclusion
The existing data on EGIST is insufficient to make a final conclusion regarding treatment, prognosis and recurrence. Thus follow-up for a long period of time is required. Also EGISTs should be kept in mind for the differential diagnosis in patients presenting with solid mass of the abdominal wall.
Conflict of interest statement
None declared.
Funding
None.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this Journal.
Author contributions
Loiy Alkhatib, and Kamal Gharaibeh participated in the care of the patient. Loiy Alkhatib performed the literature review and drafted the manuscript. Omar Albtoush, Nesreen Bataineh, Ismail Matalka, Yasuharu Tokuda assisted in the review of the literature and in revising the manuscript. All authors read and approved the final manuscript.
Contributor Information
Loiy Alkhatib, Email: Lkhatib@hotmail.com.
Omar Albtoush, Email: O.Albtoush@yahoo.com, omar83_99@yahoo.com.
Nesreen Bataineh, Email: Nisreenbataineh@yahoo.com.
Kamal Gharaibeh, Email: K_gharaibeh@yahoo.com.
Ismail Matalka, Email: Imatalka@just.edu.jo.
Yasuharu Tokuda, Email: Yasuharu.tokuda@gmail.com.
References
- 1.Tateishi U., Hasegawa T., Satake M., Moriyama N. Gastrointestinal stromal tumor: correlation of computed tomography findings with tumor grade and mortality. J Comput Assist Tomogr. 2003;27:792–798. doi: 10.1097/00004728-200309000-00018. [DOI] [PubMed] [Google Scholar]
- 2.Carlomagno G., Beneduce P. A gastrointestinal stromal tumor masquerading as an ovarian mass. World J Surg Oncol. 2004;2(15) doi: 10.1186/1477-7819-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamamoto H., Oda Y., Kawaguchi K., Nakamura N., Takahira T., Tamiya S. c-Kit and PDGFRA mutations in extragastrointestinal stromal tumor (gastrointestinal stromal tumor of the soft tissue) Am J Surg Pathol. 2004;28:479–488. doi: 10.1097/00000478-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Miettinen M., Monihan J.M., Sarlomo-Rikala M., Kovatich A.J., Carr N.J., Emory T.S. Gastrointestinal stromal tumors/smooth muscle tumors (GISTs) primary in the omentum and mesentery: clinicopathologic and immunohistochemical study of 26 cases. Am J Surg Pathol. 1999;23:1109–1118. doi: 10.1097/00000478-199909000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Gun B.D., Gun M.O., Karamanoglu Z. Primary stromal tumor of the omentum: report of a case. Surg Today. 2006;36:994–996. doi: 10.1007/s00595-004-3280-9. [DOI] [PubMed] [Google Scholar]
- 6.Thalheimer A., Meyer D., Gattenlöhner S., Timmermann W., Thiede A. Gastrointestinal stromal tumor of the abdominal wall. An unusual localization of a rare tumor. Chirurg. 2004;75:708–712. doi: 10.1007/s00104-003-0696-5. [DOI] [PubMed] [Google Scholar]
- 7.Rosai J. Stromal tumors (GISTs and related lesions) 9th edition. 2004. Surgical pathology. p. 674–680. [Google Scholar]
- 8.Li A., Lowery Nordberg M., Herrera G.A. Gastrointestinal stromal tumors: current concepts and controversies. Pathol Case Rev. 2002;7:226–233. [Google Scholar]
- 9.Ronnett B.M., Kurman R.J., Shmookler B.M., Sugarbaker P.H., Young R.H. The morphologic spectrum of ovarian metastases of appendiceal adenocarcinomas: a clinicopathologic and immunohistochemical analysis of tumors often misinterpreted as primary ovarian tumors or metastatic tumors from other gastrointestinal sites. Am J Surg Pathol. 1997;21:1144–1155. doi: 10.1097/00000478-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Reith J.D., Goldblum G.R., Lyles R.H., Weiss S.W. Extragastrointestinal (soft tissue) stromal tumors: an analysis of 48 cases with emphasis on histologic predictors of outcome. Mod Pathol. 2000;13:577–585. doi: 10.1038/modpathol.3880099. [DOI] [PubMed] [Google Scholar]
- 11.Agaimy A., Wünsch P. Gastrointestinal stromal tumours: a regular origin in the muscularis propria, but an extremely diverse gross presentation. Langenbecks Arch Surg. 2006;391(4):322–329. doi: 10.1007/s00423-005-0005-5. [DOI] [PubMed] [Google Scholar]
- 12.Thalheimer A., Meyer D., Gattenlöhner S., Timmermann W., Thiede A. Gastrointestinaler Stromatumor der Bauchwand. Der Chirurg. 2004;75(7):708–712. doi: 10.1007/s00104-003-0696-5. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher C.D., Berman J.J., Corless C., Gorstein F., Lasota J., Longley B.J. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol. 2002;10:81–89. doi: 10.1177/106689690201000201. [DOI] [PubMed] [Google Scholar]
- 14.Dorfman D.M., Bui M.M., Tubbs R.R., His E.D., Fitzgibbons P.L., Linden M.D. CD117 immunohistochemistry tissue microarray survey for quality assurance and interlaboratory comparison: a college of American pathologists cell markers committee study. Arch Pathol Lab Med. 2006;130:779–782. doi: 10.5858/2006-130-779-TCITMS. [DOI] [PubMed] [Google Scholar]
- 15.Blackstein M.E., Rankin B., Fletcher C., Heinrich M., Benjamin R., Mehren M.V. Clinical benefit of Imatinib in patients with metastatic GIST negative for the expression of CD117 in the S0033 trial. ASCO. J Clin Oncol. 2005;9010 [Google Scholar]
- 16.Raut C.P., Morgan J.A., Ashley S.W. Current issues in gastrointestinal stromal tumors: incidence, molecular biology, and contemporary treatment of localized and advanced disease. Curr Opin Gastroenterol. 2007;23:149–158. doi: 10.1097/MOG.0b013e32802086d0. [DOI] [PubMed] [Google Scholar]
- 17.Lucas D.R., Al-Abbadi M., Tabaczka P., Hamre M.R., Weaver D.W., Mott M.J. c-Kit expression in desmoid fibromatosis: comparative immunohistochemical evaluation of two commercial antibodies. Am J Clin Pathol. 2003;119:339–345. doi: 10.1309/an4e2etcj4r6jjuy. [DOI] [PubMed] [Google Scholar]
- 18.Montgomery E., Torbenson M.S., Kaushal M., Fisher C., Abraham S.C. Beta-catenin immunohistochemistry separates mesenteric fibromatosis from gastrointestinal stromal tumor and sclerosing mesenteritis. Am J Surg Pathol. 2002;26(October (10)):1296–1301. doi: 10.1097/00000478-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez J.A., Guarda L.A., Rosai J. Mesenteric fibromatosis with involvement of the gastrointestinal tract: a GIST simulator: a study of 25 cases. Am J Clin Pathol. 2004;121:93–98. doi: 10.1309/59VA-H0KV-F53W-B633. [DOI] [PubMed] [Google Scholar]


