Abstract
Background
Arcanobacterium haemolyticum is an emerging human pathogen that causes pharyngitis, wound infections, and a variety of occasional invasive diseases. Since its initial discovery in 1946, this Gram positive organism has been known to have hemolytic activity, yet no hemolysin has been previously reported. A. haemolyticum also displays variable hemolytic activity on laboratory blood agar that is dependent upon which species the blood is derived.
Results
Here we describe a cholesterol-dependent cytolysin (CDC) secreted by A. haemolyticum, designated arcanolysin (aln), which is present in all strains (n = 52) tested by DNA dot hybridization. Among the known CDCs, ALN is most closely related to pyolysin (PLO) from Trueperella (formerly Arcanobacterium) pyogenes. The aln probe, however, did not hybridize to DNA from T. pyogenes. The aln open reading frame has a lower mol %G+C (46.7%) than the rest of the A. haemolyticum genome (53.1%) and is flanked by two tRNA genes, consistent with probable acquisition by horizontal transfer. The ALN protein (~ 64 kDa) contains a predicted signal sequence, a putative PEST sequence, and a variant undecapeptide within domain 4, which is typically important for function of the toxins. The gene encoding ALN was cloned and expressed in Escherichia coli as a functional recombinant toxin. Recombinant ALN had hemolytic activity on erythrocytes and cytolytic activity on cultured cells from human, rabbit, pig and horse origins but was poorly active on ovine, bovine, murine, and canine cells. ALN was less sensitive to inhibition by free cholesterol than perfringolysin O, consistent with the presence of the variant undecapeptide.
Conclusions
ALN is a newly identified CDC with hemolytic activity and unique properties in the CDC family and may be a virulence determinant for A. haemolyticum.
Background
Arcanobacterium haemolyticum, a Gram positive, pleomorphic rod, causes wound infections and pharyngitis and can occasionally cause more severe invasive diseases such as endocarditis, meningitis, septic arthritis, pneumonia and osteomyelitis in humans [1]. There is strong epidemiologic evidence for A. haemolyticum being the only or primary isolate from throat specimens of some humans with pharyngitis [1-4] and these data suggest that the number of cases per year of A. haemolyticum-mediated pharyngitis is ~240,000-480,000 with 0.5-1 million lost work days in the United States. The organism, previously in the Corynebacterium genus, was classified as the first member of the genus Arcanobacterium [5]. The other members of the genus are uncommonly isolated and remain largely uncharacterized, with the exception of Trueperella (Arcanobacterium) pyogenes, which is an important opportunistic livestock pathogen [6].
Little is known about A. haemolyticum virulence factors with the exception of a phospholipase D (PLD) [7], which causes dermonecrosis [8]. We recently described the ability of PLD to reorganize host membrane lipid rafts, leading to enhanced bacterial adhesion [9]. Furthermore, A. haemolyticum was able to invade HeLa cells and once intracellular, PLD was able to kill host cells via direct necrosis [9]. These effects could potentially lead to bacterial dissemination to deeper tissues.
It is thought that clinical microbiology laboratories often miss A. haemolyticum in clinical specimens due to the organism's weak hemolytic activity on the commonly-used sheep blood agar, and therefore it may be misinterpreted as commensal diphtheroids and the isolate discarded. However, this organism displays more pronounced hemolysis on human and rabbit blood [10,11]. The organism has been known to have hemolytic activity since its initial discovery in 1946 [12], yet no bona fide hemolysin has been previously reported. PLD itself is not directly hemolytic, but causes synergistic hemolysis with bacteria that express cholesterol oxidase [13], prompting a search for the A. haemolyticum hemolysin. Possible clues to the identity of the A. haemolyticum hemolysin come from studies on the hemolytic bacterium T. pyogenes, which is closely related to A. haemolyticum. T. pyogenes expresses PLO, a member of the cholesterol-dependent cytolysin (CDC) toxin family, as its primary virulence factor and this molecule is a hemolysin [14]. Thus, we hypothesized that the hemolytic activity expressed by A. haemolyticum was due to the presence of an uncharacterized CDC.
