The fluorescence intensity of single molecules can change dramatically even under constant laser excitation. The phenomenon is frequently called as ‘blinking,’ and involves molecules switching between high- and low-intensity states (1). In addition to spontaneous blinking, the fluorescence of some special fluorophores, such as cyanine dyes and photoactivatable fluorescent proteins, can be switched on and off, by choice, using a second laser. Recent single-molecule spectroscopy investigations have shed light on mechanisms of single-molecule blinking and photoswitching. This ability to controllably switch single molecules led to the invention of a novel fluorescence microscopy with nanometer spatial resolution well beyond the diffraction limit.
The technique to obtain beyond diffraction-limited images by single-molecule photoswitching is called both photoactivated localization microscopy (PALM) (2) and stochastic optical reconstruction microscopy (STORM) (3). Nanometer spatial resolution is achieved by precisely locating single molecules’ positions by Gaussian fitting a series of isolated single-molecule image spots (4). Typical fluorophores can provide enough signal intensity to reach a fitting precision of 1–20 nm. However, in most biological samples, fluorophores are densely packed rather than isolated single molecules. To resolve individual molecules, the photoswitching process is applied. In this process, only one fluorophore is switched on at a time within a diffraction limited area. After the ‘on’ fluorophore is imaged, it is switched off and another molecule is switched on. By repeating this cycle thousands of times, the locations of hundreds of thousands of molecules are determined and a nanometer resolution image can be constructed.
PALM is a revolution in optical imaging of biological samples as it achieves exceptional spatial resolution with relatively simple optics and lasers. Although many biological systems and cell structures have been imaged with nanometer resolution since its invention, PALM requires fluorophores that can be reliably switched between emissive and non-emissive states. Currently, the selection of such fluorophores is very limited (5) and the switching mechanism is unclear. This poor understanding hinders the development of new photoswitching fluorophores. To date, single-molecule switching has been almost exclusively studied by fluorescence spectroscopy. For example, for many years the blinking of cresyl violet single molecules was observed and attributed to interfacial electron transfer by the fluorescence experiments (6). However, to confirm this attribution, additional experiments other than the fluorescence spectroscopy need to be conducted. Recently, a combined single-molecule fluorescence and cyclic voltammetry study provided more evidence to support the electron transfer hypothesis (7). By ramping the electrochemical potential up and down in a spectroelectrochemistry cell, the fluorescence intensity of a single cresyl violet molecule was synchronously modulated. The observation suggests the following redox reaction: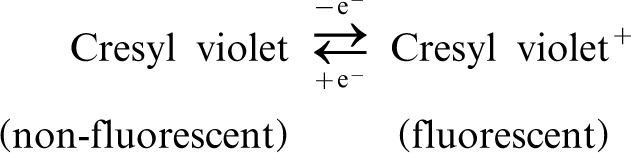 The redox potential of this single-molecule reaction was measured by cyclic voltammetry. The results support the electron transfer mechanism of cresyl violet switching.
The redox potential of this single-molecule reaction was measured by cyclic voltammetry. The results support the electron transfer mechanism of cresyl violet switching.
Reversible formation and breaking of conjugation is another important way to switch on and off molecular fluorescence. Recently, Zhuang's group discovered that the photoswitching of cyanine dye Cy5 is due to the adduction of a thiol group to the conjugated C=C bond resulting in conjugation breaking (8). In this work, the dark and bright species were isolated by chromatography and mass spectrometry. The conjugation breaking mechanism explains the importance of the thiol group in the photoswitching process and the unique photoswitching ability of Cy5.
