Abstract
Hybrid micro-/nanogels are playing an increasing important part in a diverse range of applications, due to their tunable dimensions, large surface area, stable interior network structure, and a very short response time. We review recent advances and challenges in the developments of hybrid micro-/nanogels toward applications for optical sensing of pH, temperature, glucose, ions, and other species as well as for intracellular imaging. Due to their unique advantages, hybrid micro-/nanogels as optical probes are attracting substantial interests for continuous monitoring of chemical parameters in complex samples such as blood and bioreactor fluids, in chemical research and industry, and in food quality control. In particular, their intracellular probing ability enables the monitoring of the biochemistry and biophysics of live cells over time and space, thus contributing to the explanation of intricate biological processes and the development of novel diagnoses. Unlike most other probes, hybrid micro-/nanogels could also combine other multiple functions into a single probe. The rational design of hybrid micro-/nanogels will not only improve the probing applications as desirable, but also implement their applications in new arenas. With ongoing rapid advances in bionanotechnology, the well-designed hybrid micro-/nanogel probes will be able to provide simultaneous sensing, imaging diagnosis, and therapy toward clinical applications.
Keywords: stimuli-responsive polymer, microgel, nanogel, hybrids, optical biosensor, cell imaging, intracellular detection
Gels are soft materials that combine the properties of solids and fluids. Typically, polymer gel is comprised of a three-dimensional cross-linked polymer network that has a high number of hydrophilic groups or domains (1–6). This network has a high affinity for water, but is prevented from dissolving due to the chemical or physical bonds formed between the polymer chains. It is the high water content that gives rise to the fluid-like transport properties for the molecules significantly smaller than the gel pore size. The unique physical properties resulted from this unusual state of matter make gels ideal candidates for a number of applications. One of the most promising uses comes in the form of a three-dimensional matrix for optical sensors, in which optical moieties such as organic dye, conjugated polymer or fluorophore-contained polymer, semiconductor quantum dots (QDs), and noble metal nanoparticles (NMNPs) could be incorporated into the polymer gel network (7–12).
To be applied in biomedical areas such as in vivo biological sensors and imaging diagnostics, stable colloidal particles made from gels (also referred to micro- or nanogels) are highly desirable, particularly in an optical sensor based on the volume changes of stimuli-responsive polymers. The swelling and shrinking processes of gels are determined by the collective diffusion of the polymer networks in a fluid, which is associated with the bulk counterflow of the fluid through the polymer networks. The characteristic time period that describes the volume change of gels has been found to be approximately proportional to the square of the characteristic length of the gel (13, 14). Thus, the response speed can be improved predominantly by two methods: the introduction of porosity into the gel and the downsize approach (12). The latter approach is preferable for the development of optical sensors/labels toward in vivo sensing and cell imaging. In contrast to bulk gel, the micro-/nanogels could be dispersed in water but preserving a high stable structure. In this review, we will focus on those hybrid micro-/nanogels that are constructed by immobilization of dye molecules, QDs, and/or NMNPs in the polymeric micro-/nanogels. Technically, the systems of polymer micro-/nanogels containing conjugated polymer or fluorophore-contained polymer are not hybrids, but we will still refer to the relative works here.
Apart from the tunable dimension and the rapid response, the hybrid micro-/nanogels as optical probes offer several advantages over the individual component of dye molecule, QD, or NMNP for optical sensing and cellular imaging. Although Au NPs are usually addressed as biocompatible, the cytotoxicity of the available dyes, QDs and NMNPs, is sometimes a problem. These optical moieties may also affect the immunological response of cells (15). Furthermore, the QDs and NMNPs are insensitive to local environmental change to reversibly convert the signals. In an attempt to solve these problems, several successful approaches have been developed to link ligands to these optical moieties, including derivation, non-specific adsorption, electrostatic interaction, mercapto (−SH) exchange, and so on (16–21). A recent work has linked the pH-responsive polymer to Au NPs, resulting in nanoconjugates that are responsive to pH (22). Although significant benefits have been achieved, a general concern is that the binding is dynamic and may become not very strong after long circulation. Unlike these methods, the hybrid micro-/nanogels exploit the polymer gel networks as platforms for immobilizing optical moieties. Gels have some physical properties common to living tissues, including a soft and rubbery consistency and low interfacial tension with water or biological fluids. The elastic nature of hydrated gels has been found to minimize irritation to the surrounding tissues after implantation. The low interfacial tension between the gel surface and body fluid minimizes protein adsorption and cell adhesion, which reduces the chances of a negative immune reaction (3, 23, 24). The inert protective matrix of the gel networks also eliminates interferences such as protein binding and/or membrane/organelle sequestration (25, 26).
The hybrid micro-/nanogels with the optical moieties immobilized in polymer gel networks, combining the properties from both optical moieties and polymer gels, can offer the possibilities for external switching and manipulation when applied to sensors and cell labeling agents. Since the report of the first single NP-based dye sensor called PEBBLE (Photonic Explorer for Biomedical use with Biologically Localized Embedding) (27, 28), novel nanoscale materials have attracted increasing attention and extended widely toward multiple functions. The high surface-to-volume ratio of micro-/nanogels allows not only high accessibility of analytes to the signal receivers but also attachment of targeting ligands toward the detection at specific cells or components of cells. Micro-/nanogels can load a large amount of components (single or multiple) within the gel network as well as on the surface, resulting in a variety of detection methods with respect to photonic/plasmonic intensity, energy distribution (wavelength), and temporal distribution (lift time) (29). Highly loaded optical moieties in close proximity to each other either within the restricted gel network or on the surface also allow the multiple interactions with the sensing components, resulting in signal amplification (26, 30). In addition, the micro-/nanogels are very promising as drug-delivery carriers because of their high loading capacity, good stability, and reversible volume change in response to environmental stimuli, such as pH, temperature, and glucose level that are unprecedented for common pharmaceutical carriers (4). Our group has first developed a series of inorganic-polymer hybrid nanogels for integration of optical sensing, cell imaging, and therapy (31–35). The capability of simultaneous optical diagnosis and therapy with a single nanoplatform may prove to be advantageous over conventional medicine.
The present review provides an updated overview of hybrid micro-/nanogels, especially the inorganic-polymer hybrid micro-/nanogels, for optical sensing and cell imaging applications. It covers only the untethered (free) NP probes suitable for in vitro and in vivo detections. It excludes silica gels (36, 37) or mechanically fixed sensors like thin film even when they utilize the NPs (38–41). We also recommend recent reviews for an additional reference on NP-based sensors, in which dye-polymer NP-based sensors have been discussed in detail (42, 43). The focus on polymer gel-based hybrid NPs is important as these nanostructures bridge the gap between inorganic nanoscience and the soft matter and have attracted growing interests in the developments toward emerging applications for in situ diagnosis from low-toxic polymer gels.
Hybrid micro-/nanogel design
Classification of hybrid nanogel-based optical probes
Generally, a hybrid micro-/nanogel-based optical probe is composed of two components: optical moieties and a three-dimensional polymer scaffold. Under this consideration, such hybrid micro-/nanogel probes can be classified into three types (Fig. 1).
Fig. 1.
Schematic diagrams showing three types of hybrid micro-/nanogel-based optical probes: (A) Type 1 where the antibody or specific targeting ligand acts as a chemical/biochemical signal receiver; (B) Type 2 where an optical moiety acts directly as the chemical/biochemical signal receiver; and (C) Type 3 where a responsive polymer gel network chain acts as the chemical/biochemical signal receiver, which will undergo a volume phase transition, change the physicochemical environment of the optical moieties, and convert the received signal into an optical signal.
In a Type 1 probe the specific antibodies, aptamers, or other selective analyte-recognition ligands are covalently linked to the hybrid micro-/nanogels. Binding of the analyte to the hybrid micro-/nanogels would not affect the optical properties of the optical moieties, but the apparent color may change upon colocalization of the probes. The basic concept is to develop smart platforms that have not only molecular recognition abilities but also built-in optical codes for rapid target identification. By integrating molecular recognition and optical coding, each hybrid micro-/nanogel could be considered a ‘chemical lab’ that detects and analyzes a unique sequence or compound in a complex mixture. Such encoded hybrid micro-/nanogels should find broad application in gene expression studies, high-speed screening, and medical diagnostics. In comparison with planar DNA chips, such an encoded-gel technology is expected to be more flexible in target selection (e.g. adding new genes or single-nucleotide mutations), faster in binding kinetics (similar to that in the homogeneous solution), and less expensive to produce (44).
In a Type 2 probe the optical moiety acts directly as the chemical/biochemical signal receiver. The optical properties of the optical moieties (such as indicator dye and conjugated polymer) would change upon the binding of analyte. The sensitivity and selectivity of the probe as a sensor mostly depend on the optical properties of these optical moieties and may be also affected by the gel network, which however may simply act as a three-dimensional polymer scaffold in most cases. Specifically, PEBBLEs have been developed to fill the niche that lies between pulled micro-optodes and free molecular probes (naked indicator dye molecules) (42).
In a Type 3 probe the stimuli-responsive polymer gel network chains act as the chemical/biochemical signal receiver. Upon receiving the external stimulus, the gel network will undergo a volume phase transition and change the physicochemical environment of the optical moieties, converting the received signal into optical signal.
A similar classification of optical NP sensors has been proposed by Lee and Kopelman (42, 43). Although some gel-based sensors have been included in their excellent reviews, responsive polymer gel-based probes are not discussed. Various responsive polymer gel-based probes have been recently developed, particularly for optical probing of pH, temperature, and glucose level. The pH and temperature of a living cell is changeable during many cellular events (46–50). Many pathological processes in various tissues and organs are also accompanied with local pH decrease by 1–2.5 pH units (acidosis) and/or temperature increase by 1–5°C (51–53). Thus, the development of responsive polymer micro-/nanogel-based probes for pH/temperature-responsive optical tracing can contribute to the explanation of intricate biological processes and the development of advanced diagnoses. On the other hand, there is an urgent need to develop a sensor for continuous in vivo glucose monitoring at physiological pH in subjects with diabetes mellitus (8), which represents one of the largest health concerns of the 21st century with worldwide prevalence. The discussion of these hybrid micro-/nanogel-based probes will be exemplified in the following sections.
Construction methods
With the working mechanism of the hybrid micro-/nanogel-based optical probes in mind, we will compare different construction strategies toward the hybrid micro-/nanogels. The approaches are based on (1) the type of cross-links in the gel network, (2) the linkage between the optical moieties and the polymer chains, and (3) the stable and reversible photonics and/or plasmonics of the optical moieties. Among them, it should be emphasized that the stable and reversible photonics and/or plasmonics is the most important and essential issue for preparing reliable optical probes. Reproducibility and robustness are two of the key characteristics (the others are selectivity, resistance to interference, and response time mentioned above) of any probes.
