Abstract
Iron oxide-based nanomagnets have attracted a great deal of attention in nanomedicine over the past decade. Down to the nanoscale, superparamagnetic iron oxide nanoparticles can only be magnetized in the presence of an external magnetic field, which makes them capable of forming stable colloids in a physio-biological medium. Their superparamagnetic property, together with other intrinsic properties, such as low cytotoxicity, colloidal stability, and bioactive molecule conjugation capability, makes such nanomagnets ideal in both in-vitro and in-vivo biomedical applications. In this review, a chemical, physical, and biological synthetic approach to prepare iron oxide-based nanomagnets with different physicochemical properties was illustrated and compared. The growing interest in iron oxide-based nanomagnets with multifunctionalities was explored in cancer diagnostics and treatment, focusing on their combined roles in a magnetic resonance contrast agent, hyperthermia, and magnetic force assisted drug delivery. Iron oxides as magnetic carriers in gene therapy were reviewed with a focus on the sophisticated design and construction of magnetic vectors. Finally, the iron oxide-based nanomagnet also represents a very promising tool in particle/cell interfacing in controlling cellular functionalities, such as adhesion, proliferation, differentiation, and cell patterning, in stem cell therapy and tissue engineering applications.
Keywords: iron oxide, coprecipitation, thermal decomposition, microemulsion, magnetosome, lithography, cancer targeting, stem cell, gene delivery, tissue engineering, cell actuation
A brief history of iron oxide research
Iron oxides are a collective term for oxides, hydroxides, and oxy-hydroxides composed of Fe(II) and/or Fe(III) cations and O2− and/or OH− anions. The understanding and applications of iron oxide as an important mineral originated thousands of years ago (1). In ancient China, magnetic compass was invented and used by many navigators to determine the direction of north pole of Earth. The term ‘magnetite’ originated from the district of Magnesia in Asia Minor, where plenty of such mineral exist.
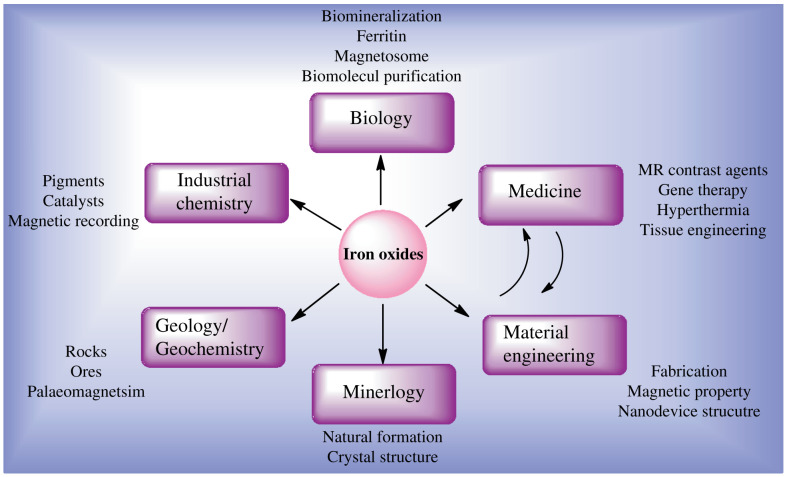
Nowadays, there is widespread research on iron oxides across many scientific disciplines, including both fundamental research and applications, such as mineralogy, biology, geology, chemistry, and medicine, as shown in Fig. 1. In modern mineralogy, for instance, the crystal structures, properties, and formation of iron oxide nanoparticles are well characterized. In industrial chemistry technology, iron oxide applications in painting pigments, catalysts, and magnetic recording are explored and refined. Interestingly, bio-mineralization of crystalline magnetite in Magnetobactericum sp. attracted enormous attention in nanomedicine due to its narrow size distribution and magnetic properties (2). Such multidisciplinary research has led to a very fruitful and much deeper understanding of iron oxides and many potential applications have been proposed and studied, from which this work originated and benefited. In this review, we focus on the synthesis of iron oxide nanoparticle-based multifunctional nanomagnets in nanomedicine, to illustrate how the advanced nano-device benefits the development of nanomedicine and how fundamental biomedical research with nanoparticles influences nanotechnology.
Fig. 1.
The multidisciplinary nature of modern research in iron oxides, with a focus on the strong influential effect between medical applications and material engineering.
Synthesis of nanomagnets
Chemical-based synthesis
Coprecipitation
Alkaline coprecipitation of Fe(III) and Fe(II) salts in aqueous media is the most universally adopted synthetic approach to produce iron oxide nanoparticles, due to its versatility, relatively low budget, feasibility to scale up, and the hydrophilic surface character of the resultants. It is possible to fabricate pure-phase magnetite by controlling the reaction factors, resulting in controlled particle size and morphology (3). Kang et al. (4) reported the preparation of monodispersed magnetite nanoparticles with an average size of 10 nm in aqueous solution. The reaction of magnetite in aqueous media can be written as follows:
| 1 |
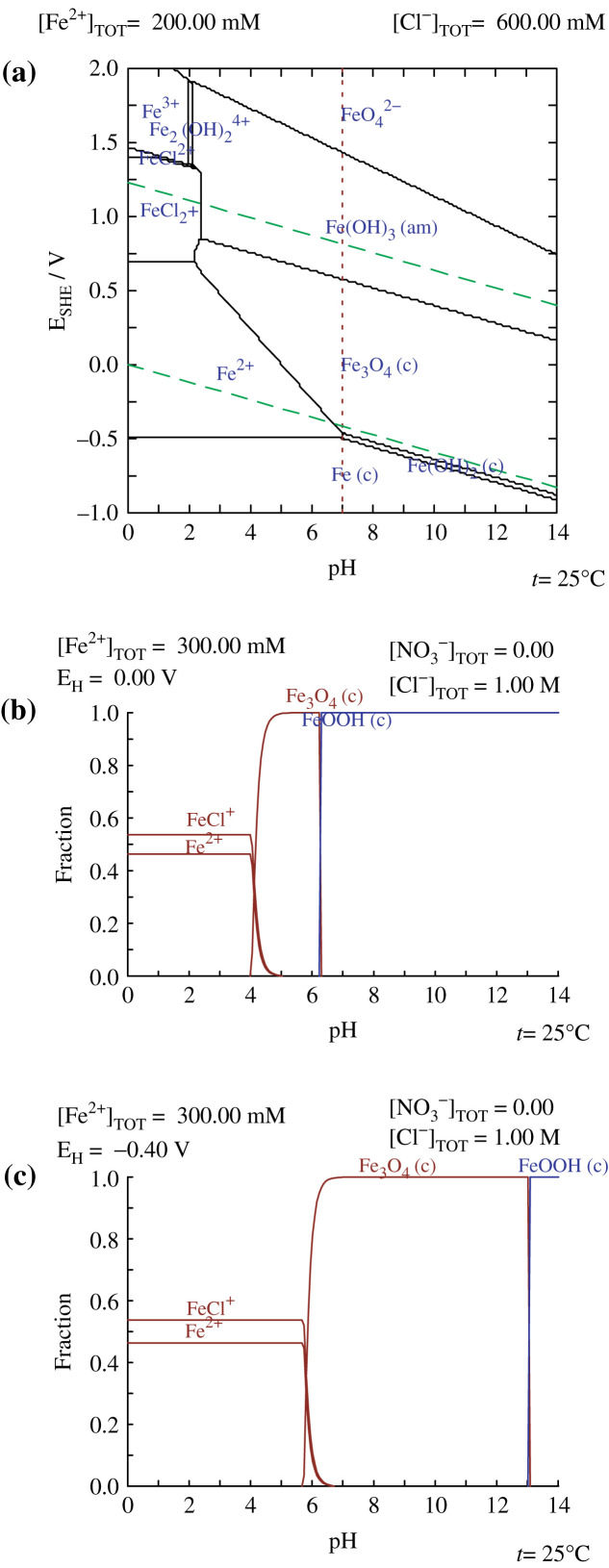
In general, the coprecipitation method was established by ‘trial and error’ approaches in the past decades; only a few research groups reported the systematic study of the chemistry of the reaction system. Babes et al. (5) performed a comprehensive quantitative analysis of different reaction parameters, including composition of the alkaline media, pH, temperature, concentration of iron species, etc. Kim et al. (6, 7) took a step forward to perform a thermodynamic modeling study of the reaction equilibrium involved in the coprecipitation of magnetite in aqueous solution, with theoretical considerations of changes in free energy and redox potential of the iron species. The thermodynamic modeling diagram is shown in Fig. 2.
fig. 2.
Thermodynamic modeling diagram showing: (a) the pH-pE predominance diagram of the Fe-Cl-H2O system at 25°C; (b) the distribution of the fraction of [Fe x+]/[Fe2++Fe3+]vs. pH calculated at 25°C for 0.3 M Fe2+ and 1 M HCl solution: E = 0 V; and (c) E = -0.4 V. Reprinted from Srcripta Mater, Vol. 44, Kim DK et al., Superparamagnetic iron oxide nanoparticles for bio-medical applications, 1714, Copyright (2001), with permission from Elsevier. Reprinted from Chem Mater, Vol. 15, Kim DK et al., Starch-coated superparamagnetic nanoparticles as MR contrast agents, 4350, Copyright (2003), with permission from ACS Publications.
According to these reports, it is possible to obtain magnetite by oxidation of Fe(II) in solution, as the reaction can be written as follows:
| 2 |
| 3 |
It is essential to adjust the stoichiometric ratio of Fe(II)/2Fe(III) at the initial stage because it is quite difficult to control the oxidation kinetics of Fe(II) afterward. Oxygen also plays an important role in the formation of single-phase magnetite; thus, bubbling the nitrogen gas directly into the media prior to reaction is efficient enough for removal of oxygen. Kang et al. deliberately transformed magnetite nanoparticles into maghemite by aeration (oxidation) at 100°C, which can be written as follows (4):
| 4 |
Interestingly, the morphologies of the particle could be tuned by different types of alkaline media: Babes et al. reported the preparation of well-faceted particles using the weak alkaline of tetramethylammonium hydroxide (TMAOH) (5); while Kang et al. prepared spherical magnetite particles using the strong alkaline of ammonium hydroxide or sodium hydroxide (4). Babes et al. demonstrated that the formation of spherical particles is because the nucleation rate per unit area is isotopic at the interface of the iron oxides and reactant solution (5). Therefore, particle shape can be tuned by controlling the rate of nucleation per unit area with the alkaline ionic strength.
