Abstract
Cysts of the adrenal gland are rare and are usually discovered incidentally. Large adrenal cysts can however present with severe abdominal pain and can be complicated by haemorrhage, rupture or infection. Adrenal pseudocysts appear to result from haemorrhage within a normal adrenal gland and can expand to accommodate massive amounts of fluid.
We report the case of a 39-year-old woman who presented with worsening right upper quadrant pain. An ultrasound scan of the abdomen confirmed a large 29 cm × 20 cm × 17 cm cyst that appeared to originate in the upper pole of the right kidney causing displacement of the liver and right kidney.
Following complete aspiration the cyst re-accumulated and an MRI scan demonstrated a thickened and irregular cyst wall with haemorrhagic fluid. Laparoscopic right adrenalectomy was performed and the histopathological diagnosis was confirmed as an adrenal pseudocyst.
Keywords: Adrenal, Pseudocyst, Laparoscopic, Adrenalectomy, Diagnosis
1. Introduction
The majority of adrenal pseudocysts are benign cystic masses originating within the adrenal cortex or medulla that are enclosed by a fibrous wall. It appears that their pathogenesis may lie in repeated episodes of trauma, infection or bleeding. There are four categories of adrenal gland cyst: epithelial, endothelial, parasitic and pseudocysts.1
Epidemiologically, adrenal cysts are more common in women and typically are found between the ages of 30 and 60.2 They account for just under 6% of all newly discovered incidentalomas.3 Clinically they are rarely palpable but there is usually tenderness on abdominal examination.
We report the case of a 39-year-old woman who presented as an acute abdomen and was diagnosed with a large unilateral adrenal pseudocyst.
2. Presentation of case
A 39-year-old female with a past medical history of Grave‘s disease was referred by her GP with right sided abdominal pain and swelling that had increased in size over a 4 month period. She described the pain as dull in nature originating in the right upper abdomen. There was no history of trauma or malignancy.
On examination she was found to have a mass in the upper right quadrant that was dull to percussion and tender to palpation. Blood investigations were normal except for a mildly reduced albumin of 28 g/L.
She underwent an abdominal ultrasound scan that demonstrated a large simple cyst measuring 29 cm × 20 cm × 17 cm in the right upper quadrant that appeared to originate in the upper pole of the right kidney. The liver and right kidney were displaced towards the midline.
The cyst was then aspirated under ultrasound guidance and cytology did not show atypical cells. Three weeks later the cyst partially re-accumulated. An MRI scan of the abdomen was then performed and the report confirmed a 10 cm × 7.7 cm × 9 cm cyst with generalised irregular thickening of the wall and with fluid that appeared to be of high signal on T1 suggestive of the presence of blood. The right kidney was found to be displaced inferiorly but of normal size and shape (Fig. 1). The posterior aspect of the right lobe of the liver was distorted. The right adrenal appeared to be splayed by the cyst and was otherwise of normal size and shape. The conclusion was that the cyst was likely to originate from the adrenal or liver.
Fig. 1.
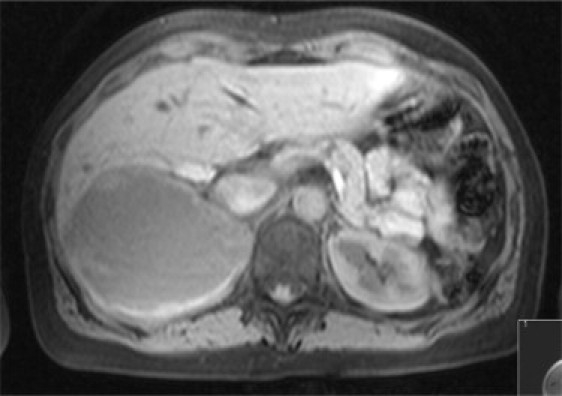
MRI abdomen demonstrating right adrenal cystic mass with a thickened and irregular wall. The posterior aspect of the right lobe of the liver is distorted.
The patient was placed in the French position with opened legs with the surgeon standing between them. Two 10 mm and two 5 mm ports were inserted (Fig. 2). A large cyst intimately related to the posterior aspect of the right lobe of the liver and retrohepatic vena cava, was found to be arising from the lateral part of the right adrenal gland.