To form stable linkages among the polymer chains and the immobilized optical moieties, polymers should have functional groups. The most widely utilized functional polymers for nanogels include poly(ethylene glycol) (PEG), poly(N-isopropylacrylamide) (PNIPAM), poly(acrylic acid) (PAA), poly(methacrylic acid) (PMAA), polyacrylamide (PAAm), poly(lactic-co-glycolic acid) (PLGA), poly(glycidyl methacrylate) (PGMA), and biopolymers like chitosan, hydroxypropylcellulose (HPC), and cholesterol-bearing pullulan modified with amino groups. The carboxyl groups, amino groups, hydroxyl groups, and ether oxygen groups are efficient stabilizers to link QDs and NMNPs (26, 54–58). Untethered dye molecules are mainly anchored onto the gels via their hydrophobic interactions with carbon backbones and π-π stacking (42, 43, 59, 60). Cross-links are important to further maintain the network structure of the gel, which can prevent dissolution of the polymer network and release of the immobilized optical moieties after a long circulation or the repeated binding/disbanding cycles to analytes.
Cross-linking in micro-/nanogels
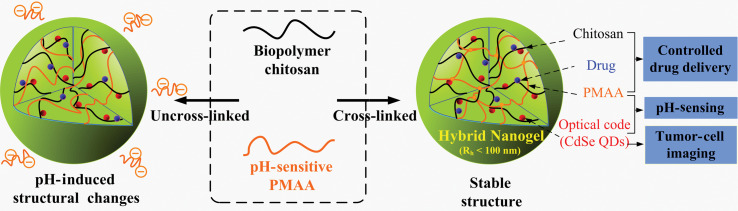
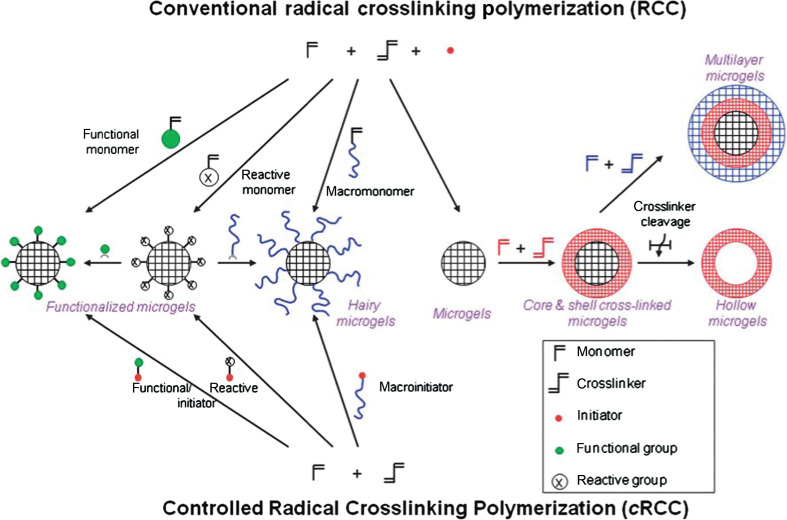
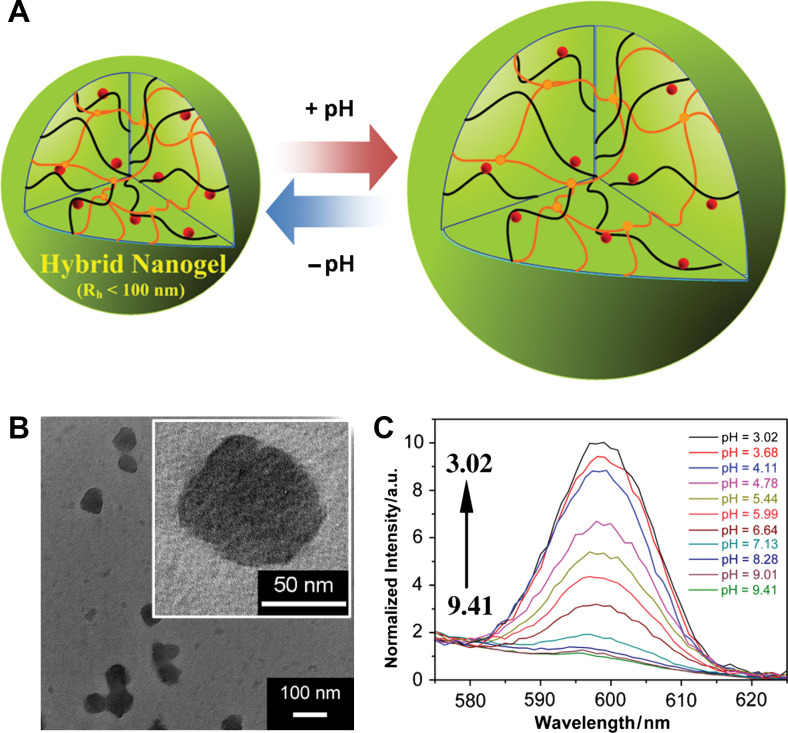
The cross-linking in micro-/nanogels is accomplished either by non-covalent physical associations, covalent chemical cross-linkages, or their combinations (1–6). The cross-links in the physically cross-linked gels arise from non-covalent attractive forces such as hydrophobic interactions, hydrogen bonding, and ionic interactions between the polymers or blending and interpenetrating networks of two dissimilar polymers. For example, chitosan chains form physical interactions with PMAA chains, which results in formation of nanogels (33). The nanogels presented negative surface charge at pH ≈ 5.0. The zeta potential tended to be neutral as the molar ratio of MAA-to-glucosamine decreased. This result is consistent with the complexation mechanism based on electrostatic interactions between the carboxyl groups of MAA and amino groups of chitosan. The interplay of other interactions, such as hydrophobic association of methyl groups of PMAA chains, may also contribute to the stability of the nanogels. Recently, we present the first direct observation of the pH-induced expulsion of PMAA chains from the physically associated chitosan-PMAA-CdSe QD hybrid nanogels during a time period of 32 h (Fig. 2) (33), which is longer than the time period (20 h) required for macromolecular colloids to accumulate in solid tumors through the blood stream (61). The mechanism of chain expulsion of the weak polyelectrolyte nanogels in response to a pH variation may involve pH-induced accumulation of excess charge within the nanogels (62–65). The constructed physically associated hybrid nanogels would not be an ideal candidate for optical sensing. In contrast, the chemically cross-linked hybrid nanogels are very stable. Chemically cross-linked gel networks were formed by polymerizing MAA monomers in the presence of chitosan chains and cross-linking agents. The gel networks formed by such cross-links, also called semi-interpenetrating (semi-IPN), can exhibit a reversible structural change in response to a pH change, leading to a reversible optical response of the constructed hybrid nanogels. These results provided fundamental guidance for the construction of feasible and reliable hybrid micro-/nanogel-based optical probes. Chemically cross-linked micro-/nanogels can also be formed by cross-linking of the functional groups presented on the polymer backbone. Both free radical precipitation polymerization and controlled/living radical polymerization techniques (2, 4, 5), as outlined in Fig. 3, can be used to prepare chemically cross-linked micro-/nanogels. While the conventional radical cross-linking polymerization technique provides a facial and simple synthetic pathway, the controlled/living radical polymerization technique such as the catalytic atom (group) transfer radical polymerization (ATRP) (66, 67), degenerative chain transfer polymerization represented by iodine-mediated polymerization (RITP) (68, 69), and reversible addition-fragmentation chain transfer polymerization (RAFT) (70, 71) can be considered as a breakthrough toward the synthesis of complex structures with a high degree of functionality and compositional variety.
Fig. 2.
Schematic diagrams showing the difference between a physically and chemically cross-linked chitosan-PMAA-CdSe hybrid nanogel. Whereas the covalently cross-linked nanogels are very stable in both structure and composition upon pH variation, the hybrid nanogels based on the physical associations exhibit a significant change in the structure and composition in response to a pH increase to physiological condition. This distinction in the stability of hybrid nanogels is important for the designed multiple functions. Adapted from Reference (33). Reprinted with permission from Elsevier Publishing Group, Copyright (2010).
Fig. 3.
Synthetic methods for the preparation of pure polymer micro-/nanogels via conventional and controlled radical cross-linking polymerization. Adapted from Reference (5). Reprinted with permission from RSC Publishing Group, Copyright (2010).
Incorporation of optical moieties into the gel network
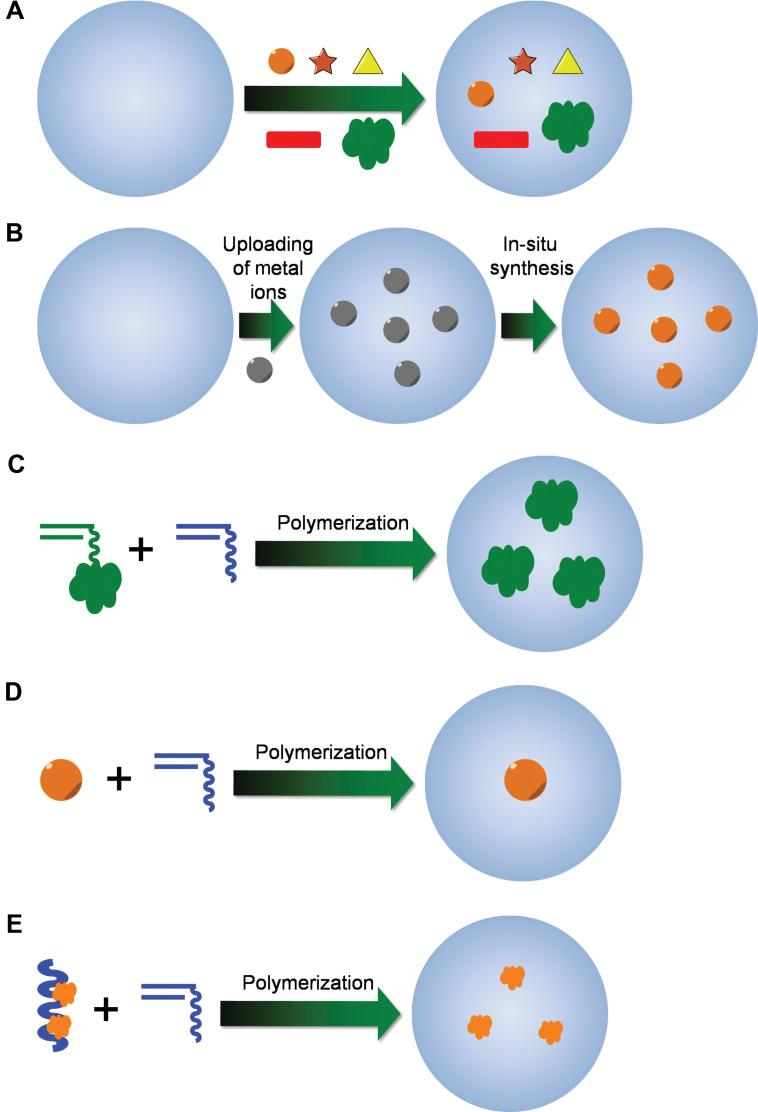
Totally based on the synthetic methods for polymer micro-/nanogels, various strategies for the construction of chemically cross-linked hybrid micro-/nanogels have been described (Fig. 4). The synthetic technique being employed is typically dictated by the desired application or the type of study to be carried out. Absorption of indictor dye molecules into the preformed polymer networks of micro-/nanogels (Fig. 4A) could be considered the oldest and simplest method for the construction of hybrid micro-/nanogel-based optical probes (42, 43, 60). In addition, we found that the Calcon dye molecules can also be directed to assemble on the surface of a pH-responsive microgel of poly(NIPAM-AA-AAm) (59). It is possibly that Calcon dye molecules were first bound to the nanogel through the binding between the sulfonate groups in Calcon with the amino groups in the nanogels to form a well-oriented monomolecular layer of Calcon dye molecules. The hydrophobic well-oriented Calcon layer binds the subsequent layers of Calcon dye molecules through the hydrophobic interactions involving the π-electron rich structures. The Calcon dye domains are ‘glued’ by non-covalent π-π interactions between Calcon dye molecules, which may be further stabilized by water species bridges (72). These dye absorption methods are commonly used in the construction of Type 2 probes. On the other hand, the copolymerization of fluorophore-containing monomers with functional monomers like NIPAM is another strategy to incorporate organic fluorophores into the micro-/nanogels, which usually results in Type 3 probes. For example, copolymerization of NIPAM with fluorescent monomers, 4-N-(2-acryloyloxyethyl)-N-methylamino-7-N,N-dimethylaminosulfonyl-2,1,3-benzoxadiazole (DBD-AA) (73–76) and dansylaminoethylacrylamide (DAEAM) (77), respectively, via emulsion polymerization has been applied to develop fluorescent nanogel sensors.