Better size, morphology, and colloidal dispersion was realized based on a modified coprecipitation synthesis, by applying the principle of nucleation in highly constrained domains. Kim et al. (8) prepared spherical-shaped superparamagnetic iron oxide nanoparticles (SPIONs) with an average size of 7.2 nm within a polymeric starch matrix, in contrast with uncoated SPIONs with an average size of 12 nm. Lin et al. reported completely separated dextran-coated magnetic nanoparticles by chemical cleavage of the dextran structure with diamine molecules, resulting in monodispersed SPIONs with an average diameter of 4.5 nm in aqueous media.(3)
Microemulsion/nanoemulsion (nE)
Microemulsion (µE) is defined as the thermodynamically stable isotropic dispersion of two immiscible liquids, stabilized by a monolayer of surfactant at the interface of the two liquids. In the water/oil (w/o) µE (or referred to as inverse µE) system, small aqueous nanodroplets are dispersed in the organic phase, and vice versa. The nano-sized water droplets containing iron ions and the alkaline source, act as confined reactors that undergo rapid coalescence and mixing to allow the chemical reaction. Attributed to the confinement of particles nucleation and growth within nano-sized droplets, the µE approach is capable of providing good control over particle size and size distribution. Santra et al. (9) performed the parametric study on the effect of different surfactants and alkalines in the µE reaction system and reported synthesis of SPIONs as small as 1–2 nm with very narrow size distribution. They had speculated that the absorption of the surfactant onto the particle surfaces was based on weak hydrogen bonding between the terminal hydroxyl group of the non-ionic surfactant and oxygen atoms on iron oxide surfaces. However, the hydrophobic tail of the surfactant often causes interconnection and aggregation of particles in an ordered fashion due to hydrophobic interaction with each other (9).
The µE approach has been explored as a polymerization process for coating polymer or silica onto pre-synthesized iron oxide cores to produce magnetic spheres with tunable shell thickness. Dresco et al. (10) demonstrated the fabrication process of magnetic hydrogel particles consisting of iron oxide cores and poly(methacrylic acid)-co-poly(2-hydroxyethyl methacrylate) (PMA-PHEMA) copolymer shells, with an average size of 80–320 nm, by a two-stage µE method. Yi et al. (11) reported the synthesis of mesoporous silica-coated SPIONs by a single-step reverse µE process, taking advantage of the small size and spherical nature of nanodroplets to produce magnetic silica spheres with tunable silica shell thickness of 1.8–30 nm.
Thermal decomposition
Inspired by the advanced fabrication of semiconductor and metallic nanocrystals (12), the decomposition of organometallic complexes at high temperature was adopted to fabricate high quality and monodispersed metal oxide nanocrystals. Rockenberger et al. (13) reported fabrication of near-monodispersed γ-Fe2O3 nanocrystals by thermal decomposition of iron cupferron complex (FeCup3) in octylamine. Hyeon et al. reported the synthesis of highly crystalline and monodispersed γ-Fe2O3 nanoparticles with one-nanometer-scale size control from 4 to 16 nm, by oxidative decomposition of iron pentacarbonyl (Fe(CO)5) in the presence of oleic acid (OA) as a capping agent and trimethylamine oxide (TMAO) as an oxidative agent in octyl ether as a non-coordinating solvent without size selection process (14, 15). Sun and Zeng (16) succeeded in producing monodispersed magnetite nanocrystals with a controllable size, from 4 to 16 nm, by thermolysis of iron acetylacetonate (Fe(acac)3) in the presence of OA, oleylamine, and alcohol in phenyl ether. Jana et al. (17) reported the synthesis of high-quality magnetite nanocrystals, from a few nanometers up to 50 nm, by adjusting the concentration of the stabilizing ligands and iron precursors during the reaction.
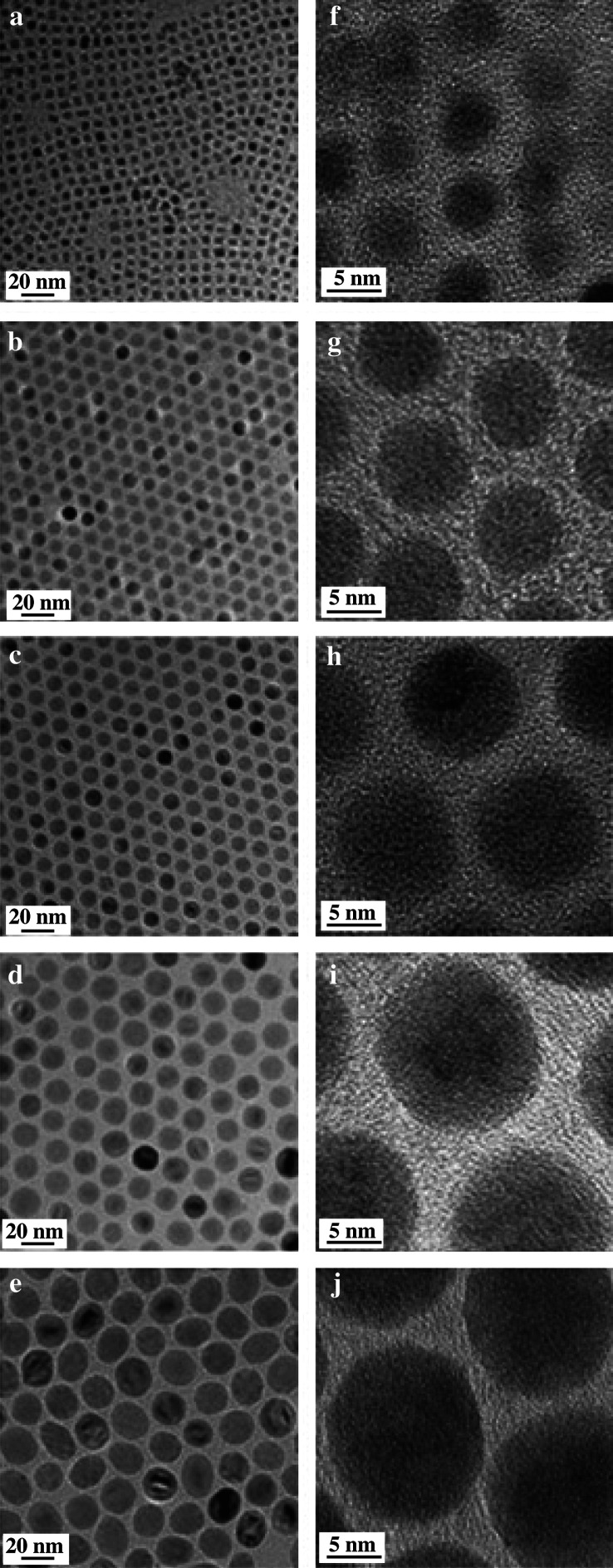
Of all the iron-containing organometallic complexes, both ferric and ferrous fatty acid complexes are the most preferred iron precursors because of their low preparation cost, low toxicity, and feasibility for mass production (18–20). The iron carboxylate can be prepared by neutralization reaction with Fe(III) salts and sodium carboxylate (19) or dissolution of iron oxides or hydroxides in carboxylic acid (18). Hyeon et al. reported an economical and environmentally friendly synthetic approach to prepare size-tunable γ-Fe2O3 nanocrystals up to 22 nm in non-coordinating solvent by adjusting the reaction time and temperature, as shown in Fig. 3.
fig. 3.
TEM images and HR-TEM of iron oxide nanocrystals prepared from thermolysis of iron (III) oleate complexes in non-coordinating solvent with average diameters of (a, f) 5 nm; (b, g) 9 nm; (c, h) 12 nm; (d, i) 16 nm; and (e, j) 22 nm, respectively. Reprinted from Nat Mater, Vol. 3, Park J et al., Ultra-large-scale synthesis of monodisperse nanocrystals, 892, Copyright (2004), with permission from Nature Publishing Group.
Attempts at thermolysis of organometallic complexes in the water miscible solvent at high temperature were made, although scarification of particles occurred. Li et al. (21, 22) chose 2-pyrrrolidone as both passivating ligands and solvents, owing to its ability to form a strong coordination bond with the surface of metallic oxides, relatively high boiling point, and miscibility with water. The same group had improved the particle dispersion and biocompatibility by adding monocarboxyl-terminated poly(ethylene glycol) (PEG) (23).
Physical-based synthesis
Lithography and sputtering
The definition of ‘top-down approach’ in nanomaterials synthesis is to reduce the starting block materials to a desirable nanoscale by controlled etching, elimination, and layering of the materials, which mainly involves lithography (24). Despite being a well-developed method in semiconductor microchip fabrication, lithography techniques are far from satisfactory in the fabrication of nanomaterials. Firstly, such a top-down approach often results in surface crystallographic defect of the prepared nano-patterns, which may seriously affect their physico-chemical properties. Secondly, lithography has its own dimensional limitations because of the limited wavelengths of the ‘light source.’ A nano-feature less than 100 nm is difficult to produce by conventional photolithography, and nano-patterns smaller than 10 nm cannot be produced even by electron beam lithography.
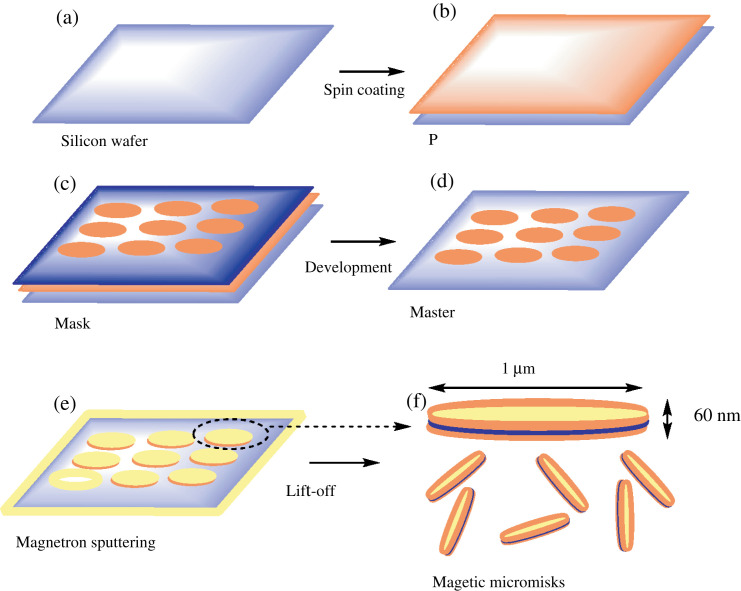
Fig. 4 illustrates a typical step-wise fabrication of a magnetic disk by photolithography and sputtering techniques (25, 26). Firstly, a photoresist layer with a thickness of 1 µm was spin-coated onto a silicon wafer, followed by placement of a mask (1 µm diameter circular dot-arrayed patterns) in contact with the pre-packed photoresist layer. Secondly, UV light was illuminated though the mask for development, and the unexposed photoresist layer was dissolved and removed by the addition of an organic solvent. Finally, magnetron sputtering was used to deposit 5 nm underlayered gold, followed by 60 nm of permalloy (Fe20Ni80), and topped with another 5 nm of gold layer. The disks were released from the wafer by the lift-off process via acetone wash. This approach allows low-cost production of uniformly sized microdisks (MDs) with magnetic spin state in remanence (26).
fig. 4.
The fabrication of microdisks (MDs) by optical lithography and magnetron sputtering. (a,b) The process starts with photoresist spin coating on a silicon wafer; (c) a mask is placed in contact with the layer of pre-packed photoresist and illuminated with UV light. (d) An organic solvent dissolves and removes unexposed photoresist. (e) Finally, magnetron sputtering is used to deposit 5 nm underlayer gold, followed by 60 nm of permalloy, and topped with another 5 nm of gold layer. (f) The disks are released from the wafer by lift-off process. Reprinted from NatMater, Vol. 9, Kim D-H et al., Biofunctionalized magnetic-vortex microdiscs for targeted cancer-cell destruction, supplementary information P3. Copyright (2010), with permission from Nature Publishing Group.
