Abstract
Over the years, biological imaging has seen many advances, allowing scientists to unfold many of the mysteries surrounding biological processes. The ideal imaging resolution would be in nanometres, as most biological processes occur at this scale. Nanotechnology has made this possible with functionalised nanoparticles that can bind to specific targets and trace processes at the cellular and molecular level. Quantum dots (QDs) or semiconductor nanocrystals are luminescent particles that have the potential to be the next generation fluorophores. This paper is an overview of the basics of QDs and their role as fluorescent probes for various biological imaging applications. Their potential clinical applications and the limitations that need to be overcome have also been discussed.
Keywords: quantum dots, in vitro and in vivo imaging
Nanotechnology is the manipulation of matter in dimensions <100 nm. It is based on the fact that the physical and chemical properties of matter change considerably at the nanoscale compared to those that they exhibit at the macroscale. Richard Feynman's famous talk ‘There is plenty of room at the bottom’, which first introduced the concept of nanotechnology, was inspired by the complexity of biological machinery, as most processes in living matter occur at the nanoscale. Consider the size of a DNA molecule, which approximates 2 nm, and the nuclear pores being ∼9 nm allow substances of less than this size to enter the nucleus. It can therefore be assumed that nanoscience finds its origin at the interface of the three basic sciences of physics, chemistry and biology, leading to completely new arenas of scientific discovery. The combination of nanotechnology with biology is obvious as nanobiotechnology allows the use of nano-tools and nano-devices to interact with, detect and alter biological processes at the cellular and molecular level (1). The most important application of nanobiotechnology is in nanomedicine, which may be defined as the application of nanotechnology to the diagnosis, monitoring and control of disease at a molecular level using engineered nanoscale devices and structures. Of the many nanoparticles that are currently being investigated, semiconductor nanocrystals or quantum dots (QDs) have a potential to lead to major advancement in biological imaging as the next generation fluorophores. This paper reviews the basics of QDs, the process of biofunctionalisation along with their role as fluorescent probes for in vitro and in vivo imaging. Their potential clinical applications, limitations and future perspectives are also discussed.
Historical background
Colloidal semiconductor QDs were first prepared by Professor Louis Brus in 1982 (2) and this marked the birth of a nanoscience building block (3). Their photophysical properties have been explored in great detail over the past two decades. Weller (4) first described the quantum confinement effects of the colloidal semiconductor Q-particles in 1993. In the same year, Murray and colleagues (5) developed a novel, reproducible method for the synthesis of high quality, monodisperse nanocrystals. QDs were first introduced to biological imaging in 1998 (6, 7) as fluorophores with properties that give them tremendous advantages over the traditional organic dyes (Table 1). Since then, much progress has been made in exploring their role in nanobiotechnology and nanomedicine as tools for diagnostic and therapeutic applications.
Table 1.
Comparison between the optical properties of traditional organic dyes and QDs for biological application
| Properties | Organic dyes | QDs | Advantages of QD |
|---|---|---|---|
| Excitation spectrum | Narrow | Broad | Organic dyes can only be excited by light of a specific wavelength due to the narrow excitation spectrum vs QDs that may be excited by light of a range of wavelengths, allowing multicolour QDs to be excited by a single wavelength of light. |
| Emission spectra | Broad and asymmetrical | Narrow and symmetrical | The broad emission spectra of conventional dyes may overlap and this limits the number of fluorescent probes that can be tagged to biomolecules for simultaneous imaging in a single experiment. QDs have narrow emission spectra that can be controlled by altering the size, composition and surface coatings of the dots. Hence, multiple QDs emitting different colours can be excited by a single wavelength of light, making them ideal for multiplexed imaging. |
| Photobleaching threshold | Low | High | Organic dyes bleach within a few minutes on exposure to light whereas QDs are extremely photostable due to their inorganic core, which is resistant to metabolic degradation and can maintain high brightness even after undergoing repeated cycles of excitation and fluorescence for hours. Hence, they can be used for long-term monitoring and cell-tracking studies. |
| Decay lifetime | Fast (<5 ns) | Slow (30–100 ns) | The fluorescence lifetime of QDs is considerably longer than typical organic dyes that decay within a few nanoseconds. This is valuable in overcoming the autofluorescence of background tissues, hence improving signal to noise ratio. |
| Quantum yield | Low | High | QDs have higher quantum yields, a larger absorbance cross section and a larger saturation intensity than organic fluorophores in aqueous environments, making them much brighter probes for in vivo studies and continuous tracking experiments over extended periods of time. |
| Absorbance cross section | Low | High | |
| Saturation intensity | Low | High |
Quantum dots (QDs) - basics
QDs are fluorescent semiconductor nanocrystals, composed of materials from the elements in the periodic groups of II–VI, III–V or IV–VI, e.g. cadmium telluride (Cd from group II and Te from group VI) and indium phosphamide (In from group III and P from group V). They range in size from 2 to 10 nm in diameter and contain approximately 200–10,000 atoms (8). Owing to the effects of quantum confinement, QDs are highly photostable, with broad absorption, narrow and symmetric emission spectra, slow excited-state decay rates and broad absorption cross-sections. Their emission colour depends on their size, chemical composition and surface chemistry and can be tuned from the ultraviolet to the visible and near-infrared (NIR) wavelengths.
Structure
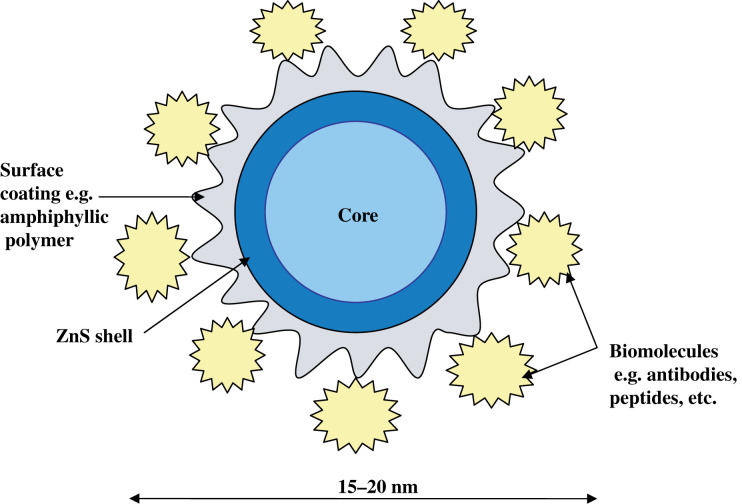
The general structure of a QD (Fig. 1) comprises an inorganic core semiconductor material, e.g. CdTe or CdSe, and an inorganic shell of a different band gap semiconductor material, e.g. ZnS. This is further coated by an aqueous organic coating to which biomolecules can be conjugated. Choice of the shell and coating is important as the shell stabilises the nanocrystal core and also alters the photophysical properties, while the coating confers properties that allow its allocation to various applications, such as determining solubility in aqueous media, providing reactive groups for binding to biological molecules as well as nullifying the toxicity.
Fig. 1.
Structure of a quantum dot.
A bare nanocrystal core is highly reactive and toxic, resulting in a very unstable structure that is prone to photochemical degradation (9). Also, the core crystalline structure has surface irregularities that lead to emission irregularities like blinking. Capping the core with a semiconductor material of a higher band gap, e.g. ZnS, not only increases the stability and quantum yield (QY), but also passivates the toxicity of the core by shielding reactive Cd2+ and Te2− ions from being exposed to photo-oxidative environments, e.g. exposure to UV and air (9). However, a ZnS coating is not sufficient to stabilise the core in biological solutions and therefore a further aqueous coating is required to ensure solubility in biological media. QDs have been coated with a shell of functionalised silica, phospholipid micelles, or linkers like mercaptoacetic acid, mercaptoundecanoic acid, dihydrolipoic acid (DHLA), or amphiphyllic polymers like modified polyacrylic acid, to render them soluble in aqueous media (10). The aqueous coating can then be tagged with various biomolecules of interest, e.g. antibodies, peptides, nucleic acids, etc., and different methods of bioconjugation have been described.
Mechanism of fluorescence
In the bulk form of the semiconductor material, the electrons exist in a range of energy levels, described as continuous. At the nanoscale, these levels become discrete owing to the effects of quantum confinement. Following a stimulus, the electron jumps from the valence to the conduction band across the band gap, leaving behind a positively charged hole. The coulomb correlated electron/hole bound pair in a semiconductor material is called an exciton and the quantum confinement effect occurs from the physical confinement of the electrons in 3D (11). The average physical distance between the electron-hole pair is the exciton bohr radius and this distance differs for the different types of semiconductor materials. As the size of the semiconductor material approaches the size of its exciton bohr radius, its properties cease to resemble the bulk material and the nanocrystal is called a QD. After being excited to the conduction band, the valence electron drops back to its valence position, emitting electromagnetic radiation, which is different from the original stimulus. This emission frequency is perceived as fluorescence and depends on the size of the band gap, which can be altered by changing the size of the QD as well as changing the surface chemistry. It is important to note that the smaller the QD, the higher the band gap energy.