Fig. 2.
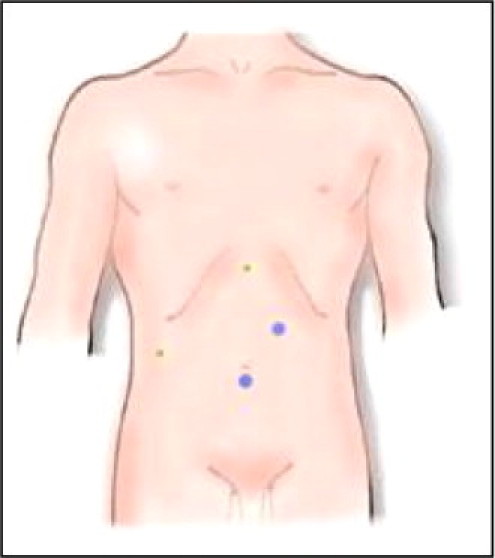
Laparoscopic port sites: 10 mm port sites in blue, 5 mm in green. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
The operation began with mobilising the hepatic flexure of the colon in order to access the inferior pole of the cyst. The cyst was dissected away from the duodenum towards the inferior vena cava and was separated from the liver to which it was very adherent. A harmonic scalpel was used to achieve this. Although it was clear that the cyst was originating from a normally looking adrenal, it was decided that the adrenal gland should be excised, given the unclear nature of the cyst.The right adrenal vein was identified and clipped. The dissection was finally completed from the posterior aspect of the cyst towards the posterior abdominal wall and the specimen removed in a 15 mm Endocatch bag and extracted through one of the 10 mm ports.
The operative time was about 1 h and 20 mins with around 200 ml of blood loss. Post-operatively, the patient made an uncomplicated recovery and was discharged on day 3.
The histopathological report confirmed an irregular peritonealised collapsed cyst approx 12 cm × 11 cm × 4.5 cm in size. The cyst was unilocular with an irregular internal surface and a tough white wall of variable thickness.
Microscopically, then cyst appeared to arise in continuity with the adrenal cortex and medullary tissues. The wall was found to contain mostly bland fibrous tissue with some smooth muscle and no epithelial lining. The lining was composed of vacuolated fibrinoid material focally with lightly pigmented foamy macrophages. There was no evidence of malignancy and no extramedullary haematopoiesis was seen (Fig. 3).
Fig. 3.
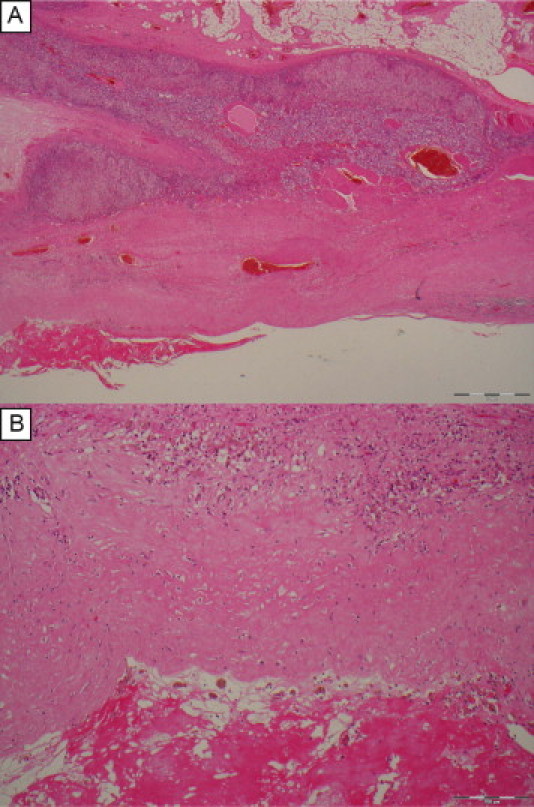
Histopathology of the adrenal pseudocyst. (A) Low power view showing adrenocortical tissue above and fibromuscular cyst wall below with fibrinoid lining lower left. (B) Higher magnification of the cyst wall showing variably cellular fibrous tissue above and fibrinoid lining material below with pigmented macrophages (siderophages) inbetween indicative of previous haemorrhage. Note the lack of an epithelial lining.