Biological-based synthesis
Magnetosome in magnetotactic bacteria
Magnetotactic bacteria (MTB) and magnetosomes have attracted much attention across many disciplines in the past decade and have been intensively studied as a model system in bio-mineralization mechanism and evolutionary bacteriology (27). Magnetosome, intracellular magnetite (Fe3O4) or greigite (Fe3S4) nanocrystals surrounded by lipid membranes in MTB are of particular interest in bio-nanomedicine, because of their strain-dependent shape and size, intrinsic high saturation magnetization, inherent biocompatibility, and perfect dispersion in biological medium (24). Interestingly, within certain bacteria strains, the particle morphology and size is highly uniform. It has been proposed that the interaction of the magnetosome membrane with the growing nanocrystals facilitates crystal growth in a certain direction, while crystal growth in other directions is retarded, resulting in cuboctahedral, hexagonal, or bullet-shaped magnetosomes (27, 28).
The bio-mineralization process of magnetosomes has been intensively studied and reported by several groups. To generalize the magnetosomes formation procedures, iron ions are firstly transported into the cell via both specific siderophores and/or non-specific mechanisms and then actively deposited within the magnetosome membrane to form a saturated iron ion solution, followed by the redox reaction of iron within the magnetosome membrane (24, 27). It is important to note that Fe(III)/Fe(II) were strictly controlled at a stoichiometric molar ratio of 2/1 to yield magnetite nanocrystals. Although certain redox mechanisms are unknown, it is well accepted that the cellular uptake of Fe(III) was firstly reduced to Fe(II) to be transported into magnetosome membrane vesicles, followed by the redox process into hydrous Fe(III) oxides. Afterward, one third of Fe(III) in hydrous oxides was reduced to Fe(II) to form magnetite followed by dehydration.
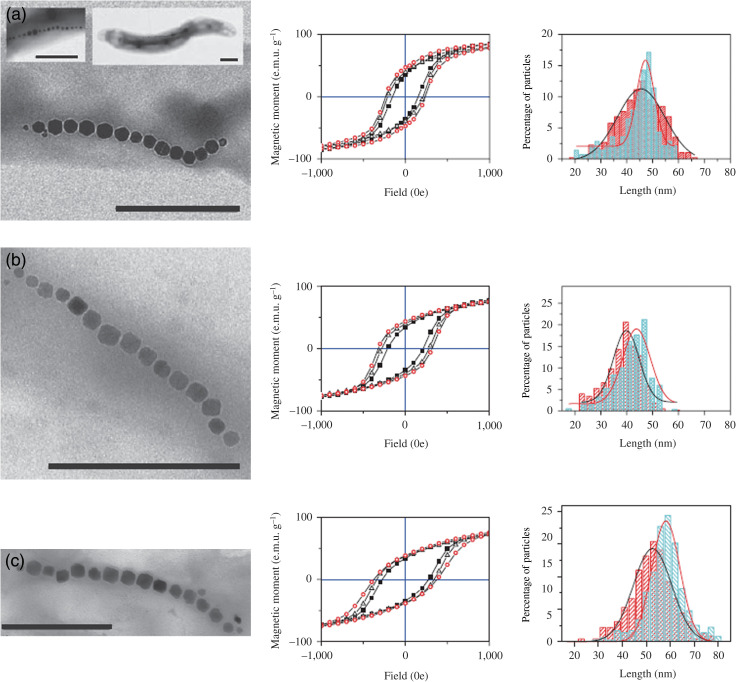
Recently, to overcome the magnetic softness problems of magnetite magnetosomes in nanomedicine applications, an in-vivo cobalt-doping method has been introduced to increase the coercive field that is needed to reverse the magnetization without significant loss in saturation magnetization of magnetosomes (29). Fig. 5 shows transmission electron microscopic images and hysteresis loops of undoped and cobalt-doped magnetosomes within three different MTB strains (Magnetospirillum gryphiswaldense MG, M. magnetotactricum MS1, and M. magneticum AMB1). It is possible to expand this method into other metal ions, such as titanium, copper, and nickel, to improve significantly the biologically controlled synthesis of magnetic particles with tunable physico-chemical propertiesin vivo.
fig. 5.
Magnetization properties of doped and undoped magnetosomes from different bacterial strains. (a–c) TEM micrographs, hysteresis loops, and length histograms for M. gryphiswaldense MG (a), M. magnetotacticum MS1 (b), and M. magneticum AMB1 (c). The TEM micrographs correspond to doped magnetosome chains unless otherwise indicated. Insets to (a) TEM images showing undoped [MG-Fe] magnetosome chains (left inset) and a whole cell of [MG-Fe] (right inset). The hysteresis loops were measured at 300 K. Closed squares, open triangles, and open circles indicate the magnetosomes corresponding to [Fe], [FeCo], and [Co] growth conditions. The histograms indicate the length distribution of [Fe] (red) and [Co] (blue) particles. The scale bars are 500 nm. Reprinted from Nat Nanotechnol, Vol. 3, Staniland S et al., Controlled cobalt doping of magnetosome in vivo, 159. Copyright (2008), with permission from Nature Publishing Group.
Nanomagnets in nanomedicine
When the particle size reduces to a certain size (a few nanometers), the formation of a domain wall is not favorable, hence the particle only contains a single magnetic domain in which all atomic magnetic moments align with each other. Even at ambient temperature, the thermal energy is still comparable to the magnetic anisotropy to change the direction of the magnetic moment of each individual particle (1). The fundamental criteria of magnetic nanoparticles in nanomedicine are superparamagnetism. In the absence of an external magnetic field, the magnetic moment of each particle is randomly oriented due to thermal fluctuation, resulting in zero net magnetization; in the presence of an external magnetic field, they tend to align with the field and exhibit a very strong magnetization in the direction of the external field, hence they can be targeted by an external magnetic field in an on-off fashion.
Nanotoxicology
Safety issues of nanoparticles in any clinical applications are a major concern. Although iron oxides are relatively less toxic compared to other transition metal or semiconductor nanomaterials, concerns regarding toxicity still remain in iron oxide-based nanomedicine, such as magnetic resonance imaging (MRI) and magnetic force drive drug/gene delivery. The term ‘non-invasive’ is controversial, because originally it implied that ‘neither surgical procedures nor scissions were involved,’ however, recent studies have shown the possibilities of iron oxide nanoparticles affecting normal cell functionalities, instead of simply being carriers (30, 31). It has been widely accepted that uncoated iron oxide nanoparticles elicit massive cellular internalization associated with significant cell death, however, iron oxide nanoparticles coated with hydrophilic and biocompatible substances have shown less cytotoxicity in a range of cell lines (32). Gupta (33) demonstrated a dose-dependent reduction in cell adhesion and viability of bare iron oxide nanoparticles, while pullulan, lactoferrin, and ceruloplasmin (32) coated nanoparticles showed no significant cell detachment or morphology changes on human fibroblasts.
Although the mechanism of nanoparticle-mediated cytotoxicity is not fully understood, it has been proposed that it is attributed to the generation of reactive oxidative species (ROS) and cellular internalization (30). Transition metal or transition metal oxide nanoparticles can generate ROS as catalysts in a Fenton-type reaction, in which hydrogen peroxide is reduced by ferrous ions to form extremely active hydroxyl-free radicals and lead to biological damage within the diffusion range (30).
| 5 |
Studies of the intracellular destination of iron oxide nanoparticles are particularly important in determining the cytotoxicity of such nanomaterials and designing effective and bio-safe nano-devices for biomedical applications. Most of the work has focused on receptormediated internalization and the endocytic pathway of such nanostructures, including the effect of the physico-chemical properties of nanoparticles, i.e. size, shape, chemical composition, surface-to-volume ratio, and surface charges, on the cellular response of different cells (34). It has been established that receptor-mediated internalization strongly depends on the size of the particles (31). Both clathrin- and caveolae-mediated endocytosis has been reported, however, controversial results on the effect of particle size and surface coating on the endocytosis pathway has also been reported (31).
Nanomagnets in cancer diagnostics and therapy
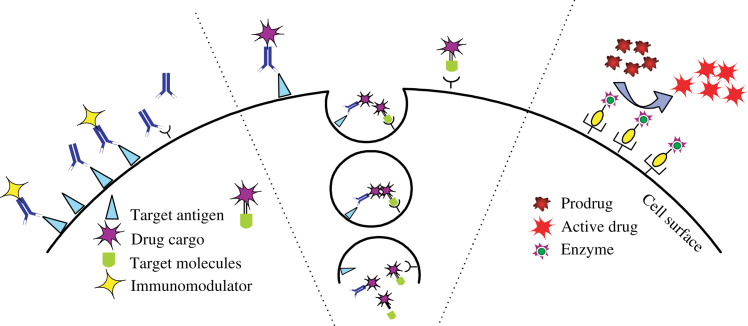
The excellent features of nanomagnets are their multifunctional properties and the potential combination of neoplastic diagnostics and therapeutics (35). In target-oriented drug delivery systems (DDS), more sophisticated designs are required to achieve targeted delivery by both physical and biochemical means and controlled release if necessary. Fig. 6 illustrates the various approaches of cancer-specific DDSs. Drugs such as protein toxins are transported to intracellular sites via receptor-mediated endocytosis (shown in the center). On the left, immunomodulators (e.g. cytokines) are coupled to a tumor-specific ligand (e.g. antibodies, folic acid) and localized on the cell surface to elicit an immune response. On the right, prodrug-activating enzymes are concentrated on the cell surface and can subsequently convert the prodrug into an active drug (36). Stimuli-activated drug release, once combined with the nanomagnet, is of particular interest in anti-cancer drug delivery. Qin et al. (37) recently developed a system consisting of hydrophobic nanocrystals and amphiphilic thermosensitive copolymers, which exhibit a sharp and reversible low critical solution temperature. An MRI technique with tumor-specific nanomagnets as contrast agents was used to locate the diseased site prior to magnetic fluid hyperthermia (MFH) treatment, which is also mediated by nanomagnets and triggered by an external AC magnetic field or active drug release from the nanomagnet carriers via an external stimuli, such as pH or temperature change, and enzymatic cleavage (38). Jian et al. tested the dual functionalities of Pluronic-stabilized magnetic particles as doxorubicin and/or paclitaxel carriers and MR contrast agent to show the high clinical significance of the multifunctional magnetic carrier in cancer treatment (35). Similarly, Jarzyna et al. (39) developed oil-in-water emulsions encapsulating magnetite nanoparticle cores as a dual-functional platform, in which magnetite particles act as MR probes and hydrophobic drugs can be loaded and released in the soybean oil core. Julian-Lopez et al. (40) reported facile fabrication of the hybrid silica-spinel iron oxide composite with magnetite for hyperthermia and MRI and the mesoporous matrix enabling the transport of therapeutic molecules in vivo. Despite potential toxicity, metallic iron nanoparticles coated with carboxyl-terminated PEG with a diameter of 10 nm have also been reported to exert local hyperthermia under an oscillating magnetic field and have a stronger T2 shortening effect as another possible multifunctional nano-platform (41). Both the outer coating substances and the interaction between the polymeric coating and particle surface often significantly influence the characteristics of the resulting MR probes. Jain et al. (42) reported that the Pluronic F127 stabilized magnetic particles sustained and enhanced accumulation in tumor tissues compared with commercial Feridex IV. Muhammed et al. also reported the use of Pluronic F127 as a phase transfer agent for hydrophobic magnetic nanocrystals prepared by thermolysis; and Pluronic F127 modified nanoparticles had shown a significant T2 shortening effect as MR contrast agents and controlled released behavior of hydrophobic anti-cancer drugs (43, 44).However, the translation of nanomagnets from laboratory into clinical setting remains complicated. The major issue regarding magnetic force assisted DDS and MFH is the relatively high concentration of magnetic carriers required for sufficient accumulation of anti-cancer drugs (magnetic force-based DDS) or induction of heat-induced apoptosis, which may result in undesirable side effects and the requirement for a strong external magnetic field generated by coil/power supply systems (38, 45).
fig. 6.