Fig. 4.
Synthetic methods for the preparation of hybrid micro-/nanogels: (A) uploading of presynthesized optical moieties with well-defined size and shape into the preformed gels; (B) uploading of metal ions and in situ synthesis of QDs/NMNPs inside the preformed nanogels; (C) copolymerization of fluorescent monomers with other functional monomers; (D) synthesis of polymer gel shell with QD/NMNP as core template; and (E) synthesis of gel particles with preformed conjugated polymer chains semi-interpenetrated in the gel network.
Following a similar procedure for loading of indicator dye molecules, presynthesized QDs/NMNPs with well-controlled shape and size can be uploaded into the preformed polymer networks of micro-/nanogels (Fig. 4A). The Type 1 probe based on the incorporation of the multicolor ZnS-capped CdSe QDs into the poly(styrene-co-acrylic acid) beads at precisely controlled ratios was first reported by Nie's group (44). The embedded QDs can be spatially separated from each other and do not undergo fluorescence resonance energy transfer. Presynthesized QDs/NMNPs have also been loaded into the stimuli-responsive polymer networks to form different Type 3 probes (26, 78–83). It is found that electrostatic interactions are not the only governing force to drive the loading of micro-/nanogels with QDs/NMNPs. The affinity of QDs/NMNPs to micro-/nanogels also allows their sequestering by cationic, anionic, and close-to-neutral-state gels. The strong binding forces between QDs/NMNPs and micro-/nanogels can even overcome electrostatic repulsion between the QDs/NMNPs and gels, which should be very important to keep the hybrid micro-/nanogels stable for the applications as sensors and cell labels in different environments (33). Moreover, with the neutral stabilizing ligands specifically designed in polymer micro-/nanogels, for example, neutral AAm groups designed in the glucose-responsive micro-/nanogels as CdS QD stabilizer (26), the anionic functional groups in the nanogels may not be necessary to upload the QDs/NMNPs. Thus, the unwanted shift of the volume phase transitions of nanogels due to the ionization can be avoided and the compositions used for micro-/nanogel synthesis can be expanded more diversely.
Although many probes have been developed via aforementioned absorption loading method, it should be mentioned that the incorporation of presynthesized formed QDs/NMNPs into the interior of the preformed nanogels generally does not allow one to achieve a high loading capacity of QDs/NMNPs because of the potential aggregation of QDs/NMNPs and/or the hybrid nanogels. Moreover, the QDs/NMNPs could be gradually released from the gel networks upon the repeated swelling/deswelling cycles during the analytical process. For example, the glucose sensor (Type 3) constructed by such an absorption method (26) demonstrated a gradual decrease in fluorescent response degree due to the escape of the QDs from the host microgels after the repeated swelling/deswelling process of gel networks in response to the change of glucose concentration. This result indicates that such constructed hybrid micro-/nanogels may have the risk of signal distortion in the sensing application that requires fully reversible optical signal intensity. To address this issue, it would be better to construct the hybrid micro-/nanogels through an in situ synthesis method (Fig. 4B), in which the preloaded metal ions could be transformed to QDs/NMNPs directly in the interior of the host micro-/nanogel templates (32, 54, 57, 58, 84–87). The covalent incorporation of ZnO QDs (88–90) and polymerizable dye molecules (73–77) onto the gel network chains can further confine the optical moieties (Fig. 4C).
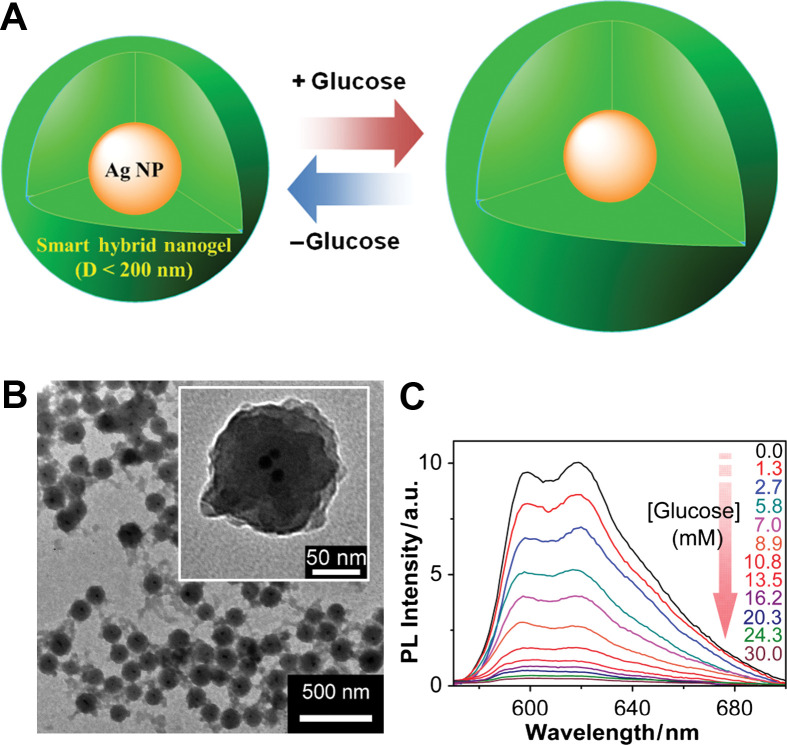
To combine the merits of an absorption method (controllable morphology and thus unique spectroscopic properties of QDs/NMNPs) and the in situ synthesis method (stability and reversibility of hybrid nanogels during analytical cycles), an alternative method is to synthesize polymer gel shell directly onto QDs/NMNPs templates (Fig. 4D). So far, this method has been widely used in the construction of Type 3 probes. In this methodology, two main approaches have been developed based on ‘grafting-onto’ and ‘grafting-from’ the core template. In the ‘grafting-from’ approach, polymers are grown directly from the surface of substrates (91, 92). Generally, a polymerization initiator was immobilized onto the surface of substrates and monomers were then polymerized from the immobilized initiator. On the other hand, in the ‘grafting-onto’ approach, polymers were directly immobilized to the surface of substrates by coupling reactions, chemical bonding, hydrophobic interactions, or electrostatic interactions. A general approach for the encapsulation of NMNPs with different sizes and shapes into the nanogels is based on the capping of surfactants (31, 34, 35, 93–101). Surfactants, such as cetyltrimethylammonium bromide (CTAB) and sodium dodecyl sulfate (SDS), are extremely efficient for the synthesis of NMNPs with a wide variety of compositions, sizes, and shapes. These surfactant-coated NMNPs can further serve as substrates for the free-radical polymerization. For example, the inorganic and polymeric synthetic procedures can be combined in such a way that involves the first synthesis of SDS-capped Ag NPs (10±3 nm), followed by the free-radical precipitation polymerization of the monomers to form a glucose responsive nanogel shell of poly(4-vinylphenylboronic acid-co-2-(dimethylamino)ethyl acrylate) [p(VPBA-DMAEA)] coated onto the Ag NPs templates, so that the glucose-sensitive and fluorescent components can be integrated into a single nano-object (100). Furthermore, the restricted environment and the high porosity of the gel shell offer the possibility to carry out selective chemical reactions on the encapsulated NMNPs, such as the growth of Au clusters onto Ag NP cores via galvanic replacement reaction between Ag and AuCl4− (34, 57). The simple procedures allow the build-up of other temperature, pH, or glucose sensitive polymer shells onto the NMNPs of desirable properties.
At last, the fluorescent hybrid micro-/nanogels can be produced through the in situ polymerization of functional monomers in the presence of conjugated polymers that can interpenetrate or semi-interpenetrate into the gel network (Fig. 4E) (102). Semiconducting polymer nanospheres (SPNs) have been synthesized and encapsulated in phospholipid micelles by a solvent evaporation technique (103). Conjugated polymers are known to possess high absorption coefficients and high fluorescence efficiency. A variety of conjugated polymer-based chemical sensors have been developed (7). Although conjugated polymers are usually hydrophobic, the formation of an interpenetrating or semi-interpenetrating gel network makes it possible to fabricate water-dispersible fluorescent micro-/nanogels containing conjugated polymers.
Hybrid micro-/nanogel for optical sensing
A variety of hybrid micro-/nanogels have been exploited for optical sensing of pH, temperature, glucose, ions, and other species.
Type 1 probe
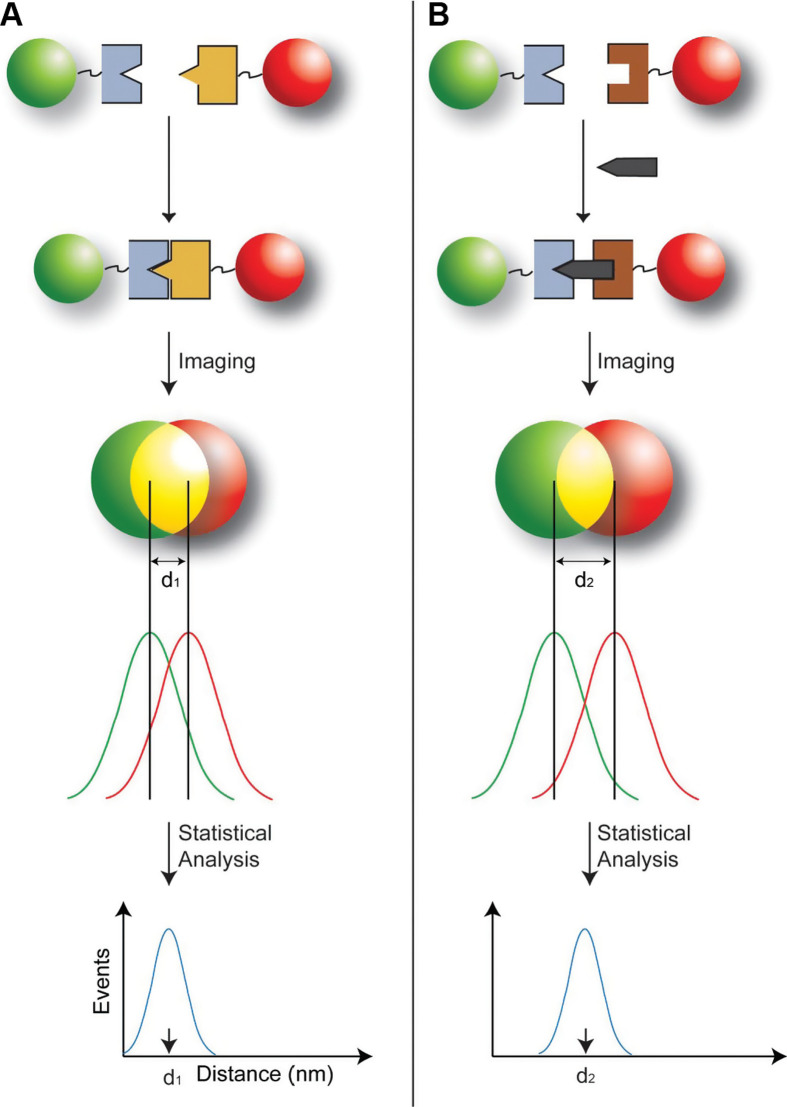
The history of a Type 1 probe can be traced back to the work reported by Nie's group in 2001 (44). Multicolor optical coding for biological assays has been achieved by embedding different-sized QDs into polymeric beads at precisely controlled ratios. Their novel optical properties (e.g. size-tunable emission and simultaneous excitation) render these highly luminescent QDs ideal fluorophores for wavelength-and-intensity multiplexing. The use of 10 intensity levels and six colors could theoretically code one million nucleic acid or protein sequences. Imaging and spectroscopic measurements indicate that the QD-tagged beads are highly uniform and reproducible, yielding bead identification accuracies as high as 99.99% under favorable conditions. DNA hybridization studies demonstrate that the coding and targeting signals can be simultaneously read at the single-bead level. Recently, Nie's group has further extended this spectral coding technology for single-molecule detection and biomolecular structural mapping (Fig. 5) (45).