On 6 month follow up the patient remained asymptomatic and was discharged from clinic.
3. Discussion
Given the increased availability of CT scanning, adrenal pseudocysts are now being encountered at higher levels than previously.4
However, adrenal pseudocysts are still rare and account for 32–80% of all adrenal cysts.5 Only 7% of all reported adrenal pseudocysts are malignant or potentially malignant and the risk increases with size, in particular if over 6 cm.3,6
Commonly adrenal pseudocyst will present to the surgeon once they have reached a significantly large size as to cause pressure effects on nearby organs. Epigastric pain, nausea and vomiting are typical symptoms and a palpable mass on examination may be found.7,8 They rarely cause adrenal hypofunction, Cushing's syndrome or pheochromocytoma.8
Following a thorough history and examination of the patient, laboratory blood investigations to include full blood count, liver function tests, renal function, serum cortisol, aldosterone, calcium and urinary catecholamines, 5-HIAA and metanephrines is required.
Although USS, MRI and CT scan can all be used to evaluate abdominal cysts, CT scanning is the gold standard for adrenal masses being able to identify small tumours with 100% sensitivity.9,10 The CT appearance of adrenal pseudocysts are typically unilocular or multilocular cystic lesions, but they can also appear to be mixed or solid masses and may also demonstrate areas of central calcification which can mimic adrenal tumours.4
MRI scanning enables excellent anatomical detail and can often differentiate pseudocysts from malignancy.11,12
The literature appears to favour surgical excision of all lesions greater than 5 cm, any suspicion of malignancy or if the tumour is hormonally active.11,13 However, there is evidence to recommend surgical excision of adrenal tumours 4 cm in size.14 Patients with smaller tumours less than 4 cm should have repeat CT scan at 3 months after diagnosis and should be monitored for 18 months.3,15
4. Conclusion
This case report has demonstrated that even large adrenal cysts are amenable to laparoscopic resection. The literature on laparoscopic versus open adrenalectomy appears to favour the laparoscopic approach regardless of tumour size.3,5,6,16 An evaluation of 242 laparoscopic adrenalectomies over a 15 year period in one centre, demonstrated a low complication rate, low conversion rate and rapid functional recovery.17 Furthermore, there appears to be no significant difference in outcome for all tumour sizes.18
This case report of a large rare adrenal pseudocyst (10 cm × 7.7 cm × 9 cm) provides further evidence and support to the laparoscopic resectability of such large adrenal masses.
Funding
None.
Conflict of interest statement
None.
Author contributions
Mr. Atheer B. Ujam MBBS, BDS, MFDS, MRCS contributed to data collection, data analysis and writing. Mr. Christopher J. Peters MBChB, BSc, MRCS contributed to data analysis and editing. Dr Paul J. Tadrous, MBBS, MSc, PhD, FRCPath contributed to data analysis, diagnosis of pathology and report. Mr John Jeff Webster MBBS, FRCS contributed to data analysis, diagnosis of disease and editing surgical aspects of report. Dr Keith Steer MBBS, PhD contributed to editing and revising report critically for important intellectual content. Mr Alberto Martinez-Isla MBBS, MSc, FRCS contributed to editing and critically revising report, head surgeon.
References
- 1.Carvounis E. Vascular adrenal cysts a brief review of the literature. Arch Pathol Lab Med. 2006;130(November):1722–1724. doi: 10.5858/2006-130-1722-VACABR. [DOI] [PubMed] [Google Scholar]
- 2.Wieneke JA, Thompson LDR. Non-neoplastic lesions of the adrenal gland. In: Thompson LDR, editor. Endocrine pathology. In: Goldblum JR, series editor. Foundations in diagnostic pathology, vol. 5. Philadelphia, PA: Churchill Livingstone Elsevier; 2006. p. 183–204.