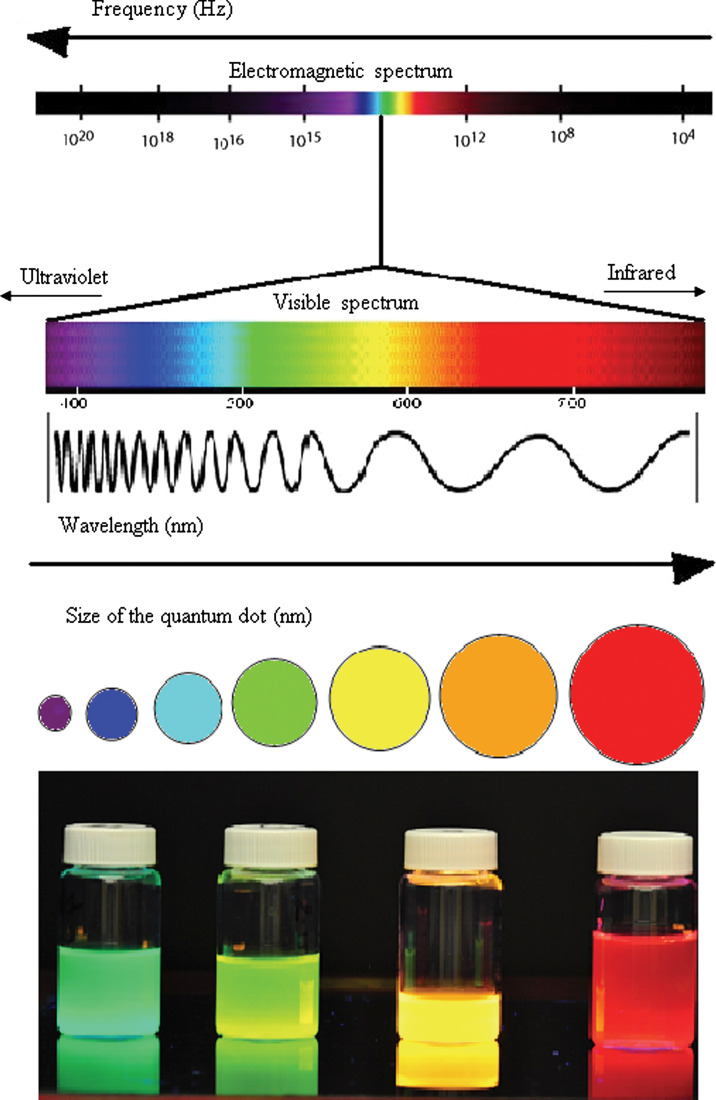
This size tunable absorption and emission property of QDs is an extremely valuable property for biological imaging as they can be tuned all the way from the UV to the NIR of the spectrum such that smaller dots emit in the blue range and larger dots in the red and NIR region (Fig. 2). QDs have a broad excitation and narrow, discrete emission spectra. The peak emission wavelength of the QD is slightly longer than the first exciton peak or absorption onset, and this energy separation is known as the Stoke's shift. Additionally, their peak emission wavelength is independent of the wavelength of excitation light. This means that variable-sized QDs can be excited by a single wavelength of light, as long as this wavelength is shorter than the absorption onset. This property finds application in multiplexed imaging where a number of different-sized QDs with discrete emission peaks and hence different colours can be excited by a single wavelength of light.
Fig. 2.
Size tunable emission.
Process of biofunctionalisation
The synthesis of colloidal QDs and their application to the biological scenario is a multistep process (Fig. 3). Each step has its own challenges and involves the integration of quantum physics, materials science, synthetic chemistry and, most importantly, biology.
Fig. 3.
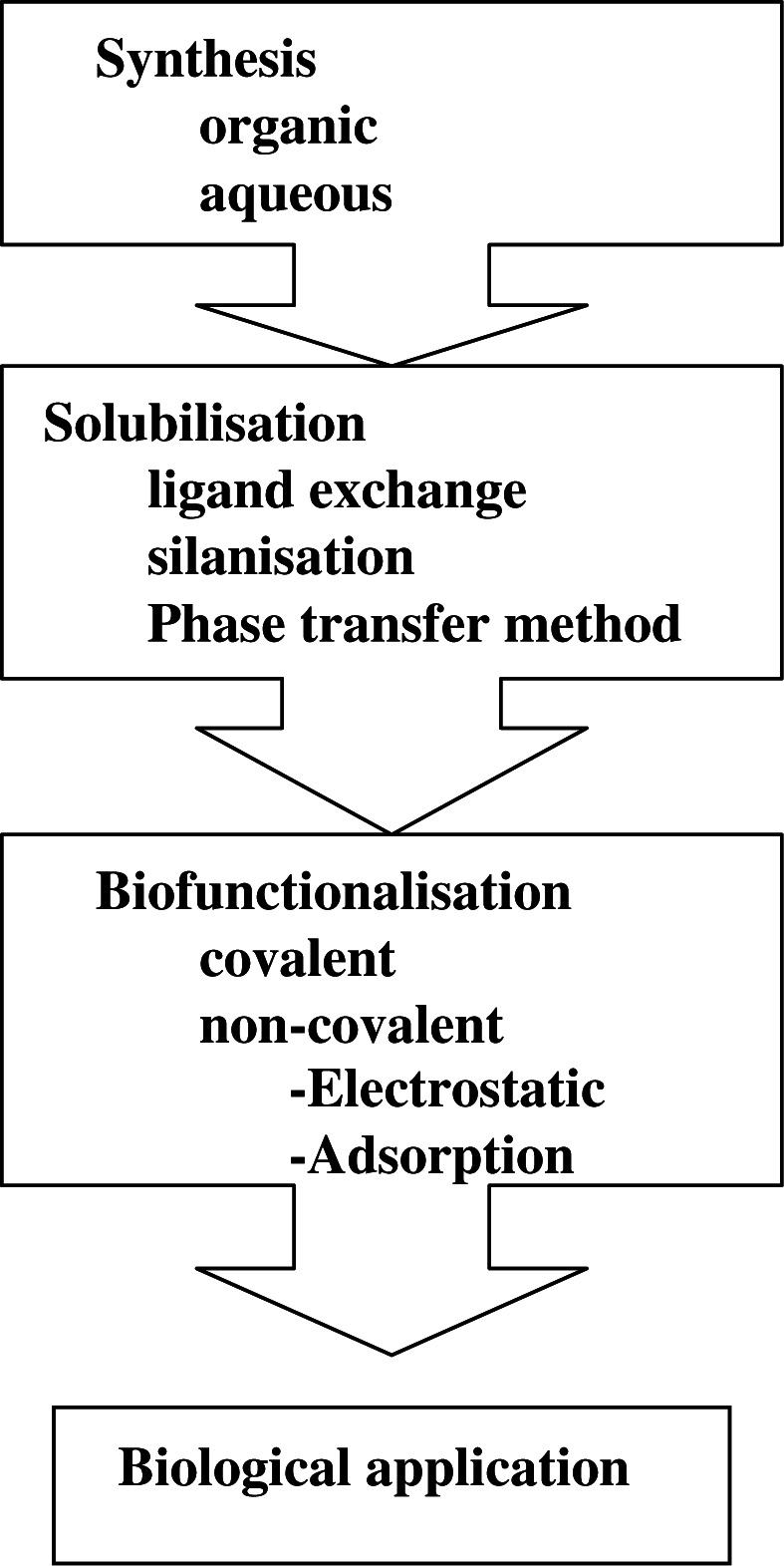
Pathway of processing quantum dots for biological application.
Synthesis
QDs can be synthesised by an organic or aqueous route and, recently, a microwave-assisted method has also been described. Currently, most available QDs have been synthesised using the organic route involving high temperatures and in the presence of surfactants to yield monodisperse and stable particles (5, 12). However, the QDs produced by the organic phase are insoluble in aqueous media and therefore not applicable to biological systems. A number of methods to solubilise organically synthesised QDs in aqueous media have been developed with good success and are described below. Aqueous synthesis, on the other hand, produces water soluble QDs through a simpler, inexpensive and reproducible method that can easily be scaled up. However, aqueously synthesised QDs do not have good crystallinity, have low QYs and full width half maximum (FWHM), and long reaction times, making preparation a time consuming and tedious process (13). Recently, various groups have improved the QY of water soluble QDs by optimising the synthetic methods and post synthetic treatment, e.g. by illuminating under room light for 20 days (13). Li and colleagues (14) have recently described a new method of QD synthesis based on microwave irradiation with controllable temperatures. This allowed a rapid production of size tunable QDs in 5–45 min, based on the reaction between Cd2+ and NaHTe solution (13). This method showed significant advantages over the traditional aqueous synthesis, such as reduced toxicity, good reproducibility, inexpensive, excellent water solubility, stability and biological compatibility and a comparable QY to the organic synthetic route.
Solubilisation
This is the biggest challenge prior to the biological application of QDs. As most QDs are synthesised by the organic route, their hydrophobic surface chemistry makes them insoluble in aqueous biological media. Over the years, scientists have discovered a wide array of surface coatings for solubilisation of QDs (15). The aim is to achieve an ideal surface chemistry that is stable in biological media and does not alter the photophysical properties of the QDs, while retaining a small size and providing free reactive surface groups for binding and recognition of biomolecules. The techniques used to achieve solubilisation include ligand exchange, surface silanisation and phase transfer methods. The ligand exchange method is based on the exchange of the hydrophobic surfactant molecules with bifunctional molecules, which are hydrophilic on one side and hydrophobic on the other, to bind to the ZnS shell on the QD (16). Most often, thiols (-SH) are used to bind to the ZnS and carboxyl (-COOH) groups are used as hydrophilic ends. The resulting QDs are soluble in both aqueous and polar solvents. This is by far the simplest method of solubilisation. Surface silanisation involves the growth of a silica shell around the nanocrystal. As silica shells are highly cross linked, they are very stable. However, the drawback is that the process is laborious and the shell may be hydrolysed (6). The phase transfer method uses amphiphyllic (17, 18) polymers to coat the QD surface. The hydrophobic alkyl chains of the polymer interdigitate with the alkyl groups on the QD surface, while the hydrophilic groups point outwards to attain water solubility. However, coating with a polymer may increase the overall diameter of the QD and this may pose a limitation in biological applications (19). Various reports of coating using phospholipids micelles, dithioretol, organic dendrons and oligomeric ligands are present in the literature (20–23).