Fig. 5.
Schematic diagrams showing the design of the color-coded probe, a typical Type 1 probe, for detecting single biomolecules in two different binding modes. (A) Direct binding between two bioconjugated particles leading to a separation distance of d 1. This mode of binding was used to construct rigid molecular structures (molecular rulers) for verification/validation studies of the precision in distance measurements. (B) Indirect sandwich-type binding in which two particles recognize the same target molecule at two different sites. This indirect mode of binding allows native biomolecules such as genes to be recognized and detected at the single-molecule level. Adapted from Reference (45). Reprinted with permission from National Academy of Sciences Publishing Group, Copyright (2008).
Type 2 probe
Type 2 probes for pH (H+), cobalt, copper, nickel, potassium, silver, sodium, zinc, and chloride ions have been developed and well summarized in literatures (42, 43). These probes are generally made of non-ionic polymer gels, such as PAAm, PEG, and polystyrene, embedded with three components, including a non-fluorescent ionophore that binds selectively to the ion of interest (may not include for pH sensing), a fluorescent hydrogen ion-selective dye that plays the role of a signal receiver, and a lipophilic additive that maintains ionic strength. The operation of the entire system is based on a thermodynamic equilibrium that controls ion exchange (for sensing cations) or ion co-extraction (for sensing anions), i.e. an equilibrium-based correlation between different ion species. The degree of protonation measured from the fluorescence change of the H+-selective dye is related to the concentration of the analyte ion according to the theory developed for optical absorption-based ion-correlation sensors (104, 105). The non-ionic polymer gel is selected to ensure a local chemical equilibrium among the embedded components in the interior of gel networks in aqueous phase. When oxygen-sensitive dye was used, sensors for molecular reactive oxygen species (ROS; single oxygen and hydrogen peroxide) and radical ROS (hydroxyl radical) could be developed (42, 43). A glucose sensor could also be developed by utilizing the enzyme glucose oxidase (106). Rubio-Retama's group developed an interpenetrated microgel of PNIPAM with poly(thiophene-ethyl butyl sulfonate; PTEBS) for picric acid detection (102). At temperatures below the low critical solution temperature (LCST) of PNIPAM-PTEBS, the picric acid was able to interact with the π-conjugated PTEBS via π-π interactions, thus quenching the PL emission of PTEBS. When the temperatures were above the LCST of polymer network chains, the π-π interactions were weakened, resulting in the recovery of the initial PL emission. Liu's group reported a NIPAM-based microgel for Cu2+ detection via fluorescence quenching effect of Cu2+ on DAEAM units (77). Interestingly, they found that the detection sensitivity for Cu2+ sensitivity can be dramatically enhanced via the thermo-induced shrinking of microgels at elevated temperatures.
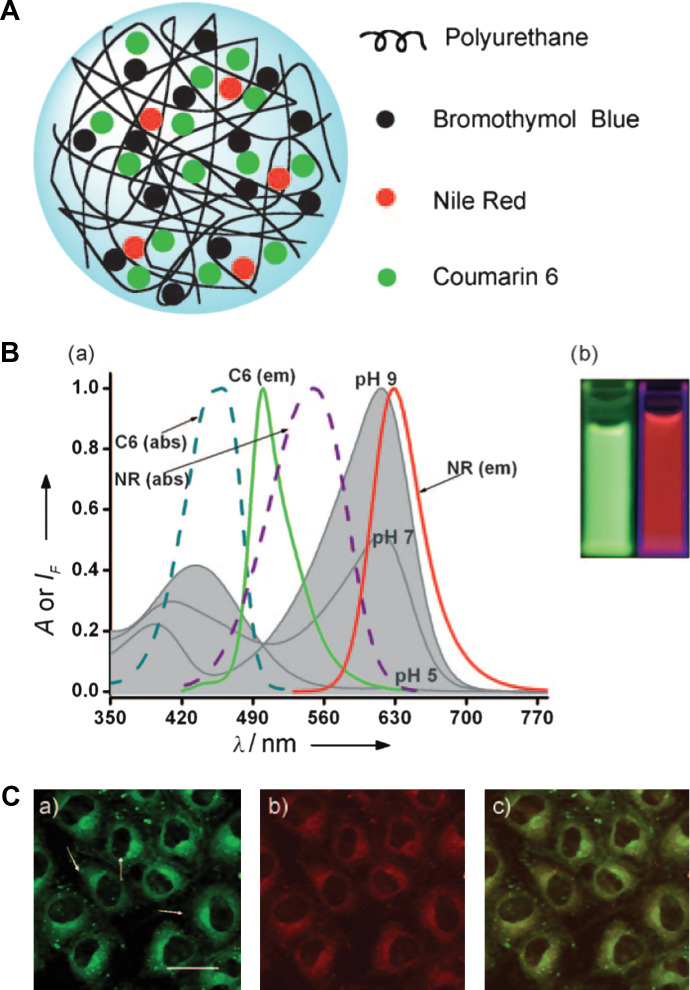
While most of the above probes contain single spectral peak for optical signaling, Wolfbeis's group loaded the polyurethane nanogel with the pH indicator bromothymol blue (BTB) as well as two other standard fluorophores of coumarin (C6) and Nile Red (NR) (60). The sensing capability of the Wolfbeis's probe relies on two specific features. The first is the spectral overlap of the absorption of the pH indicator BTB with the dual emission of the fluorophores C6 and Nile Red (Fig. 6). The second feature is the efficient fluorescence resonance energy transfer (FRET; predominantly red fluorescence at pH 7) that occurs between C6 and NR in the aqueous suspension of nanogel but not in aqueous solution alone where they are too far apart. The mechanism of the pH-dependent FRET can be explained on the basis of the spectra (Fig. 6B). Upon photoexcitation of C6 at 440 nm, green fluorescence is induced with a peak at 520 nm but part of the emission is transferred to NR by FRET. The red fluorescence of NR resulting from FRET has a peak at 620 nm. Sensitivity to pH is imparted because BTB is yellow at pH below 6, with an absorption peak at around 435 nm. Therefore, a good fraction of the green emission of C6 (the energy not transferred to NR) is absorbed by the yellow form of BTB (present at low pH), whereas the FRET-induced emission of NR is preserved. In fact, the red fluorescence of NR is easily visible and detectable. However, BTB is blue at pH values above 8 with an absorption peak at 628 nm, which strongly overlaps the red emission of NR. As a result, it absorbs most of the red fluorescence of NR. Correspondingly, the visible fluorescence of the hybrid nanogels is dominated by the green fluorescence of C6.
Fig. 6.
(A) Model of a typical Type 2 probe, a ratiometric pH sensing nanogel, which is constructed by uploading of the pH probe dye molecules into the gel networks. (B) pH-dependent absorption of bromothymol blue in aqueous solution at pH 5.0, 7.0, and 9.0 (gray curves), and absorption and emission spectra of coumarin 6 (C6) and Nile Red (NR) in ethanol. The picture in (B) shows the green fluorescence of a mixture of C6 and NR in ethanol/water solvent (left), and the same components in the nanogel (NG) in aqueous suspension under 365 nm illumination (right). (C) Fluorescence micrographs of NRK cells incubated with a pH 7.4 buffer and loaded with the pH-responsive nanogel: (a) Fluorescence of the C6 acquired in the green channel (scale bar=20 mm); (b) fluorescence of NR acquired in the red channel; (c) overlay of (a) and (b). Adapted from Reference (60). Reprinted with permission from Wiley-VCH Publishing Group, Copyright (2010).
Type 3 probe
The signal receivers in Type 3 probes are stimuli-responsive polymer gels that can undergo conformational and chemical changes upon receiving an external signal. These changes are accompanied by variations in the physical properties of the polymer. The signal is either derived from the changes in the materials’ environment such as a change in temperature, pH, glucose concentration, chemical composition, and applied mechanical force, or triggered exogenously by irradiation with light or exposure to an electrical and magnetic field (1–6). The efficient transduction mechanisms of the stimuli-responsive polymer systems make them suitable for sensor applications (40, 41, 107). In the form of hybrid micro-/nanogels, temperature (34, 35, 54, 74–76, 78, 80, 82, 83, 85, 93–99), pH (31–33, 59, 79, 82, 84, 57, 92, 93), and glucose (26, 58, 100) probes have been developed.
Two factors may mainly account for the signal conversion. One is the change in the refractive index of the medium around the optical moieties during the stimuli-induced swelling/shrinking transition of the polymer gels. For example, an increase in the refractive index of nanogel has been observed during its collapsing process (94, 108), which results in an increase in the Rayleigh scattering (because of a larger refractive index contrast between the collapsed gels and solvent) and the local refractive index around the NMNPs, leading to a red-shift and enhancement of the surface plasmon band of NMNPs (Fig. 7) as theoretically reported and predicted by Mie theory (109, 110). The change in refractive index could also affect the efficient photonics transmission and thus the quantum efficiency of optical moieties (74–76). Another is related to the variation of the non-radiative energy loss paths. For QDs/NMNPs, the non-radiative energy loss paths are highly dependent on the environmental nature around the QDs/NMNPs (31–35, 111). For example, at the temperatures below the LCST of polymers, the polymer network chains tend to expand in water. However, the bonding of the polymer chains with the QDs/NMNPs hinders the chain expansion at a highly swollen state, creating an elastic tension in the bonds at the polymer/inorganics interface, thus creating surface states that could quench the PL. This phenomenon is similar to the temperature-induced PL quenching of colloidal CdTe QDs dispersed in water (112). The freezing of water induces strain in the capping shell, which can further propagate the strain to the surface of the QDs, creating surface quenching states. In contrast, at high temperatures above the LCST of polymers, the gel network chains are in shrunk states, which diminish the elastic tension and consequently reduce the number of surface trap states acting as emission quenching centers. A similar relationship between the PL response and volume phase transition has been found in glucose-responsive hybrid nanogels (Fig. 8) and pH-responsive hybrid nanogels (Fig. 9), respectively.
