Abstract
Functional nanomaterials have recently attracted strong interest from the biology community, not only as potential drug delivery vehicles or diagnostic tools, but also as optical nanomaterials. This is illustrated by the explosion of publications in the field with more than 2,000 publications in the last 2 years (4,000 papers since 2000; from ISI Web of Knowledge, ‘nanoparticle and cell’ hit). Such a publication boom in this novel interdisciplinary field has resulted in papers of unequal standard, partly because it is challenging to assemble the required expertise in chemistry, physics, and biology in a single team. As an extreme example, several papers published in physical chemistry journals claim intracellular delivery of nanoparticles, but show pictures of cells that are, to the expert biologist, evidently dead (and therefore permeable). To attain proper cellular applications using nanomaterials, it is critical not only to achieve efficient delivery in healthy cells, but also to control the intracellular availability and the fate of the nanomaterial. This is still an open challenge that will only be met by innovative delivery methods combined with rigorous and quantitative characterization of the uptake and the fate of the nanoparticles. This review mainly focuses on gold nanoparticles and discusses the various approaches to nanoparticle delivery, including surface chemical modifications and several methods used to facilitate cellular uptake and endosomal escape. We will also review the main detection methods and how their optimum use can inform about intracellular localization, efficiency of delivery, and integrity of the surface capping.
Keywords: gold nanoparticles, cell delivery, bionanotechnology, nanomaterials, photothermal microscopy, cell imaging, intracellular fate
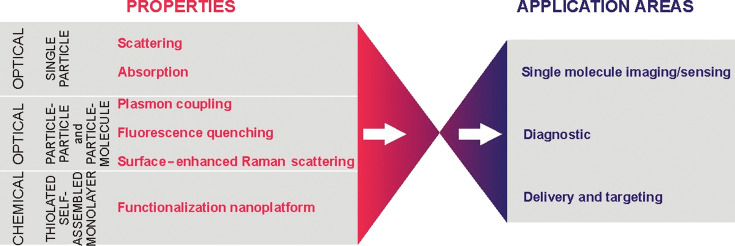
Gold nanoparticles have emerged as attractive nanomaterials for biological and biomedical applications because of their physical and chemical properties (1–13) (Fig. 1). The gold core is inert and essentially non-toxic to cells (14). The particles absorb and resonantly scatter visible and near-infrared light upon excitation of their surface plasmon oscillation. The plasmon resonance band can be tuned over a wide spectral range by changing intrinsic parameters such as the material (bi-metallic or hybrid particles), the size, or the shape (sphere, rod, cube, triangle, cage, etc.) (15). The light-scattering signal is intense and much brighter than chemical fluorophores and does not photobleach or blink (16). This constitutes an advantage for their applications in single molecule imaging, where the use of dyes, fluorophores, or quantum dots is limited by low signal intensities, complex blinking phenomena, and photobleaching. For particles below 30 nm, the absorption becomes dominant over scattering and can be used for detection by photothermal microscopy (17). Gold nanoparticles with varying core size are prepared by the reduction of gold salts in the presence of stabilizing agents to prevent nanoparticle agglomeration and control growth (18–22). Particle suspensions are also commercially available. Furthermore, gold nanoparticles can be easily functionalized by anchoring thiol linkers in their monolayers. A wide variety of functional bionanoconjugates has been obtained, including nanoparticles modified with peptides, proteins, antibodies, oligosaccharides, and nucleic acids (23–29) (for review, see References 6, 12). This allows the nanomaterials to act as multifunctional platforms for both therapeutic and diagnostic purposes (5, 30, 31).
Fig. 1.
Properties and potential applications of gold nanoparticles in biology and medicine.
The use of functionalized gold nanoparticles for biological and biomedical applications includes bio-imaging, single molecule tracking, biosensing, drug delivery, transfection, and diagnostic. For example, through proper functionalization, the particles can be engineered to accumulate preferentially in tumor cells using targeting ligands, providing a tool for cancer diagnosis and gene therapy (32). Sensor arrays have been developed to differentiate normal, cancerous, and metastatic cells using the fluorescence quenching properties of gold nanoparticles (30). The interactions and fate of a broad range of functionalized nanoparticles is currently under investigation in a wide diversity of biological models, ranging from whole organisms to tissues to cells in culture, and also to yeast (33) and prokaryote bacteria (34). The needs of intracellular delivery depend on the applications. For cancer cell targeting and killing, the requirement is proper cell recognition and uptake independently of the ultimate localization (e.g. vesicular localization is not a problem in this context). However, for intracellular imaging and sensing, cell recognition is less important, whereas the ultimate intracellular localization of the nanomaterial is crucial and needs to be fully addressed.
Here, we will focus on the delivery and detection of nanoparticles (with a particular emphasis on gold) into mammalian cells. Many biomedical applications will ultimately necessitate a targeted intracellular delivery and availability of the nanomaterial, not only to specific cells, but also to specific subcellular compartments. Currently, the main challenge is to avoid endosomal localization and to control the stability of the functional capping after cellular uptake. This review discusses how the physico-chemical properties of the particles affect cellular uptake and the current approaches under study for intracellular delivery and control of subcellular localization. We will review the different techniques used for gold nanoparticles imaging and quantification inside fixed and living cells and the possible methods to probe the integrity of the functional capping inside the cellular compartment.
Non-specific cellular uptake
The plasma membrane defines the separation between the interior of a cell and the outside environment. It is semi-permeable and allows free diffusion of small and non-polar molecules. However, bigger objects such as nanomaterials are incapable of crossing the plasma membrane and require uptake mechanisms such as endocytosis (for review, see References (35) and (36). Most gold nanoparticle bioconjugates are easily taken up by the cells through endocytotic mechanisms, but they remain trapped in endosomal vesicles and are incapable of reaching the cytosol. Although endocytotic uptake is the norm for a broad range of nanomaterials, its efficiency is dependent on the nanoparticle surface chemistry and the physical properties (size and shape) of the material.
Surface chemistry
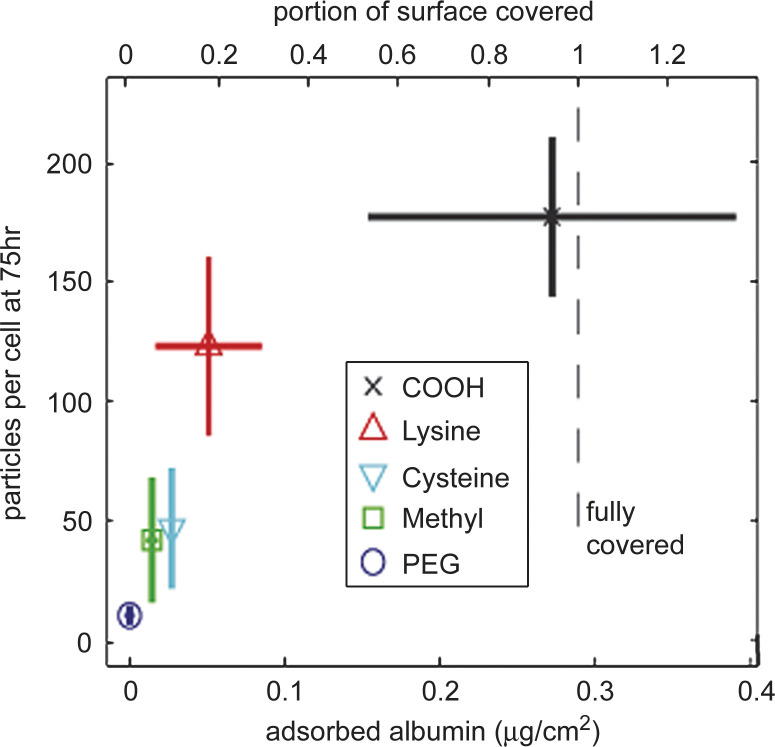
Incubation of nanomaterials with live cells implicates their exposure to cell culture medium often containing serum. There are evidences suggesting that non-specific interaction of serum proteins has a major effect on uptake. Increase in uptake after 12 h incubation in fetal calf serum (FCS) was observed for superparamagnetic iron oxide nanoparticles (SPIONs) covered with a silane layer (37). Significant difference of uptake was observed for different surface chemistry, such as layer terminated with COOH, NH2, and PEG, but this difference essentially vanished when the particles had been exposed to FCS for several hours (37). In another study, an excellent correlation was found between adsorption of albumin and uptake of a series of functionalized polystyrene particles (Fig. 2) (38). In addition, after removing selected components from the serum, the authors concluded that the uptake does not depend on the identity of proteins adsorbed to particle surfaces, but rather on the capacity of the surfaces to bind protein and they propose that an assessment of adsorptive capacity could be used to predict nanoparticle-cell interactions.
Fig. 2.
Uptake of functionalized polystyrene particles in HUVEC cells correlate with protein adsorption capacity (adapted from Reference 38). The lines indicate standard deviation from three independent experiments. Reproduced from Ehrenberg MS, Friedman AE, Finkelstein JN, Oberdorster G, McGrath JL. The influence of protein adsorption on nanoparticle association with cultured endothelial cells. Biomaterials 2009; 30: 603–10. Copyright 2009 with permission from Elsevier.
Using mixed monolayers containing oligonucleotides on 13 nm gold nanoparticles, Giljohan et al. observed a correlation between oligonucleotide loading and cell uptake. They also observed an increase in size after exposure to serum correlated with the amount of oligonucleotide and concluded that serum proteins play a key role in mediating nanoparticle-cell interaction and uptake of oligonucleotide-capped nanoparticles (39).
One way to prevent non-specific interactions of serum proteins with nanoparticles is surface functionalization with polyethylene glycol (PEG) (40). There is a consensus on the role of PEG to strongly reduce nanomaterial interaction with cells, thereby impairing their intracellular uptake. PEGylation of nanorods was used to reduce non-specific binding of herceptin-coated nanorods, leading to specific recognition of the cancer cell lines presenting the antigen (41). No significant uptake could be obtained for 10 nm gold nanoparticles decorated with PEG 5000, however an increase of more than a factor 10 was obtained by including a small proportion of a signaling peptide in the layer through ligand exchange (42). Nativo et al. found that uptake in HeLa cells of 16 nm nanoparticles prepared by the Turkevich-Frens method was completely stopped if these particles were protected by a ligand shell terminated with tetraethylene glycol (43). Even after prolonged incubation for 24 h or after a 10-fold increase in concentration, no particles were found inside the cells by transmission electron microscopy (TEM), and no gold was detected by atomic emission spectroscopy (AES) (43). Similarly, the uptake of large core-shell polymer beads was reduced by PEGylation (44).
However, two studies seem to contradict the apparent consensus on the effect of PEG. In the first study, Liu et al. reported high uptake of 4.7 nm PEGylated gold nanoparticles in CT26 cells and subsequent X-ray irradiation cell damage (42). One possible explanation resides in the fact that the PEG molecule used is not thiolated and the nanoparticles may therefore not be functionalized with PEG. In the second study, Gu et al. observed a very slow (plateau after ∼30 h) but significant uptake of 3–4 nm gold nanoparticles that had been functionalized with PEG in a two-step functionalization process (45). Even more surprisingly, the paper presents convincing evidence of localization of the nanoparticles in the nucleosomes. We suggest that the end-group of the particular PEG used (amino-propyl) may play a role, but a definite interpretation of this observation will require further investigation.
Surface structure
In 2004, Jackson et al. reported the synthesis of stripy nanoparticles based on scanning tunneling microscopy observation (46). In subsequent papers, a number of unusual properties were claimed for these new materials, including, most recently, their ability to penetrate the plasma membrane without disruption (47). Both the structure and the properties of these particles are, however, a matter of controversy (48).