- 3.Aloraifi F., O‘Brien G., Broe P. Giant adrenal pseudocyst treated laparoscopically: case report and review of the literature. Open Surg J. 2008;2:39–42. [Google Scholar]
- 4.Wang L.J., Wong Y.C., Chen C.J. Imaging spectrum of adrenal pseudocysts on CT. Eur Radiol. 2003;13:531–535. doi: 10.1007/s00330-002-1537-5. [DOI] [PubMed] [Google Scholar]
- 5.Kim B.S., Joo S.H., Choi S.I. Laparoscopic resection of an adrenal pseudocyst mimicking a retroperitoneal mucinous cystic neoplasm. World J Gastroenterol. 2009;15(June (23)):2923–2926. doi: 10.3748/wjg.15.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kar M., Pucci E., Brody F. Laparoscopic resection of an adrenal pseudocyst. J Laparoendosc Adv Surg Tech A. 2006;16(October (5)):478–481. doi: 10.1089/lap.2006.16.478. [DOI] [PubMed] [Google Scholar]
- 7.Papaziogas B., Katsikas B., Psaralexis K. Adrenal pseudocyst presenting as acute abdomen during pregnancy. Acta Chir Belg. 2006;106:722–725. doi: 10.1080/00015458.2006.11679993. [DOI] [PubMed] [Google Scholar]
- 8.Demir A., Tanidir Y., Kaya H. A giant adrenal pseudocyst – case report and review of the literature. Int Urol Nephrol. 2006;38(1):167–169. doi: 10.1007/s11255-005-3422-z. [DOI] [PubMed] [Google Scholar]
- 9.Yip L., Tublin M.E., Falcone J.A. The adrenal mass: correlation of histopathology with imaging. Ann Surg Oncol. 2010;17(March (3)):846–852. doi: 10.1245/s10434-009-0829-2. [Epub 2009 December 4] [DOI] [PubMed] [Google Scholar]
- 10.Sroujreh A.S., Farah G.R., Haddad M.J. Adrenal cyst: diagnosis and treatment. Br J Urol. 1990;65:570–575. doi: 10.1111/j.1464-410x.1990.tb14822.x. [DOI] [PubMed] [Google Scholar]
- 11.Wedmid A., Palese M. Diagnosis and treatment of the adrenal cyst. Curr Urol Rep. 2010;11(February (1)):44–50. doi: 10.1007/s11934-009-0080-1. [DOI] [PubMed] [Google Scholar]
- 12.Elsayes K.M., Mukundan G., Narra V.R. MR imaging features with pathologic correlation. RadioGraphics. 2004;(October (24)):S73–S86. doi: 10.1148/rg.24si045514. [DOI] [PubMed] [Google Scholar]
- 13.Kasperlik-Zeluska A.A., Rosłonowska E., Słowinska-Srzednicka J. Incidentally discovered adrenal mass (incidentaloma): investigation and management of 208 patients. Clin Endocrinol (Oxf) 1997;46(January (1)):29–37. doi: 10.1046/j.1365-2265.1997.d01-1751.x. [DOI] [PubMed] [Google Scholar]
- 14.Herrera M.F., Grant C.S., van Heerden Incidentally discovered adrenal tumors: an institutional perspective. Surgery. 1991;110(December (6)):1014–1021. [PubMed] [Google Scholar]
- 15.Rao A, Majmudar B, Bumpers H. Giant adrenal pseudocyst. http://www.hcplive.com/publications/surgical-rounds/2007/2007-05/2007-05_07.
- 16.Sharma R., Ganpule A., Veeramani M. Laparoscopic management of adrenal lesions larger than 5 cm in diameter. Urol J. 2009;6(Fall (4)):254–259. [PubMed] [Google Scholar]
- 17.Kazaryan A.M., Marangos I.P., Rosseland A.R. Laparoscopic adrenalectomy: Norwegian single-center experience of 242 procedures. J Laparoendosc Adv Surg Tech A. 2009;19(April (2)):181–189. doi: 10.1089/lap.2008.0286. [DOI] [PubMed] [Google Scholar]
- 18.Parnaby C.N., Chong P.S., Chisholm L. The role of laparoscopic adrenalectomy for adrenal tumours of 6 cm or greater. Surg Endosc. 2008;22(March (3)):617–621. doi: 10.1007/s00464-007-9709-7. [Epub 2007 December 11] [DOI] [PubMed] [Google Scholar]