Bioconjugation
Once solubilisation has been achieved, QDs can be functionalised by conjugation to a number of biological molecules, including avidin, biotin, oligonucleotides, peptides, antibodies, DNA and albumin (16), through surface reactive groups for specific targeted action. Methods of bioconjugation broadly fall into two categories: non-covalent and covalent conjugation (24). Non-covalent interactions include adsorption, electrostatic interaction and mercapto exchange. Biomolecules like oligonucleotides and various serum albumins (25) can be adsorbed on the surface of the water soluble QD. This process is non-specific and depends on the pH, temperature, ionic strength and surface charge of the molecule (16). QDs may be cationic or anionic and may interact with biomolecules through electrostatic interaction. The surface charge plays an important role in the cellular interaction of QDs and is determined by the free surface reactive groups. It has been demonstrated that proteins engineered with positively charged domains can interact with the negative charges on the QD surface coated with DHLA through electrostatic interaction (26). These conjugates have greater photoluminescence but are also more stable than unconjugated dots. However, electrostatic interactions are non-specific and relatively weaker compared to covalent bonding and this may pose a problem in the biological environment. Many biological molecules have a thiol group that can be tagged on to the surface of a QD by a mercapto exchange process (16). However, the resulting bond between thiol and Zn is not only weak but also dynamic, and this may lead to precipitation of the biomolecules in solution as they easily detach from the QD surface. Covalent linkage is the most stable of all the bioconjugation methods and utilises functional groups on the QD surface, such as primary amines, carboxylic acids, and thiols, to form a covalent bond with similar groups present on the biomolecules or through the use of crosslinker molecules (16). The commonest method of bioconjugation is via the avidin-biotin interaction. This is based on the high affinity interaction between avidin and biotin through a generic key-lock mechanism. Avidin is attached to antibodies and biotin can be covalently bound to the surface of QDs and vice versa. As most of the commercially available biomolecules are avidin or biotin linked, it makes this process most convenient as well (13, 26).
Bioimaging applications
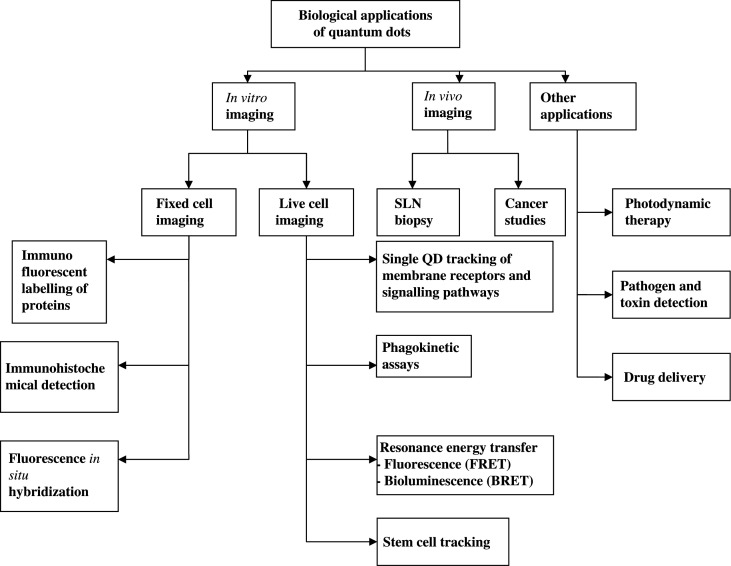
Fluorescence microscopy is an established method of visualising the structures and molecules within cells and QDs are increasingly being used as fluorescent biological labels for cellular and molecular imaging. QD application to this field is similar to that of organic fluorophores but with various advantages. They can be used to visualise cellular structures and receptors in both fixed and live cells. Although QDs have been used for a broad range of biological applications (Fig. 4), their vast potential for biomedical imaging and therapeutics remains unexplored.
Fig. 4.
Biological applications.
In vitro imaging - fixed cells
QD conjugates can be used for several immunohistochemical applications and have various advantages over organic fluorophores, including increased photoluminescence, photostability, broad excitation and narrow emission spectra allowing multiplexed imaging. Mutlilabelling QD protocols for an extremely sensitive immunohistochemical detection have been described (27). Immunohistochemical detection of target molecules in fixed cells involves the use of labelled primary antibodies that specifically bind to the antigen of interest followed by the application of a secondary antibody. Secondary antibodies are conjugated to organic fluorophores or enzymes that catalyse the deposition of fluorescent substrates at or near the site of the primary antibody, demonstrating that enzyme-based amplification can greatly increase the sensitivity of immunohistochemical detection (27).
Detection of target molecules in their natural distribution requires fixation followed by permeabilisation of the cell wall by fluorescent probes. As QDs are larger than organic dyes, they require different methods of cell fixation and permeabilisation (28). The timing of cell fixation, i.e. before or after exposure to QDs, determines their stability, localisation within the cell as well as emission properties. While in prefixed permeabilised cells, QDs are readily internalised regardless of the cell type, in live cells previously incubated with QDs, the choice of fixative influences the fluorescence characteristics. A comparative analysis of gluteraldehyde, methanol and paraformaldehyde demonstrated that 2% paraformaldehyde was the fixative of choice (29).
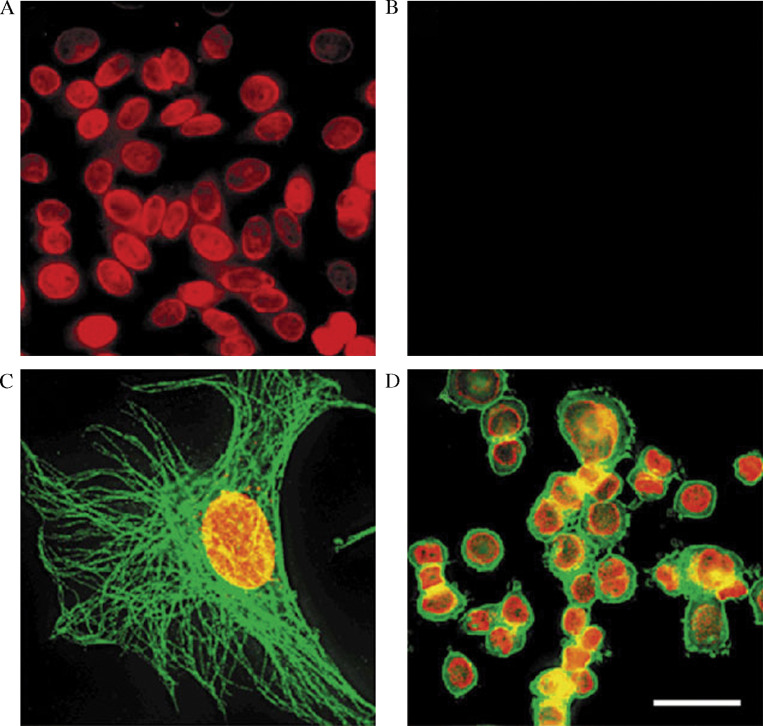
QD-Ab conjugates have accurately identified membrane-bound protein p-gp (glycoprotein) in fixed MCF7r breast adenocarcinoma cells (13). The distribution of p-gp was displayed by confocal reconstruction of 3D imaging and this showed that Q-Ab conjugate labelling was highly sensitive and photostable compared to organic dyes like FITC, AlexaFluor488 and R-phycoerythrin. Using QD conjugates, actin and microtubule fibres in the cytoplasm (6) and various nuclear antigens of fixed cancer cells have been stained (30). Indirect immunofluorescence has been used to identify Her2, microtubules and nuclear antigens in fixed cancer cells by first incubating fixed cells with a primary Ab, then a biotinylated secondary Ab and finally with QD-labelled streptavidin. Apart from single-target labelling, double labelling of nuclear antigens and Her2/microtubules with two different QDs has also been demonstrated (Fig. 5). The QDs were shown to be several fold more photostable than the organic dye Alexafluor 488 (30).
Fig. 5.
Fixed cell imaging and simultaneous detection of multiple cellular targets using QD conjugates. (A) Nuclear antigens in the nuclei of human epithelial cells labelled with ANA, anti-human IgG-biotin and QD 630-streptavidin. (B) When normal human IgGs were used, no detectable stain was observed. (C) Simultaneous labelling of nuclear antigens (red) and microtubules (green) using different QD conjugates in a 3T3 cell. (D) Her2 on the surface of SK-BR-3 cells was stained green with mouse anti-Her2 antibody and QD 535-IgG (green). Nuclear antigens were labelled with ANA, anti-human IgG-biotin and QD 630-streptavidin (red). Reprinted with permission from Macmillan Publishers Ltd: Nature Biotechnology (30) copyright 2003.
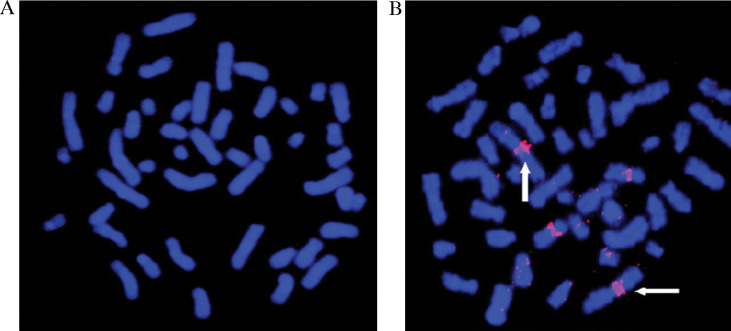
Fluorescence in situ hybridisation (FISH) technology to detect nucleic acid sequences in fixed biological structures has been well established (31). It uses fluorescently labelled DNA probes for gene mapping and identification of subtle chromosomal abnormalities and can provide diagnostic and prognostic results for particular chromosomal disorders. It utilises the concept of detection of a fluorescent signal at the site of hybridisation of a fluorescent dye-labelled probe with its homologous chromosomal target. The drawbacks of using rapidly photobeaching and multicolour organic dyes with problems of spectral overlap can be overcome with QDs with their high photostability and discrete spectra allowing multiplexed imaging. Xiao and Barker (32) used a QD-FISH probe to analyse human metaphase chromosomes (Fig. 6) and found that compared to organic dyes like Texas-Red and FITC, QDs were more photostable and significantly brighter, making them a more stable and quantitative mode of FISH for research and clinical applications. QDs are also likely to probe single DNA molecules and their interaction with proteins, allowing the study of dynamic processes. The application of QD conjugates to visualise single-copy sequence DNA probes as short as 1,500 nucleotides in length has been demonstrated (33). This should find application in rapid gene mapping and DNA-protein interactions (13).