Schematic presentation of targeted delivery of therapeutic proteins and peptides to antigen-positive tumor cells or tumor vasculature. Reprinted from AAPS J, Vol. 8, Lu Y et al., Issues related to targeted delivery of proteins and peptides, E476, Copyright (2006), with permission from SpringerLink.
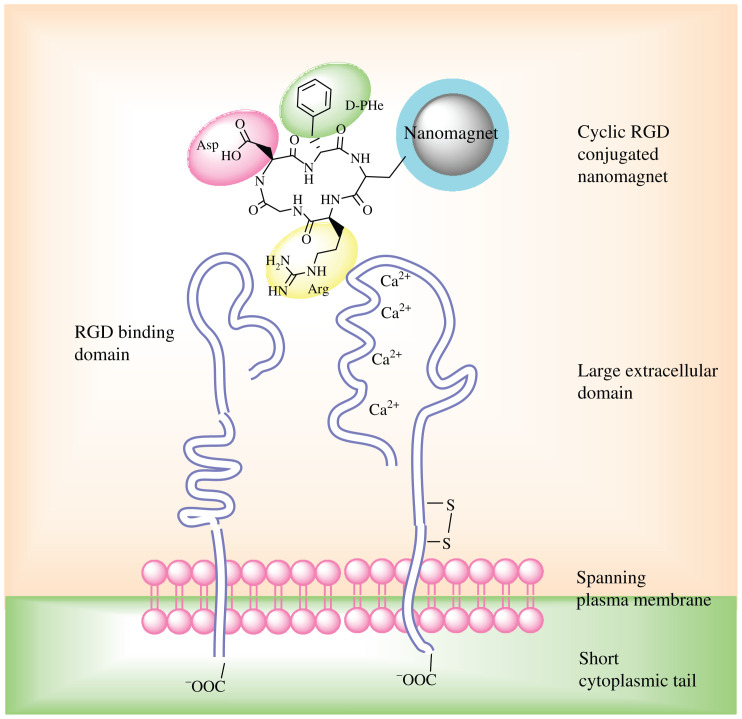
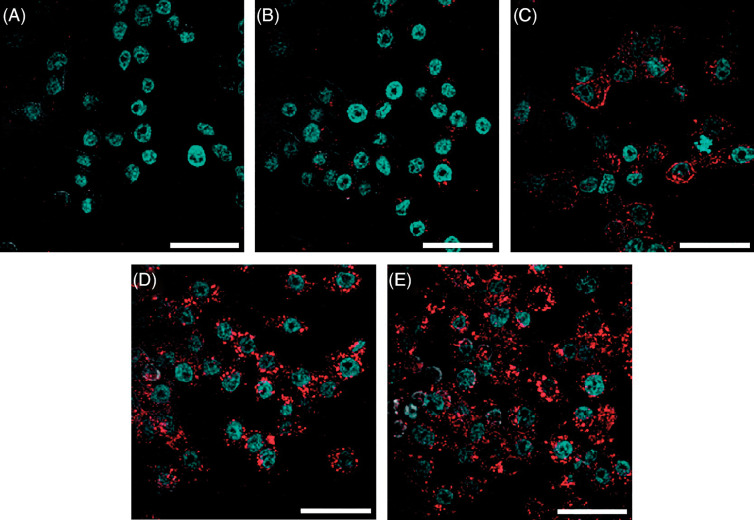
Sufficient delivery of local hyperthermia and anti-cancer drugs is strongly dependent on the cellular internalization competence of magnetic nanoparticles. Active cancer targeting can be achieved by further conjugations with particular targeting ligand on nanoparticles, as shown in Table 1. Numerous potential malignancy targeting ligands have been identified as considerable improvements have been made in cancer biology in the past decade, among which the Arginine-Glycine-Aspartic acid (RGD) peptide sequence is of particular concern in active cancer targeting, attributed to the abundance of integrin receptors in various carcinomas. Fig. 7 shows the binding effects of magnetic nanoparticles to cancer cell via RGD-integrin interaction, which leads to receptor-mediated endocytosis of RGD-modified magnetic nanoparticles (46, 47). Folic acid, also called vitamin B9, has also been widely used to target different types of cancer; Fig. 8 shows confocal microscope images of the cellular uptake of magnetic nanoparticles functionalized with folic acid (48).
Table 1.
Active cancer-targeting strategies of magnetic nanoparticle biomedical applications
| Active ligand | Surface receptors | Cancer cells | Applications |
|---|---|---|---|
| RGD peptide | Integrin αVβ3 | Breast carcinomas | siRNA delivery (47) |
| Glioblastoma | Brain tumor diagnosis by MRI and near-infrared fluorescent (NIRF) imaging (79) | ||
| Activated platelets | Thrombus visualization by MRI (80) | ||
| CTX (chlorotoxin) | MMP-2 (matrix metalloproteinase-2) | Gliomas medulloblastomas | Brain tumor diagnosis by MRI (81, 82) |
| Herceptin | HER2 (human epidermal growth factor receptor 2) | Breast cancer | Platin delivery (83, 84) Breast cancer diagnosis by MRI (7) Localized hyperthermia (85) |
| Anti-TfR (transferrin receptor) MAb | TfR (transferrin receptor) | Hematopoietic and neural progenitor cells | MR tracking of cell differentiation and migration (86, 87) |
| Gliosarcomas | MR detection of gene expression (88) | ||
| Tf (transferrin) | Cervical cancer | Gene delivery (89) | |
| LHRH (luteinizing hormone-releasing hormone) | LHRHR (luteinizing hormone-releasing hormone receptor) | Breast cancer Ovarian cancer | Targeted Fe-induced apoptosis in cancer treatment (90) Breast tumor and metastases detection by MRI (91, 92) |
| FA (folic acid) | FAR (folic acid receptor) | Breast cancer Epithelial carcinomas | Early diagnosis by MRI (93, 94) |
fig. 7.
Schematic illustration of receptor-mediated binding event of magnetic particles and cell. cRGD peptide was conjugated to the nanomagnet through a protective coating layer (e.g. dextran or PEG); cRGD motif recognize the RGD binding domain of the integrin αvβ3 receptors that overexpressed on tumor cells.
fig. 8.
Confocal microscopy images of KB cells treated with FA functionalized SPIONs for 1 h at 37°C, 5% CO2. Nuclei was stained with DAPI to give blue fluorescence, SPIONs were conjugated with 6-TAMRA dye to give red fluorescence. The scale bars are 40 µm (A) Negative control KB cells without any treatment; KB cells with (B) FAR blocked; (C) with G5-Ac(102)-FA(5)-6T3; (D) with FA-SPIONs at 50 nM; and (E) 100 nM. Reprinted from ACS Nano, Vol. 2, Landmark KJ, Synthesis, characterization, and in vivo testing of superparamagnetic iron oxide nanoparticles targeted using folic acid-conjugated dendrimers, 778. Copyright (2008), with permission from ACS Publishing Group.
Nanomagnets in stem cell therapy
Stem cells are characterized by their self-renewal capability via mitotic cell division and differentiation into different specialized cell types. Transplantation of stem cells is the administration of stem cells in vivo to regenerate, repair, or restore the functionality of various organs or tissues. It has attracted enormous attention for the treatment of various diseases, such as leukemia, neural degenerative diseases, cancer, spinal cord injuries, and muscle damage, etc. It is essential to understand the fundamental and practical aspects of stem cell transplantation, thus non-invasive tracking of final destination and differentiation of administered stem cells is important in stem cell research. Among the existing cell labeling and monitoring techniques, such as with cell membrane dyes, fluorescent dyes, and positron emission tomography (PET) scanning, MRI has been focused as a potential technique for tracking the transplanted stem cells due to its non-invasive and real-time cellular tracking abilities with high spatial resolution (49). It has been proved that the magnetic labeling of human mesenchymal stem cells (hMSCs) and CD34+ hematopoietic stem cells with iron oxide nanoparticles allows tracking of labeled cells by MRI at single cellular level (50).
The prerequisite for achieving the MR signal of administered objective cells in vivo is loading sufficient contrast agents without influencing cell viability, proliferation, and functionalities (including differentiation ability). The effective and selective magnetic labeling of stem cells can be achieved by sophisticated surface conjugation on nanomagnets. Dunning et al. (51) reported the successful magnetic labeling and in-vivo MR tracking of Schwann cells and olfactory ensheathing cells (OEC) after transplantation into the central nervous system (CNS). Arab et al. (52) reported an evaluation of the loading efficiency, cell toxicity, capability of differentiation, and phenotypic changes of hMSCs and CD34+ hematopoietic stem cells, after labeling with ferumoxides complexed with protamine sulfate and conjugated with a polycationic peptide for transfection. Lewin et al. (53) demonstrated the effective endocytosis of human immunodeficiency virus (HIV) derived membrane translocation signal Tat peptide-linked iron oxide nanoparticles into CD34+ hematopoietic and neural progenitor cells. Matuszewski et al. also studied the effect of lipophilic transfection medium and particle size on labelling efficiency of cells, which shed some light on rational design of nanomagnets (49). Magnetic labeling of stem cells not only enables non-invasive tracking and quantification of cells, but also facilitates the targeted deposition of stem cells into certain targeted areas in vivo by magnetic resonance fluoroscopy. Alexander et al. (54) demonstrated the precisely guided administration of magnetic labeled MSCs into the area between the myocardial infarcted and normal tissue by a clinical magnetic resonance fluoroscopy procedure.
Nanomagnets in gene therapy
Gene therapy involves the insertion of genetic materials, including plasmid DNA, small interference RNA (siRNA), double-stranded DNA (dsDNA), messenger RNA (mRNA), and oligonucleotides (ODNs), into target cells or tissues to express heterogeneous gene products, knockdown mutated deleterious genes, and replace with functional ones. Although gene therapy has shown promising results for the treatment of hereditary diseases, such as cystic fibrosis, thalassemia, Parkinson's disease, Huntington's disease, inherited color blindness, etc., development of effective non-viral vectors remains one of the biggest challenges to replace potentially toxic and immunogenic viral vectors. For this reason, magnetofection was established to take advantage of both the biochemical (cationic lipids or polymers) and physical means (magnetic forces) for accelerated nucleic acid (NA) delivery to the target cells (55).