Size and a few small complications
Size
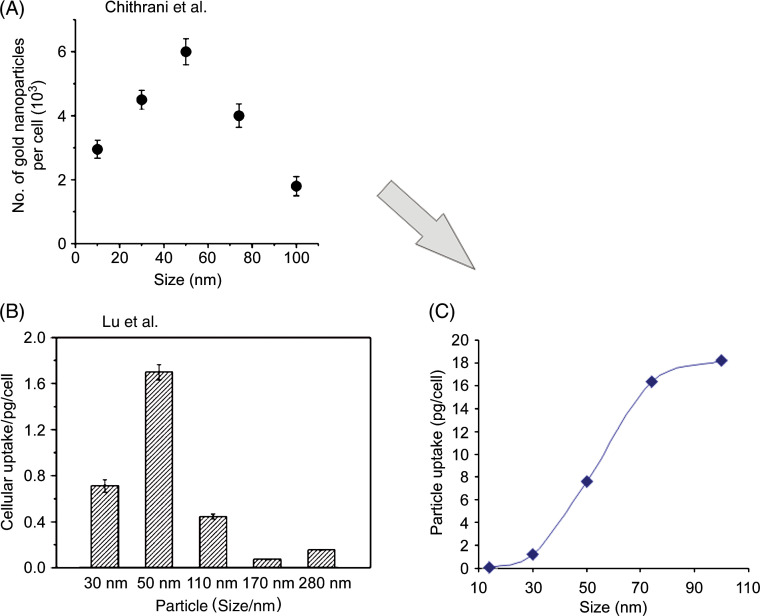
The synthesis of gold nanoparticles can be controlled to obtain particles in a large range of sizes. This has allowed Chithrani et al. to evaluate nanoparticle entry into HeLa cells for particles in the 14–100 nm size range (49). The authors found that maximum entry is observed for a 50-nm diameter sphere (Fig. 3A). More recently, building on progress in the size-controlled synthesis of silica, Lu et al. have measured in the same cell line how entry of spherical mesoporous silica beads vary in the 30–280 nm size range (50) (Fig. 3B). The latter paper concluded that their results are in agreement with Chithrani et al., but careful observation of the results indicates a more complicated picture. While in the first paper, uptake is measured in number of particles per cell, in the second, it is measured in picogram of materials per cell. If expressed in pg/cell, the results by Chithrani et al. indicate a plateau around 100 nm rather than a peak at 50 nm (Fig. 3C).
Fig. 3.
Comparison of nanoparticle uptake as a function of size reported by (a) Chithrani et al. (49) and (b) Lu et al. (50). (c) The results of Chithrani et al. are re-plotted with the particle uptake expressed in pg/cell (as in Reference 50) instead of number of particles per cell. (A and C) Adapted with permission from Chithrani et al. (49). Copyright 2006 American Chemical Society. (B) Lu F, Wu SH, Hung Y, Mou CY. Size effect on cell uptake in well-suspended, uniform mesoporous silica nanoparticles. Small 2009; 5: 1408–13. Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission.
The debate on a maximum size for internalization is still open. Some authors report a maximum size for internalization by non-phagocyte cells, e.g. 1 µm latex beads are not taken by mouse melanoma B16 cells (51). However, gold nanowires that have a 200-nm diameter and a length of several micrometers have been observed to be taken up by fibroblast and HeLa cells (52).
Kinetics of entry
One difficulty in comparing various studies is that most papers present a still picture for a given time of incubation. There have been very few comprehensive quantitative studies of the kinetics of interaction and uptake of nanomaterials in cells. According to some reports, equilibrium (or saturation) takes several hours and is size dependent. Rejman et al. reports significant changes in the dynamics of uptake for latex beads between 50 and 500 nm in murine melanoma cell line B16-F10. The saturation time increased with size from ∼30 min (50–100 nm beads) to several hours (200 nm beads) (51). Hartig et al. present a detailed kinetic analysis of 140 nm positively charged fluorescent polymer beads interactions with endothelial cells (53). They observe extensive cooperativity and do not approach saturation uptake in the range of concentration accessible. At the complete opposite, a report on carbon nanotubes indicates an almost instantaneous equilibrium with the cell having a similar endo- and exocytosis rate that keeps the intracellular concentration constant (54).
Clustering
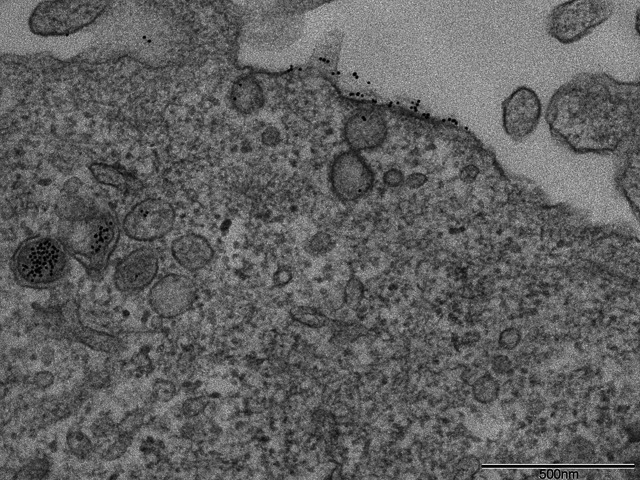
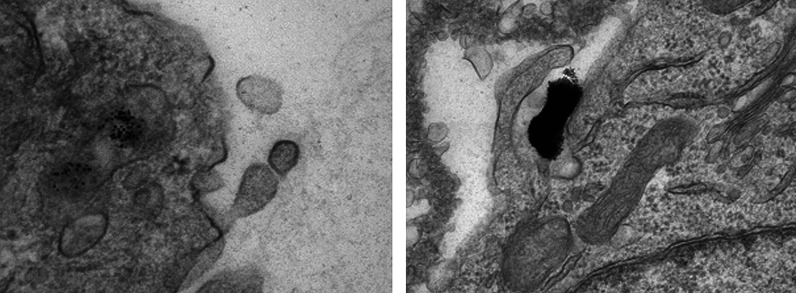
Another difficulty in comparing various published studies is to know exactly which species are taken up by the cell. Is it single nanoparticles or aggregates formed prior to, or during interaction with the membrane? Salmaso et al. used particles capped with a thermoresponsive polymer to control aggregation. The results showed an increase in uptake upon aggregation (55). Surface presentation has been proposed as an original approach aiming at better controlled delivery conditions (56). Chithrani et al. have reported that single 50 nm transferrin-coated gold nanoparticles are able to enter cells, while 14 nm transferrin-coated nanoparticles were only taken up when clustered in groups of at least six particles (57). However, in our own experiments on the entry of peptide-capped nanoparticles in HeLa cells, we have observed endosomes containing single 10 nm nanoparticles (Fig. 4). A possible explanation for these apparently conflicting results may be the functionalization of the nanoparticles (transferrin versus peptide/PEG) and its effect on uptake mechanism. Real-time single particle imaging will be necessary to better understand this phenomenon and its impact on uptake.
Fig. 4.
Transmission electron microscopy image of the uptake of 10 nm gold nanoparticles coated with 10% CALNN-HA2 – 20% CALNN-PEG – 70% CALNN. The particles (6 nM) were incubated in the presence of serum with HeLa cells for 3 h before fixation and TEM imaging. The scale bar is 500 nm.
Shape and rigidity
It has been suggested that uptake was higher for gold nanoparticles than for gold nanorods (49). This was based on the observation of enrichment of the number of spheres in endosomes when HeLa cells were exposed to a suspension of particles containing a mixture of rods and a small proportion of spheres. Similar trends were reported in a study of the influence of shape and rigidity on uptake by macrophages using polystyrene particles (58). Polystyrene particles of identical volumes but different shapes were obtained by stretching the particles embedded in a polymer film. The extended flexible (‘worm-like’) particles exhibited negligible phagocytosis when compared to spherical particles (58). However, the same question was also addressed using a series of size-controlled nanohydrogel particles and in these conditions the high aspect ratio particles (d=150 nm, h=450 nm) were internalized by HeLa cells approximately four times faster than the more symmetric low aspect ratio particles (d=200 nm, h=200 nm). In the latter study, the particles were being dosed at constant particle mass (15 µg/ml) (59).
It is remarkable that after several thousand publications on the interaction of particles with cells, simple questions such as the effect of size or particle chemistry on nanoparticle uptake in cells are not settled. The most likely explanation is that while the question is simple, the answer is not: the effect of size is not independent of other parameters such as surface chemistry, cell line, colloidal stability, non-specific interactions, etc. It is therefore possible that high-throughput approaches with systematic variations of material parameters will be required to reach a better understanding of nanomaterials-cell interactions. Passive uptake of nanomaterials always results in endosomal localization. This is a stringent limitation for biological and biomedical applications and therefore considerable efforts are being made to escape or bypass the endocytotic pathway. In the next section, we examine facilitated and active delivery routes for nanoparticle entry into cells.
Facilitated delivery
A number of strategies for biomolecule delivery, especially anti-sense oligonucleotides and DNA have been developed (for review, see Reference (60). They include the use of penetrating peptides, polycationic molecules, liposomes, and methods involving physical transient disruption of the plasma membrane. Most of these methods have been applied to nanomaterials and gold nanoparticles and their efficiency for intracellular delivery and cytoplasmic availability of the cargo will be discussed.
Cell-penetrating peptides
Over the past 15 years, carrier delivery methods have been developed to facilitate the entry of short oligonucleotides, plasmids, peptides, and proteins. Since the discovery of the Antennapedia homeodomain (61), short cationic peptides that are able to translocate the cell membrane without disruption have caused much excitement. These peptides were called cell-penetrating peptides (CPPs) (62, 63). Since then, different CPPs have been described, including Tat, Sweet Arrow peptide, transportan, and polyarginines (for review, see Reference (64), and their method of internalization has been extensively studied, discussed, and has raised some controversy (65–69). To summarize, CPPs utilize more than one mechanism of endocytosis to translocate through the plasma membrane. Imaging of Tat peptide-conjugated quantum dots in living cells has shown that the peptide-conjugated nanomaterials are entering cells via macropinocytosis. Tracking data demonstrated that the quantum dotsloaded vesicles were actively transported by molecular machines to an asymmetric perinuclear region (70). Although this study was performed with quantum dots, it is likely that the same mechanisms occur with gold nanoparticles. Direct translocation of CPPs through the membrane has also been reported, especially when they are present in higher concentration and in the presence of endocytotic inhibitors (67, 68). CPPs are attractive as a transfection method, because only incubation is needed and no physical disruption and manipulation of the membrane is necessary.
Gold nanoparticles functionalized with CPPs have been prepared and their efficiency for delivery has been evaluated. There is a clear consensus that intracellular uptake is increased by CPPs. However, the subcellular localization and escape from endosomes is inconsistent. Gold nanoparticles (3 nm) functionalized with Tat peptide were shown by electron microscopy to be localized in the nucleus and in the cytoplasm (71). However, other studies using gold nanoparticles functionalized with amphipatic proline-rich peptide (12 nm gold nanoparticles-C-SAP) (72), Tat, or penetratin (10 nm gold nanoparticles) (43) showed the nanoparticles trapped in endosomes. Similar vesicular localization was also observed with Tat-quantum dots (70). The large discrepancy obtained between different studies can be due to the cell lines used, the size of the cargoes (73), the chemical composition of the capping layers (74), and could also originate from artifacts coming from the fixation required for electron microscopy (75). Careful consideration of cell shape and viability must also be taken into account. A number of studies claim intracellular uptake and even nuclear localization, but show pictures of dead/dying cells; such studies are not significant as membrane permeability is a hallmark of dying cells.
Nuclear localization has been observed using nuclear localization sequences (NLS) in combination with CPPs and PEG (43). However, only a few percent of nuclear localization was achieved and most of the gold nanoparticles remained trapped in endosomes. Mandal et al. also observed endosomal localization using gold nanoparticles capped with a monolayer composed of CPP and NLS (76). Successful nuclear localization using microinjection of gold nanoparticles (∼20 nm) capped with nucleoplasmin (containing a NLS) was observed by Feldherr et al. However, no nuclear import was observed with the NLS sequence only (77). Tkachenko et al. have also observed endosomal trapping and no nuclear localization using the same NLS sequence from the SV40 large T antigen with 20 nm gold nanoparticles incubated on Hep2G cells (78, 79). The same team eventually managed to visualize, by video-enhanced color differential interference contrast (VEC-DIC) microscopy, nuclear delivery of gold nanoparticles using an adenoviral fiber protein containing receptor-mediated endocytosis (RME) and NLS sequences. However, the unique cell shown as proof of the successful delivery doesn't look very healthy and is likely to be engaged in a death program. This re-emphasizes the requirement to assess the cell shape and viability for these types of experiments to avoid artifacts. Taken together, and excluding a number of studies showing nuclear localization in dead cells, no conditions where the majority of particles had a nuclear localization has been achieved using NLS, indicating that endosomal escape constitutes a prerequisite for successful intracellular targeting.