Fig. 6.
Detection of chromosome region 1q12 in human metaphase chromosomes by FISH using a QD-labelled probe. (A) Control (no QD conjugate); (B) streptavidin-Qdot 605 detection of chromosome 1q12 region in homologous chromosomes (vertical and horizontal arrows); bar in panel C is 10 µm. (32). Printed by permission of Oxford University Press.
In vitro imaging - live cells
One of the biggest challenges of cell biology is to explore the molecular dynamics of various cellular events in live cells. This would aid the understanding of cellular and molecular interactions and monitor them over prolonged periods at high resolution. QDs introduced to live cells can be used for various applications like cell tracking, which are crucial to stem cell research and determining the metastatic potential of cancer cells. Their prolonged photostability allows long-term imaging applications like single-molecule tracking in living cells, hence unfolding many cellular and molecular processes which have never been done before.
Labelling of live cells with QDs is a crucial step prior to various imaging applications. QDs can be loaded inside the cells or be liganded to the extracellular surface.
Intracellular labelling could be achieved by incubating cells with QDs via non-specific endocytosis (34, 35) or they may be bound to peptides on the cell surface that are specifically endocytosed (7). Alternatively, they can be introduced into the cell via microinjection (20, 33, 36), which though laborious, is the only technique that ensures uniform cytoplasmic distribution. By far, peptide-mediated intracellular delivery of QDs has been most commonly used. This approach is based on the fact that protein transduction domains (PTD) can allow the passive delivery of drugs across the cell membranes as well as the blood brain barrier. A number of biomolecules, including proteins, oligonucleotides, liposomes and magnetic nanoparticles, have been delivered into cells using PTDs (37). It has been shown that QDs can be delivered into cells using similar techniques. QDs have been coupled to PTDs via a streptavidin-biotin link (38), covalently (39), by electrostatic adsorption or adsorption to synthetic PTDs like pep-1 (40). Of all the approaches for intracellular delivery, coupling PTDs to QDs via a streptavidin-biotin is most easily performed.
Extracellular labelling via membrane receptors and membrane-associated proteins are good targets for QD imaging. Various biological processes and pathways, such as chemotaxis, synaptic regulation or signal transduction, rely on the transmembrane receptors and signalling pathways. Plasma membranes have a complex and dynamic architecture with various components that affect the diffusion of endogenous proteins and lipids. Pinaud et al. (41) used biotinylated peptide-coated QDs to study the organisation of the plasma membrane and the influence of lipid raft microdomains on the diffusion of raft-associated proteins in HeLa cells. Labelling with QDs allowed high-resolution and long-term tracking of an individual glycosyl-phosphatidyl-inositol-anchored avidin test probe (Av-GPI), and the classification of their various diffusive behaviours.
Single-particle tracking
This is a technique of following single molecules in real time to visualise the actual molecular dynamics in their habitat environment. This can be used to track various biological molecules like lipids, membrane-associated proteins and cytosolic motor proteins, as well as detailed descriptions of the compartment sizes of micro-domains and the time that individual macromolecules reside in each compartment. Apart from genetically encoded fluorescent proteins and organic dyes, various materials have been used for single-particle tracking studies, including latex or fluorescent microspheres (∼20–500 nm) and colloidal gold nanoparticles (40 nm). Fluorescent proteins and organic dyes have low photoluminescence in a background of high cell autofluorescence and a low photobleaching threshold making long-term tracking difficult. Gold nanoparticles are good agents for single-particle tracking, but do not allow multiplexed imaging.
QD probes have been used to demonstrate the dynamics of glycine receptors (GlyRs) in neuronal membranes (42). QDs were used to track individual GlyRs and analyse their lateral dynamics in neuronal membranes in living cells over periods of time ranging from milliseconds to minutes. QD labelling enabled imaging for 20 min as compared to the organic dye Cy3, which allowed imaging for only 5 sec. Tracking individual dots allowed characterisation of multiple diffusion domains showing that QDs are ideal probes for single-molecule studies in live cells. Using single-QD tracking, the dynamics of individual GABA receptors in the axonal growth of spinal neurons has also been demonstrated (43). Biotinylated epidermal growth factor (EGF)-conjugated QDs have been used to study the endocytosis and trafficking of erbB receptors, showing a previously unreported mechanism of retrograde transport to the cell body (44).
Stem cell tracking
As one of the most fascinating areas of contemporary biology, stem cell research has a vast potential to treat a myriad of medical conditions. Currently, magnetic and superparamagnetic iron oxide (SPIO) nanoparticles are being used to achieve this objective through the use of MRI. However, the gradual loss of the MRI cell signal due to cell division poses a hindrance to long-term imaging studies. The application of QDs as nanomaterials for monitoring stem cell survival, distribution, differentiation and regenerative impact either in vitro or in vivo due to their inherent long-term fluorescence intensity has been shown by several groups. Shah et al. (45) reported the long-term labelling of human bone marrow-derived mesenchymal stem cells (hMSC) with RGD peptide-conjugated QDs during self-replication and differentiation in osteogenic cell lineages. The same group also demonstrated that RGD-conjugated QD-labelled hMSCs remained viable following multilineage differentiation as did the unlabelled hMSCs (46). The regenerative potential of stem cells to repair damaged cardiac tissue is being investigated, but attempts at tracking the differentiation and distribution of stem cells in vivo have been hampered by the inherent autofluorescence of damaged cardiac tissue. QD-labelled hMSC can illuminate stem cells in histological sections for at least 8 weeks following delivery, allowing a 3D reconstruction of the location of all stem cells following injection into the heart (47). While QD-labelled tracking of stem cells promises great advancements in the field of stem cell research, the effects of QDs on stem cell self-renewal and differentiation are largely unknown and need to be explored.
Phagokinetic assays
QDs have been used to demonstrate the metastatic potential of cancer cells to distinguish between invasive and non-invasive cancer cell lines (48). Gu et al. (49, 50) have demonstrated a -2D in vitro cell motility assay based on the phagokinetic uptake of QDs by cells as they move across a homogenous layer of QDs, leaving behind a fluorescent free trail. The ratio of the trail area to the cell area distinguishes between invasive and non-invasive tumour cells (49, 50).
Biosensing applications
Fluorescence resonance energy transfer (FRET) is a process in which energy is transferred from an excited donor to an acceptor particle, leading to a reduction in the donor's emission and excited-state lifetime and an increase in the acceptor's emission intensity. This happens whenever the distance between the donor and acceptor is smaller than the critical radius, known as the Förster radius (9). FRET is sensitive to the distance between the donor and the acceptor and is used to measure changes in distances rather than absolute distances. It is therefore suitable for studying biomolecule conformation, dynamics and interactions, e.g. monitoring protein conformational changes, protein interactions and assaying enzyme activity (9). Using organic dyes for FRET poses the problems of early photobleaching and significant emission overlap between the donor and acceptor and QDs provide an excellent alternative.
QD-based FRET technology has found application in monitoring other processes, such as DNA replication and telomerisation, for fast and sensitive DNA detection and DNA array analyses (51). Most QD-based FRET probes are based on QDs as donors and organic fluorophores as acceptors. A single QD-based nanosensor capable of detecting extremely low concentrations of DNA (50 copies) in a separation-free format has been demonstrated (52). QDs were linked to DNA probes to capture DNA targets. The target strand binds to a dye-labelled reporter strand, thus forming a FRET donor-acceptor ensemble.
QD FRET-based sensing can also be used to detect receptor-ligand binding. QDs conjugated to maltose-binding protein (MBP) were used to demonstrate the association of maltose with MBP by a competitive binding mechanism that induced a FRET change and was hence able to detect the presence of maltose in solution (53). QD-linked MBP interacted with QSY-9 (organic dye) conjugate in the absence of maltose, resulting in quenching of fluorescence of the QDs by the dye. Adding maltose displaced the dye and restored QD fluorescence. Another application of QD-FRET is the detection of enzyme activity in particular protease sensing. This is based on inserting peptide sequences of various proteases between QDs and quenchers (or acceptor dyes). The QD fluorescence is quenched by the acceptor dye, but cleavage of the peptide sequences by specific proteases causes removal of these acceptor molecules, and hence the QDs are switched on. A good example is rhodamine Red-X dye-labelled peptides linked to CdSe/ZnS QDs used as FRET probes to monitor the proteolytic ability of collagenases in normal and cancer cells. Hydrolytic cleavage of the peptide-linked dye by the collagenases restored QD fluorescence allowing detection of specific enzyme activity over short periods of time (54).
Bioluminescence resonance energy transfer (BRET) is a naturally occurring phenomenon, whereby a light-emitting protein that acts as a donor (e.g. Renilla luciferase) non-radiatively transfers energy to a fluorescent protein as an acceptor (e.g. GFP) in close proximity. The process is similar to FRET except that the energy comes from a chemical reaction catalysed by the donor enzyme (e.g. Renilla luciferase-mediated oxidation of its substrate coelenterazine) rather than absorption of excitation photons. So et al. (55) prepared self-illuminating QDs by covalently coupling QDs (as acceptors) to a bioluminescent protein Renilla reniformis luciferase (as a donor). The protein emits a blue light at 480 nm on addition of a substrate, coelenterazine. The QDs can be excited if they are in close proximity to the protein and emit at their emission maximum. These conjugates emit long-wavelength (from red to NIR) bioluminescent light in cells and in animals, even in deep tissues, and are suitable for multiplexed in vivo imaging.