Li et al. (56) reported that transfection efficiency of PEI/DNA polyplexes, which are covalently linked to dextran-modified magnetic beads (200 nm), shows 35- to 85-fold higher values under magnetic field. Similarly, Xenariou et al. (57) demonstrated reporter gene expression of Lipofectamine 2000/pDNA lipoplex increase 300-fold under a magnetic field, when complexed with TransMAGPEI (consisting of iron oxide cores and PEI shell) at suboptimal pDNA concentrations. Mykhaylyk et al. (58) recently described self-assembly of DNA and cationic lipid or polymer-associated magnetic nanoparticles for in-vitro magnetic force-assisted transfection of both adherent and suspended cells.
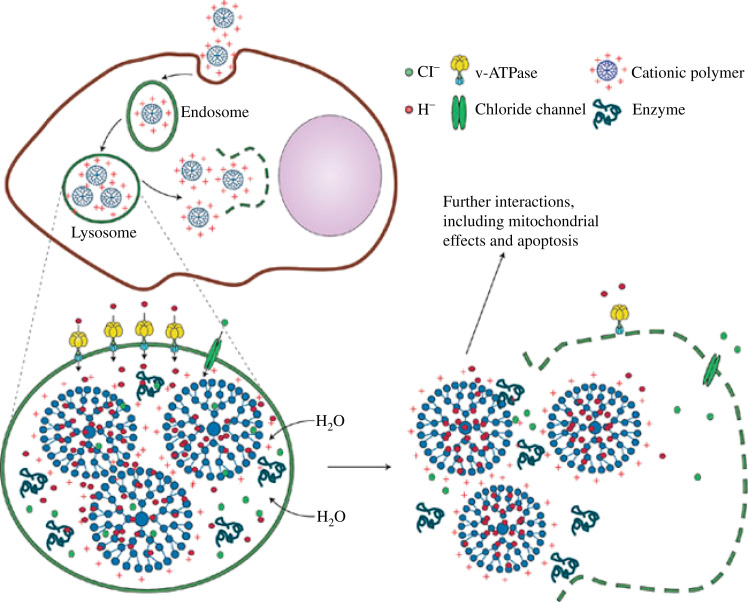
Huth et al. (59) systematically investigated the mechanisms of magentofection of PEI-based magnetic nanoparticles. The cellular uptake pattern of PEI-modified magnetic nanoparticles is virtually the same as polyplexes via the endocytic pathway. Fig. 9 illustrates the mechanisms of the proton sponge effect, in which genetic materials are released from cationic lipid- or polymer-coated magnetic particles in acidic endosomes. Cationic magnetic particles are endocytosed in the tight-fitting vesicles, attributed to electrostatic interaction between negatively charged cell membrane and cationic magnetic particles. Once the particles are endocytosed in the endosomal environment, the unsaturated amino groups (e.g. PEI coating) sequester the pumped-in-protons by the ATPase residing on the endosomal membrane, which leads to retention of Cl− ions and water molecules in the endosome. As the water retention builds up, the endosomes swell and burst, resulting in the release of genetic material and magnetic particles into the cytoplasm (60). It has been reported that the accelerated sedimentation of DNA is the main driving force for increased transfection efficiency when it is complexed with magnetic vectors (59). Namiki et al. (61) reported effective magnetic field-guided siRNA delivery with LipoMag®, which consists of an OA-coated iron oxide core and cationic lipid shells, in mice gastric tumor models. Cohorny et al. (62) reported polylactide-induced small clusters of OA-coated magnetic nanoparticles and subsequent PEI/oleate ion pair by emulsion-evaporation method.
fig. 9.
Schematic illustration of the proton sponge effect leading to endosomal or lysosomal burst and release of the cationic magnetic particles into the cytoplasm. Reprinted from Nat Mater, Vol. 8, Nel AE, Understanding biophysiochemical interactions at the nano-bio interface, 552. Copyright (2009), with permission from Nature Publishing Group.
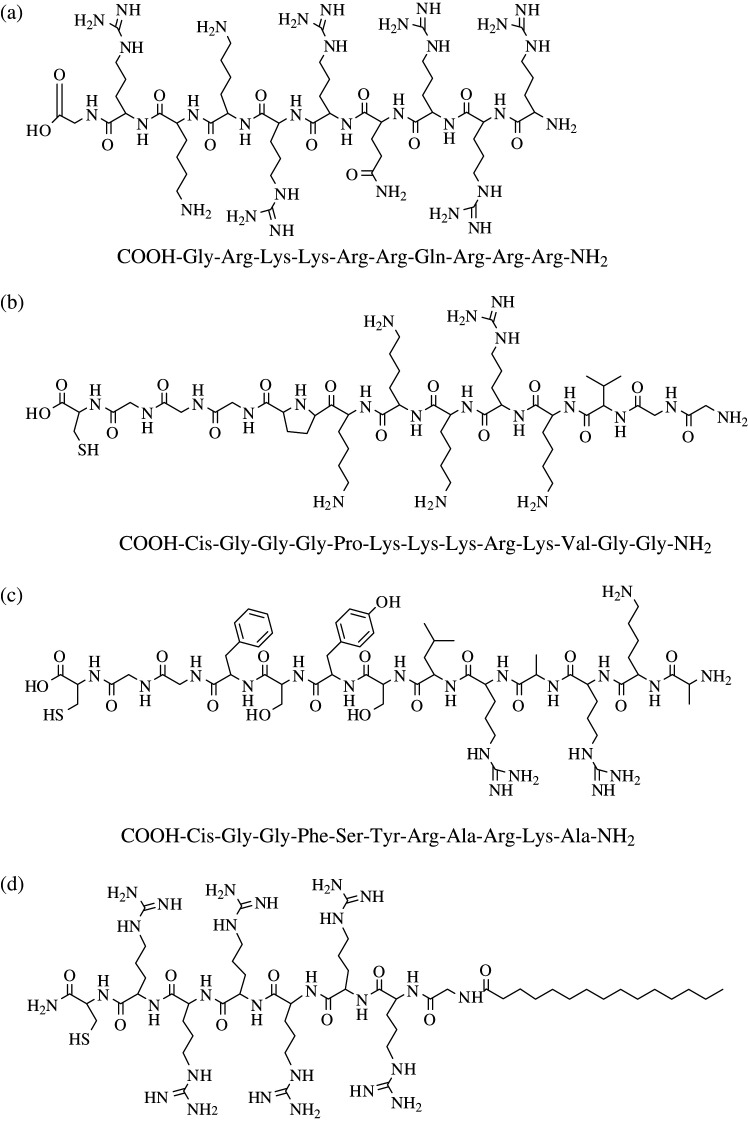
Subcellular targeting with magnetic gene carriers represents another avenue for gene therapy. The discovery of HIV Tat protein transduction domains (PTD) and nucleus localization signals (NLS) has opened up a new approach to using cell-penetrating peptides (CPPs) to direct in-vitro and in-vivo delivery of therapeutic agents on target sites (63, 64). Lewin et al. (53) labeled CD34+ neural progenitor cells with Tat peptide-derivative magnetic NPs for in-vivo MR tracking and recovery of progenitor cells. During the past decade, tremendous progress has been made in protein transduction study, various CPPs and peptidomimetics have been indentified for subcellular and specific delivery of proteins, DNA, drugs, and imaging probes; however, the internalization mechanism of CPPs are not well investigated (65). A common feature of such CPPs is the high incidence of basic amino acid residues, such as Arg and Lys (Fig. 10); it can be considered that the guanidine groups of Arg is a critical component for the biological activity of CPPs, and hydrogen bonding between highly basic Arg (or Lys) and phospholipids bi-layer may be involved in transduction into cells (66, 67). Fig. 10 shows a list of molecular structures and amino acid sequences of CPPs from different origins.
fig. 10.
Molecular structure and peptide sequence of common CPPs: (a) HIV-derived TAT49–57; (b) SV40 Large T NLS; (c) adenoviral NLS; and (d) myristoylated polyarginine peptide. [Figure adopted from References (63–65).]
Nanomagnets in tissue engineering
In the early 1990s, Langer and Vacanti (68) first gave the definition of tissue engineering: ‘an interdisciplinary field that applies the principles of engineering and life science toward the development of biological substitute that restore, maintain, or improve tissue function or a whole organ.’ Significant achievements are reported in the construction of bio-functional tissues inside the scaffold matrices in vitro via a combination of the development in biological active scaffold, tissue-culturing techniques (e.g. bio-reactors), and in-depth understanding of cell biology, including the physiology of the tissue-scaffold microenvironment and the ability to induce of cellular functionality of some bioactive molecules.
Several researches have demonstrated the feasibility of constructing 2D or 3D tissue integrities by orientating the cells under an external magnetic field. Ito et al. reported the construction of monotypic and heterotypic cell multilayer sheets of liver endothelial cells, skeletal muscle cells, and mesenchymal stem cells by magnetic-induced forces (69–71). The same group also demonstrated increased cell-seeding density via magnetic forces, resulting in the formation of bone-like tissues within 3D scaffold matrices (72, 73). Recently, micro-patterning of target cells with relatively precise spatial control on the cell-adhesive surface in vitro has been achieved by selectively labeling the target cells with peptide-modified magnetite liposome with a computer-aided precise positioning of the steel plate under an external magnetic field (47). Such magnetic force-based micro-patterning techniques show promising results in skin regeneration (74).
Advanced magnetic actuation of cells
Remote manipulation of cells and cellular components in vitro and in vivo provides an important tool to understanding the cell functional and cellular signaling pathway. In the past few years, significant efforts have been made in magnetic force targeting for the mechano-sensitive ion channels of the cells in vitro, the ligand-receptor binding, receptors activation and clustering, downstream signaling pathway, and subsequent phenotype changes have been investigated with a variety of cell lineages (75–77). Compared to other remote control techniques, such as optical tweezers, the magnetic manipulation via nanomagnets allows ‘action at distance’ while maintaining the precise localization of nanomagnets interfacing the target cells (78). Moreover, such distance between the external magnetic field and the nanomagnet represents the possibility of overcoming interfering with the tissue structures by remote cell control in vivo (78).