Fig. 7.
TEM images of Au-PNIPAM core-shell hybrid nanogels of spherical (A) and flower-like shaped Au NP cores (B), and (C) their corresponding AFM images (A, top in C; B, down in C), respectively. (D) UV-Vis spectra of aqueous dispersion of the two indicated samples at the temperature below (15°C, solid lines) and above (50°C, dot lines) of PNIPAM. Adopted from Reference (95). Reprinted with permission from Wiley-VCH Publishing Group, Copyright (2008).
fig. 8.
(A) Model of a ratiometric glucose sensing nanogel (Type 3 probe), which is constructed by the growth of the glucose-sensitive nanogel onto SDS-capped Ag NPs templates (10±3 nm). (B) TEM images. (C) PL response of the hybrid nanogels as a function of the glucose concentration in PBS at physiological pH and temperature. Adapted from Reference (100). Reprinted with permission from ACS Publishing Group, Copyright (2010).
Fig. 9.
(A) Model of a ratiometric pH sensing nanogel (Type 3 probe), which is constructed by in situ synthesis of CdSe QDs into the pH-sensitive nanogel. (B) TEM images. (C) PL response of the hybrid nanogels as a function of the pH values. Adapted from Reference (33). Reprinted with permission from Elsevier Publishing Group, Copyright (2010).
Hybrid micro-/nanogel for integration of optical sensing and cell imaging
Intracellular probing enables the monitoring of many biochemistry and biophysics processes of live cells over time and space. Numerous groups have focused on the development of hybrid micro-/nanogels for intracellular optical probing (31–35, 42, 43, 50, 76), particularly toward the real-time monitoring on the change of pH, temperature, glucose concentration, and common ions, which are found to be closely associated with cancer, diabetes, and other diseases (8, 46–53). Intracellular probing on these events can contribute to the explanation of intricate biological processes and the development of novel diagnoses. However, among the large number of the probes developed, only a few of them are suitable for ‘ratiometric’ detection.
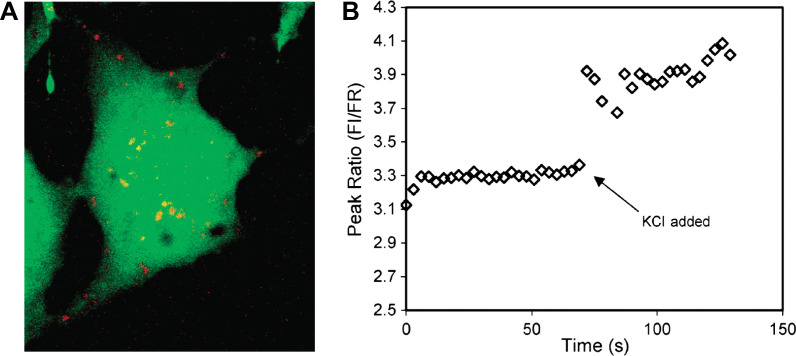
Type 2 probes have been applied successfully for intracellular measurements of pH, oxygen, K+, Cl-, Mg2+, and Ca2+ ions (42, 43). For example, intracellular measurements of pH and Ca2+ ion were made by PEBBLE probes (113–115). Each of the pH-sensitive and Ca2+-selective probes combines an ion-selective internal standard entrapped within a PAAm nanogel (20–200 nm). The use of PEBBLE probes permits the quantitative discrimination of subtle differences between the ability of human SY5Y neuroblastoma and C6 glioma cells to respond to the challenge with m-dinitrobenzene. Specifically, measurement of intracellular calcium, the precursor to cell death, has been achieved. The real-time measurement of Mg2+ ions has also been achieved by PEBBLE probes loaded with Coumarin 343 dye (Fig. 10) (116). Wolfbeis's group have introduced their Type 2 pH probes into living epithelial normal rat kidney cells (Fig. 6C) (50).
Fig. 10.
(A) Compressed z stack of confocal image of a single C6 glioma cell injected with magnesium-selective PEBBLE probes (Type 2 probe). The probes, depicted in red, reside in the cytosol and do not enter the nucleus at the center of the cell. (B) Spectra of the intercellular probes acquired on a fluorescence microscope. An aliquot of KCl was added at t=69 s. Ion channels open, resulting in an increase in intracellular free magnesium indicated by the increase in peak ratio of the probes. Adapted from Reference (116). Reprinted with permission from ACS Publishing Group, Copyright (2003).
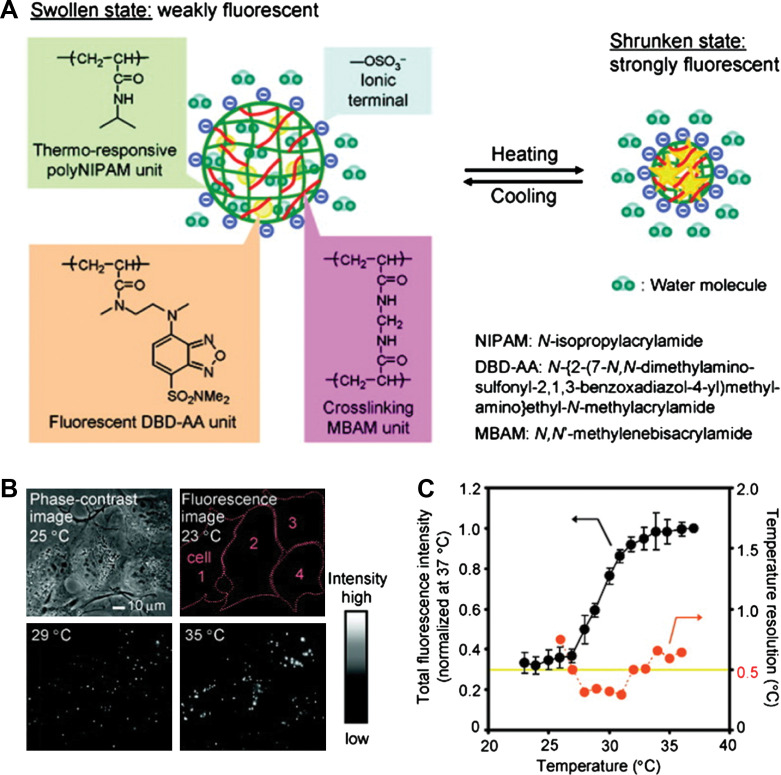
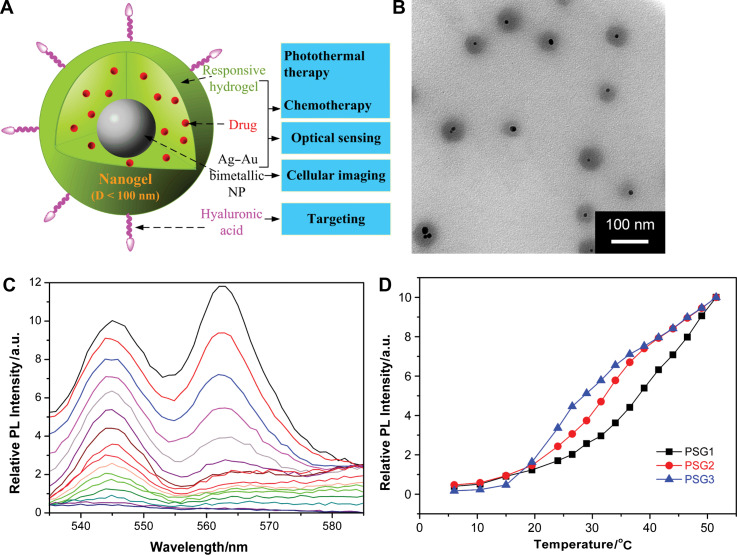
Recently, nanoscale sensing of temperature in the cytoplasm of living cells was demonstrated by Uchiyama's group (Fig. 11) (76). To form the temperature-sensitive nanogel (Type 3 probe), a water-sensitive fluorophore DBD-AA was copolymerized with NIPAM. The fluorescence of the developed hydrophilic fluorescent nanogel thermometer dispersed within the cytoplasm of living COS7 cell was enhanced with increasing the temperature. A temperature resolution of 0.29–0.5°C over the range of 27 and 33°C can be achieved without any interference due to precipitation or interaction with cellular components. We recently introduced Type 3 pH probes (31–33) and temperature probes (Fig. 12) (34, 35) into living mouse melanoma B16F10 cells to demonstrate the feasibility of intracellular sensing of pH and temperature, respectively. The QDs/NMNPs were used as optical moieties in our probes. The most important difference between our probes and others is the tunable sensitivity of our probes, which can be achieved through engineering the stimuli-responsive properties of the gel networks. In addition, we have further integrated multiple functions into a single probe. In a typical temperature probe (34), the core-shell structured hybrid nanogels were constructed by coating the Ag-Au bimetallic NP core with a thermo-responsive non-linear PEG-based hydrogel as shell, and then semi-interpenetrating the targeting ligands of hyaluronic acid chains into the surface networks of gel shell. The Ag-Au NP core can emit strong visible fluorescence for imaging of mouse melanoma B16F10 cells. The reversible thermo-responsive volume phase transition of the non-linear PEG-based nanogel shell can not only modify the physicochemical environment of the Ag-Au NP core to manipulate the fluorescence intensity for sensing the environmental temperature change, but also provide a high loading capacity for a model anticancer drug temozolomide and offer a thermo-triggered drug release. The drug release can be induced by both the heat generated by external NIR irradiation and the temperature increase of local environmental media. The ability of the hybrid nanogels to combine the local specific chemotherapy with external NIR photothermal treatment significantly improved the therapeutic efficacy due to a synergistic effect. These small, stable, and non-toxic hybrid nanogels that can integrate multiple functionalities into a single NP will offer broad opportunities for sensing, imaging, and monitoring the biological environments as well as for high efficacy treatment of various diseases.
Fig. 11.
(A) Model of a ratiometric temperature sensing nanogel (Type 3 probe), which is constructed by copolymerization of a water-sensitive fluorophore DBD-AA into a temperature-sensitive PNIPAM-based nanogel. (B) Phase-contrast and fluorescence images of living COS7 cells containing the fluorescent nanogel thermometer. (C) Calibration cure and temperature resolution. Adapted from Reference (76). Reprinted with permission from ACS Publishing Group, Copyright (2009).
Fig. 12.
(A) Model of a ratiometric temperature sensing nanogel (Type 3 probe), which is constructed by coating a thermo-responsive non-linear PEG-based gel shell onto the Ag-Au bimetallic NP core. (B) Typical TEM image. (C) Typical PL profiles taken at 2.5°C intervals from bottom to top except the bottom 5 curves (every 4.5°C), from 6.0 to 51.5°C. (D) Tunable temperature sensing in terms of PL intensity of the hybrid nanogels. Adapted from Reference (34). Reprinted with permission from Elsevier Publishing Group, Copyright (2010).