In vivo imaging
Fluorescence imaging of live animals is limited by the poor transmission of visible light through the living tissues as well as by the intense autofluorescence of tissue chromophores. QDs can overcome these limitations through their high photoluminescence, enhanced photostability under prolonged laser illumination and size tunability to emit at longer wavelengths like the NIR, which is not subjected to scattering and absorption as light in the visible range. Also, QDs have a two-photon absorption cross section several times greater than organic dyes and this property makes them more efficient at probing thick tissue specimens by multiphoton microscopy (56). Green-emitting QDs were injected in living mice intravenously and dynamically visualised by two-photon microscopy as they perfused through the skin capillaries several micrometers deep. Two-photon excitation allows greater tissue penetration due to excitation in the NIR range (56). Voura et al. (57) used spectral imaging to trace intravenously injected tumour-labelled QDs into mice as they extravastated into lung tissue, and found that the behaviour of QD-labelled tumour cells in vivo was indistinguishable from that of unlabelled cells. Also QDs and spectral imaging allowed the simultaneous identification of five different populations of cells using an interesting multiphoton laser excitation application (57).
Sentinel lymph node biopsy (SLNB)
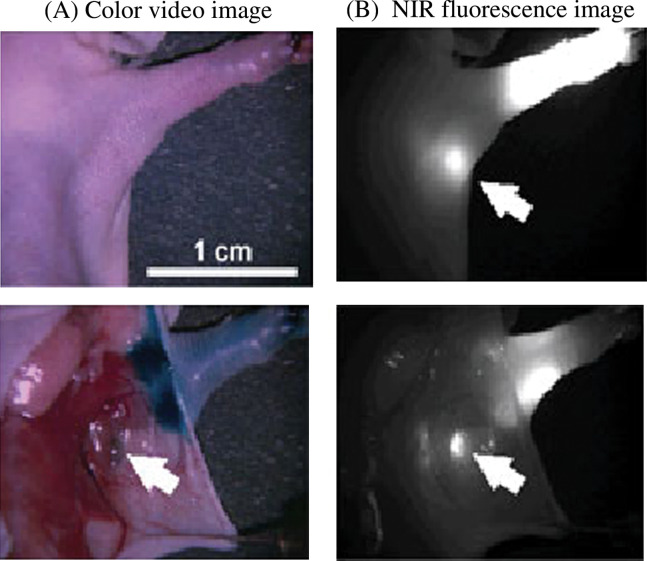
This is a means of ultra-staging cancer metastasis and is now the standard of care in breast cancer surgery. It is based on targeting the first draining lymph node, also called the sentinel lymph node (SLN) of a lymphatic basin at the cancer site to determine the extent of disease spread. Absence of metastasis in the SLN means that the disease is limited and extensive surgery can be avoided. Current tracers for SLN biopsy include the blue dye and radio-isotope. However, these have various limitations that can be overcome by the use of QDs that emit in the NIR range (>700 nm). The main problem with live animal imaging is to overcome the background tissue autofluorescence. NIR imaging can overcome this problem based on the concept that normal tissue chromophores do not absorb or scatter light in the NIR range. NIR light can therefore penetrate deeper tissues without being scattered and is ideal for imaging in real time. NIR QDs have successfully been used to demonstrate in vivo SLN biopsy in mice and pigs (21) (Fig. 7). An intradermal injection of picomolar quantities of NIR QDs entered the lymphatics and the fluorescence could be traced to the SLN in real time by the surgeon using an NIR imaging system. This allowed an accurate and sensitive localisation and biopsy of the SLN with minimal tissue dissection.
Fig. 7.
Detection of sentinel lymph node using NIR QDs in a mouse model. NIR QDs injected intradermally into the foot pad of a mouse migrate to the sentinel lymph node 5 min post injection. (A) Colour video image; (B) NIR fluorescence image; isosulphan blue dye colocalised to the same node (indicated by the arrows). Reprinted by permission from Macmillan Publishers Ltd: Nature Biotechnology (21), copyright 2004.
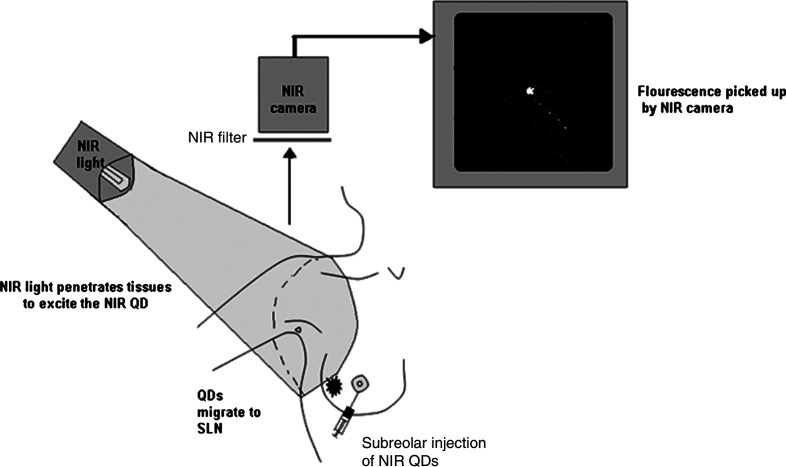
Fig. 8 is a schematic diagram of an NIR imaging system for SLNB in breast cancer surgery, as this has potential to be one of the major clinical applications of QDs in the future. The NIR light penetrates deep tissues with minimal scatter and excites the QDs that emit in the NIR range. The fluorescence from these dots is detected by an NIR camera, which is basically a regular CCD camera without an infrared (IR) filter. These images can be superimposed with images from the colour camera on a PC to anatomically locate the exact position of the QDs.
Fig. 8.
Schematic diagram of a near-infrared imaging system for SLNB in breast cancer surgery.
Cancer localisation and therapy
In vivo localisation of cancer antigens using QDs bound to tumour-specific antibodies has been demonstrated in molecular imaging studies. Antibody specific to prostate cancer cell marker PSMA was conjugated to QDs and injected into mice transplanted with human prostate cancer. This accurately localised the tumour, which was clearly imaged in vivo. Their bright luminescence and long lifetime allowed a more accurate and sensitive imaging compared to green fluorescent protein (GFP) (38). QDs linked to alpha-fetoprotein (AFP) monoclonal antibody were injected intravenously and successfully targeted human hepatocellular carcinoma xenograft growing in nude mice (58). QDs conjugated to anti-EGFR antibodies were able to detect increased expression of EGFR levels, which corresponds to early change from cervical dysplasia to cancer (59). This can have a huge impact on the early diagnosis and treatment of cancer.
Other applications
Drug delivery
Targeted QD imaging may find application as an ultrasensitive tool for early cancer diagnosis as well as image-guided drug delivery of chemotherapeutic agents to overcome systemic side effects. Drugs can be targeted to tumours by an enhanced permeability and retention (EPR) effect and this concept has been applied to anticancer agents (10). QD probes can target and accumulate in tumours by both the EPR effect and recognition of cancer cell surface biomarkers. Chemotherapeutic agents bound to QD probes that will recognise and bind to cancer cells, might offer a new strategy for molecular cancer therapy by avoiding systemic toxicity. QDs are one of the many nanoscale platforms being developed as novel drug delivery systems (60) based on their ability to target specific sites at a molecular level and unique photophysical properties (61).
Photodynamic therapy (PDT)
One of the major advances in minimally invasive therapies for cancer is photodynamic therapy (PDT). First discovered in the early 1900s, it is now an approved cancer treatment for various superficial malignancies, including basal cell carcinoma, oral, oesophageal and lung cancers (62). It is based on the targeted localised destruction of diseased tissues via the generation of cytotoxic singlet oxygen (1O2) using a non-toxic photosensitiser (PS), activated by light of a specific wavelength in the presence of molecular oxygen (3O2). Singlet oxygen leads to cellular necrosis and apoptosis of target cells via oxidation and degradation of cellular components.
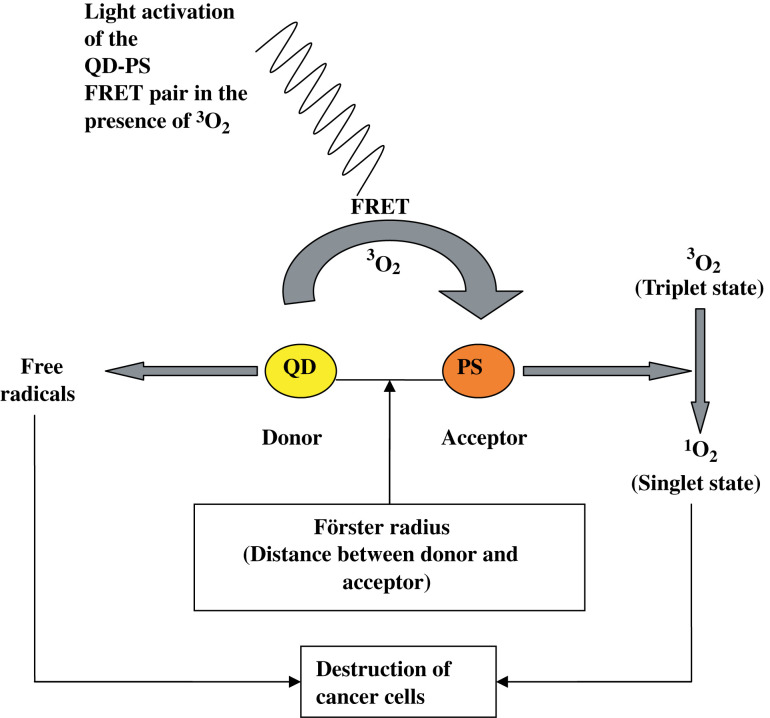
Photofrin is the most commonly used PS for PDT and suffers from various drawbacks like instability in aqueous solution, prolonged cutaneous sensitivity, chemical impurities and weak absorption at therapeutic wavelengths of >630 nm, which is essential for deep tissue penetration (63, 64). The application of QDs-PS complexes as therapeutic PDT agents was first reported by Samia et al. (65). QDs can be used in PDT either indirectly as energy donors to conventional PSs by FRET mechanism or directly as they react with molecular oxygen by energy transfer mechanisms to generate reactive oxygen species (ROS) (Fig. 9). With their high photoluminescence quantum efficiency, prolonged photostability, high molar extinction coefficients and tunable emission spectra at NIR wavelengths, they form ideal donors for the FRET process (66) in PDT. Also, functionalisation of the QD surface gives the advantage of enhanced solubility, biocompatibility and localisation of the QD-PS pair to the exact cancer site for specific targeted action (66).