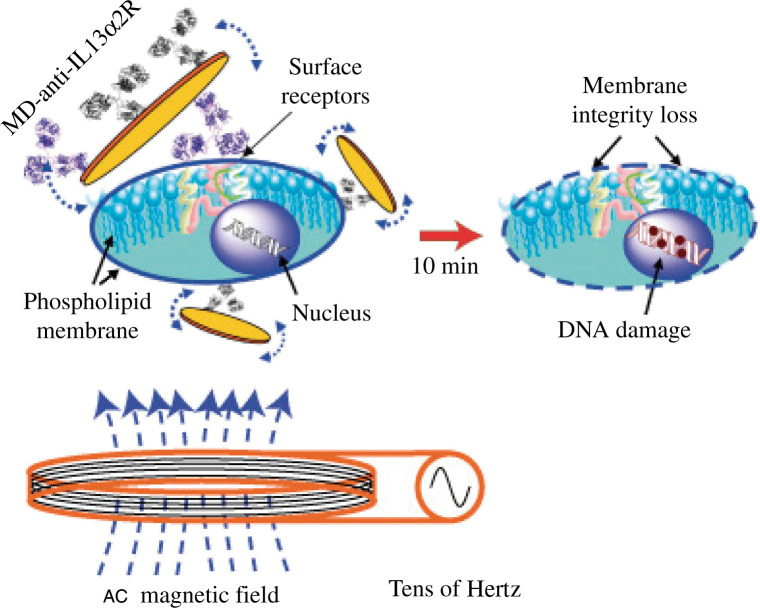
Recently, a top-down approach by photolithography and etching has been reported to fabricate 2D magnetic MDs with a spin-vortex ground state. Such ferromagnetic MDs have two advantages in nanomedicine applications: zero remanance and the ability to respond external frequency. Kim et al. (25) reported the induced apoptosis of several cancer cells by the mechanical forces induced by the spin-vortex at very low frequency (tens of Hz) for a short time (10 min). Fig. 11 schematically shows that the antibody functionalized magnetic MDs selectively bind to the cancer cells, followed by transduction of the mechanical force to the phospholipids membrane, causing membrane destruction and eventually DNA damage (25).
fig. 11.
The concept of targeted magneto-mechanical cancer cell destruction using disc-shaped magnetic particles possessing a spin-vortex ground state. Reprinted from Nat Mater, Vol. 9, Kim D-H et al., Biofunctionalized magnetic-vortex microdiscs for targeted cancer-cell destruction, 166. Copyright (2010), with permission from Nature Publishing Group.
Future perspective and outlook
In the past decade, nanomedicine in nanotechnology has been rapidly growing as an active research field because of its adaptable application in targeting, drug delivery, diagnosis, and treatment of various diseases and ailments. In this paper, several advanced fabrication techniques for the synthesis of multifunctional nanomagnets with novel physico-chemical properties have been reviewed, together with their promising applications in nanomedicine, such as cancer treatments, stem cell tracking and therapy, gene therapy, and tissue engineering. The challenge remains to translate nanomagnets from the laboratory into clinical applications. One of the major obstacles in magnetic drug/gene delivery is that the currently available magnets are not strong enough to magnetize the magnetic nanoparticles to counterbalance the blood flow for effective accumulation of nanomagnets at the target site. The development of high-quality magnetic nanoparticles with a strong magnetization and powerful external magnetic field is equally essential in potential magnetic drug/gene delivery applications. Moreover, iron oxide-based nanomagnets have been described as ‘non-invasive’ contrast agents as MR probes, more practical confirmation on the effect of such particles on normal cellular functionalities have been reported in recent years. Therefore, the direction of future research should be highlighted to elevate the techniques into other areas, such as nanoarticulation and nanomodulation of cells. The combination of advanced synthetic technology of nanomagnets and drug delivery techniques will enable researchers to explore the cellular events under certain conditions, such as magnetic-vortex-mediated apoptosis and controlled release of therapeutic agents in a controlled manner.
Acknowledgements
This work was financially supported by the Engineering and Physical Sciences Research Council (EPSRC) CoDDS projects (EP/E016944/1).
Biographies
 Meng Meng Lin received a BSc in biotechnology at the University of Hong Kong, China in 2004 and an MSc in biomedical nanotechnology at Newcastle University, UK, in 2005. She is currently working toward her PhD at the Institute of Science and Technology in Medicine, Keele University, UK. She was a visiting student at the Royal Institute of Technology, Sweden, in 2006. Her research interests include nanoparticles preparation, cell/nanomaterials interface, and cancer-oriented drug delivery.
Meng Meng Lin received a BSc in biotechnology at the University of Hong Kong, China in 2004 and an MSc in biomedical nanotechnology at Newcastle University, UK, in 2005. She is currently working toward her PhD at the Institute of Science and Technology in Medicine, Keele University, UK. She was a visiting student at the Royal Institute of Technology, Sweden, in 2006. Her research interests include nanoparticles preparation, cell/nanomaterials interface, and cancer-oriented drug delivery.
 Hyung-Hwan Kim received an MSc degree in chemistry from Korea University in 1998 and a PhD in oriental medicine from Kyung Hee University, Korea, in 2003. He was a Postdoctoral Fellow at the Vascular Medicine Research Unit, Brigham & Women's Hospital, Harvard Medical School, in 2003. He has been working as an instructor at the Harvard Medical School since 2007 and is Vice Director of the International Research Center of Bioscience and Biotechnology, Jungwon University, Korea. His research interests include cells and signal pathways in vascular biology field.
Hyung-Hwan Kim received an MSc degree in chemistry from Korea University in 1998 and a PhD in oriental medicine from Kyung Hee University, Korea, in 2003. He was a Postdoctoral Fellow at the Vascular Medicine Research Unit, Brigham & Women's Hospital, Harvard Medical School, in 2003. He has been working as an instructor at the Harvard Medical School since 2007 and is Vice Director of the International Research Center of Bioscience and Biotechnology, Jungwon University, Korea. His research interests include cells and signal pathways in vascular biology field.
 Hyuck Kim received a PhD in oriental medicine from Kyung Hee University, Korea, in 2004. He is currently adjunct Professor at the Faculty of Herb Industry, Jungwon University, Korea, and Director of the International Research Center of Bioscience and Biotechnology, Jungwon University, Korea, since 2008. Additionally, he is President of Jein Medical Center since 2006. He is establishing a collaboration with Harvard Medical School on Asian Hybrid Medicine.
Hyuck Kim received a PhD in oriental medicine from Kyung Hee University, Korea, in 2004. He is currently adjunct Professor at the Faculty of Herb Industry, Jungwon University, Korea, and Director of the International Research Center of Bioscience and Biotechnology, Jungwon University, Korea, since 2008. Additionally, he is President of Jein Medical Center since 2006. He is establishing a collaboration with Harvard Medical School on Asian Hybrid Medicine.
 Mamoun Muhammed received a BSc and MSc from Cairo University, and a PhD from the Royal Institute of Technology in 1975. He is Chairman of the International Committee on Nanostructured Materials since 2005 and is currently Chair Professor, Head of Functional Materials Division at the Royal Institute of Technology, Sweden. He has published over 200 papers, co-edited one book, and has been a guest editor for two special volumes in the Nanostructured Materials Journal.
Mamoun Muhammed received a BSc and MSc from Cairo University, and a PhD from the Royal Institute of Technology in 1975. He is Chairman of the International Committee on Nanostructured Materials since 2005 and is currently Chair Professor, Head of Functional Materials Division at the Royal Institute of Technology, Sweden. He has published over 200 papers, co-edited one book, and has been a guest editor for two special volumes in the Nanostructured Materials Journal.
 Do Kyung Kim received a PhD in nanobio from the Royal Institute of Technology, Sweden in 2002. He was a Postdoctoral Fellow at the Massachusetts Institute of Technology (MIT), in 2003. He is currently a lecturer in the Institute of Science and Technology in Medicine, Keele University, UK, and Professor at the International Research Center of Bioscience and Biotechnology, Jungwon University, Korea. His research interests include target-oriented drug delivery systems, contrast agents, and quantum dots for nanomedicine.
Do Kyung Kim received a PhD in nanobio from the Royal Institute of Technology, Sweden in 2002. He was a Postdoctoral Fellow at the Massachusetts Institute of Technology (MIT), in 2003. He is currently a lecturer in the Institute of Science and Technology in Medicine, Keele University, UK, and Professor at the International Research Center of Bioscience and Biotechnology, Jungwon University, Korea. His research interests include target-oriented drug delivery systems, contrast agents, and quantum dots for nanomedicine.
Conflict of interest and funding
There is no conflict of interest in the present study for any of the authors.
References
- 1.Cornell RM, Schewertmann U. The iron oxides: structure, properties, reactions, occurences and uses. Weinheim and New York: Wiley-VCH Verlag GmbH; 2003. [Google Scholar]
- 2.Schuler D. Formation of magnetosomes in magnetostactic bacteria. J Mol Microbiol Biotechnol. 1999;1:79–86. [PubMed] [Google Scholar]
- 3.Lin MM, Li S, Kim H-H, Kim H, Lee H-B, Muhammed M, et al. Complete separation of magnetic nanoparticles via chemical cleavage of dextran by ethylenediamine for intracellular uptake. J Mater Chem. 2010;20:444–7. [Google Scholar]
- 4.Kang YS, Risud S, Radolt JF, Stroeve P. Synthesis and characterization of nanometer-size Fe3O4 and gamma-Fe2O3 particles. Chem Mater. 1996;8:2209–11. [Google Scholar]
- 5.Babes L, Denizot B, Tanguy G, Le Jeune JJ, Jallet P. Synthesis of iron oxide nanoparticles used as MRI contrast agents: a parametric study. J Colloid Inter Sci. 1999;212:474–82. doi: 10.1006/jcis.1998.6053. [DOI] [PubMed] [Google Scholar]
- 6.Kim DK, Zhang W, Voit W, Rao KV, Kehr J, Bjelke B, et al. Superparamagnetic iron oxide nanoparticles for bio-medical applications. Scripta Mater. 2001;44:1713–7. [Google Scholar]
- 7.Kim DK, Mikhaylova M, Zhang Y, Muhammed M. Protective coating of superparamagnetic iron oxide nanoparticles. Chem Mater. 2003;15:1617–27. [Google Scholar]
- 8.Kim DK, Mikhaylova M, Wang FH, Kehr J, Bjelke B, Zhang Y, et al. Starch-coated superparamagnetic nanoparticles as MR contrast agents. Chem Mater. 2003;15:4343–51. [Google Scholar]
- 9.Santra S, Tapec R, Theodoropoulou N, Dobson J, Hebard A, Tan W. Synthesis and characterization of silica-coated iron oxide nanoparticles in microemulsion: the effect of nonionic surfactants. Langmuir. 2001;17:2900–6. [Google Scholar]