Here we report the identification and characterization of a CDC from A. haemolyticum, designated arcanolysin (ALN). We show that ALN has several distinct structural features among the CDC family and demonstrate that ALN is cholesterol-dependent and provide evidence that ALN has variable hemolytic and cytotoxic activity against mammalian cells from different species. We propose ALN is the long, sought-after hemolysin.
Methods
Bacteria and growth conditions
ATCC 9345 is the A haemolyticum type strain. The other A. haemolyticum strains used in this study were archival isolates obtained from diverse human clinical cases (Table 1). A. haemolyticum and Escherichia coli strains were grown as previously described [9].
Table 1.
Arcanobacterium strains used in this study.
| Strain (all A. haemolyticum except as noted) |
Relevant characteristics | Source |
|---|---|---|
| AhS1 | Biotype S*; wound infection; 73 year old male; 1991 | Petteri Carlson |
| AhS2 | Biotype S; paronychia; 16 year old male; 1991 | Petteri Carlson |
| AhS3 | Biotype S; wound infection; 11 year old male; 1991 | Petteri Carlson |
| AhS4 | Biotype S; infected leg ulcer; 47 year old male; 1991 | Petteri Carlson |
| AhS5 | Biotype S; wound infection; 64 year old male; 1991 | Petteri Carlson |
| AhS6 | Biotype S; wound infection; 43 year old male; 1991 | Petteri Carlson |
| AhS7 | Biotype S; infected leg ulcer; 68 year old female; 1991 | Petteri Carlson |
| AhS8 | Biotype S; wound infection; 62 year old male; 1991 | Petteri Carlson |
| AhS9 | Biotype S; wound infection; 38 year old male; 1991 | Petteri Carlson |
| AhS10 | Biotype S; paronychia; 21 year old male; 1991 | Petteri Carlson |
| AhS11 | Biotype S; pharyngitis; 3 year old male; 1991 | Petteri Carlson |
| AhS12 | Biotype S; pharyngitis; 23 year old female; 1992 | Petteri Carlson |
| AhS13 | Biotype S; pharyngitis; 28 year old female; 1992 | Petteri Carlson |
| AhS14 | Biotype S; pharyngitis; 23 year old female; 1992 | Petteri Carlson |
| AhS15 | Biotype S; pharyngitis; 20 year old male; 1992 | Petteri Carlson |
| AhS16 | Biotype S; sinusitis; 41 year old male; 1990 | Petteri Carlson |
| AhS17 | Biotype S; sinusitis; 65 year old female; 1991 | Petteri Carlson |
| AhS18 | Biotype S; pharyngitis; 12 year old male; 1992 | Petteri Carlson |
| AhS19 | Biotype S; pharyngitis; 20 year old female; 1992 | Petteri Carlson |
| AhS20 | Biotype S; pharyngitis; 34 year old male; 1992 | Petteri Carlson |
| AhS21 | Biotype S; peritonsillar abscess; 15 year old male; 1996 | Petteri Carlson |
| AhS22 | Biotype S; pharyngitis, pneumonia; 42 year old male; 1996 | Petteri Carlson |
| AhS23 | Biotype S; diabetic foot gangrene; 45 year old male; 1997 | Petteri Carlson |
| AhS24 | Biotype S; tonsillitis; 16 year old female; 1998 | Petteri Carlson |
| AhS25 | Biotype S; metatarsal osteitis; 37 year old male; 1998 | Petteri Carlson |
| AhR26 | Biotype R; wound infection; 43 year old male; 1991 | Petteri Carlson |
| AhR27 | Biotype R; wound infection; 53 year old male; 1991 | Petteri Carlson |
| AhR28 | Biotype R; pharyngitis; 13 year old female; 1991 | Petteri Carlson |
| AhR29 | Biotype R (uncertain); peritonsillar abscess; 18 year old male; 1991 | Petteri Carlson |
| AhR30 | Biotype R; sinusitis; 14 year old male; 1992 | Petteri Carlson |
| AhR31 | Biotype R; peritonsillar abscess; 21 year old male; 1986 | Petteri Carlson |
| AhR32 | Biotype R; peritonsillar abscess; 15 year old female; 1992 | Petteri Carlson |