Protein-mediated delivery: use of transferrin
Proteins have also been used as vectors to facilitate delivery of gold nanoparticles. The interaction between transferrin and its receptor has been used as a potential pathway for cellular uptake of drugs and genes (80). Transferrin plays an important role in iron transport for hemoglobin synthesis. It has been successfully applied for enhanced internalization of 20 nm gold nanoparticles capped with transferrin (96 nm hydrodynamic radius) as monitored by atomic force microscopy (AFM) (81). The uptake of 25 nm gold particles capped with transferrin was also studied by laser scanning confocal microscopy (82). The subcellular localization is not detailed in these studies, yet the receptor mediated endocytosis observed by AFM (81) and the spotty fluorescent signal detected by confocal microscopy (82) suggests endosomal localization. In a third study with particles of various sizes and shapes, TEM and fluorescence imaging indicate endosomal localization as well (57).
Transfection reagents
Polyethylenimine (PEI) is a polycationic molecule that has been used as a DNA transfection agent due to the charge-based interaction with negatively charged DNA. It has been used to cap gold nanoparticles and enhance siRNA and DNA delivery (83, 84). However, these studies were not focused on the delivery of the gold nanoparticles. Other cationic molecules, such as cationic lipids, have been developed and are commercially available for the purpose of oligonucleotide transfection. These compounds include Fugene-6, Effectene, GeneSHUTTLE, lipofectamine, etc. Again, gold nanoparticles have been used in combination with these compounds to enhance DNA transfection efficiency (85). We have used Fugene-6 for gold nanoparticle delivery, and found that it increased the aggregation of 10 nm CALNN-capped gold nanoparticles (86). Although a high-level cellular uptake was observed, all gold nanoparticles were localized inside endosomes (Fig. 5). Similar results were obtained with the protein delivery reagent BioPorter (86).
Fig. 5.
TEM images of 10 nm CALNN-capped gold nanoparticles internalized with Fugene-6 (15 µl Fugene-6, 15 µM CALNN-capped gold nanoparticles). TEM images clearly show endosomal localization as well as some large aggregates taken up by the cells by macropinocytosis (right panel).
Liposomes
A liposome is a vesicle made out of a bilayer of phospholipids that envelops a core aqueous compartment. They can be composed of naturally derived phospholipids or surfactant components. They have been developed for drug delivery as they can carry either hydrophilic (in the center) or hydrophobic (in the membrane) molecules. Initially, the suggested mechanism for delivery was their fusion with the plasma membrane to deliver their contents into the cytosol, but it is now accepted that the main delivery mechanism is endocytosis (for review about cationic liposomes-based delivery, see Reference (87). One of the approaches to achieving gold nanoparticles delivery is to incorporate the nanoparticles inside or on the surface of the liposome. Liposome intracellular uptake occurs via endocytosis leading to the gold cargo delivery inside endosomes (43, 88). Small gold nanoparticles (1.4 nm) have been successfully delivered by liposomes into cancer cells (89). In this case, the delivery efficiency was increased by 1,000 fold for the small gold nanoparticles. Yet, again, the investigation of intracellular distribution clearly showed lysosomal localization (final degrading organelles) within 40 min of incubation (89).
Bacterial toxins
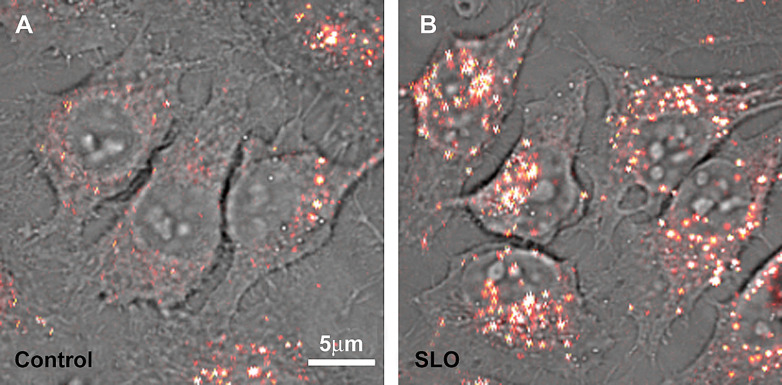
The use of toxins of microbial origin constitutes a different approach for intracellular delivery. Fifteen years ago, it was used for oligonucleotides delivery, especially anti-sense oligonucleotides. They allow reversible permeabilization by transiently forming pores in the cell membrane (90–92). Streptolysin-O (SLO) increased by more than 100 times the intracellular delivery of oligodeoxynucleotides in the KY01 myelogenous leukemia cells compared to conditions without SLO (93, 94). More recently, SLO has been successfully used for protein (95) and peptide nucleic acid (PNA) delivery (96). We have therefore tested this method on 10% CALNN-PEG – 90% CALNN functionalized gold nanoparticles (10 and 5 nm), and obtained improvement of cellular uptake (Fig. 6), suggesting that this route has to be further explored.
Fig. 6.
SLO enhances intracellular uptake of 10 nm gold nanoparticles. Overlay of a bright field and a photothermal image of 5 nm gold nanoparticles (coated with 10% CCALNN-PEG – 90% CALNN, 600 nM) delivered in HeLa cells with SLO (B) compared to a control without SLO (A).
Listeriolysin O, another pore-forming toxin, is a thiol-activated, cholesterol-dependent toxin protein. It differs from other thiol-activated toxins, since its cytosolytic activity is maximized at pH 5.5 (for review, see Reference (97). Its activity in low pH vesicles allows release of the endosome content into the cytosol (98). This toxin loses its activity in the neutral cytosol and has therefore minimal toxicity (99). The use of these toxins is currently not described for nanoparticles delivery, yet they might offer attractive properties for cytoplasmic delivery.
Active delivery using physical methods
Several methods for active delivery involving disruption of the cell membrane, physically or chemically, have been tested with nanoparticles.
Sonoporation
Sonoporation uses ultrasound to generate transient non-selective pores on the cell membrane and has been exploited as an intracellular drug and gene delivery strategy. The technique is based on ultrasound in conjunction with microbubbles to drive the cargo into the cell by causing violent bubble cavitation and jet streaming, resulting in pore formation that may be followed by rapid annealing if the membrane damage is not too severe. The size of the pores has been estimated to be ∼110±40 nm in a Xenopus laevis oocytes model (102). A combination of ultrasound and microbubbles has been used for chemotherapeutic drug (103), siRNA (104), DNA (105), and peptide (106) delivery. Ultrasound has been used for delivery of 100 nm nanogold-dipalmitoyl phosphatidyl ethanolamine (DPPE) that are not spontaneously internalized by endocytosis (101). It clearly increased intracellular uptake, without causing significant cell death. However, TEM images showed once again a vesicular localization.
Genegun
Genegun is a physical means of molecule delivery. It uses a high pressure helium gas burst to accelerate the cargo and deliver it into the cells and was first described by Klein et al. (107). It has primarily been used to transfect cells with DNA or plasmids (in particular in plant cells). Nanoparticles are frequently used as an adjuvant in these studies, but their fate and localization is not reported because the focus is on DNA transfection efficiency. It has even been used in vivo for gene delivery into superficial hepatocytes (108). It has recently been used to transfer nanosensors into several adherent cell lines. The cells displayed a good nuclear uptake of the nanosensor according to the fluorescence levels and distribution measured by confocal microscopy, but the viability remained questionable according to the phase contrast images (109).
Microinjection
Another mechanical delivery method is microinjection to single cells. It allows control of the delivery dosage and precise timing of delivery. It has been widely used in many research areas, including the transfection of cells refractory to common transfection reagents such as primary cells (for review, see Reference (110). However, inappropriate manipulation (injection pressure, needle positioning) can lead to cytotoxicity or cellular stress. Surprisingly, only a few papers show micro-injected gold nanoparticles and their intracellular localization. It has been reported with nucleoplasmin-capped gold nanoparticles in BALB/c 3T3 cells (77). Targeted optical injection of gold nanoparticles has recently been described by Dholakia et al. (111). They have used a combination of optical tweezing and opto-injection to deliver single 100 nm nanoparticles into the nucleus of single mammalian cells. Although the approach is very neat and well controlled, it seems to be technically extremely challenging, requires sophisticated lasers, and is limited to large nanoparticles. Laser irradiation has also been used with 15–30 nm gold nanoparticles to increase membrane permeabilization and nanoparticle delivery without causing cell death (112), but the intracellular distribution was not evaluated.
Assisted endosomal escape
The main limitation with all the techniques described above is the ultimate vesicular localization and absence of availability in the cytosol or in the nucleus in a healthy living cell (for a summary, see Table 1). The same limitations have already been discussed for quantum dots (for review, see Reference (113). In the field of DNA and plasmid delivery, it has previously been shown that lysosomotropic agents, such as chloroquine or sucrose (114), can improve DNA delivery and subsequent exogenous gene expression. Chloroquine is known to induce vesicular disruption by elevating the intravesicular pH of lysosomes and endosomes. We and others have shown that chloroquine induces endosomal disruption and increases the availability and stability of gold nanoparticles inside the cells (85, 86), yet the effects are not massive and the nanoparticles seem to stay in the region of broken endosomes. Maiolo et al. have shown a specific redistribution of fluorescently labeled cell-penetrating peptides from endosomes to cytoplasm using laser illumination. The phototreatment is supposed to open the endosomes without causing cell death for 24 h (115). As yet, this attractive method has not been evaluated for gold nanoparticles.
Table 1.
Comparison of gold nanoparticles subcellular localization using different methods of cellular delivery
| Method | Gold core size (nm) | Capping | Subcellular localization | Toxicity | Reference |
|---|---|---|---|---|---|
| CPP | 16 | 94% PEG, 2% Tat, 2% NLS, 2% penetratin | Cytoplasmic and nuclear | Not reported | 43 |
| CPP | ∼20 | Nuclear localization sequence and receptor-mediated endocytosis peptides | Nucleus | ∼5% death | 78 |
| CPP | 12 | Sweet arrow peptide | Endosomal | Not reported | 72 |
| CPP | 2.8 | Tat peptide | Cytosolic around the mitochondria and in nucleus | Low cytotoxicity below 10 µM | 71; 68 |
| CPP | 20 | Biotinylated Tat-HA2, PEG-SH, anti-actin antibodies | Cytoskeleton (cytoplasm) | Not reported | 100 |
| PEI | 4 | PEI | Mainly endosomal and some nuclear localization | 20–30% death | 83 |
| SLO toxin | 5 and 10 | 90% CALNN – 10% CALNN-PEG | Endosomal and cytosolic | Toxicity controlled by protocol optimization | Shaheen et al., unpublished data |
| Liposomes | 1.4 | Phospholipid | Lysosomes near the nuclear membrane | Not reported | 89 |
| Transferrin | 14–100 | Tranferrin | Endosomal | Non-toxic | 57; 81 |
| Ultrasound | 100 | DDPE | Endosomal | Non-toxic | 101 |
| Microinjection | 11–32 | Nucleoplasmin | Nuclear and cytoplasmic | Not reported | 77 |
In the CPP field, the HA2 peptide has been used in combination with penetrating peptides to increase cytoplasmic delivery. HA2 is a 20 amino acid fusogenic peptide from the influenza virus. It is a highly conserved pH-sensitive sequence, which destabilizes plasma membrane at low pH (116) and is therefore expected to escape endosomal compartments (117). It has been used in combination with polyarginine to increase transfection efficiency (118). Gold nanoparticles capped with Tat-HA2 have recently been successfully used for actin filaments labeling with gold nanoparticles and live cell detection of cytoskeleton dynamics (100).
Some strategies have been developed to avoid endosomal uptake, by designing pH sensitive liposomes combined with fusogenic peptides (119) or with the bacterial toxin listerolysin O for DNA-nanoparticles delivery (119). Interestingly, successful mitochondrial targeting of 10 nm gold colloids was achieved using functionalized liposomes containing octa-arginine on the surface, which enter cells via macro-pinocytosis and then fuse with the mitochondrial membrane to deliver the cargo (MITO-Porter) (120).
Measurements and detection in mammalian cells
To understand the fate of gold nanoparticles in live cells and their interactions with intracellular molecules or compartments, it is necessary to combine complementary techniques to obtain a detailed view, which includes quantitative assessment of uptake, nanoparticle intracellular localization, biochemical environment, and the fate of the nanoparticle capping layer.