- 10.Dresco PA, Zaitsev VS, Gambino RJ, Chu B. Preparation and properties of magnetite and polymer magnetite nanoparticles. Langmuir. 1999;15:1945–51. doi: 10.1006/jcis.1998.5993. [DOI] [PubMed] [Google Scholar]
- 11.Yi DK, Lee SL, Papaefthymiou GC, Ying JY. Nanoparticle architectures templated by SiO2/Fe3O4 nanocomposites. Chem Mater. 2006;18:614–9. [Google Scholar]
- 12.Wang X, Zhuang J, Peng Q, Li Y. A general strategy for nanocrystal synthesis. Nature. 2005;437:121–4. doi: 10.1038/nature03968. [DOI] [PubMed] [Google Scholar]
- 13.Rockenberger I, Scher EC, Alivisator AP. A new nonhydrolytic single-precursor approach to surfactant-capped nanocrystals of transition metal oxides. J Am Chem Soc. 1999;121:11595–6. [Google Scholar]
- 14.Hyeon T, Lee SS, Park J, Chung Y, Na HB. Synthesis of highly crystalline and monodisperse maghemite nanocrystallites without a size-selection process. J Am Chem Soc. 2001;123:12798–801. doi: 10.1021/ja016812s. [DOI] [PubMed] [Google Scholar]
- 15.Park J, Lee E, Hwang N-M, Kang M, Kim SC, Hwang Y, et al. One-nanometer-scale size-controlled synthesis of monodisperse magnetic iron oxide nanoparticles. Angew Chem Int Ed. 2005;44:2872–7. doi: 10.1002/anie.200461665. [DOI] [PubMed] [Google Scholar]
- 16.Sun S, Zeng H. Size-controlled synthesis of magnetite nanoparticles. J Am Chem Soc. 2002;124:8204–5. doi: 10.1021/ja026501x. [DOI] [PubMed] [Google Scholar]
- 17.Jana NR, Chen Y, Peng X. Size- and shape-controlled magnetic (Cr, Mn, Fe, Co, Ni) oxide nanocrystals via a simple and general approach. Chem Mater. 2004;16:3931–5. [Google Scholar]
- 18.Yu WW, Falkner JC, Yavuz CT, Colvin VL. Synthesis of monodisperse iron oxide nanocrystals by thermal decomposition of iron carboxylate salts. Chem Commun. 2004;21:2306–7. doi: 10.1039/b409601k. [DOI] [PubMed] [Google Scholar]
- 19.Park J, An K, Hwang Y, Park J-G, Noh H-J, Kim J-Y, et al. Ultra-large-scale synthesis of monodisperse nanocrystals. Nat Mater. 2004;3:891–5. doi: 10.1038/nmat1251. [DOI] [PubMed] [Google Scholar]
- 20.Bao N, Shen L, Wang Y, Padhan P, Gupta A. A facile thermolysis route to monodisperse ferrite nanocrystals. J Am Chem Soc. 2007;129:12374–5. doi: 10.1021/ja074458d. [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Chen H, Bao H, Gao M. One-pot reaction to synthesize water-soluble magnetic nanocrystals. Chem Mater. 2004;16:1391–3. [Google Scholar]
- 22.Li Z, Sun Q, Gao M. Preparation of water-soluble magnetic nanocrystals from hydrated ferric salts in 2-pyrrolidone: mechanism leading to Fe3O4 . Angew Chem Int Ed. 2005;44:123–6. doi: 10.1002/anie.200460715. [DOI] [PubMed] [Google Scholar]
- 23.Li Z, Wei L, Gao M, Lei H. One-pot reaction to synthesize biocompatible magnetic nanoparticles. Adv Mater. 2005;17:1001–5. [Google Scholar]
- 24.Varadan VK, Chen L, Xie J. Nanomedicine: design and applications of magnetic nanomaterials, nanosensors and nanosystems. Chichester, West Sussex: Wiley; 2008. [Google Scholar]
- 25.Kim D-H, Rozhkova EA, Ulasov IV, Bader SD, Rajh T, Lesniak MS, et al. Biofunctionalized magnetic-vortex microdiscs for targeted cancer-cell destruction. Nat Mater. 2010;9:165–71. doi: 10.1038/nmat2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rozhkova EA, Novosad V, Kim D-H, Pearson J, Divan R, Rajh T, et al. Ferromagnetic microdisks as carriers for biomedical applications. J Appl Phys. 2009;105:07B306–9. [Google Scholar]
- 27.Bauerlein E. Biomineralization of unicellular organisms: an unusual membrane biochemistry for the production of inorganic nano- and microstructures. Angew Chem Int Ed. 2003;42:614–41. doi: 10.1002/anie.200390176. [DOI] [PubMed] [Google Scholar]
- 28.Sarikaya M, Tamerler C, Jen AKY, Schulten K, Baneyx F. Molecular biomimetics: nanotechnology through biology. Nat Mater. 2003;2:577–85. doi: 10.1038/nmat964. [DOI] [PubMed] [Google Scholar]
- 29.Staniland S, Williams W, Telling N, Van Der Laan G, Harrison A, Ward B. Controlled cobalt doping of magnetosome in vivo. Nat Nanotechnol. 2008;3:158–62. doi: 10.1038/nnano.2008.35. [DOI] [PubMed] [Google Scholar]
- 30.Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. Small. 2008;4:26–49. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- 31.Jiang W, Kim BYS, Rutka JT, Chan WCW. Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol. 2008;3:145–50. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 32.Gupta AK, Curtis ASG. Lactoferrin and ceruloplasmin derivatized superparamagnetic iron oxide nanoparticles for targeting cell surface receptors. Biomaterials. 2004;25:3029–40. doi: 10.1016/j.biomaterials.2003.09.095. [DOI] [PubMed] [Google Scholar]
- 33.Gupta AK, Gupta M. Cytotoxicty suppression and cellular uptake enhancement of surface modified magnetic nanoparticles. Biomaterials. 2004;26:1565–73. doi: 10.1016/j.biomaterials.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 34.Buzea C, Pacheco I, Robbie K. Nanomaterial and nanoparticles: sources and toxicity. Biointerphases. 2007;2:MR17–MR71. doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- 35.Jain TK, Richey J, Strand M, Leslie-Pelecky DL, Flask CA, Labhasetwar V. Magnetic nanoparticles with dual functional properties: drug delivery and magnetic resonance imaging. Biomaterials. 2008;29:4012–21. doi: 10.1016/j.biomaterials.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu Y, Yang J, Sega E. Issues related to targeted delivery of proteins and peptides. AAPS J. 2006;8:E466–78. doi: 10.1208/aapsj080355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qin J, Jo SK, Muhammed M. Coating nanocrystals with amphiphilic thermosensitive copolymers. Angew Chem Int Ed. 2009;48:7845–9. doi: 10.1002/anie.200900489. [DOI] [PubMed] [Google Scholar]
- 38.Lin MM, Kim HH, Kim H, Dobson J, Kim D-K. Surface activation and targeting strategies of superparamagnetic iron oxide nanoparticles in cancer-oriented diagnosis and therapy. Nanomedicine. 2010;5:109–33. doi: 10.2217/nnm.09.96. [DOI] [PubMed] [Google Scholar]
- 39.Jarzyna PA, Skajaa T, Gianella A, Cormode DP, Samber DD, Dickson SD, et al. Iron oxide core oil-in-water emulsions as a multifunctional nanoparticle platform for tumor targeting and imaging. Biomaterials. 2009;30:6947–54. doi: 10.1016/j.biomaterials.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Julian-Lopez B, Boissiere C, Chaneac C, Grosso D, Vasseur S, Miraux S, et al. Mesoporous maghemite-organosilica microspheres: a promising route towards multifunctional platforms for smart diagnosis and therapy. J Mater Chem. 2007;17:1563–9. [Google Scholar]
- 41.Hadjipanayis CG, Bonder MJ, Balakrishnan S, Wang X, Mao H, Hadjipanayis GC. Metallic iron nanoparticles for MRI contrast enhancement and local hyperthermia. Small. 2008;4:1925–9. doi: 10.1002/smll.200800261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jain TK, Foy SP, Erokwu B, Dimitrijevic S, Flask CA, Labhasetwar V. Magnetic resonance imaging of multifunctional pluronic stabilized iron oxide nanoparticles in tumor bearing mice. Biomaterials. 2009;30:6748–56. doi: 10.1016/j.biomaterials.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qin J, Asempah I, Laurent S, Fornara A, Muller RN, Muhammed M. Injectable superparamagnetic ferrogels for controlled release of hydrophobic drugs. Adv Mater. 2009;21:1354–7. [Google Scholar]
- 44.Qin J, Laurent S, Jo SK, Roch A, Mikhaylova M, Bhujwalla ZM, et al. A high-performance magnetic resonance imaging T-2 contrast agent. Adv Mater. 2007;19:1874–8. [Google Scholar]
- 45.Dobson J. A twist on tumour targeting. Nat Mater. 2010;9:95–6. doi: 10.1038/nmat2604. [DOI] [PubMed] [Google Scholar]
- 46.Lee J-H, Lee K, Moon SH, Lee Y, Park TG, Cheon J. All-in-one target-cell-specific magnetic nanoparticles for simultaneous molecular imaging and siRNA delivery. Angew Chem Int Ed. 2009;48:4174–9. doi: 10.1002/anie.200805998. [DOI] [PubMed] [Google Scholar]
- 47.Ito A, Akiyama H, Kawabe Y, Kamihira M. Magnetic forcebased cell patterning using Arg-Gly-Asp (RGD) peptideconjugated magnetite cationic liposomes. Journal of Bioscience and Bioengineering. 2007;104:288–93. doi: 10.1263/jbb.104.288. [DOI] [PubMed] [Google Scholar]
- 48.Landmark KJ, DiMaggio S, Ward J, Kelly C, Vogot S, Hong S, et al. Synthesis, characterization, and in vitro testing of superparamagnetic iron oxide nanoparticles targeted using folic acidconjugated dendrimers. ACS Nano. 2008;2:773–83. doi: 10.1021/nn800034w. [DOI] [PubMed] [Google Scholar]
- 49.Matuszewski L, Persigehl T, Wall A, Schwindt W, Tombach B, Fobker M, et al. Cell tagging with clinically approved iron oxides: feasibility and effect of lipofection, particle size, and surface coating on labeling efficiency. Radiology. 2005;235:155–61. doi: 10.1148/radiol.2351040094. [DOI] [PubMed] [Google Scholar]
- 50.Hinds KA, Hill JM, Shapiro EM, Laukkanen MO, Silva AC, Combs CA, et al. Highly efficient endosomal labeling of progenitor and stem cells with large magnetic particles allows magnetic resonance imaging of single cells. Blood. 2003;102:867–72. doi: 10.1182/blood-2002-12-3669. [DOI] [PubMed] [Google Scholar]
- 51.Dunning MD, Lakatos A, Loizou L, Kettunen M, Frech-Constant C, Brindle KM, et al. Superparamagnetic iron oxide-labeled Schwann cells and olfactory ensheathing cells can be traced in vivo by magnetic resonance imaging and retain functional properties after transplantation into the CNS. J Neurosci. 2004;24:9799–810. doi: 10.1523/JNEUROSCI.3126-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arab AS, Yocum GT, Kalish H, Jordan EK, Anderson SA, Khakoo AY, et al. Efficient magnetic cell labeling with protamine sulfate complexed to ferumoxides for cellular MRI. Blood. 2004;104:1217–23. doi: 10.1182/blood-2004-02-0655. [DOI] [PubMed] [Google Scholar]
- 53.Lewin M, Carlesso N, Tung C-H, Tang X-W, Cory D, Scadden DT, et al. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat Biotechnol. 2000;18:410–14. doi: 10.1038/74464. [DOI] [PubMed] [Google Scholar]