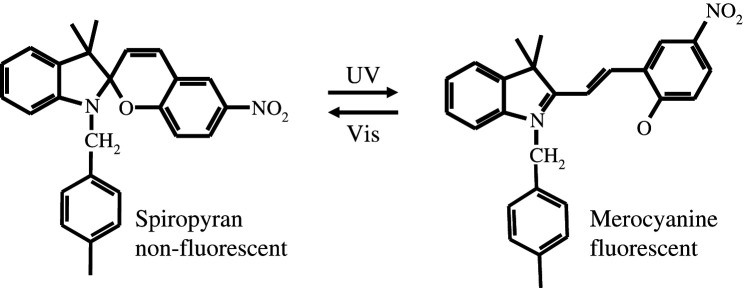
Besides thiol adduction, another way to form and break conjugation is through ring opening and closing (9, 10). Spiropyran has a reversible ring opening and closing photochemical reaction. Upon UV excitation, spiropyran converts to merocyanine containing a conjugation chain by ring opening (Scheme 1). The ring can be formed again by thermal activation or visible excitation. The photoswitching between non-fluorescent spiropyran and fluorescent merocyanine is useful for nanometer imaging because it is a uni-molecular reaction and less sensitive to oxygen than the Cy5-thiol reaction. Spiropyran/merocyanine showed excellent photoswitching properties and strong fluorescence when embedded into polymer nanoparticles. We recently demonstrated nanometer resolution imaging using spiropyran nanoparticles (10).
Scheme 1.
The reversible photochemical reaction between spiropyran and merocyanine
The above are two examples of how conjugation is formed and interrupted through photochemical reactions. Understanding photochemical reaction mechanism and molecular structural change opens the door for developing new photoswitchable fluorophores. Currently, the photoswitchable fluorophores suffer from either low brightness or slow switching efficiency. In the future, new fluorophores with improved brightness and switching speed will greatly enhance the nanometer resolution fluorescence microscopy.
Acknowledgements
The authors acknowledge the support from EMSL (a user facility of DOE-BER) and DOE-BES.
References
- 1.Jau T. Random on-off telegraphic signaling in single nanoparticles and molecules. Nano Rev. 2010;1:5031. doi: 10.3402/nano.v1i0.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313:1642–5. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- 3.Rust MJ, Bates M, Zhuang X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM) Nat Meth. 2006;3:793. doi: 10.1038/nmeth929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson RE, Larson DR, Webb WW. Precise nanometer localization analysis for individual fluorescent probes. Biophys J. 2002;82:2775–83. doi: 10.1016/S0006-3495(02)75618-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez-Suarez M, Ting AY. Fluorescent probes for super-resolution imaging in living cells. Nat Rev Mol Cell Biol. 2008;9:929–43. doi: 10.1038/nrm2531. [DOI] [PubMed] [Google Scholar]
- 6.Biju V, Micic M, Hu D, Lu HP. Intermittent single-molecule interfacial electron transfer dynamics. J Am Chem Soc. 2004;126:9374–81. doi: 10.1021/ja040057b. [DOI] [PubMed] [Google Scholar]
- 7.Lei C, Hu D, Ackerman EJ. Single-molecule fluorescence spectroelectrochemistry of cresyl violet. Chem Commun. 2008:5490–92. doi: 10.1039/b812161c. [DOI] [PubMed] [Google Scholar]
- 8.Dempsey GT, Bates M, Kowtoniuk WE, Liu DR, Tsien RY, Zhuang XW. Photoswitching mechanism of cyanine dyes. J Am Chem Soc. 2009;131:18192. doi: 10.1021/ja904588g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folling J, Belov V, Kunetsky R, Medda R, Schonle A, Egner A, et al. Photochromic rhodamines provide nanoscopy with optical sectioning. Angew Chem Int Ed. 2007;46:6266–70. doi: 10.1002/anie.200702167. [DOI] [PubMed] [Google Scholar]
- 10.Hu D, Tian Z, Wu W, Wan W, Li ADQ. Photoswitchable nanoparticles enable high-resolution cell imaging: the pulsar microscopy. J Am Chem Soc. 2008;130:15279. doi: 10.1021/ja805948u. [DOI] [PMC free article] [PubMed] [Google Scholar]