Direct measurements of the gold content: TEM, ICP-AES, and ICP-MS
TEM is widely used for intracellular detection of metal nanoparticles as it allows their direct visualization due to their high electron density. In conventional TEM (as opposed to high resolution TEM), nanoparticles above 5 nm can easily be detected inside cells because of the good contrast provided by the high electron density of metal nanoparticles. It is a powerful technique that permits the visualization of single nanoparticles in cellular compartments and organelles. However, it has rather low throughput since it necessitates time-consuming processing of the samples (cell fixation and resin embedding). It also requires many images taken from a large number of sliced cells to obtain significant results about localization. Many studies reporting TEM experiments show a single representative image, but important statistical information, such as number of nanoparticles found, number of images analyzed, number of different cells imaged, are often missing (for review on quantification of intracellular gold nanoparticles, see Reference 10).
TEM has been used to quantify the number of citrate-coated gold nanoparticles in vesicles after uptake in mammalian cells (49). Here, TEM results have been further corroborated by inductively coupled plasma atomic emission spectroscopy (ICP-AES). This technique enables a count of the number of gold atoms, thereby providing a more precise quantification of the number of gold spheres, though following the same trend as the TEM estimate. As for ICP-AES, inductively coupled plasma mass spectrometry (ICP-MS) gives an elemental analysis and, therefore, an estimate of the number of nanoparticles in the sample can be calculated. It has been successfully used to study the uptake of GdIII-enriched polyvalent Cy3-DNA-gold nanoparticles conjugate (Cy3-DNA-GdIII@Au-NP) in NIH/3T3 and HeLa cells (121). These methods require cell lysis of a large number of cells. They provide, quantitatively, the average population uptake at a given time point, but do not reveal cell-to-cell variations, dynamics, and intracellular localization.
Fluorescence
To detect gold nanoparticles by fluorescence, two options can be used: a fluorescent label attached to the gold core or a fluorescent gold core.
Fluorescent molecules
The use of fluorescent molecules attached to the gold core is the most widely used. However, attention must be drawn to the quenching effect of the gold core. Depending on the distance between the fluorescent label and the gold core (122), the size of the nanoparticle, and the loading of the fluorescent dye on the nanoparticle (123, 124), energy transfer may prevent photons from being emitted (or enhance their emission). The molecule should therefore be far enough from the gold core, and if the fluorescent signal is to report on the localization of the particle, independent proof that the conjugate is intact should be provided. It has been shown that a ligand can be removed on or after cell entry either by ligand exchange (125) or proteolysis (86). The fluorescence can be measured by wild field microscopy (126) or, for a better resolution, by laser scanning confocal microscopy. The latter technique provides an image of a thin section of the sample, thereby providing better localization information. For example, the uptake of 3.5 nm lysine and FITC functionalized gold nanoparticles was investigated in RAW264.7 macrophage cells by laser confocal scanning microscopy (127).
Fluorescent gold clusters
Gold clusters have been shown to exhibit intrinsic fluorescence that varies with the size of the cluster (128). Chang's group reported the synthesis of intrinsically fluorescent gold nanoclusters (AuNC@DHLA) of less than 4 nm. These particles displayed less photobleaching than organic fluorophores and successful conjugations with PEG, PEG-biotin, and streptavidin were achieved (129). They have been used to specifically label endogenous biotin of HepG2 fixed cells and, in a preliminary study, unconjugated AuNC@DHLA have been imaged inside live HAEC cells as a ‘proof of principle’ of their potential in live cell imaging (129). Two other studies have reported internalization of fluorescent gold clusters in living cells (130, 131). However, controls in the absence of nanoparticles are needed (131), and in both cases fluorescence microscopy images indicate endosomal localization, although Lin et al. claim nuclear localization.
Scattering and absorption
Several recent papers have reviewed the optical properties of metal nanoparticles and their applications in biosensing (2–4). Here, we focus on selected examples of reports that use these techniques in the context of live cells.
Dark field
Curry et al. studied the epidermal growth factor receptor (EGFR) by dark-field microspectrometry and 60 nm gold nanoparticles conjugated to anti-EGFR (132). Here, the reflected dark-field imaging allowed visualization in live cells of the receptor-mediated uptake of gold nanoparticles and spectrometry allowed recording of the scattering spectra of gold nanoparticles. Yet, the fact that individual nanoparticles of this size can be distinguished in a scattering environment like a cell requires further evidences. Anti-EGFR conjugated and unconjugated gold nanoparticles were compared and different distributions of peak wavelengths were found from the scattering data. The unconjugated gold nanoparticles had a red-shifted and broader distribution as compared to the conjugated ones. The red-shift can be explained by the aggregation of the nanoparticles, and indeed TEM images showed that unconjugated gold nanoparticles were in endosomes in close proximity to one another, when the anti-EGFR conjugated particles were isolated. As mentioned in Assisted endosomal escape section, Kumar et al. have used 20 nm gold nanoparticles functionalized with an anti-actin antibody for labeling actin in live cells (100). The paper does not report single particle detection (and the TEM shows a very high density of labels), but clearly demonstrates the potential of dark field to improve the understanding of nanoparticle interaction with cells. Louit et al. have reported single particle imaging, tracking, and spectroscopy using dark-field microscopy on live cells (133). For this purpose, they used large 80 nm nanoparticles, which allowed overcoming the scattering background of the cells. To extract robust information from single particle or single molecule studies, it is normally necessary to look at hundreds of traces and to carry out rigorous statistical analysis. Louit et al. did not present such analysis, but their paper is a clear proof of principle demonstration of the potential of this technique to follow the interaction and uptake of single large gold nanoparticles in live cells. They suggested that information on the environment of the particle could be deduced from an observed modulation in the scattering spectrum.
Plasmon coupling
Detection of biological events at the single molecule level has been used for biosensing by exploiting the distance-dependent scattering properties of the gold nanoparticles. Using these properties, optical contrast between isolated and closely spaced gold nanoparticles can be achieved (differences in peak intensity and wavelength) (134–136). Inducing the close proximity of plasmonic particles in the presence of a specific target can therefore provide a sensor to probe molecular interactions (135). Nanoparticle plasmon resonance coupling has allowed dynamic imaging of growth factor states in living cells (137). A recent application of the plasmon coupling in living cells has been the detection of protease activation at single molecule level. The signal provided by the plasmon rulers based on optical imaging of crown gold nanoparticles allowed continuous monitoring of caspase-3 activity in live cells (138). Other ways of achieving sensor detection in cells is to exploit the fluorescence quenching properties of gold nanoparticles. This has successfully been developed to detect mRNA in living cells using nano-flares (139). Plasmonic resonance energy transfer (PRET) has been developed for ion metal sensing in living HeLa cells (140).
Raman scattering
Surface Raman scattering (SERS) has also been used for live cell probing. SERS can fulfill the requirements of dynamic in vivo systems by using low laser powers and short data acquisition times. Raman scattering occurs during collision of photons with molecules. During this process, photons gain or lose energy and this change in energy results in scattered photons. A Raman spectrum is generated by scattering from different molecular vibrations and provides a vibrational fingerprint of a molecule (for a review on SERS principle and applications, see Reference 141). In SERS, Raman scattering signals can be enhanced by several orders of magnitude for molecules in the vicinity of metal nanostructures. For example, gold nanoparticles can amplify the efficiencies of adsorbed molecules by 1014- to 1015-fold, allowing spectroscopic detection of single molecules. Applications are multiple and range from chemical probing in intracellular compartments such as pH (141), to subcellular detection of 20 nm gold nanoparticles decorated with large T antigen NLS (142) or in vivo tumor detection using PEGylated SERS nanotags (143). Recently, time-resolved acquisition of the SERS spectra has also allowed observation of the transport of gold nanoparticles through a living cell (144).
Differential interference contrast
Tkachenko et al. have studied the uptake of 20 nm gold nanoparticles functionalized with different NLS sequences using VEC-DIC. The principle of the technique seems appealing, as the gold nanoparticles are supposed to appear as colored areas together with the shape and topography of the cells provided by DIC on a single image recorded at video rate (79). However, there is no evidence of the possibility of individual nanoparticles imaging and it is unclear how much uptake is required to obtain a significant signal. More recently, a dual-wavelength DIC microscopy has been developed to image 20–80 nm silver or/and gold nanoparticles, which is based on the wavelength-dependent contrast of metal nanoparticles (145). The technique has also been used to image the entry of Tat-functionalized 40 nm gold nanoparticles in live HeLa cells at video rates (145). The principle relies on comparing two DIC images taken at the same time at two different wavelengths; one at 540 nm corresponding to the maximum contrast wavelength of gold nanoparticles to detect the nanoparticles and the cells, and one at 720 nm as a control image to detect cells only. The DIC contrast is not only depending on the size of the nanoparticles, but also on the complex refractive indexes of metal nanoparticles (145).
Photothermal microscopy (absorption)
In 2002, Boyer et al. introduced photothermal imaging of gold nanoparticles (17). It is an absorption-based imaging technique that allows the direct imaging of metal nanoparticles (146, 147), nanotubes (148), and quantum dots (149). The technique relies on the overlay of two laser beams, one that induces a small localized temperature change (∼1–2°C) if a nano-absorber is present, the other that detects the eventual change of temperature. It has further been used to image membrane proteins using gold nanoparticles as labels (150). It allowed detection of single small nanoparticles (10 nm) for a long period of time without blinking or photobleaching. The sensitivity of the technique has since been improved to allow imaging of 5 nm gold nanoparticles in living cells and the technique was renamed laser-induced scattering around a nano absorber (LISNA). Single nanoparticle photothermal tracking (SNaPT) has been developed by the same group and is able to achieve the tracking of metal nanoparticles at video rate for long periods of time. They have recorded the lateral movement of a membrane protein AMPA receptor in live neurons via the tracking of 5 nm AMPA receptor-conjugated gold nanoparticles for more than 5 min (151). Photothermal absorption correlation spectroscopy (PhACS), the equivalent of fluorescence correlation spectroscopy (FCS) with a detection based on the absorption of metal nanoparticles, would also allow the measurement of diffusion constants of metal nanoparticles and the hydrodynamic radii of functionalized nanoparticles (152), but it has not yet been applied to live cells.
Optoacoustic and photoacoustic tomography (absorption)
Optoacoustic tomography (OAT) uses a pulsed laser that produces a change of temperature localized in the vicinity of any absorbing tissue or object. It creates an acoustic wave that is detected and further reconstructed in a 2-D or 3-D image. It has been reported in in vitro experiments with 40 nm gold nanoparticles conjugated to an antibody that targets human SK-BR-3 breast cancer cells (153). Photoacoustic tomography (PAT) is also based on the absorption of a pulsed laser by an absorbing object, which creates a temperature change generating photoacoustic waves (154). Due to the dark-field configuration of its illumination, the extracted signal produces better quality images. PAT has been used with PEGylated gold nanoparticles as a contrast agent for in vivo tumor imaging in mice (155). One advantage of this technique is the ability to image deep tissue and, therefore, has a potential in ex vivo and in vivo cancer diagnostics and therapeutics.