Fig. 9.
Mechanism of PDT using quantum dots. QD-PS FRET pair localises to the site of cancer and is activated by light of a specific wavelength in the presence of molecular oxygen (3O2) to generate singlet oxygen, which is toxic to cancer cells. Free radicals are also generated directly by activation of the QD by light.
Micro-organism and toxin detection
Using the concept of multiplexed imaging, different food-borne pathogenic bacteria have been simultaneously detected at low concentrations of 10−3 cfu/ml within 2 h by using antibody-conjugated QDs and magnetic microparticles (67). When compared with organic green dye fluorescein isothiocyanate, CdSe/ZnS core/shell bioconjugates displayed brighter fluorescence intensities, lower detection thresholds and better accuracy in analysing bacterial cell mixtures composed of pathogenic Escherichia coli O157:H7 and harmless E. coli DH5alpha using flow cytometry. A novel method for an ultrasensitive, fluorescent detection of DNA and antigen molecules based on self-assembly of multiwalled carbon nanotubes and CdSe QDs via oligonucleotide hybridisation has also been described (68). Overall, the QD technology demonstrates great potential for a rapid and cost-effective detection of pathogen and toxin contamination of food samples.
Limitations
Introduction of QDs to the biological milieu involves elaboration of various aspects, such as toxicity, thrombogenicity, immunogenicity as well as ADME characteristics (absorption, metabolism, distribution and excretion). QD toxicity can arise from different levels, including composition of the core, surface coating, size and surface charge (69–71). The core nanocrystal is composed of heavy metals like cadmium, tellurium and selenium, which are known to have acute and chronic toxicities. Characterisation of in vitro and in vivo toxicity of QDs has been a daunting task due to the complex nature of these nanomaterials. Over the years, a number of studies have emerged with conflicting results, which makes it difficult to evaluate, generalise and predict the important aspects of toxicity. Most research is based on in vitro cytotoxicity, which involves exposure to very high doses. Fewer in vivo studies looking at ADME characteristics in small animals have been published. As the in vivo toxicity of QDs evolves from changes induced at the molecular level, it is crucial to first characterise the cellular interaction in relation to various representative cell lines. Little is known about the mechanism of QD uptake by cells and its interaction with the different cellular organelles. It is crucial to understand and explore these mechanisms for progress in the field of nanomedicine, cancer diagnosis and treatment.
Other limitations to the QD application are based on photophysical properties like blinking and photobrightening. Blinking occurs when the QD rapidly alternates between an emitting and non-emitting state. This may cause problems in single-molecule imaging or tracking. Photobrightening on the other hand, is the increase in the intensity of fluorescence of the QD on excitation. Although this may be advantageous, in certain cases it poses a problem in fluorescence quantisation studies (16). Both blinking and photobrightening result from the mobile charges present on the surface of the QDs. A significant amount of research is underway to evaluate and overcome these limitations (56, 72, 73).
Future perspectives
QDs have found vast application in biological and biomedical research as the next generation fluorescent probes. They are a powerful tool for illuminating many of the mysteries that encompass signal transduction pathways and biomolecular interaction within cells. Through extrapolating their properties for in vivo molecular and cellular imaging, QDs have a potential to lead to major advances in nanomedicine. They can revolutionise cancer diagnosis and therapy through early pre-symptomatic diagnosis and image-guided drug delivery of chemotherapeutic agents. NIR QDs may replace the current tracers for SLN biopsy. The most promising aspect of QD application is in their use as PSs for PDT. This application is unique as it utilises their inherent toxicity via the generation of ROS to target cancer cells and micro-organisms. Overall, there are relentless possibilities for the application of QDs in biology and nanomedicine. However, QD technology is still in its infancy and extensive research is still required to resolve many of the issues that are limiting their safe application in clinical medicine.
Acknowledgements
We would like to acknowledge the financial support of The Sebba Trust, UK, for development of QDs for detection of breast cancer.
Biographies
Dr. Sarwat B. Rizvi is currently a PhD student at the Division of Surgical and Interventional Sciences, University College London. She graduated with honours (MBBS) from The Aga Khan University Medical College, Pakistan and completed her Basic Surgical Training in the United Kingdom. She passed the membership exam of the Royal College of Surgeons of Edinburgh (MRCSed) and pursued further training in Critical Care and Emergency Medicine at the Addenbrooke's University Hospital, Cambridge. Her research interests include the assessment of nanotoxicology and development of a biocompatible semiconductor nanocrystal for sentinel lymph node biopsy.
 Miss Shirin Ghaderi received her BSc degree in Biochemistry at Queen Mary College, University of London and MSc in Drug Discovery from University of London's School of Pharmacy. She is currently a PhD student in nanotechnology, concentrating on using nanoparticles (quantum dots) as drug delivery and imaging in cancer studies under supervision of Professor Seifalian.
Miss Shirin Ghaderi received her BSc degree in Biochemistry at Queen Mary College, University of London and MSc in Drug Discovery from University of London's School of Pharmacy. She is currently a PhD student in nanotechnology, concentrating on using nanoparticles (quantum dots) as drug delivery and imaging in cancer studies under supervision of Professor Seifalian.
 Dr. Mo Keshtgar (BSc, MBBS, FRCSI, FRCS(Gen), PhD) is a Consultant Surgical Oncologist, working at the Royal Free Hospitals and honorary senior lecturer at UCL. His main area of specialist interest is management of breast cancer patients and his main research interest is application of nanotechnology for diagnostic and treatment of breast cancer. He has co-authored three textbooks and over 50 peer-reviewed publications. He has also delivered in excess of 60 invited lectures in the UK and over 20 countries. He is currently conducting several research programmes on sentinel lymph node biopsy and intraoperative radiotherapy and a clinical research on endoscopic surgery for breast cancer. He has supervised/supervising 10 postgraduate students to MD/PhD programmes. He is one of the national trainers on sentinel lymph node biopsy.
Dr. Mo Keshtgar (BSc, MBBS, FRCSI, FRCS(Gen), PhD) is a Consultant Surgical Oncologist, working at the Royal Free Hospitals and honorary senior lecturer at UCL. His main area of specialist interest is management of breast cancer patients and his main research interest is application of nanotechnology for diagnostic and treatment of breast cancer. He has co-authored three textbooks and over 50 peer-reviewed publications. He has also delivered in excess of 60 invited lectures in the UK and over 20 countries. He is currently conducting several research programmes on sentinel lymph node biopsy and intraoperative radiotherapy and a clinical research on endoscopic surgery for breast cancer. He has supervised/supervising 10 postgraduate students to MD/PhD programmes. He is one of the national trainers on sentinel lymph node biopsy.
 Professor Alexander M. Seifalian is Professor of Nanotechnology and Regenerative Medicine and Director of Centre for Nanotechnology & Regenerative Medicine at University College London. He is based within Division of Surgery & Interventional Science. He has completed his undergraduate and postgraduate education at University of London and University College London Medical School. He is Fellow of the Institute of Nanotechnology (FIoN) and has published over 310 peer-reviewed research papers, 24 book chapters and four families of patents.
Professor Alexander M. Seifalian is Professor of Nanotechnology and Regenerative Medicine and Director of Centre for Nanotechnology & Regenerative Medicine at University College London. He is based within Division of Surgery & Interventional Science. He has completed his undergraduate and postgraduate education at University of London and University College London Medical School. He is Fellow of the Institute of Nanotechnology (FIoN) and has published over 310 peer-reviewed research papers, 24 book chapters and four families of patents.
During his career, he has led and managed many large projects with multidisciplinary teams with very successful outcomes in terms of commercialisation and translation to patients, including development and commercialisation of a bypass graft for vascular access for haemodialysis; development of bioreactors with fluid dynamic system; developed non-invasive technique to monitor shear stress in vivo as well as on cells in physiological flow circuit using RF ultrasound signal; technique extraction of RNA from porous scaffold; laser-activated vascular sealants that have been commercialised for vascular, liver and brain surgery. His current projects are development of cardiovascular implants using nanomaterials and stem cells technology, development of organs using tissue engineering and development of nanoparticle for detection and treatment of cancer. He also has developed a family for nanomaterials and nanocomposite polymer for biomedical application.