- 54.Alexander JD, Guttman MA, Raman VK, Peters DC, Pessanha BSS, Hill JM, et al. Magnetic resonance fluoroscopy allows targeted delivery of mesenchymal stem cells to infarct borders in swine. Circulation. 2003;108:2899–904. doi: 10.1161/01.CIR.0000095790.28368.F9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Plank C, Scherer F, Bergemann C, Remy JS, Krotz C, Anton M, et al. The magnetofection method: using magnetic force to enhance gene delivery. Bio Chem. 2003;384:737–47. doi: 10.1515/BC.2003.082. [DOI] [PubMed] [Google Scholar]
- 56.Li W, Ma N, Ong LL, Kaminski A, Skrabal C, Ugurlucan M, et al. Enhanced thoracic gene delivery by magnetic nanobead-mediated vector. J Gene Med. 2008;10:897–909. doi: 10.1002/jgm.1208. [DOI] [PubMed] [Google Scholar]
- 57.Xenariou S, Griesenbach U, Ferrari S, Dean D, Scheule RK, Cheng SH, et al. Using magnetic forces to enhance non-viral gene transfer to airway epithelium in vivo. Gene Ther. 2006;13:1545–52. doi: 10.1038/sj.gt.3302803. [DOI] [PubMed] [Google Scholar]
- 58.Mykhaylyk O, Antequera YS, Vlaskou D, Plank C. Generation of magnetic nonviral gene transfer agents and magnetofection in vitro. Nat Protoc. 2007;2:2391–411. doi: 10.1038/nprot.2007.352. [DOI] [PubMed] [Google Scholar]
- 59.Huth S, Lausier J, Gersting SW, Rudolph C, Plank C, Welsch U, et al. Insight into the mechanism of magnetofection using PEI-based magnetofectins for gene transfer. J Gene Med. 2004;6:923–36. doi: 10.1002/jgm.577. [DOI] [PubMed] [Google Scholar]
- 60.Nel AE, Madler L, Velegol D, Xia T, Hoek EM, Somasundaran P, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8:543–57. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 61.Namiki Y, Namiki T, Yoshida H, Ishii Y, Tsubota A, Koido S, et al. A novel magnetic crystal-lipid nanostructure for magnetically guided in vivo gene delivery. Nat Nanotechnol. 2009;4:598–606. doi: 10.1038/nnano.2009.202. [DOI] [PubMed] [Google Scholar]
- 62.Cohorny M, Polyak B, Aferiev IS, Walsh K, Friedman G, Levy RJ. Magnetically driven plasmid DNA delivery with biodegradable polymeric nanoparticles. FASEB J. 2007;21:2510–19. doi: 10.1096/fj.06-8070com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–72. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 64.Cutrona G, Carpaneto EM, Ulivi M, Roncella S, Landt S, Ferrarini M, et al. Effects in live cells of a c-myc anti-gene PNA linked to a nuclear localization signal. Nat Biotechnol. 2000;18:300–3. doi: 10.1038/73745. [DOI] [PubMed] [Google Scholar]
- 65.Pham W, Nabuurs RJ, Van Buchem MA, Moore A. Design and synthesis of novel myristoylated polyarginine peptides for in vivo molecular neuroimaging. Proc Int Soc Mag Reson Med. 2006;14:1851–2. [Google Scholar]
- 66.Nishihara M, Perret F, Takeuchi T, Futaki S, Lazar AN, Coleman AW, et al. Arginin magic with new counterions up with sleeve. Org Biomol Chem. 2005;3:1659–69. doi: 10.1039/b501472g. [DOI] [PubMed] [Google Scholar]
- 67.Chauhan A, Tikoo A, Kapur AK, Singh M. The taming of the cell penetrating domain of the HIV Tat: myths and realities. Journal of Controlled Release. 2007;117:148–62. doi: 10.1016/j.jconrel.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 69.Ito A, Jitsunobu H, Kawabe Y, Kamihira M. Construction of heterotypic cell sheets by magnetic force-based 3-D coculture of HepG2 and NIH3T3 cells. J Biosci Bioeng. 2007;104:371–8. doi: 10.1263/jbb.104.371. [DOI] [PubMed] [Google Scholar]
- 70.Ito A, Takizawa Y, Honda H, Hata KI, Kagami H, Ueda M, et al. Tissue engineering using magnetite nanoparticles and magnetic force: heterotypic layers of cocultured hepatocytes and endothelial cells. Tissue Eng. 2004;10:833–40. doi: 10.1089/1076327041348301. [DOI] [PubMed] [Google Scholar]
- 71.Yamamoto Y, Ito A, Kawabe Y, Shimizu K, Fujita H, Nagamori E, et al. Preparation of artificial skeletal muscle tissues by a magnetic force-based tissue engineering technique. J Biosci Bioeng. 2009;108:538–43. doi: 10.1016/j.jbiosc.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 72.Ito A, Ino K, Hayashida M, Kobayashi T, Matsunuma H, Kagami H, et al. Novel methodology for fabrication of tissue-engineered tubular constructs using magnetite nanoparticles and magnetic force. Tissue Eng. 2005;11:1553–61. doi: 10.1089/ten.2005.11.1553. [DOI] [PubMed] [Google Scholar]
- 73.Shimizu K, Ito A, Honda H. Mag-seeding of rat bone marrow stromal cells into porous hydroxyapatite scaffolds for bone tissue engineering. J Biosci Bioeng. 2007;104:171–7. doi: 10.1263/jbb.104.171. [DOI] [PubMed] [Google Scholar]
- 74.Akiyama H, Ito A, Kawabe Y, Kamihira M. Fabrication of complex three-dimensional tissue architectures using a magnetic force-based cell patterning technique. Biomed Microdevices. 2009;11:713–21. doi: 10.1007/s10544-009-9284-x. [DOI] [PubMed] [Google Scholar]
- 75.Cartmell SH, Dobson J, Verschueren SB, El Haj AJ. Development of magnetic particle techniques for long term culture of bone cells with intermittent mechanical activation. IEEE Trans NanoBiosci. 2002;1:92–7. doi: 10.1109/tnb.2002.806945. [DOI] [PubMed] [Google Scholar]
- 76.Dobson J, Cartmell SH, Keramane A, El Haj A. Principles and design of a novel magnetic force mechanical conditioning bioreactor for tissue engineering, stem cell conditioning, and dynamic in vitro screening. IEEE Trans NanoBiosci. 2006;5:173–7. doi: 10.1109/tnb.2006.880823. [DOI] [PubMed] [Google Scholar]
- 77.Hughes S, McBain S, Dobson J, El Haj A. Selective activation of mechanosensitive ion channels using magnetic particles. J R Soc Interface. 2008;5:855–63. doi: 10.1098/rsif.2007.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dobson J. Remote control of cellular behaviour with magnetic nanoparticles. Nat Nanotechnol. 2008;3:139–43. doi: 10.1038/nnano.2008.39. [DOI] [PubMed] [Google Scholar]
- 79.Chen K, Xie J, Xu H, Behera D, Michalski MH, Biswai S, et al. Triblock copolymer coated iron oxide nanoparticle conjugate for tumor integrin targeting. Biomaterials. 2009;30:6912–9. doi: 10.1016/j.biomaterials.2009.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Johansson LO, Bjornerud A, Ahlstrom HK, Ladd DL, Fujii DK. A targeted contrast agent for magnetic resonance imaging of thrombus: implications of spatial resolution. J Magn Reson Imaging. 2001;13:615–8. doi: 10.1002/jmri.1086. [DOI] [PubMed] [Google Scholar]
- 81.Veiseh O, Gunn JW, Kievit FM, Sun C, Fang C, Lee JSH, et al. Inhibition of tumor-cell invasion with Chlorotoxin-bound superparamagnetic nanoparticles. Small. 2009;5:256–64. doi: 10.1002/smll.200800646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Veiseh O, Sun C, Gunn J, Kohler N, Gabikian P, Lee D, et al. Optical and MRI multifunctional nanoprobe for targeting gliomas. Nano Lett. 2005;5:1003–8. doi: 10.1021/nl0502569. [DOI] [PubMed] [Google Scholar]
- 83.Xu C, Wang B, Sun S. Dumbbell-like Au-Fe3O4 nanoparticles for target-specific platin delivery. J Am Chem Soc. 2009;131:4216–7. doi: 10.1021/ja900790v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen T-J, Cheng T-H, Chen C-Y, Hsu SCN, Cheng T-L, Liu G-C, et al. Targeted herceptin-dextran iron oxide nanoparticles for noninvasive imaging of HER2/neu receptors using MRI. J Biol Inorg Chem. 2009;14:253–60. doi: 10.1007/s00775-008-0445-9. [DOI] [PubMed] [Google Scholar]
- 85.Wuang SC, Neoh KG, Kang E-T, Pack DW, Leckband DE. HER-2-mediated endocytosis of magnetic nanospheres and the implications in cell targeting and particle magnetization. Biomaterials. 2008;29:2270–9. doi: 10.1016/j.biomaterials.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bulte JM, Zhang S-C, van Gelderen P, Herynek V, Jordan EK, Duncan ID, et al. Neurotransplantation of magnetically labeled oligodendrocyte progenitors: magnetic resonance tracking of cell migration and myelination. PNAS. 1999;96:15256–61. doi: 10.1073/pnas.96.26.15256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Daldrup-Link HE, Rudelius M, Oostendorp RAJ, Settles M, Piontek G, Metz S, et al. Targeting of hematopoietic progenitor cells with MR contrast agents. Radiology. 2003;228:760–7. doi: 10.1148/radiol.2283020322. [DOI] [PubMed] [Google Scholar]
- 88.Hogemann D, Josephson L, Weissleder R, Basilion JP. Improvement of MRI probes to allow efficient detection of gene expression. Biocong Chem. 2000;11:941–6. doi: 10.1021/bc000079x. [DOI] [PubMed] [Google Scholar]
- 89.Pan X, Guan K, Yo J-W, Epstein AJ, Lee LJ, Lee RJ. Cationic lipid-coated magnetic nanoparticles associated with transferrin for gene delivery. Int J Pharm. 2008;358:263–70. doi: 10.1016/j.ijpharm.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu C, Yuan Z, Kohler N, Kim J, Chung MA, Sun S. FePt nanoparticles as an Fe reservoir for controlled Fe release and tumor inhibition. J Am Chem Soc. 2009;131:15346–51. doi: 10.1021/ja905938a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leuschner C, Kumar CSSR, Hansel W, Soboyejo W, Zhou JK, Hormes J. LHRH-conjugated magnetic iron oxide nanoparticles for detection of breast cancer metastases. Breast Cancer Res Treat. 2006;99:163–76. doi: 10.1007/s10549-006-9199-7. [DOI] [PubMed] [Google Scholar]
- 92.Zhou JK, Leuschner C, Kumar C, Hormes JF, Soboyejo WO. Sub-cellular accumulation of magnetic nanoparticles in breast tumors and metastases. Biomaterials. 2006;27:2001–8. doi: 10.1016/j.biomaterials.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 93.Galanzha EI, Shanshkov EV, Kelly T, Kim J-W, Yang L, Zharov VP. In vivo magnetic enrichment and multiplex photoacousitc detection of circulation tumour cells. Nat Nanotechnol. 2009;4:855–60. doi: 10.1038/nnano.2009.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shi X, Wang SH, Swanson SD, Ge S, Cao Z, van Antwerp ME, et al. Dendrimer-functionalized shell-crosslinked iron oxide nanoparticles for in-vivo magnetic resonance imaging of tumors. Adv Mater. 2007;20:1671–8. [Google Scholar]