Integrity of the capping layer
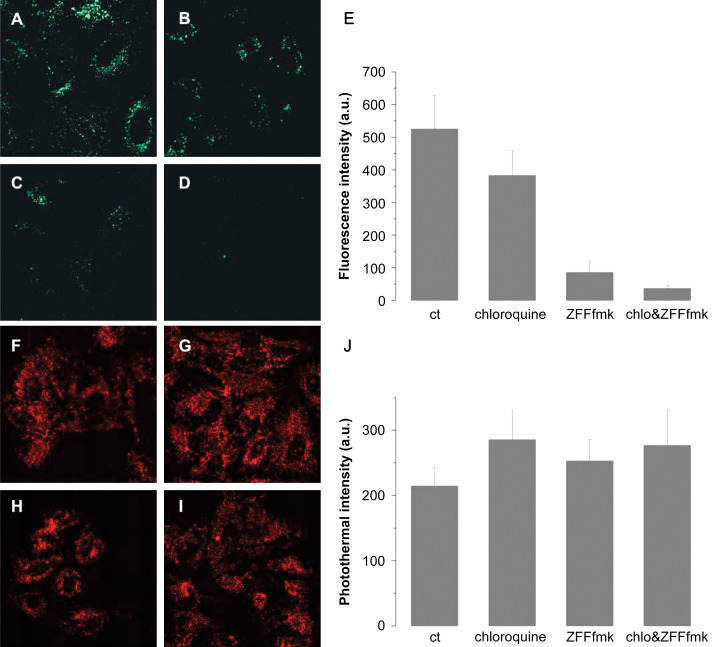
To go beyond proofs of principle and develop robust applications of bionanotechnology, a critical piece is currently missing: very little is known about the fate of the nanoparticle capping layer inside living cells. This knowledge gap is due partly to the technical difficulty of investigating nanoparticle surface properties after it has been exposed to the complex environment of the cell. One pioneering study in this field was the analysis of the capping composition using laser desorption/ionization mass spectrometry of cell lysates (156). This technique provides a high level of chemical information, but is time consuming and limited to fixed time points. As shown in Fig. 7, we have used real-time fluorescence confocal imaging on single living cells combined with photothermal imaging on fixed cells to simultaneously report on the localization of the gold core and on the integrity of the capping layer (in this case a peptide ligand shell) (86). We demonstrated that peptide cleavage occurs during nanoparticle uptake and we determined that Cathepsin L was the protease responsible for the degradation. This result is of general significance because one third of all human proteins are potentially cleaved at least once by Cathepsin L (86) and most applications of nanomaterials require conjugation with peptides or proteins to encode for specific recognition, targeting, or actuation. The probability that such bionanoconjugates would degrade during internalization is very high and yet has rarely been discussed in the literature.
Fig. 7.
Cathepsin-dependent cleavage of the peptide monolayer. Adapted with permission from See et al. (86). Copyright 2009 American Chemical Society. HeLa cells were incubated for 4 h with 6 nM of fluorescently labeled 10 nm peptide-capped gold nanoparticles. (A–D) Confocal images of fluorescence release inside cells after 4 h incubation, (A) nanoparticles only, (B) nanoparticles in the presence of 100 µM chloroquine, (C) nanoparticles in the presence of 20 µM Z-FF-fmk (irreversible cathepsin inhibitor), (D) nanoparticles in the presence of both 100 µM chloroquine and 20 µM Z-FF-fmk. (E) Quantification of five different fields for each condition described in A–D. (F–I) Photothermal images of nanoparticle uptake, (F) nanoparticles only, (G) nanoparticles in the presence of 100 µM chloroquine, (H) nanoparticles in the presence of 20 µM Z-FF-fmk, (I) gold nanoparticles in the presence of both 100 µM chloroquine and 20 µM ZFF-fmk. (K) Quantification of photothermal intensity for each condition described in A–D. Average intensity of at least 40 cells is shown for each condition. Uptake is not affected by chloroquine or Z-FF-fmk, but degradation of the monolayer is reduced by chloroquine and suppressed by Z-FF-fmk.
In summary, there is a range of methods available to probe the amount of nanoparticles internalized, their intracellular localization, their capping stability, and their potential interactions. To progress towards the rational design of nanoparticle-based sensors and actuators, it is now necessary to systematically combine these tools to obtain a comprehensive view of the bionanoconjugates uptake, localization, and biochemical fate.
Conclusion
In this review, we have focused on intracellular delivery of nanomaterials. We observe that in spite of the increasing number of published papers, controlling the intracellular delivery, fate, and intracellular localization of nanoparticles remains a major challenge and even simple questions such as the effect of surface charge, chemical functionalization, and size are still controversial. Clearly, one of the major challenges is the endosomal trapping of the nanomaterials taken up. For in vitro applications such as nanoparticle-based imaging, disruptive methods such as temporary permeation by physical or biochemical means can be used. Even with such methods, particles are generally found in endosomes demonstrating the powerful mechanisms that cells use to protect themselves from foreign materials and organisms. Biology, however, also teaches us that it is possible to escape or bypass the endosomes: some micro-organisms, such as viruses, are able to disrupt the endosomal membrane to reach the cytosol. Thus, there is hope that the challenge can be overcome in a robust way using astutely designed nanomaterials. This will require progress in understanding in real time what happens to the particles and to their coating immediately after incubation in cell medium, during contact with the cellular membrane, and after intracellular uptake. Combining existing and emerging methods of investigations, with strong interdisciplinary inputs from biology, chemistry and physics, we may well achieve ‘virus-like’ nanoparticles in the near future. Other issues will include control of the intracellular mobility, targeting to specific proteins and DNA targets, but it will only be possible to start addressing these challenges seriously once a robust path to the cytosol is found.
Biographies
 Raphaël Lévy is a BBSRC David Phillips Research Fellow at the University of Liverpool. He graduated in Physics at the University Louis Pasteur in Strasbourg (France). In 2002, after a Master in Soft Condensed Matter Physics, he obtained a PhD in Physics at the University Louis Pasteur. He then moved to the University of Liverpool as a Post-doctoral Marie Curie Research Fellow. In 2006, he obtained a prestigious David Phillips Fellowship, to develop single particle-based imaging in living cells (photothermal microscopy). His research interests include the design and characterization of nanomaterials and their interactions with living cells.
Raphaël Lévy is a BBSRC David Phillips Research Fellow at the University of Liverpool. He graduated in Physics at the University Louis Pasteur in Strasbourg (France). In 2002, after a Master in Soft Condensed Matter Physics, he obtained a PhD in Physics at the University Louis Pasteur. He then moved to the University of Liverpool as a Post-doctoral Marie Curie Research Fellow. In 2006, he obtained a prestigious David Phillips Fellowship, to develop single particle-based imaging in living cells (photothermal microscopy). His research interests include the design and characterization of nanomaterials and their interactions with living cells.
 Umbreen Shaheen completed her Master in Zoology and then lectured at the University of Balochistan. She studied biotechnology at the National Institute of Biotechnology and Genetic Engineering (NIBGE, Pakistan) and is currently doing her PhD at the University of Liverpool, on intracellular delivery of peptide-capped gold nanoparticles.
Umbreen Shaheen completed her Master in Zoology and then lectured at the University of Balochistan. She studied biotechnology at the National Institute of Biotechnology and Genetic Engineering (NIBGE, Pakistan) and is currently doing her PhD at the University of Liverpool, on intracellular delivery of peptide-capped gold nanoparticles.
 Yann Cesbron is a PhD student at the University of Liverpool, developing photothermal microscopy for biological imaging. He graduated at the University Louis Pasteur (Strasbourg, France) with a Master of Science in Condensed Matter Physics and a second Master of Science in Polymer Materials. He moved to Liverpool in 2006 to start his PhD.
Yann Cesbron is a PhD student at the University of Liverpool, developing photothermal microscopy for biological imaging. He graduated at the University Louis Pasteur (Strasbourg, France) with a Master of Science in Condensed Matter Physics and a second Master of Science in Polymer Materials. He moved to Liverpool in 2006 to start his PhD.
 Violaine Sée is a BBSRC David Phillips Research Fellow at the University of Liverpool. She graduated in Chemistry and Molecular and Cellular Biology at the University Louis Pasteur in Strasbourg (France). After a Master in Pharmacology, in 2001 she obtained her PhD in Pharmacology and Neurobiology at the University Louis Pasteur. She was then assistant lecturer and subsequently moved to the University of Liverpool as a Post-doctoral Research Fellow. In 2005, she obtained a prestigious David Phillips Fellowship, to develop her work on intracellular signaling dynamics. She is focusing on the imaging of single living cells in order to understand regulation of gene transcription and cell fate. She has recently been interested in using new techniques for single molecule imaging in live cells based on the use of gold nanoparticles.
Violaine Sée is a BBSRC David Phillips Research Fellow at the University of Liverpool. She graduated in Chemistry and Molecular and Cellular Biology at the University Louis Pasteur in Strasbourg (France). After a Master in Pharmacology, in 2001 she obtained her PhD in Pharmacology and Neurobiology at the University Louis Pasteur. She was then assistant lecturer and subsequently moved to the University of Liverpool as a Post-doctoral Research Fellow. In 2005, she obtained a prestigious David Phillips Fellowship, to develop her work on intracellular signaling dynamics. She is focusing on the imaging of single living cells in order to understand regulation of gene transcription and cell fate. She has recently been interested in using new techniques for single molecule imaging in live cells based on the use of gold nanoparticles.
Conflict of interest and funding
There is no conflict of interest in the present study for any of the authors.
References
- 1.Faraji AH, Wipf P. Nanoparticles in cellular drug delivery. Bioorg Med Chem. 2009;17:2950–62. doi: 10.1016/j.bmc.2009.02.043. [DOI] [PubMed] [Google Scholar]
- 2.Anker JN, Hall WP, Lyandres O, Shah NC, Zhao J, Van Duyne RP. Biosensing with plasmonic nanosensors. Nat Mater. 2008;7:442–53. doi: 10.1038/nmat2162. [DOI] [PubMed] [Google Scholar]
- 3.Jain PK, Huang XH, El-Sayed IH, El-Sayed MA. Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc Chem Res. 2008;41:1578–86. doi: 10.1021/ar7002804. [DOI] [PubMed] [Google Scholar]
- 4.Stewart ME, Anderton CR, Thompson LB, Maria J, Gray SK, Rogers JA, et al. Nanostructured plasmonic sensors. Chem Rev. 2008;108:494–521. doi: 10.1021/cr068126n. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh P, Han G, De M, Kim CK, Rotello VM. Gold nanoparticles in delivery applications. Adv Drug Deliv Rev. 2008;60:1307–15. doi: 10.1016/j.addr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Boisselier E, Astruc D. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem Soc Rev. 2009;38:1759–82. doi: 10.1039/b806051g. [DOI] [PubMed] [Google Scholar]
- 7.Chourpa I, Lei FH, Dubois P, Manfait M, Sockalingum GD. Intracellular applications of analytical SERS spectroscopy and multispectral imaging. Chem Soc Rev. 2008;37:993–1000. doi: 10.1039/b714732p. [DOI] [PubMed] [Google Scholar]
- 8.Eustis S, El-Sayed MA. Why gold nanoparticles are more precious than pretty gold: noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem Soc Rev. 2006;35:209–17. doi: 10.1039/b514191e. [DOI] [PubMed] [Google Scholar]
- 9.Gao JH, Xu B. Applications of nanomaterials inside cells. Nano Today. 2008;4:37–51. [Google Scholar]