He has been awarded the top prize in the field of development of nanomaterials and technologies in development of cardiovascular implants in 2007 by Medical Future Innovation and in 2009 received a Business Innovation Award from UK Trade & Investment (UKTI) in the Life Sciences and Healthcare category
References
- 1.West JL, Halas NJ. Applications of nanotechnology to biotechnology commentary. Curr Opin Biotechnol. 2000;11:215–7. doi: 10.1016/s0958-1669(00)00082-3. [DOI] [PubMed] [Google Scholar]
- 2.Rossetti R, Brus L. Electron-hole recombination emission as a probe of surface-chemistry in aqueous CdS colloids. J Phys Chem. 1982;86:4470–2. [Google Scholar]
- 3.Alivisatos AP. Birth of a nanoscience building block. ACS Nano. 2008;2:1514–6. doi: 10.1021/nn800485f. [DOI] [PubMed] [Google Scholar]
- 4.Weller H. Colloidal semiconductor Q-particles - chemistry in the transition region between solid-state and molecules. Angew Chem Int Ed Eng. 1993;32:41–53. [Google Scholar]
- 5.Murray CB, Norris DJ, Bawendi MG. Synthesis and characterization of nearly monodisperse Cde (e=S, Se, Te) semiconductor nanocrystallites. J Am Chem Soc. 1993;115:8706–15. [Google Scholar]
- 6.Bruchez M, Jr, Moronne M, Gin P, Weiss S, Alivisatos AP. Semiconductor nanocrystals as fluorescent biological labels. Science. 1998;281:2013–6. doi: 10.1126/science.281.5385.2013. [DOI] [PubMed] [Google Scholar]
- 7.Chan WC, Nie S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science. 1998;281:2016–8. doi: 10.1126/science.281.5385.2016. [DOI] [PubMed] [Google Scholar]
- 8.Smith AM, Duan H, Mohs AM, Nie S. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv Drug Deliv Rev. 2008;60:1226–40. doi: 10.1016/j.addr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jamieson T, Bakhshi R, Petrova D, Pocock R, Imani M, Seifalian AM. Biological applications of quantum dots. Biomaterials. 2007;28:4717–32. doi: 10.1016/j.biomaterials.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Iga AM, Robertson JH, Winslet MC, Seifalian AM. Clinical potential of quantum dots. J Biomed Biotechnol. 2007;2007:76087. doi: 10.1155/2007/76087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arya H, Kaul Z, Wadhwa R, Taira K, Hirano T, Kaul SC. Quantum dots in bio-imaging: revolution by the small. Biochem Biophys Res Commun. 2005;329:1173–7. doi: 10.1016/j.bbrc.2005.02.043. [DOI] [PubMed] [Google Scholar]
- 12.Peng ZA, Peng X. Formation of high-quality CdTe, CdSe, and CdS nanocrystals using CdO as precursor. J Am Chem Soc. 2001;123:183–4. doi: 10.1021/ja003633m. [DOI] [PubMed] [Google Scholar]
- 13.Weng J, Ren J. Luminescent quantum dots: a very attractive and promising tool in biomedicine. Curr Med Chem. 2006;13:897–909. doi: 10.2174/092986706776361076. [DOI] [PubMed] [Google Scholar]
- 14.Li L, Qian H, Ren J. Rapid synthesis of highly luminescent CdTe nanocrystals in the aqueous phase by microwave irradiation with controllable temperature. Chem Commun (Camb) 2005;4:528–30. doi: 10.1039/b412686f. [DOI] [PubMed] [Google Scholar]
- 15.Pinaud F, Michalet X, Bentolila LA, Tsay JM, Doose S, Li JJ, et al. Advances in fluorescence imaging with quantum dot bio-probes. Biomaterials. 2006;27:1679–87. doi: 10.1016/j.biomaterials.2005.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alivisatos AP, Gu W, Larabell C. Quantum dots as cellular probes. Annu Rev Biomed Eng. 2005;7:55–76. doi: 10.1146/annurev.bioeng.7.060804.100432. [DOI] [PubMed] [Google Scholar]
- 17.Wang XS, Dykstra TE, Salvador MR, Manners I, Scholes GD, Winnik MA. Surface passivation of luminescent colloidal quantum dots with poly(dimethylaminoethyl methacrylate) through a ligand exchange process. J Am Chem Soc. 2004;126:7784–5. doi: 10.1021/ja0489339. [DOI] [PubMed] [Google Scholar]
- 18.Nann T. Phase-transfer of CdSe@ZnS quantum dots using amphiphilic hyperbranched polyethylenimine. Chem Commun (Camb) 2005;13:1735–6. doi: 10.1039/b414807j. [DOI] [PubMed] [Google Scholar]
- 19.Uyeda HT, Medintz IL, Jaiswal JK, Simon SM, Mattoussi H. Synthesis of compact multidentate ligands to prepare stable hydrophilic quantum dot fluorophores. J Am Chem Soc. 2005;127:3870–8. doi: 10.1021/ja044031w. [DOI] [PubMed] [Google Scholar]
- 20.Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science. 2002;298:1759–62. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 21.Kim S, Lim YT, Soltesz EG, Grand AM De, Lee J, Nakayama A, et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat Biotechnol. 2004;22:93–7. doi: 10.1038/nbt920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pathak S, Choi SK, Arnheim N, Thompson ME. Hydroxylated quantum dots as luminescent probes for in situ hybridization. J Am Chem Soc. 2001;123:4103–4. doi: 10.1021/ja0058334. [DOI] [PubMed] [Google Scholar]
- 23.Wang YA, Li JJ, Chen H, Peng X. Stabilization of inorganic nanocrystals by organic dendrons. J Am Chem Soc. 2002;124:2293–8. doi: 10.1021/ja016711u. [DOI] [PubMed] [Google Scholar]
- 24.Xing Y, Xia Z, Rao J. Semiconductor quantum dots for biosensing and in vivo imaging. IEEE Trans Nanobiosci. 2009;8:4–12. doi: 10.1109/TNB.2009.2017321. [DOI] [PubMed] [Google Scholar]
- 25.Lakowicz JR, Gryczynski I, Gryczynski Z, Nowaczyk K, Murphy CJ. Time-resolved spectral observations of cadmium-enriched cadmium sulfide nanoparticles and the effects of DNA oligomer binding. Anal Biochem. 2000;280:128–36. doi: 10.1006/abio.2000.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clapp AR, Goldman ER, Mattoussi H. Capping of CdSe-ZnS quantum dots with DHLA and subsequent conjugation with proteins. Nat Protoc. 2006;1:1258–66. doi: 10.1038/nprot.2006.184. [DOI] [PubMed] [Google Scholar]
- 27.Akhtar RS, Latham CB, Siniscalco D, Fuccio C, Roth KA. Immunohistochemical detection with quantum dots. Methods Mol Biol. 2007;374:11–28. doi: 10.1385/1-59745-369-2:11. [DOI] [PubMed] [Google Scholar]
- 28.Ornberg RL, Liu H. Immunofluorescent labeling of proteins in cultured cells with quantum dot secondary antibody conjugates. Methods Mol Biol. 2007;374:3–10. doi: 10.1385/1-59745-369-2:3. [DOI] [PubMed] [Google Scholar]
- 29.Williams Y, Byrne S, Bashir M, Davies A, Whelan A, Gun'ko Y, et al. Comparison of three cell fixation methods for high content analysis assays utilizing quantum dots. J Microsc. 2008;232:91–8. doi: 10.1111/j.1365-2818.2008.02083.x. [DOI] [PubMed] [Google Scholar]
- 30.Wu X, Liu H, Liu J, Haley KN, Treadway JA, Larson JP, et al. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat Biotechnol. 2003;21:41–6. doi: 10.1038/nbt764. [DOI] [PubMed] [Google Scholar]
- 31.Levsky JM, Singer RH. Fluorescence in situ hybridization: past, present and future. J Cell Sci. 2003;116:2833–8. doi: 10.1242/jcs.00633. [DOI] [PubMed] [Google Scholar]
- 32.Xiao Y, Barker PE. Semiconductor nanocrystal probes for human metaphase chromosomes. Nucleic Acids Res. 2004;32:e28. doi: 10.1093/nar/gnh024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knoll JH. Human metaphase chromosome FISH using quantum dot conjugates. Methods Mol Biol. 2007;374:55–66. doi: 10.1385/1-59745-369-2:55. [DOI] [PubMed] [Google Scholar]
- 34.Hoshino A, Hanaki K, Suzuki K, Yamamoto K. Applications of T-lymphoma labeled with fluorescent quantum dots to cell tracing markers in mouse body. Biochem Biophys Res Commun. 2004;314:46–53. doi: 10.1016/j.bbrc.2003.11.185. [DOI] [PubMed] [Google Scholar]
- 35.Hanaki K, Momo A, Oku T, Komoto A, Maenosono S, Yamaguchi Y, et al. Semiconductor quantum dot/albumin complex is a long-life and highly photostable endosome marker. Biochem Biophys Res Commun. 2003;302:496–501. doi: 10.1016/s0006-291x(03)00211-0. [DOI] [PubMed] [Google Scholar]
- 36.Jaiswal JK, Mattoussi H, Mauro JM, Simon SM. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nat Biotechnol. 2003;21:47–51. doi: 10.1038/nbt767. [DOI] [PubMed] [Google Scholar]
- 37.Lagerholm BC. Peptide-mediated intracellular delivery of quantum dots. Methods Mol Biol. 2007;374:105–12. doi: 10.1385/1-59745-369-2:105. [DOI] [PubMed] [Google Scholar]