Conclusions and outlook
In conclusion, hybrid micro-/nanogels form a distinct class of hydrophilic dispersed probes with promising properties for optical sensing and cell imaging. The advantages of these hybrid micro-/nanogels include their simplicity of formation, biocompatibility, and high stability for chemical/biochemical sensing and disease diagnosis. As more and more research is carried out in this field, it becomes clear that these hybrid nanogel systems hold great promise as a nanoscale platform toward multifunctional biomaterials. Hybrid nanogels that respond to a change in their environment are potentially useful in the combined sensing, imaging, and therapy that may enable natural biosystems (pathological tissues and organs) to self-manipulate in a rational fashion.
Although a great deal of research has been carried out in this field, we believe that most of the potential resources are untapped. It is clear that there is no universal system that can address all current needs of sensing and imaging applications. In this regard, the capabilities of hybrid micro-/nanogel-based probes as well as other classes of probes are not infinite, but it would be interesting to develop probes for dual, triple, and even multiple sensing and imaging. There are also opportunities for new efforts in advanced synthetic approaches to improve both selectivity and sensitivity of the hybrid micro-/nanogels for optical sensing in complex environment and intracellular probing. The rational design of multifunctional nanoplatforms will allow the improvement of the sensing applications as well as the implementation of the hybrid micro-/nanogels in new arenas. In the case of core-shell structured inorganic-responsive polymer hybrid micro-/nanogels reported by our group and others, the range of potential applications of such probes is just at the beginning to be uncovered. Early proof-of-concept studies of these probes for simultaneous sensing, imaging, and therapy can provide guidance for the development of clinical application-oriented bionanomaterials. We certainly hope that future application of hybrid micro-/nanogels will exceed our expectations and believe that many capable scientists across the globe will contribute outstanding work to advance these novel probes toward the smart age.
Biographies
 Weitai Wu received a BS in applied chemistry (2003) and a PhD in Condensed Matter Physics (2008) from the University of Science and Technology of China (USTC), P.R. China. In 2006 and 2007, he worked on the research staff in the Department of Electrical Engineering at the Hong Kong Polytechnic University, Hong Kong S.A.R., to develop polymer-bonded magnetic devices. He has been working as a postdoctoral research associate in the Department of Chemistry at the College of Staten Island, The City University of New York, since 2008. He is currently interested in the chemistry and physics of smart polymers, conjugated polymers, polymer-inorganic hybrids, and their applications in biomedicine.
Weitai Wu received a BS in applied chemistry (2003) and a PhD in Condensed Matter Physics (2008) from the University of Science and Technology of China (USTC), P.R. China. In 2006 and 2007, he worked on the research staff in the Department of Electrical Engineering at the Hong Kong Polytechnic University, Hong Kong S.A.R., to develop polymer-bonded magnetic devices. He has been working as a postdoctoral research associate in the Department of Chemistry at the College of Staten Island, The City University of New York, since 2008. He is currently interested in the chemistry and physics of smart polymers, conjugated polymers, polymer-inorganic hybrids, and their applications in biomedicine.
 Shuiqin Zhou received a BS (1988) and an MS (1991) from Xiamen University, China and a PhD from The Chinese University of Hong Kong in 1996. During 1996–2000, she worked in the State University of New York at Stony Brook as a postdoctoral research associate. She then worked at Union Carbide/The Dow Chemical Company as a senior chemist in research and development until 2002 when she moved to The City University of New York at the College of Staten Island as an associate professor. She is currently a professor of chemistry and has over 80 journal publications and several book chapters in the field of polymer physics, colloidal science, nanomaterials, and biomaterials. Her research group is currently working in the smart biomaterials and supramolecular assembled nanomaterials.
Shuiqin Zhou received a BS (1988) and an MS (1991) from Xiamen University, China and a PhD from The Chinese University of Hong Kong in 1996. During 1996–2000, she worked in the State University of New York at Stony Brook as a postdoctoral research associate. She then worked at Union Carbide/The Dow Chemical Company as a senior chemist in research and development until 2002 when she moved to The City University of New York at the College of Staten Island as an associate professor. She is currently a professor of chemistry and has over 80 journal publications and several book chapters in the field of polymer physics, colloidal science, nanomaterials, and biomaterials. Her research group is currently working in the smart biomaterials and supramolecular assembled nanomaterials.
Conflict of interest and funding
There is no conflict of interest in the present study for any of the authors. This work is supported from the USAID under the US-Pakistan Science and Technology Cooperative Program (PGA-P280422).
References
- 1.Hoffman AS. Hydrogels for biomedical applications. Adv Drug Delivery Rev. 2002;43:3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 2.Nayak S, Lyon LA. Soft nanotechnology with soft nanoparticles. Angew Chem Int Ed. 2005;44:7686–708. doi: 10.1002/anie.200501321. [DOI] [PubMed] [Google Scholar]
- 3.Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv Mater. 2006;18:1345–60. [Google Scholar]
- 4.Kabanov AV, Vinogradov SV. Nanogels as pharmaceutical carriers: finite networks of infinite capabilities. Angew Chem Int Ed. 2009;48:5418–29. doi: 10.1002/anie.200900441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanson N, Rieger J. Synthesis of nanogels/microgels by conventional and controlled radical crosslinking copolymerization. Polym Chem. 2010;1:965–77. [Google Scholar]
- 6.Motornov M, Roiter Y, Tokarev I, Minko S. Stimuli-responsive nanoparticles, nanogels and capsules for integrated multifunctional intelligent systems. Prog Polym Sci. 2010;35:174–211. [Google Scholar]
- 7.McQuade DT, Pullen AE, Swager TM. Conjugated polymer-based chemical sensors. Chem Rev. 2000;100:2537–74. doi: 10.1021/cr9801014. [DOI] [PubMed] [Google Scholar]
- 8.Pickup JC, Hussain F, Evans ND, Rolinski OJ, Birch DJS. Fluorescence-based glucose sensors. Biosens Bioelectron. 2005;20:2555–65. doi: 10.1016/j.bios.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Matsubara K, Watanabe M, Takeoka Y. A thermally adjustable multicolor photochromic hydrogel. Angew Chem Int Ed. 2007;119:1718–22. doi: 10.1002/anie.200603554. [DOI] [PubMed] [Google Scholar]
- 10.McDonagh C, Burke CS, MacCraith BD. Optical chemical sensors. Chem Rev. 2008;108:400–22. doi: 10.1021/cr068102g. [DOI] [PubMed] [Google Scholar]
- 11.Borisov SM, Wolfbeis OS. Optical biosensors. Chem Rev. 2008;108:423–61. doi: 10.1021/cr068105t. [DOI] [PubMed] [Google Scholar]
- 12.Stich MIJ, Fischer LH, Wolfbeis OS. Multiple fluorescent chemical sensing and imaging. Chem Soc Rev. 2010;39:3102–14. doi: 10.1039/b909635n. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Tanaka T. Kinetics of swelling and shrinking of gels. J Chem Phys. 1990;92:1365–71. [Google Scholar]
- 14.Reese CE, Mikhonin AV, Kamenjicki M, Tikhonov A, Asher SA. Nanogel nanosecond photonic crystal optical switching. J Am Chem Soc. 2004;126:1493–6. doi: 10.1021/ja037118a. [DOI] [PubMed] [Google Scholar]
- 15.Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. Small. 2008;4:26–49. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- 16.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, li JJ, et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538–44. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosi NL, Mirkin CA. Nanostructures in biodiagnostics. Chem Rev. 2005;105:1547–62. doi: 10.1021/cr030067f. [DOI] [PubMed] [Google Scholar]
- 18.Jian PK, Huang X, El-Sayed IH, El-Sayed MA. Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc Chem Res. 2008;21:1578–86. doi: 10.1021/ar7002804. [DOI] [PubMed] [Google Scholar]
- 19.Biju V, Itoh T, Anas A, Sujith A, Ishikawa M. Semiconductor quantum dots and metal nanoparticles: syntheses, optical properties, and biological applications. Anal Bioanal Chem. 2008;391:2469–95. doi: 10.1007/s00216-008-2185-7. [DOI] [PubMed] [Google Scholar]
- 20.Costi R, Saunders AE. Colloidal hybrid nanostructures: a new type of functional materials. Angew Chem Int Ed. 2010;49:4878–97. doi: 10.1002/anie.200906010. [DOI] [PubMed] [Google Scholar]
- 21.Wu W, Zhou T, Berliner A, Banerjee P, Zhou SQ. Glucose-mediated assembly of phenylboronic acid modified CdTe/ZnTe/ZnS quantum dots for intracellular glucose probing. Angew Chem Int Ed. 2010;49:6554–8. doi: 10.1002/anie.201001508. [DOI] [PubMed] [Google Scholar]
- 22.Qian X, Li J, Nie SM. Stimuli-responsive SERS nanoparticles: conformational control of plasmonic coupling and surface Raman enhancement. J Am Chem Soc. 2009;131:7540–1. doi: 10.1021/ja902226z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamidi M, Azadi A, Rafiei P. Hydrogel nanoparticles in drug delivery. Adv Drug Deliv Rev. 2008;60:1638–49. doi: 10.1016/j.addr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Bhattarai N, Gunn J, Zhang M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv Drug Deliv Rev. 2010;62:83–99. doi: 10.1016/j.addr.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 25.Graber ML, Dilillo DC, Friedman BL, Pastorizamunoz E. Characteristics of fluoroprobes for measuring intracellular pH. Anal Biochem. 1986;156:202–12. doi: 10.1016/0003-2697(86)90174-0. [DOI] [PubMed] [Google Scholar]