- 10.Mayhew TM, Muhlfeld C, Vanhecke D, Ochs M. A review of recent methods for efficiently quantifying immunogold and other nanoparticles using TEM sections through cells, tissues and organs. Ann Anat. 2009;91:153–70. doi: 10.1016/j.aanat.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Nel AE, Madler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8:543–57. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 12.Sperling RA, Rivera gil P, Zhang F, Zanella M, Parak WJ. Biological applications of gold nanoparticles. Chem Soc Rev. 2008;37:1896–908. doi: 10.1039/b712170a. [DOI] [PubMed] [Google Scholar]
- 13.Willets KA. Surface-enhanced Raman scattering (SERS) for probing internal cellular structure and dynamics. Anal Bioanal Chem. 2009;394:85–94. doi: 10.1007/s00216-009-2682-3. [DOI] [PubMed] [Google Scholar]
- 14.Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1:325–7. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- 15.Sonnichsen C, Franzl T, Wilk T, von Plessen G, Feldmann J, Wilson O, et al. Drastic reduction of plasmon damping in gold nanorods. Phys Rev Lett. 2002;88:077402. doi: 10.1103/PhysRevLett.88.077402. [DOI] [PubMed] [Google Scholar]
- 16.Yguerabide J, Yguerabide EE. Light-scattering submicroscopic particles as highly fluorescent analogs and their use as tracer labels in clinical and biological applications - I. theory. Anal Biochem. 1998;262:137–56. doi: 10.1006/abio.1998.2759. [DOI] [PubMed] [Google Scholar]
- 17.Boyer D, Tamarat P, Maali A, Lounis B, Orrit M. Photothermal imaging of nanometer-sized metal particles among scatterers. Science. 2002;297:1160–3. doi: 10.1126/science.1073765. [DOI] [PubMed] [Google Scholar]
- 18.Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman R. Synthesis of thiol-derivatized gold nanoparticles in a 2-phase liquid-liquid system. J Chem Soc - Chem Commun. 1994:801–2. [Google Scholar]
- 19.Frens G. Controlled nucleation for regulation of particle-size in monodisperse gold suspensions. Nat Phys Sci. 1973;241:20–2. [Google Scholar]
- 20.Goubet N, Ding Y, Brust M, Wang ZL, Pileni MP. A way to control the gold nanocrystals size: using seeds with different sizes and subjecting them to mild annealing. ACS Nano. 2009;3:3622–8. doi: 10.1021/nn9007274. [DOI] [PubMed] [Google Scholar]
- 21.Pileni MP. Control of the size and shape of inorganic nanocrystals at various scales from nano to macrodomains. J Phys Chem C. 2007;111:9019–38. [Google Scholar]
- 22.Turkevich J, Stevenson PC, Hillier J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc. 1951;11:55–75. [Google Scholar]
- 23.Alivisatos AP, Johnsson KP, Peng XG, Wilson TE, Loweth CJ, Bruchez MP, et al. Organization of ‘nanocrystal molecules’ using DNA. Nature. 1996;382:609–11. doi: 10.1038/382609a0. [DOI] [PubMed] [Google Scholar]
- 24.Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature. 1996;382:607–9. doi: 10.1038/382607a0. [DOI] [PubMed] [Google Scholar]
- 25.Levy R. Peptide-capped gold nanoparticles: towards artificial proteins. Chembiochem. 2006;7:1141–5. doi: 10.1002/cbic.200600129. [DOI] [PubMed] [Google Scholar]
- 26.Schofield CL, Haines AH, Field RA, Russell DA. Silver and gold glyconanoparticles for colorimetric bioassays. Langmuir. 2006;22:6707–11. doi: 10.1021/la060288r. [DOI] [PubMed] [Google Scholar]
- 27.Levy R, Thanh NTK, Doty RC, Hussain I, Nichols RJ, Schiffrin DJ, et al. Rational and combinatorial design of peptide capping ligands for gold nanoparticles. J Am Chem Soc. 2004;126:10076–84. doi: 10.1021/ja0487269. [DOI] [PubMed] [Google Scholar]
- 28.Skidmore MA, Patey SJ, Thanh NTK, Fernig DG, Turnbull JE, Yates EA. Attachment of glycosaminoglycan oligosaccharides to thiol-derivatised gold surfaces. Chem Commun. 2004;23:2700–1. doi: 10.1039/b411726c. [DOI] [PubMed] [Google Scholar]
- 29.Kumar S, Aaron J, Sokolov K. Directional conjugation of antibodies to nanoparticles for synthesis of multiplexed optical contrast agents with both delivery and targeting moieties. Nat Protoc. 2008;3:314–20. doi: 10.1038/nprot.2008.1. [DOI] [PubMed] [Google Scholar]
- 30.Bajaj A, Miranda OR, Kim IB, Phillips RL, Jerry DJ, Bunz UHF, et al. Detection and differentiation of normal, cancerous, and metastatic cells using nanoparticle-polymer sensor arrays. Proc Nat Acad Sci USA. 2009;106:10912–6. doi: 10.1073/pnas.0900975106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghosh PS, Kim CK, Han G, Forbes NS, Rotello VM. Efficient gene delivery vectors by tuning the surface charge density of amino acid-functionalized gold nanoparticles. ACS Nano. 2008;2:2213–8. doi: 10.1021/nn800507t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Sayed IH, Huang XH, El-Sayed MA. Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancer. Nano Lett. 2005;5:829–34. doi: 10.1021/nl050074e. [DOI] [PubMed] [Google Scholar]
- 33.Maheshwari V, Fomenko DE, Singh G, Saraf RF. Ion mediated monolayer deposition of gold nanoparticles on microorganisms: discrimination by age. Langmuir. 2010;26:371–7. doi: 10.1021/la9021195. [DOI] [PubMed] [Google Scholar]
- 34.Amin RM, Mohamed MB, Ramadan MA, Verwanger T, Krammer B. Rapid and sensitive microplate assay for screening the effect of silver and gold nanoparticles on bacteria. Nanomedicine. 2009;4:637–43. doi: 10.2217/nnm.09.50. [DOI] [PubMed] [Google Scholar]
- 35.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 36.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 37.Chen ZP, Xu RZ, Zhang Y, Gu N. Effects of proteins from culture medium on surface property of silanes-functionalized magnetic nanoparticles. Nanoscale Res Lett. 2009;4:204–9. doi: 10.1007/s11671-008-9226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ehrenberg MS, Friedman AE, Finkelstein JN, Oberdorster G, McGrath JL. The influence of protein adsorption on nanoparticle association with cultured endothelial cells. Biomaterials. 2009;30:603–10. doi: 10.1016/j.biomaterials.2008.09.050. [DOI] [PubMed] [Google Scholar]
- 39.Giljohann DA, Seferos DS, Patel PC, Millstone JE, Rosi NL, Mirkin CA. Oligonucleotide loading determines cellular up-take of DNA-modified gold nanoparticles. Nano Lett. 2007;7:3818–21. doi: 10.1021/nl072471q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Free P, Shaw CP, Levy R. PEGylation modulates the interfacial kinetics of proteases on peptide-capped gold nanoparticles. Chem Commun. 2009:5009–11. doi: 10.1039/b910657j. [DOI] [PubMed] [Google Scholar]
- 41.Eghtedari M, Liopo AV, Copland JA, Oraevslty AA, Motamedi M. Engineering of hetero-functional gold nanorods for the in vivo molecular targeting of breast cancer cells. Nano Lett. 2009;9:287–91. doi: 10.1021/nl802915q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu CJ, Wang CH, Chien CC, Yang TY, Chen ST, Leng WH, et al. Enhanced X-ray irradiation-induced cancer cell damage by gold nanoparticles treated by a new synthesis method of polyethylene glycol modification. Nanotechnology. 2008;(19):295104. doi: 10.1088/0957-4484/19/29/295104. [DOI] [PubMed] [Google Scholar]
- 43.Nativo P, Prior IA, Brust M. Uptake and intracellular fate of surface-modified gold nanoparticles. ACS Nano. 2008;2:1639–44. doi: 10.1021/nn800330a. [DOI] [PubMed] [Google Scholar]
- 44.Zahr AS, Davis CA, Pishko MV. Macrophage uptake of coreshell nanoparticles surface modified with poly(ethylene glycol) Langmuir. 2006;22:8178–85. doi: 10.1021/la060951b. [DOI] [PubMed] [Google Scholar]
- 45.Gu YJ, Cheng JP, Lin CC, Lam YW, Cheng SH, Wong WT. Nuclear penetration of surface functionalized gold nanoparticles. Toxicol Appl Pharmacol. 2009;237:196–204. doi: 10.1016/j.taap.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 46.Jackson AM, Myerson JW, Stellacci F. Spontaneous assembly of subnanometre-ordered domains in the ligand shell of monolayer-protected nanoparticles. Nat Mater. 2004;3:330–6. doi: 10.1038/nmat1116. [DOI] [PubMed] [Google Scholar]
- 47.Verma A, Uzun O, Hu YH, Hu Y, Han HS, Watson N, et al. Surface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticles. Nat Mater. 2008;7:588–95. doi: 10.1038/nmat2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cesbron Y, Shaw CP, Birchall JP, Free P, Levy R. Stripy nanoparticles revisited; 2010. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chithrani BD, Ghazani AA, Chan WCW. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662–8. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 50.Lu F, Wu SH, Hung Y, Mou CY. Size effect on cell uptake in well-suspended, uniform mesoporous silica nanoparticles. Small. 2009;5:1408–13. doi: 10.1002/smll.200900005. [DOI] [PubMed] [Google Scholar]
- 51.Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J. 2004;377:159–69. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuo CW, Lai AJ, Wei KH, Chen P. Studies of surface-modified gold nanowires inside living cells. Adv Funct Mater. 2007;17:3707–14. [Google Scholar]
- 53.Hartig SM, Greene RR, Carlesso G, Higginbotham JN, Khan WN, Prokop A, et al. Kinetic analysis of nanoparticulate polyelectrolyte complex interactions with endothelial cells. Biomaterials. 2007;28:3843–55. doi: 10.1016/j.biomaterials.2007.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jin H, Heller DA, Strano MS. Single-particle tracking of endocytosis and exocytosis of single-walled carbon nanotubes in NIH-3T3 cells. Nano Lett. 2008;8:1577–85. doi: 10.1021/nl072969s. [DOI] [PubMed] [Google Scholar]
- 55.Salmaso S, Caliceti P, Amendola V, Meneghetti M, Magnusson JP, Pasparakis G, et al. Cell up-take control of gold nanoparticles functionalized with a thermoresponsive polymer. J Mater Chem. 2009;19:1608–15. [Google Scholar]
- 56.Alberola AP, Radler JO. The defined presentation of nanoparticles to cells and their surface controlled uptake. Biomaterials. 2009;30:3766–70. doi: 10.1016/j.biomaterials.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 57.Chithrani BD, Chan WCW. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007;7:1542–50. doi: 10.1021/nl070363y. [DOI] [PubMed] [Google Scholar]
- 58.Champion JA, Mitragotri S. Shape induced inhibition of phagocytosis of polymer particles. Pharm Res. 2009;26:244–9. doi: 10.1007/s11095-008-9626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gratton SEA, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, et al. The effect of particle design on cellular internalization pathways. Proc Nat Acad Sci USA. 2008;105:11613–8. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao X, Kim KS, Liu DX. Nonviral gene delivery: what we know and what is next. AAPS J. 2007;9:E92–E104. doi: 10.1208/aapsj0901009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Derossi D, Joliot AH, Chassaing G, Prochiantz A. The 3rd helix of the antennapedia homeodomain translocates through biological-membranes. J Biol Chem. 1994;269:10444–50. [PubMed] [Google Scholar]
- 62.Vives E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997;272:16010–17. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- 63.Fischer R, Fotin-Mleczek M, Hufnagel H, Brock R. Break on through to the other side - biophysics and cell biology shed light on cell-penetrating peptides. Chembiochem. 2005;6:2126–42. doi: 10.1002/cbic.200500044. [DOI] [PubMed] [Google Scholar]