- 38.Gao X, Cui Y, Levenson RM, Chung LW, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol. 2004;22:969–76. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 39.Hoshino A, Fujioka K, Oku T, Nakamura S, Suga M, Yamaguchi Y, et al. Quantum dots targeted to the assigned organelle in living cells. Microbiol Immunol. 2004;48:985–94. doi: 10.1111/j.1348-0421.2004.tb03621.x. [DOI] [PubMed] [Google Scholar]
- 40.Mattheakis LC, Dias JM, Choi YJ, Gong J, Bruchez MP, Liu J, et al. Optical coding of mammalian cells using semiconductor quantum dots. Anal Biochem. 2004;327:200–8. doi: 10.1016/j.ab.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 41.Pinaud F, Michalet X, Iyer G, Margeat E, Moore HP, Weiss S. Dynamic partitioning of a glycosyl-phosphatidylinositol-anchored protein in glycosphingolipid-rich microdomains imaged by single-quantum dot tracking. Traffic. 2009;10:691–712. doi: 10.1111/j.1600-0854.2009.00902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dahan M, Levi S, Luccardini C, Rostaing P, Riveau B, Triller A. Diffusion dynamics of glycine receptors revealed by single-quantum dot tracking. Science. 2003;302:442–5. doi: 10.1126/science.1088525. [DOI] [PubMed] [Google Scholar]
- 43.Bouzigues C, Levi S, Triller A, Dahan M. Single quantum dot tracking of membrane receptors. Methods Mol Biol. 2007;374:81–91. doi: 10.1385/1-59745-369-2:81. [DOI] [PubMed] [Google Scholar]
- 44.Lidke DS, Nagy P, Heintzmann R, Arndt-Jovin DJ, Post JN, Grecco HE, et al. Quantum dot ligands provide new insights into erbB/HER receptor-mediated signal transduction. Nat Biotechnol. 2004;22:198–203. doi: 10.1038/nbt929. [DOI] [PubMed] [Google Scholar]
- 45.Shah B, Clark P, Stroscio M, Mao J. Labeling and imaging of human mesenchymal stem cells with quantum dot bioconjugates during proliferation and osteogenic differentiation in long term. Conf Proc IEEE Eng Med Biol Soc. 2006;1:1470–3. doi: 10.1109/IEMBS.2006.260082. [DOI] [PubMed] [Google Scholar]
- 46.Shah BS, Clark PA, Moioli EK, Stroscio MA, Mao JJ. Labeling of mesenchymal stem cells by bioconjugated quantum dots. Nano Lett. 2007;7:3071–9. doi: 10.1021/nl071547f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosen AB, Kelly DJ, Schuldt AJ, Lu J, Potapova IA, Doronin SV, et al. Finding fluorescent needles in the cardiac haystack: tracking human mesenchymal stem cells labeled with quantum dots for quantitative in vivo three-dimensional fluorescence analysis. Stem Cells. 2007;25:2128–38. doi: 10.1634/stemcells.2006-0722. [DOI] [PubMed] [Google Scholar]
- 48.Pellegrino T, Parak WJ, Boudreau R, Gros MA Le, Gerion D, Alivisatos AP, et al. Quantum dot-based cell motility assay. Differentiation. 2003;71:542–8. doi: 10.1111/j.1432-0436.2003.07109006.x. [DOI] [PubMed] [Google Scholar]
- 49.Gu W, Pellegrino T, Parak WJ, Boudreau R, Gros MA Le, Alivisatos AP, et al. Measuring cell motility using quantum dot probes. Methods Mol Biol. 2007;374:125–31. doi: 10.1385/1-59745-369-2:125. [DOI] [PubMed] [Google Scholar]
- 50.Gu W, Pellegrino T, Parak WJ, Boudreau R, Gros MA Le, Gerion D, et al. Quantum-dot-based cell motility assay. Sci STKE. 2005;2005:15. doi: 10.1126/stke.2902005pl5. [DOI] [PubMed] [Google Scholar]
- 51.Patolsky F, Gill R, Weizmann Y, Mokari T, Banin U, Willner I. Lighting-up the dynamics of telomerization and DNA replication by CdSe-ZnS quantum dots. J Am Chem Soc. 2003;125:13918–9. doi: 10.1021/ja035848c. [DOI] [PubMed] [Google Scholar]
- 52.Zhang CY, Yeh HC, Kuroki MT, Wang TH. Single-quantum-dot-based DNA nanosensor. Nat Mater. 2005;4:826–31. doi: 10.1038/nmat1508. [DOI] [PubMed] [Google Scholar]
- 53.Medintz IL, Clapp AR, Mattoussi H, Goldman ER, Fisher B, Mauro JM. Self-assembled nanoscale biosensors based on quantum dot FRET donors. Nat Mater. 2003;2:630–8. doi: 10.1038/nmat961. [DOI] [PubMed] [Google Scholar]
- 54.Shi L, Paoli V De, Rosenzweig N, Rosenzweig Z. Synthesis and application of quantum dots FRET-based protease sensors. J Am Chem Soc. 2006;128:10378–9. doi: 10.1021/ja063509o. [DOI] [PubMed] [Google Scholar]
- 55.So MK, Xu C, Loening AM, Gambhir SS, Rao J. Self-illuminating quantum dot conjugates for in vivo imaging. Nat Biotechnol. 2006;24:339–43. doi: 10.1038/nbt1188. [DOI] [PubMed] [Google Scholar]
- 56.Larson DR, Zipfel WR, Williams RM, Clark SW, Bruchez MP, Wise FW, et al. Water-soluble quantum dots for multiphoton fluorescence imaging in vivo. Science. 2003;300:1434–6. doi: 10.1126/science.1083780. [DOI] [PubMed] [Google Scholar]
- 57.Voura EB, Jaiswal JK, Mattoussi H, Simon SM. Tracking metastatic tumor cell extravasation with quantum dot nanocrystals and fluorescence emission-scanning microscopy. Nat Med. 2004;10:993–8. doi: 10.1038/nm1096. [DOI] [PubMed] [Google Scholar]
- 58.Chen LD, Liu J, Yu XF, He M, Pei XF, Tang ZY, et al. The biocompatibility of quantum dot probes used for the targeted imaging of hepatocellular carcinoma metastasis. Biomaterials. 2008;29:4170–6. doi: 10.1016/j.biomaterials.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 59.Nida DL, Rahman MS, Carlson KD, Richards-Kortum R, Follen M. Fluorescent nanocrystals for use in early cervical cancer detection. Gynecol Oncol. 2005;99:S89–S94. doi: 10.1016/j.ygyno.2005.07.050. [DOI] [PubMed] [Google Scholar]
- 60.Cuenca AG, Jiang H, Hochwald SN, Delano M, Cance WG, Grobmyer SR. Emerging implications of nanotechnology on cancer diagnostics and therapeutics. Cancer. 2006;107:459–66. doi: 10.1002/cncr.22035. [DOI] [PubMed] [Google Scholar]
- 61.Hild WA, Breunig M, Goepferich A. Quantum dots - nano-sized probes for the exploration of cellular and intracellular targeting. Eur J Pharm Biopharm. 2008;68:153–68. doi: 10.1016/j.ejpb.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 62.Hopper C. Photodynamic therapy: a clinical reality in the treatment of cancer. Lancet Oncol. 2000;1:212–9. doi: 10.1016/s1470-2045(00)00166-2. [DOI] [PubMed] [Google Scholar]
- 63.Pandey RK. Recent advances in photodynamic therapy. J Porphyr Phthalocya. 2000;4:368–73. [Google Scholar]
- 64.Samia AC, Dayal S, Burda C. Quantum dot-based energy transfer: perspectives and potential for applications in photodynamic therapy. Photochem Photobiol. 2006;82:617–25. doi: 10.1562/2005-05-11-IR-525. [DOI] [PubMed] [Google Scholar]
- 65.Samia AC, Chen X, Burda C. Semiconductor quantum dots for photodynamic therapy. J Am Chem Soc. 2003;125:15736–7. doi: 10.1021/ja0386905. [DOI] [PubMed] [Google Scholar]
- 66.Yaghini E, Seifalian AM, MacRobert AJ. Quantum dots and their potential biomedical applications in photosensitization for photodynamic therapy. Nanomedicine (Lond) 2009;4:353–63. doi: 10.2217/nnm.09.9. [DOI] [PubMed] [Google Scholar]
- 67.Zhao Y, Ye M, Chao Q, Jia N, Ge Y, Shen H. Simultaneous detection of multifood-borne pathogenic bacteria based on functionalized quantum dots coupled with immunomagnetic separation in food samples. J Agric Food Chem. 2009;57:517–24. doi: 10.1021/jf802817y. [DOI] [PubMed] [Google Scholar]
- 68.Cui D, Pan B, Zhang H, Gao F, Wu R, Wang J, et al. Self-assembly of quantum dots and carbon nanotubes for ultrasensitive DNA and antigen detection. Anal Chem. 2008;80:7996–8001. doi: 10.1021/ac800992m. [DOI] [PubMed] [Google Scholar]
- 69.Kirchner C, Liedl T, Kudera S, Pellegrino T, Munoz JA, Gaub HE, et al. Cytotoxicity of colloidal CdSe and CdSe/ZnS nanoparticles. Nano Lett. 2005;5:331–8. doi: 10.1021/nl047996m. [DOI] [PubMed] [Google Scholar]
- 70.Lovric J, Bazzi HS, Cuie Y, Fortin GR, Winnik FM, Maysinger D. Differences in subcellular distribution and toxicity of green and red emitting CdTe quantum dots. J Mol Med. 2005;83:377–85. doi: 10.1007/s00109-004-0629-x. [DOI] [PubMed] [Google Scholar]
- 71.Shiohara A, Hoshino A, Hanaki K, Suzuki K, Yamamoto K. On the cyto-toxicity caused by quantum dots. Microbiol Immunol. 2004;48:669–75. doi: 10.1111/j.1348-0421.2004.tb03478.x. [DOI] [PubMed] [Google Scholar]
- 72.Hohng S, Ha T. Near-complete suppression of quantum dot blinking in ambient conditions. J Am Chem Soc. 2004;126:1324–5. doi: 10.1021/ja039686w. [DOI] [PubMed] [Google Scholar]
- 73.Yao J, Larson DR, Vishwasrao HD, Zipfel WR, Webb WW. Blinking and nonradiant dark fraction of water-soluble quantum dots in aqueous solution. Proc Natl Acad Sci USA. 2005;102:14284–9. doi: 10.1073/pnas.0506523102. [DOI] [PMC free article] [PubMed] [Google Scholar]