- 26.Wu W, Zhou T, Aiello M, Zhou SQ. Construction of optical glucose nanobiosensor with high sensitivity and selectivity at physiological pH on the basis of organic-inorganic hybrid microgels. Biosens Bioelecton. 2010;25:2603–10. doi: 10.1016/j.bios.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 27.Sasaki K, Shi ZY, Kopelman R, Masuhara H. Three-dimensional pH microprobing with an optically manipulated fluorescent particle. Chem Lett. 1996;2:141–2. [Google Scholar]
- 28.Clark HA, Barker SLR, Brasuel M, Miller MT, Monson E, et al. Subcellular optochemical nanobiosensors: probes encapsulated by biologically localized embedding (PEBBLEs) Sens Actuators, B. 1998;51:12–6. [Google Scholar]
- 29.Nagl S, Stich MIJ, Schaferling M, Wolfbeis OS. Method for simultaneous luminescence sensing of two species using optical probes of different decay time, and its application to an enzymatic reaction at varying temperature. Anal Bioanal Chem. 2009;393:1199–207. doi: 10.1007/s00216-008-2467-0. [DOI] [PubMed] [Google Scholar]
- 30.Montalti M, Prodi L, Zaccheroni N. Fluorescence quenching amplication in silica nanosensors for metal ions. J Mater Chem. 2005;15:2810–4. [Google Scholar]
- 31.Wu W, Zhou T, Berliner A, Banerjee P, Zhou SQ. Smart core-shell hybrid nanogels with Ag nanoparticle core for cancer cell imaging and gel shell for pH-regulated drug delivery. Chem Mater. 2010;22:1966–76. [Google Scholar]
- 32.Wu W, Aiello M, Zhou T, Berliner A, Banerjee P, Zhou SQ. In-situ immobilization of quantum dots in polysaccharide-based nanogels for integration of optical pH-sensing, tumor cell imaging, and drug delivery. Biomaterials. 2010;31:3023–31. doi: 10.1016/j.biomaterials.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Wu W, Shen J, Banerjee P, Zhou SQ. Chitosan-based responsive hybrid nanogels for integration of optical pH-sensing, tumor cell imaging and controlled drug delivery. Biomaterials. 2010;31:8371–81. doi: 10.1016/j.biomaterials.2010.07.061. [DOI] [PubMed] [Google Scholar]
- 34.Wu W, Shen J, Banerjee P, Zhou SQ. Core-shell hybrid nanogels for integration of optical temperature-sensing, targeted tumor cell imaging, and combined chemo-photothermal treatment. Biomaterials. 2010;31:7555–66. doi: 10.1016/j.biomaterials.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 35.Wu W, Shen J, Banerjee P, Zhou SQ. Water-dispersible multifunctional hybrid nanogels for combined curcumin and photothermal therapy. Biomaterials. 2010 doi: 10.1016/j.biomaterials.2010.08.112. [DOI] [PubMed] [Google Scholar]
- 36.Xu H, Aylott JW, Kopelman R, Miller TJ, Philbert MA. A real-time ratiometric method for the determination of molecular oxygen inside living cells using sol-gel-based spherical optical nanosensors with applications to rat C6 glioma. Anal Chem. 2001;73:4124–33. doi: 10.1021/ac0102718. [DOI] [PubMed] [Google Scholar]
- 37.Lee JE, Lee N, Kim H, Kim J, Choi SH, Kim JH, et al. Uniform mesoporous dye-doped silica nanoparticles decorated with multiple magnetite nanocrystals for simultaneous enhanced magnetic resonance imaging, fluorescence imaging, and drug delivery. J Am Chem Soc. 2010;132:552–7. doi: 10.1021/ja905793q. [DOI] [PubMed] [Google Scholar]
- 38.Kabilan S, Marshall AJ, Sartain FK, Lee MC, Hussain A, Yang X, et al. Holographic glucose sensors. Biosens Bioelectron. 2005;20:1602–10. doi: 10.1016/j.bios.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Muscatello MMW, Stunja LE, Asher SA. Polymerized crystalline colloidal array sensing of high glucose concentrations. Anal Chem. 2009;81:4978–86. doi: 10.1021/ac900006x. [DOI] [PubMed] [Google Scholar]
- 40.Peng H, Stich MIJ, Yu J, Sun L, Fischer LH, Wolfbeis OS. Luminescent europium(III) nanoparticles for sensing and imaging of temperature in the physiological range. Adv Mater. 2010;22:716–9. doi: 10.1002/adma.200901614. [DOI] [PubMed] [Google Scholar]
- 41.Tokarev I, Tokareva I, Gopishetty V, Katz E, Minko S. Specific biochemical-to-optical signal transduction by responsive thin hydrogel films loaded with noble metal nanoparticles. Adv Mater. 2010;22:1412–6. doi: 10.1002/adma.200903456. [DOI] [PubMed] [Google Scholar]
- 42.Lee YEK, Kopelman R. Optical nanoparticles sensors for quantitative intracellular imaging. WIREs Nanomed Nanobiotechnol. 2008;1:98–110. doi: 10.1002/wnan.2. [DOI] [PubMed] [Google Scholar]
- 43.Lee YEK, Smith R, Kopelman R. Nanoparticle PEBBLE sensors in live cells and in vivo. Annu Rev Anal Chem. 2009;2:57–76. doi: 10.1146/annurev.anchem.1.031207.112823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han MY, Gao XH, Su JZ, Nie SM. Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules. Nat Biotechnol. 2001;19:631–5. doi: 10.1038/90228. [DOI] [PubMed] [Google Scholar]
- 45.Agrawal A, Deo R, Wang GD, Wang MD, Nie SM. Nanometer-scale mapping and single-molecule detection with color-coded nanoparticle probes. Proc Natl Acad Sci USA. 2008;105:3298–303. doi: 10.1073/pnas.0712351105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyerhof O, Lohmann K. Energy relationships in the transformation of phosphoric acid esters in muscle extract. Biochem Z. 1932;253:431–61. [Google Scholar]
- 47.Nakamura T, Matsuoka I. Calorimetric studies of heat of respiration of mitochondria. J Biochem. 1978;84:39–46. doi: 10.1093/oxfordjournals.jbchem.a132117. [DOI] [PubMed] [Google Scholar]
- 48.Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–60. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- 49.Monti M, Brandt L, Ikomi-Kumm J, Olsson H. Microcalorimetric investigation of cell metabolism in tumour cells from patients with non-Hodgkin lymphoma (NHL) Scand J Haematol. 1986;36:353–7. doi: 10.1111/j.1600-0609.1986.tb01749.x. [DOI] [PubMed] [Google Scholar]
- 50.Karnebogen M, Singer D, Kallerhoff M, Ringert RH. Microcalorimetric investigations on isolated tumorous and non-tumorous tissue samples. Thermochim Acta. 1993;229:147–55. [Google Scholar]
- 51.Tannock IF, Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989;49:4373–84. [PubMed] [Google Scholar]
- 52.Stubbs M, McSheehy PMJ, Griffiths JR, Bashford CL. Causes and consequences of tumour acidity and implications for treatment. Mol Med Today. 2000;6:15–9. doi: 10.1016/s1357-4310(99)01615-9. [DOI] [PubMed] [Google Scholar]
- 53.Coakley RD, Grubb BR, Paradiso AM, Gatzy JT, Johnson LG, Kreda SM, et al. Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc Natl Acad Sci USA. 2003;100:16083–8. doi: 10.1073/pnas.2634339100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang J, Xu S, Kumacheva E. Polymer microgels: reactors for semiconductor, metal, and magnetic nanoparticles. J Am Chem Soc. 2004;126:7908–14. doi: 10.1021/ja031523k. [DOI] [PubMed] [Google Scholar]
- 55.Hasegawa U, Nomura SIM, Kaul SC, Hirano T, Akiyoshi K. Nanogel-quantum dot hybrid nanoparticles for live cell imaging. Biochem Biophys Res Commun. 2005;331:917–21. doi: 10.1016/j.bbrc.2005.03.228. [DOI] [PubMed] [Google Scholar]
- 56.Xiong YJ, Siekkinen AR, Wang JG, Yin YD, Kim MJ, Xia YN. Synthesis of silver nanoplates at high yields by slowing down the polyol reduction of silver nitrate with polyacrylamide. J Mater Chem. 2007;17:2600–2. [Google Scholar]
- 57.Wu W, Zhou T, Zhou SQ. Tunable photoluminescence of Ag nanocrystals in multiple-sensitive hybrid microgels. Chem Mater. 2009;21:2851–61. [Google Scholar]
- 58.Wu W, Zhou T, Shen J, Zhou SQ. Optical detection of glucose by CdS quantum dots immobilized in smart microgels. Chem Comm. 2009:4390–2. doi: 10.1039/b907348e. [DOI] [PubMed] [Google Scholar]
- 59.Wu W, Zhou T, Aiello M, Zhou SQ. Optically pH and H2O2 dual responsive composite colloids through the directed assembly of organic dyes on responsive microgels. Chem Mater. 2009;21:4905–13. [Google Scholar]
- 60.Peng H, Stolwijk JA, Sun L, Wegener J, Wolfbeis OS. A nanogel for ratiometric fluorescent sensing of intracellular pH values. Angew Chem Int Ed. 2010;49:4246–9. doi: 10.1002/anie.200906926. [DOI] [PubMed] [Google Scholar]
- 61.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–92. [PubMed] [Google Scholar]
- 62.Hiller J, Mendelsohn J, Rubner MF. Reversibly erasable nanoporous anti-reflection coatings from polyelectrolyte multilayers. Nat Mater. 2002;1:59–63. doi: 10.1038/nmat719. [DOI] [PubMed] [Google Scholar]
- 63.Picart C, Mutterer J, Richert L, Luo Y, Prestwich GD, Schaaf P, et al. Molecular basis for the explanation of the exponential growth of polyelectrolyte multilayers. Proc Natl Acad Sci USA. 2002;99:12531–5. doi: 10.1073/pnas.202486099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sui ZJ, Schlenoff JB. Phase separations in pH-responsive polyelectrolyte multilayers: charge extrusion versus charge expulsion. Langmuir. 2004;20:6026–31. doi: 10.1021/la0495985. [DOI] [PubMed] [Google Scholar]
- 65.Kharlampieva E, Ankner JF, Rubinstein M, Sukhishvili SA. pH-Induced release of polyanions from multilayer films. Phys Rev Lett. 2008;100:128303. doi: 10.1103/PhysRevLett.100.128303. [DOI] [PubMed] [Google Scholar]
- 66.Wang JS, Matyjasewski K. Controlled/“living” radical polymerization: atom transfer radical polymerization in the presence of transition-metal complexes. J Am Chem Soc. 1995;117:5614–5. [Google Scholar]
- 67.Ouchi M, Terashima T, Sawamoto M. Transition metal-catalyzed living radical polymerization: toward perfection in catalysis and precision polymer synthesis. Chem Rev. 2009;109:4963–5050. doi: 10.1021/cr900234b. [DOI] [PubMed] [Google Scholar]
- 68.Matyjaszewski K, Gaynor S, Wang JS. Controlled radical polymerizations: the use of alkyl iodides in degenerative transfer. Macromolecules. 1995;28:2093–5. [Google Scholar]
- 69.Lacroix-Desmazes P, Severac R, Boutevin B. Reverse iodine transfer polymerization of methyl acrylate and n-butyl acrylate. Macromolecules. 2005;38:6299–309. [Google Scholar]
- 70.Chiefari J, Chong YK, Ercole F, Krstina J, Jeffery J, Le TPT, et al. Living free-radical polymerization by reversible addition-fragmentation chain transfer: the RAFT process. Macromolecules. 1998;31:5559–62. [Google Scholar]