- 64.Pujals S, Fernandez-Carneado J, Lopez-Iglesias C, Kogan MJ, Giralt E. Mechanistic aspects of CPP-mediated intracellular drug delivery: relevance of CPP self-assembly. Biochim Biophys Acta - Biomembranes. 2006;1758:264–79. doi: 10.1016/j.bbamem.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 65.Heitz F, Morris MC, Divita G. Twenty years of cell-penetrating peptides: from molecular mechanisms to therapeutics. Br J Pharmacol. 2009;157:195–206. doi: 10.1111/j.1476-5381.2009.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee HL, Dubikovskaya EA, Hwang H, Semyonov AN, Wang H, Jones LR, et al. Single-molecule motions of oligoarginine transporter conjugates on the plasma membrane of Chinese hamster ovary cells. J Am Chem Soc. 2008;130:9364–70. doi: 10.1021/ja710798b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duchardt F, Fotin-Mleczek M, Schwarz H, Fischer R, Brock R. A comprehensive model for the cellular uptake of cationic cell-penetrating peptides. Traffic. 2007;8:848–66. doi: 10.1111/j.1600-0854.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- 68.Berry CC. Intracellular delivery of nanopartides via the HIV-1 tat pepticle. Nanomedicine. 2008;3:357–65. doi: 10.2217/17435889.3.3.357. [DOI] [PubMed] [Google Scholar]
- 69.Derossi D, Calvet S, Trembleau A, Brunissen A, Chassaing G, Prochiantz A. Cell internalization of the third helix of the antennapedia homeodomain is receptor-independent. J Biol Chem. 1996;271:18188–93. doi: 10.1074/jbc.271.30.18188. [DOI] [PubMed] [Google Scholar]
- 70.Ruan G, Agrawal A, Marcus AI, Nie S. Imaging and tracking of tat peptide-conjugated quantum dots in living cells: new insights into nanoparticle uptake, intracellular transport, and vesicle shedding. J Am Chem Soc. 2007;129:14759–66. doi: 10.1021/ja074936k. [DOI] [PubMed] [Google Scholar]
- 71.de la Fuente JM, Berry CC. Tat peptide as an efficient molecule to translocate gold nanoparticles into the cell nucleus. Bioconj Chem. 2005;16:1176–80. doi: 10.1021/bc050033+. [DOI] [PubMed] [Google Scholar]
- 72.Pujals S, Bastus NG, Pereiro E, Lopez-Iglesias C, Punte VF, Kogan MJ, et al. Shuttling gold nanoparticles into tumoral cells with an amphipathic proline-rich peptide. Chembiochem. 2009;10:1025–31. doi: 10.1002/cbic.200800843. [DOI] [PubMed] [Google Scholar]
- 73.Webster A, Compton SJ, Aylott JW. Optical calcium sensors: development of a generic method for their introduction to the cell using conjugated cell penetrating peptides. Analyst. 2005;130:163–70. doi: 10.1039/b413725f. [DOI] [PubMed] [Google Scholar]
- 74.Liu YL, Shipton MK, Ryan J, Kaufman ED, Franzen S, Feldheim DL. Synthesis, stability, and cellular internalization of gold nanoparticles containing mixed peptide-poly(ethylene glycol) monolayers. Anal Chem. 2007;79:2221–9. doi: 10.1021/ac061578f. [DOI] [PubMed] [Google Scholar]
- 75.Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ, et al. Cell-penetrating peptides - a reevaluation of the mechanism of cellular uptake. J Biol Chem. 2003;278:585–90. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- 76.Mandal D, Maran A, Yaszemski MJ, Bolander ME, Sarkar G. Cellular uptake of gold nanoparticles directly cross-linked with carrier peptides by osteosarcoma cells. J Mater Sci - Mater Med. 2009;20:347–50. doi: 10.1007/s10856-008-3588-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feldherr CM, Lanford RE, Akin D. Signal-mediated nuclear transport in Simian-virus 40-transformed cells is regulated by large tumor-antigen. Proc Nat Acad Sci USA. 1992;89:11002–5. doi: 10.1073/pnas.89.22.11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tkachenko AG, Xie H, Coleman D, Glomm W, Ryan J, Anderson MF, et al. Multifunctional gold nanoparticle-peptide complexes for nuclear targeting. J Am Chem Soc. 2003;125:4700–1. doi: 10.1021/ja0296935. [DOI] [PubMed] [Google Scholar]
- 79.Tkachenko AG, Xie H, Liu YL, Coleman D, Ryan J, Glomm WR, et al. Cellular trajectories of peptide-modified gold particle complexes: comparison of nuclear localization signals and peptide transduction domains. Bioconj Chem. 2004;15:482–90. doi: 10.1021/bc034189q. [DOI] [PubMed] [Google Scholar]
- 80.Cotten M, Wagner E, Birnstiel ML. Receptor-mediated transport of DNA into eukaryotic cells. Method Enzymol. 1993;217:618–44. doi: 10.1016/0076-6879(93)17092-j. [DOI] [PubMed] [Google Scholar]
- 81.Yang PH, Sun XS, Chiu JF, Sun HZ, He QY. Transferrinmediated gold nanoparticle cellular uptake. Bioconj Chem. 2005;16:494–6. doi: 10.1021/bc049775d. [DOI] [PubMed] [Google Scholar]
- 82.Li JL, Wang L, Liu XY, Zhang ZP, Guo HC, Liu WM, et al. In vitro cancer cell imaging and therapy using transferrinconjugated gold nanoparticles. Cancer Lett. 2009;274:319–26. doi: 10.1016/j.canlet.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 83.Thomas M, Klibanov AM. Non-viral gene therapy: polycation- mediated DNA delivery. Appl Microbiol Biotechnol. 2003;62:27–34. doi: 10.1007/s00253-003-1321-8. [DOI] [PubMed] [Google Scholar]
- 84.Song WJ, Du JZ, Sun TM, Zhang PZ, Wang J. Gold nanoparticles capped with polyethylenemine for enhanced siRNA delivery. Small. 2009;6:239–46. doi: 10.1002/smll.200901513. [DOI] [PubMed] [Google Scholar]
- 85.Sandhu KK, McIntosh CM, Simard JM, Smith SW, Rotello VM. Gold nanoparticle-mediated transfection of mammalian cells. Bioconj Chem. 2002;13:3–6. doi: 10.1021/bc015545c. [DOI] [PubMed] [Google Scholar]
- 86.See V, Free P, Cesbron Y, Nativo P, Shaheen U, Rigden DJ, et al. Cathepsin L digestion of nanobioconjugates upon endocytosis. ACS Nano. 2009;3:2461–8. doi: 10.1021/nn9006994. [DOI] [PubMed] [Google Scholar]
- 87.Simoes S, Filipe A, Faneca H, Mano M, Penacho N, Duzgunes N, et al. Cationic liposomes for gene delivery. Expert Opin Drug Deliv. 2005;2:237–54. doi: 10.1517/17425247.2.2.237. [DOI] [PubMed] [Google Scholar]
- 88.Straubinger RM, Hong K, Friend DS, Papahadjopoulos D. Endocytosis of liposomes and intracellular fate of encapsulated molecules - encounter with a low pH compartment after internalization in coated vesicles. Cell. 1983;32:1069–79. doi: 10.1016/0092-8674(83)90291-x. [DOI] [PubMed] [Google Scholar]
- 89.Chithrani DB, Dunne M, Stewart J, Allen C, Jaffray DA. Cellular uptake and transport of gold nanoparticles incorporated in a liposomal carrier. Nanomedicine. 2009;6:161–9. doi: 10.1016/j.nano.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 90.Barry ELR, Gesek FA, Friedman PA. Introduction of antisense oligonucleotides into cells by permeabilization with streptolysin-O. Biotechniques. 1993;15:1016–20. [PubMed] [Google Scholar]
- 91.Bhakdi S, Bayley H, Valeva A, Walev I, Walker B, Weller U, et al. Staphylococcal alpha-toxin, streptolysin-O, and Escherichia coli hemolysin: prototypes of pore-forming bacterial cytolysins. Arch Microbiol. 1996;165:73–9. doi: 10.1007/s002030050300. [DOI] [PubMed] [Google Scholar]
- 92.Bhakdi S, Tranumjensen J, Sziegoleit A. Mechanism of membrane damage by streptolysin-O. Infect Immun. 1985;47:52–60. doi: 10.1128/iai.47.1.52-60.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dokka S, Rojanasakul Y. Novel non-endocytic delivery of antisense oligonucleotides. Adv Drug Deliv Rev. 2000;44:35–49. doi: 10.1016/s0169-409x(00)00082-x. [DOI] [PubMed] [Google Scholar]
- 94.Spiller DG, Tidd DM. Nuclear delivery of antisense oligodeoxynucleotides through reversible permeabilization of human leukemia-cells with streptolysin-O. Antisense Res Dev. 1995;5:13–21. doi: 10.1089/ard.1995.5.13. [DOI] [PubMed] [Google Scholar]
- 95.Walev I, Bhakdi SC, Hofmann F, Djonder N, Valeva A, Aktories K, et al. Delivery of proteins into living cells by reversible membrane permeabilization with streptolysin-O. Proc Nat Acad Sci USA. 2001;98:3185–90. doi: 10.1073/pnas.051429498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boutimah-Hamoudi F, Leforestier E, Senamaud-Beaufort C, Nielsen PE, Giovannangeli C, Saison-Behmoaras TE. Cellular antisense activity of peptide nucleic acid (PNAs) targeted to HIV-1 polypurine tract (PPT) containing RNA. Nucleic Acids Res. 2007;35:3907–17. doi: 10.1093/nar/gkm374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Provoda CJ, Lee KD. Bacterial pore-forming hemolysins and their use in the cytosolic delivery of macromolecules. Adv Drug Deliv Rev. 2000;41:209–21. doi: 10.1016/s0169-409x(99)00067-8. [DOI] [PubMed] [Google Scholar]
- 98.Beauregard KE, Lee KD, Collier RJ, Swanson JA. pH-dependent perforation of macrophage phagosomes by listeriolysin O from Listeria monocytogenes. J Exp Med. 1997;186:1159–63. doi: 10.1084/jem.186.7.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Glomski IJ, Decatur AL, Portnoy DA. Listeria monocytogenes mutants that fail to compartmentalize listerolysin O activity are cytotoxic, avirulent, and unable to evade host extracellular defenses. Infect Immun. 2003;71:6754–65. doi: 10.1128/IAI.71.12.6754-6765.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kumar S, Harrison N, Richards-Kortum R, Sokolov K. Plasmonic nanosensors for imaging intracellular biomarkers in live cells. Nano Lett. 2007;7:1338–43. doi: 10.1021/nl070365i. [DOI] [PubMed] [Google Scholar]
- 101.Soman NR, Marsh JN, Lanza GM, Wickline SA. New mechanisms for non-porative ultrasound stimulation of cargo delivery to cell cytosol with targeted perfluorocarbon nanoparticles. Nanotechnology. 2008;19:185102. doi: 10.1088/0957-4484/19/18/185102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou Y, Kumon RE, Cui J, Deng CX. The size of sonoporation pores on the cell membrane. Ultrasound Med Biol. 2009;35:1756–60. doi: 10.1016/j.ultrasmedbio.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lentacker I, Geers B, Demeester J, De Smedt SC, Sanders NN. Design and evaluation of doxorubicin-containing microbubbles for ultrasound-triggered doxorubicin delivery: cytotoxicity and mechanisms involved. Mol Ther. 2009 doi: 10.1038/mt.2009.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Otani K, Yamahara K, Ohnishi S, Obata H, Kitamura S, Nagaya N. Nonviral delivery of siRNA into mesenchymal stem cells by a combination of ultrasound and microbubbles. J Control Release. 2009;133:146–53. doi: 10.1016/j.jconrel.2008.09.088. [DOI] [PubMed] [Google Scholar]
- 105.Mehier-Humbert S, Bettinger T, Yan F, Guy RH. Ultrasoundmediated gene delivery: kinetics of plasmid internalization and gene expression. J Control Release. 2005;104:203–11. doi: 10.1016/j.jconrel.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 106.Kinoshita M, Hynynen K. Intracellular delivery of Bak BH3 peptide by microbubble-enhanced ultrasound. Pharm Res. 2005;22:716–20. doi: 10.1007/s11095-005-2586-7. [DOI] [PubMed] [Google Scholar]
- 107.Klein TM, Wolf ED, Wu R, Sanford JC. High-velocity microprojectiles for delivering nucleic-acids into living cells. Nature. 1987;327:70–3. [PubMed] [Google Scholar]
- 108.Chang ML, Chen JC, Yeh CT, Chang MY, Liang CK, Chiu CT, et al. Gene gun bombardment with DNA-coated gold particles is a potential alternative to hydrodynamics-based transfection for delivering genes into superficial hepatocytes. Hum Gene Ther. 2008;19:391–5. doi: 10.1089/hum.2007.152. [DOI] [PubMed] [Google Scholar]
- 109.Webster A, Coupland P, Houghton FD, Leese HJ, Aylott JW. The delivery of PEBBLE nanosensors to measure the intracellular environment. Biochem Soc Trans. 2007;35:538–43. doi: 10.1042/BST0350538. [DOI] [PubMed] [Google Scholar]
- 110.Zhang Y, Yu LC. Single-cell microinjection technology in cell biology. Bioessays. 2008;30:606–10. doi: 10.1002/bies.20759. [DOI] [PubMed] [Google Scholar]
- 111.McDougall C, Stevenson DJ, Brown CTA, Gunn-Moore F, Dholakia K. Targeted optical injection of gold nanoparticles into single mammalian cells. J Biophoton. 2009;2:736–43. doi: 10.1002/jbio.200910030. [DOI] [PubMed] [Google Scholar]
- 112.Yao CP, Rahmanzadeh R, Endl E, Zhang ZX, Gerdes J, Huttmann G. Elevation of plasma membrane permeability by laser irradiation of selectively bound nanoparticles. J Biomed Opt. 2005;(10):064012. doi: 10.1117/1.2137321. [DOI] [PubMed] [Google Scholar]
- 113.Luccardini C, Yakovlev A, Gaillard S, van't Hoff M, Alberola AP, Mallet JM, et al. Getting across the plasma membrane and beyond: intracellular uses of colloidal semiconductor nanocrystals. J Biomed Biotechnol. 2007:68963. doi: 10.1155/2007/68963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ciftci K, Levy RJ. Enhanced plasmid DNA transfection with lysosomotropic agents in cultured fibroblasts. Int J Pharm. 2001;218:81–92. doi: 10.1016/s0378-5173(01)00623-8. [DOI] [PubMed] [Google Scholar]
- 115.Maiolo JR, Ottinger EA, Ferrer M. Specific redistribution of cell-penetrating peptides from endosomes to the cytoplasm and nucleus upon laser illumination. J Am Chem Soc. 2004;126:15376–7. doi: 10.1021/ja044867z. [DOI] [PubMed] [Google Scholar]
- 116.Skehel JJ, Cross K, Steinhauer D, Wiley DC. Influenza fusion peptides. Biochem Soc Trans. 2001;29:623–6. doi: 10.1042/bst0290623. [DOI] [PubMed] [Google Scholar]
- 117.Wadia JS, Stan RV, Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat Med. 2004;10:310–5. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- 118.Michiue H, Tomizawa K, Wei FY, Matsushita M, Lu YF, Ichikawa T, et al. The NH2 terminus of influenza virus hemagglutinin-2 subunit peptides enhances the antitumor potency of polyarginine-mediated p53 protein transduction. J Biol Chem. 2005;280:8285–9. doi: 10.1074/jbc.M412430200. [DOI] [PubMed] [Google Scholar]
- 119.Fretz MM, Mastrobattista E, Koning GA, Jiskoot W, Storm G. Strategies for cytosolic delivery of liposomal macromolecules. Int J Pharm. 2005;298:305–9. doi: 10.1016/j.ijpharm.2005.02.040. [DOI] [PubMed] [Google Scholar]
- 120.Yamada Y, Akita H, Kamiya H, Kogure K, Yamamoto T, Shinohara Y, et al. MITO-Porter: a liposome-based carrier system for delivery of macromolecules into mitochondria via membrane fusion. Biochim Biophys Acta - Biomembranes. 2008;1778:423–32. doi: 10.1016/j.bbamem.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 121.Song Y, Xu XY, MacRenaris KW, Zhang XQ, Mirkin CA, Meade TJ. Multimodal gadolinium-enriched DNA-gold nanoparticle conjugates for cellular imaging. Angew Chem - Int Ed. 2009;48:9143–7. doi: 10.1002/anie.200904666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dulkeith E, Ringler M, Klar TA, Feldmann J, Javier AM, Parak WJ. Gold nanoparticles quench fluorescence by phase induced radiative rate suppression. Nano Lett. 2005;5:585–9. doi: 10.1021/nl0480969. [DOI] [PubMed] [Google Scholar]
- 123.Zhang J, Fu Y, Chowdhury MH, Lakowicz JR. Singlemolecule studies on fluorescently labeled silver particles: effects of particle size. J Phys Chem C. 2007;112:18–26. doi: 10.1021/jp074938r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Anger P, Bharadwaj P, Novotny L. Enhancement and quenching of single-molecule fluorescence. Phys Rev Lett. 2006;96:113002. doi: 10.1103/PhysRevLett.96.113002. [DOI] [PubMed] [Google Scholar]
- 125.Hong R, Han G, Fernandez JM, Kim BJ, Forbes NS, Rotello VM. Glutathione-mediated delivery and release using monolayer protected nanoparticle carriers. J Am Chem Soc. 2006;128:1078–9. doi: 10.1021/ja056726i. [DOI] [PubMed] [Google Scholar]
- 126.Saha A, Basiruddin SK, Sarkar R, Pradhan N, Jana NR. Functionalized plasmonic-fluorescent nanoparticles for imaging and detection. J Phys Chem C. 2009;113:18492–8. [Google Scholar]
- 127.Shukla R, Bansal V, Chaudhary M, Basu A, Bhonde RR, Sastry M. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: a microscopic overview. Langmuir. 2005;21:10644–54. doi: 10.1021/la0513712. [DOI] [PubMed] [Google Scholar]
- 128.Schaeffer N, Tan B, Dickinson C, Rosseinsky MJ, Laromaine A, McComb DW, et al. Fluorescent or not? Size-dependent fluorescence switching for polymer-stabilized gold clusters in the 1.1–1.7 nm size range. Chem Commun. 2008:3986–8. doi: 10.1039/b809876j. [DOI] [PubMed] [Google Scholar]
- 129.Lin CAJ, Yang TY, Lee CH, Huang SH, Sperling RA, Zanella M, et al. Synthesis, characterization, and bioconjugation of fluorescent gold nanoclusters toward biological labeling applications. ACS Nano. 2009;3:395–401. doi: 10.1021/nn800632j. [DOI] [PubMed] [Google Scholar]
- 130.Lin SY, Chen NT, Sum SP, Lo LW, Yang CS. Ligand exchanged photoluminescent gold quantum dots functionalized with leading peptides for nuclear targeting and intracellular imaging. Chem Commun. 2008;39:4762–4. doi: 10.1039/b808207c. [DOI] [PubMed] [Google Scholar]
- 131.Liu CL, Ho ML, Chen YC, Hsieh CC, Lin YC, Wang YH, et al. Thiol-functionalized gold nanodots: two-photon absorption property and imaging in vitro. J Phys Chem C. 2009;113:21082–9. [Google Scholar]
- 132.Curry AC, Crow M, Wax A. Molecular imaging of epidermal growth factor receptor in live cells with refractive index sensitivity using dark-field microspectroscopy and immunotargeted nanoparticles. J Biomed Opt. 2008:13. doi: 10.1117/1.2837450. [DOI] [PubMed] [Google Scholar]
- 133.Louit G, Asahi T, Tanaka G, Uwada T, Masuhara H. Spectral and 3-dimensional tracking of single gold nanoparticles in living cells studied by Rayleigh light scattering microscopy. J Phys Chem C. 2009;113:11766–72. [Google Scholar]
- 134.Aaron J, Nitin N, Travis K, Kumar S, Collier T, Park SY, et al. Plasmon resonance coupling of metal nanoparticles for molecular imaging of carcinogenesis in vivo. J Biomed Opt. 2007;12:034007. doi: 10.1117/1.2737351. [DOI] [PubMed] [Google Scholar]
- 135.Sonnichsen C, Reinhard BM, Liphardt J, Alivisatos AP. A molecular ruler based on plasmon coupling of single gold and silver nanoparticles. Nat Biotechnol. 2005;23:741–5. doi: 10.1038/nbt1100. [DOI] [PubMed] [Google Scholar]
- 136.Rechberger W, Hohenau A, Leitner A, Krenn JR, Lamprecht B, Aussenegg FR. Optical properties of two interacting gold nanoparticles. Opt Commun. 2003;220:137–41. [Google Scholar]
- 137.Aaron J, Travis K, Harrison N, Sokolov K. Dynamic imaging of molecular assemblies in live cells based on nanoparticle plasmon resonance coupling. Nano Lett. 2009;9:3612–8. doi: 10.1021/nl9018275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jun YW, Sheikholeslami S, Hostetter DR, Tajon C, Craik CS, Alivisatos AP. Continuous imaging of plasmon rulers in live cells reveals early-stage caspase-3 activation at the singlemolecule level. Proc Nat Acad Sci USA. 2009;106:17735–40. doi: 10.1073/pnas.0907367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Seferos DS, Giljohann DA, Hill HD, Prigodich AE, Mirkin CA. Nano-flares: probes for transfection and mRNA detection in living cells. J Am Chem Soc. 2007;129:15477–9. doi: 10.1021/ja0776529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Choi Y, Park Y, Kang T, Lee LP. Selective and sensitive detection of metal ions by plasmonic resonance energy transfer-based nanospectroscopy. Nat Nanotechnol. 2009;4:742–6. doi: 10.1038/nnano.2009.258. [DOI] [PubMed] [Google Scholar]
- 141.Kneipp J, Kneipp H, Wittig B, Kneipp K. Novel optical nanosensors for probing and imaging live cells; Nanomed Nanotechnol Biol Med; 2009. in press. [DOI] [PubMed] [Google Scholar]
- 142.Xie W, Wang L, Zhang YY, Su L, Shen AG, Tan JQ, et al. Nuclear targeted nanoprobe for single living cell detection by surface-enhanced Raman scattering. Bioconj Chem. 2009;20:768–73. doi: 10.1021/bc800469g. [DOI] [PubMed] [Google Scholar]
- 143.Qian XM, Peng XH, Ansari DO, Yin-Goen Q, Chen GZ, Shin DM, et al. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat Biotechnol. 2008;26:83–90. doi: 10.1038/nbt1377. [DOI] [PubMed] [Google Scholar]
- 144.Fujita K, Ishitobi S, Hamada K, Smith NI, Taguchi A, Inouye Y, et al. Time-resolved observation of surface-enhanced Raman scattering from gold nanoparticles during transport through a living cell. J Biomed Opt. 2009;14:024038. doi: 10.1117/1.3119242. [DOI] [PubMed] [Google Scholar]
- 145.Sun W, Wang GF, Fang N, Yeung ES. Wavelength-dependent differential interference contrast microscopy: selectively imaging nanoparticle probes in live cells. Anal Chem. 2009;81:9203–8. doi: 10.1021/ac901623b. [DOI] [PubMed] [Google Scholar]
- 146.Berciaud S, Cognet L, Blab GA, Lounis B. Photothermal heterodyne imaging of individual nonfluorescent nanoclusters and nanocrystals. Phys Rev Lett. 2004;93:257402. doi: 10.1103/PhysRevLett.93.257402. [DOI] [PubMed] [Google Scholar]
- 147.Berciaud S, Lasne D, Blab GA, Cognet L, Lounis B. Photothermal heterodyne imaging of individual metallic nanoparticles: theory versus experiment. Phys Rev B. 2006;73:045424. [Google Scholar]
- 148.Berciaud S, Cognet L, Poulin P, Weisman RB, Lounis B. Absorption spectroscopy of individual single-walled carbon nanotubes. Nano Lett. 2007;7:1203–7. doi: 10.1021/nl062933k. [DOI] [PubMed] [Google Scholar]
- 149.Berciaud S, Cognet L, Lounis B. Photothermal absorption spectroscopy of individual semiconductor nanocrystals. Nano Lett. 2005;5:2160–3. doi: 10.1021/nl051805d. [DOI] [PubMed] [Google Scholar]
- 150.Cognet L, Tardin C, Boyer D, Choquet D, Tamarat P, Lounis B. Single metallic nanoparticle imaging for protein detection in cells. Proc Nat Acad Sci USA. 2003;100:11350–5. doi: 10.1073/pnas.1534635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lasne D, Blab GA, Berciaud S, Heine M, Groc L, Choquet D, et al. Single nanoparticle photothermal tracking (SNaPT) of 5- nm gold beads in live cells. Biophys J. 2006;91:4598–604. doi: 10.1529/biophysj.106.089771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Octeau V, Cognet L, Duchesne L, Lasne D, Schaeffer N, Fernig DG, et al. Photothermal absorption correlation spectroscopy. ACS Nano. 2009;3:345–50. doi: 10.1021/nn800771m. [DOI] [PubMed] [Google Scholar]
- 153.Copland JA, Eghtedari M, Popov VL, Kotov N, Mamedova N, Motamedi M, et al. Bioconjugated gold nanoparticles as a molecular based contrast agent: implications for imaging of deep tumors using optoacoustic tomography. Mol Imaging Biol. 2004;6:341–9. doi: 10.1016/j.mibio.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 154.Zhang HF, Maslov K, Stoica G, Wang LHV. Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging. Nat Biotechnol. 2006;24:848–51. doi: 10.1038/nbt1220. [DOI] [PubMed] [Google Scholar]
- 155.Zhang Q, Iwakuma N, Sharma P, Moudgil BM, Wu C, McNeill J, et al. Gold nanoparticles as a contrast agent for in vivo tumor imaging with photoacoustic tomography. Nanotechnology. 2009;20:395102. doi: 10.1088/0957-4484/20/39/395102. [DOI] [PubMed] [Google Scholar]
- 156.Zhu ZJ, Ghosh PS, Miranda OR, Vachet RW, Rotello VM. Multiplexed screening of cellular uptake of gold nanoparticles using laser desorption/ionization mass spectrometry. J Am Chem Soc. 2008;130:14139–43. doi: 10.1021/ja805392f. [DOI] [PMC free article] [PubMed] [Google Scholar]