- 71.Barner-Kowollik C, editor. Handbook of RAFT Polymerization. Weinheim: Wiley; 2008. [Google Scholar]
- 72.Lin KJ. SMTP-1: the first functionalized metalloporphyrin molecular sieves with large channels. Angew Chem Int Ed. 1999;38:2730–2. [PubMed] [Google Scholar]
- 73.Uchiyama S, Matsymura Y, de Silva AP, Iwai K. Fluorescent molecular thermometers based on polymers showing temperature-induced phase transitions and labeled with polarity-responsive benzofurazans. Anal Chem. 2003;75:5926–35. doi: 10.1021/ac0346914. [DOI] [PubMed] [Google Scholar]
- 74.Iwai K, Matsumura Y, Uchiyama S, de Silva AP. Development of fluorescent microgel thermometers based on thermo-responsive polymers and their modulation of sensitivity range. J Mater Chem. 2005;15:2796–800. [Google Scholar]
- 75.Gota C, Uchiyama S, Yoshihara T, Tobita S, Ohwada T. Temperature-dependent fluorescence lifttime of a fluorescent polymeric thermometer, poly(N-isopropylacrylamide), labeled by polarity and hydrogen bonding sensitive 4-sulfamoyl-7-aminobenzofurazan. J Phys Chem B. 2008;112:2829–36. doi: 10.1021/jp709810g. [DOI] [PubMed] [Google Scholar]
- 76.Gota C, Okabe K, Funatsu T, Harada Y, Uchiyama S. Hydrophilic fluorescent nanogel thermometer for intracellular thermometry. J Am Chem Soc. 2009;131:2766–7. doi: 10.1021/ja807714j. [DOI] [PubMed] [Google Scholar]
- 77.Yin J, Guan X, Wang D, Liu SY. Metal-chelating and dansyl-labeled poly(N-isopropylacrylamde) microgels as fluorescent Cu2+ sensors with thermo-enhanced detection sensitivity. Langmuir. 2009;25:11367–74. doi: 10.1021/la901377h. [DOI] [PubMed] [Google Scholar]
- 78.Gorelikov I, Field LM, Kumacheva E. Hybrid microgels photoresponsive in the near-infrared spectral range. J Am Chem Soc. 2004;126:15938–9. doi: 10.1021/ja0448869. [DOI] [PubMed] [Google Scholar]
- 79.Das M, Mordoukhovski L, Kumacheva E. Sequestering gold nanorods by polymer microgels. Adv Mater. 2008;20:2371–5. [Google Scholar]
- 80.Hong Y, Gao M, Wang D, Mhwald H. Incorporating fluorescent CdTe nanocrystals into a hydrogel via hydrogen bonding: toward fluorescent microspheres with temperature-responsive properties. Chem Mater. 2005;17:2648–53. [Google Scholar]
- 81.Agrawal M, Rubio-Retama J, Zafeiropoulos NE, Gaponik N, Gupta S, et al. Switchable photoluminescence of CdTe nanocrystals by temperature-responsive microgels. Langmuir. 2008;24:9820–4. doi: 10.1021/la801347d. [DOI] [PubMed] [Google Scholar]
- 82.Karg M, Lu Y, Carbo-Argibay E, Pastoriza-Santos I, Perez-Juste J, Liz-Marzan LM, et al. Multiresponsive hybrid colloids based on gold nanorods and poly(NIPAM-co-allylacetic acid) microgels: temperature- and pH-tunable plasmon resonance. Langmuir. 2009;25:3163–7. doi: 10.1021/la803458j. [DOI] [PubMed] [Google Scholar]
- 83.Dou H, Yang W, Tao K, Li W, Sun K. Thermal sensitive microgels with stable and reversible photoluminescence based on covalently bonded quantum dots. Langmuir. 2010;26:5022–7. doi: 10.1021/la903667r. [DOI] [PubMed] [Google Scholar]
- 84.Zhang J, Xu S, Kumacheva E. Photogeneration of fluorescent silver nanoclusters in polymer microgels. Adv Mater. 2005;17:2336–40. [Google Scholar]
- 85.Suzuki D, Kawaguchi H. Hybrid microgels with reversibly changeable multiple brilliant color. Langmuir. 2006;22:3818–22. doi: 10.1021/la052999f. [DOI] [PubMed] [Google Scholar]
- 86.Lu Y, Mei Y, Drechsler M, Ballauff M. Thermosensitive core-shell particles as carriers for Ag nanoparticles: modulating the catalytic activity by a phase transition in networks. Angew Chem Int Ed. 2006;45:813–6. doi: 10.1002/anie.200502731. [DOI] [PubMed] [Google Scholar]
- 87.Kim JH, Lee TR. Hydrogel-templated growth of large gold nanoparticles: synthesis of thermally responsive hydrogel-nanoparticle composites. Langmuir. 2007;23:6504–9. doi: 10.1021/la0629173. [DOI] [PubMed] [Google Scholar]
- 88.Xiong H, Xu Y, Ren Q, Xia Y. Stable aqueous ZnO@polymer core-shell nanoparticles with tunable photoluminescence and their application in cell imaging. J Am Chem Soc. 2008;130:7522–3. doi: 10.1021/ja800999u. [DOI] [PubMed] [Google Scholar]
- 89.Zhang P, Liu W. ZnO QD@PMAA-co-PDMAEMA nonviral vector for plasmid DNA delivery and bioimaging. Biomaterials. 2010;31:3087–94. doi: 10.1016/j.biomaterials.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 90.Janczewski D, Tomczak N, Han MY, Vancso GJ. Stimulus responsive PNIPAM/QD hybrid microspheres by copolymerization with surface engineered QDs. Macromolecules. 2009;42:1801–4. [Google Scholar]
- 91.Kim DJ, Kang SM, Kong B, Kim WJ, Paik HJ, Choi H, et al. Formation of thermoresponsive gold nanoparticles/PNIPAm hybrids by surface-initiated, atom transfer radical polymerization in aqueous media. Macromol Chem Phys. 2005;206:1941–6. [Google Scholar]
- 92.Li D, He Q, Cui Y, Li J. Fabrication of pH-responsive nanocomposites of gold nanoparticles/poly(4-vinylpyridine) Chem Mater. 2007;19:412–7. [Google Scholar]
- 93.Kim JH, Lee TR. Thermo- and pH-responsive hydrogel-coated gold nanoparticles. Chem Mater. 2004;16:3647–51. [Google Scholar]
- 94.Karg M, Pastoriza-Santos I, Lizmarzan LM, Hellweg T. A versatile approach for the preparation of thermosensitive PNIPAM core-shell microgels with nanoparticles cores. ChemPhysChem. 2006;7:2298–301. doi: 10.1002/cphc.200600483. [DOI] [PubMed] [Google Scholar]
- 95.Contreras-Caceres R, Sanchez-Iglesias A, Karg M, Pastoriza-Santos I, Perez-Juste J, Pacifico J, et al. Encapsulation and growth of gold nanoparticles in thermoresponsive microgels. Adv Mater. 2008;20:1666–70. [Google Scholar]
- 96.Sanchez-Iglesias A, Grzelczak M, Rodriguez-Gonzalez B, Guardia-Giros P, Pastoriza-Santos I, Perez-Juste J, et al. Synthesis of multifunctional composite microgels via in situ Ni growth on pNIPAM-coated Au nanoparticles. ACS Nano. 2009;3:3184–90. doi: 10.1021/nn9006169. [DOI] [PubMed] [Google Scholar]
- 97.Alvarez-Puebla RA, Contreras-Caceres R, Pastoriza-Santos I, Perez-Juste J, Liz-Marzan LM. Au@pNIPAM colloids as molecular traps for surface-enhanced, spectroscopic, ultra-sensitive analysis. Angew Chem Int Ed. 2009;48:138–43. doi: 10.1002/anie.200804059. [DOI] [PubMed] [Google Scholar]
- 98.Carregal-Romero S, Buurma NJ, Perez-Juste J, Liz-Marzan LM, Herves P. Catalysis by Au@pNIPAM nanocomposites: effect of the cross-linking density. Chem Mater. 2010;22:3051–59. [Google Scholar]
- 99.Singh N, Lyon LA. Au nanoparticles template synthesis of pNIPAM nanogels. Chem Mater. 2007;19:719–26. [Google Scholar]
- 100.Wu W, Mitra N, Yan ECY, Zhou SQ. Multifunctional hybrid nanogel for integration of optical glucose sensing and self-regulated insulin release at physiological pH. ACS Nano. 2010;4:4831–9. doi: 10.1021/nn1008319. [DOI] [PubMed] [Google Scholar]
- 101.Qu W, Wang S, Hu Z, Cheang T, Xing Z, Zhang X, et al. In situ synthesis of gold@3,4-dihydroxy-L-phenylalanine core-shell nanospheres used for cell imaging. J Phys Chem C. 2010;114:13010–6. [Google Scholar]
- 102.Laurenti M, Lopez-Cabarcos E, Garcia-Blanco F, Frick B, Rubio-Retama J. Interpenetrated PNIPAM-polythiophene microgels for nitro aromatic compound detection. Langmuir. 2009;25:9579–84. doi: 10.1021/la900864a. [DOI] [PubMed] [Google Scholar]
- 103.Howes P, Green M, Levitt J, Suhling K, Hughes M. Phospholopid encapsulated semiconducting polymer nanoparticles: their use in cell imaging and protein attachment. J Am Chem Soc. 2010;132:3989–96. doi: 10.1021/ja1002179. [DOI] [PubMed] [Google Scholar]
- 104.Buhlmann P, Pretsch E, Bakker E. Carrier-based ionselective electrodes and bulk optodes, 2. Ionophores for potentiometric and optical sensors. Chem Rev. 1998;98:1593–687. doi: 10.1021/cr970113+. [DOI] [PubMed] [Google Scholar]
- 105.Bakker E, Simon W. Selectivity of ion-sensitive bulk optodes. Anal Chem. 1992;64:1805–12. [Google Scholar]
- 106.Xu H, Aylott JW, Kopelman R. Fluorescent nano-PEBBLE sensors designed for intracellular glucose imaging. Analyst. 2002;127:1471–7. doi: 10.1039/b202782h. [DOI] [PubMed] [Google Scholar]
- 107.Stuart MAC, Huck WTS, Genzer J, Muller M, Ober C, Stamm M, et al. Emerging applications of stimuli-responsive polymer materials. Nat Mater. 2010;9:101–13. doi: 10.1038/nmat2614. [DOI] [PubMed] [Google Scholar]
- 108.Garner BW, Cai T, Ghosh S, Hu Z, Neogi A. Refractive index change due to volume-phase transition in polyacrylamide gel nanospheres for optoelectronics and bio-photonics. App Phys Express. 2009;2:057001. [Google Scholar]
- 109.Mulvaney P. Surface plasmon spectroscopy of nanosized metal particles. Langmuir. 1996;12:788–800. [Google Scholar]
- 110.Liz-Marzan LM, Giersig M, Mulvaney P. Synthesis of nanosized gold-silica core-shell particles. Langmuir. 1996;12:4329–35. [Google Scholar]
- 111.Wilcoxon JP, Martin JE, Parsapour F, Wiedenman B, Kelley DF. Photoluminescence from nanosize gold clusters. J Chem Phys. 1998;108:9137–43. [Google Scholar]
- 112.Wuister SR, Doneg CM, Meijerink A. Luminescence temperature antiquenching of water-soluble CdTe quantum dots: role of the solvent. J Am Chem Soc. 2004;126:10397–402. doi: 10.1021/ja048222a. [DOI] [PubMed] [Google Scholar]
- 113.Clark HA, Hoyer M, Philbert MA, Kopelman R. Optical nanosensors for chemical analysis inside single living cells. 1. Fabrication, characterization, and methods for intracellular delivery of PEBBLE sensors. Anal Chem. 1999;71:4831–6. doi: 10.1021/ac990629o. [DOI] [PubMed] [Google Scholar]
- 114.Clark HA, Kopelman R, Tjalkens R, Philbert MA. Optical nanosensors for chemical analysis inside single living cells. 2. Sensors for pH and calcium and the intracellular application of PEBBLE sensors. Anal Chem. 1999;71:4837–43. doi: 10.1021/ac990630n. [DOI] [PubMed] [Google Scholar]
- 115.Monson E, Brasuel M, Philbert M, Kopelman R. PEBBLE nanosensors for in vitro bioanalysis. In: Vo-Dinh T, editor. Biomedical photonics handbook. Boca Raton, FL: CRC Press LLC; 2003. pp. 59-1–59-14. [Google Scholar]
- 116.Park EJ, Brasuel M, Behrend C, Philbert MA, Kopelman R. Ratiometric optical PEBBLE nanosensors for real-time magnesium ion concentrations inside viable cells. Anal Chem. 2003;75:3784–91. doi: 10.1021/ac0342323. [DOI] [PubMed] [Google Scholar]