| AhR33 | Biotype R; pharyngitis; 26 year old male; 1992 | Petteri Carlson |
| AhR34 | Biotype R; pharyngitis; 15 year old male; 1992 | Petteri Carlson |
| AhR35 | Biotype R; pharyngitis; 18 year old male; 1992 | Petteri Carlson |
| AhR36 | Biotype R; pharyngitis; 21 year old male; 1992 | Petteri Carlson |
| AhR37 | Biotype R; peritonsillar abscess; 15 year old female; 1992 | Petteri Carlson |
| AhR38 | Biotype R; wound infection; 21 year old female; 1992 | Petteri Carlson |
| AhR39 | Biotype R; pharyngitis; 18 year old female; 1992 | Petteri Carlson |
| AhR40 | Biotype R; pharyngitis; 17 year old male; 1992 | Petteri Carlson |
| AhR41 | Biotype R; pharyngitis; 24 year old male; 1992 | Petteri Carlson |
| AhR42 | Biotype R; pharyngitis; 16 year old female; 1992 | Petteri Carlson |
| AhR43 | Biotype R; pharyngitis; 12 year old male; 1992 | Petteri Carlson |
| AhR44 | Biotype R; pharyngitis; 18 year old male; 1992 | Petteri Carlson |
| AhR45 | Biotype R; pharyngitis; 16 year old male; 1992 | Petteri Carlson |
| AhR46 | Biotype R; pharyngitis; 14 year old female; 1992 | Petteri Carlson |
| AhR47 | Biotype R; pharyngitis; 13 year old female; 1991 | Petteri Carlson |
| AhR48 | Biotype R; pharyngitis; 15 year old male; 1992 | Petteri Carlson |
| AhR49 | Biotype R; pharyngitis; 20 year old male; 1991 | Petteri Carlson |
| AhR50 | Biotype R; pharyngitis; 19 year old female; 1992 | Petteri Carlson |
| CCUG39796 | Biotype unknown; infected leg ulcer; 52 year old male; 1998 | Petteri Carlson |
| ATCC9345; DSM 20595 | Biotype unknown, 1946 | American Type Culture Collection; [12] |
| Trueperella (Arcanobacterium) pyogenes BBR1 | plo nanH nanP cbpA fimAB tet(W); Isolated from a bovine abscess | [14] |
* S, Smooth type; R, rough type.
DNA techniques
E. coli DH5αMCR plasmid DNA extraction, transformation, DNA restriction, ligation and agarose gel electrophoresis were by standard methods [15]. DNA hybridization was performed using the DIG DNA Labeling and Detection Kit (Roche). PCR DNA amplification was performed using Vent DNA polymerase (NEB) for 35 cycles of 1 min at 94°C, 1 min at 50°C and 1 min/kb at 72°C, with a final extension step of 72°C for 7 min.
Nucleotide sequence determination and analysis
Prior to the recent GenBank deposit of the 1.986 MB genome from strain ATCC9345 (= DSM20595 = 11018) [16], we sequenced the same strain to > 20× coverage (454 Life Sciences), with ~1.945 MB of unique sequence (> 98% complete) with essentially identical sequence data. A translated ORF with amino acid similarity to CDCs, Arch_1062, was identified within this sequence. Oligonucleotide primers flanking this ORF were used to amplify the region by PCR. The nucleotide sequence was confirmed by automated DNA sequencing of both strands. The aln sequence data and flanking regions were submitted to the GenBank/EMBL/DDBJ databases under accession number FJ785427.
Database searches were performed using the BlastX and BlastP algorithms [17]. tRNA sequences were identified using the tRNAscan-SE program [18]. Signal sequence prediction was performed using SignalP [19]. Transcriptional terminators were identified using mfold [20]. Multiple sequence alignments were performed using CLUSTAL W [21], and tree construction was with the neighbor-joining algorithm and midpoint rooting, carried out in MacVector version 12.0.3 (MacVector, Inc.). PEST sequence prediction used the pestfind algorithm http://emboss.bioinformatics.nl/cgi-bin/emboss/epestfind.
Cloning and purification of a recombinant, 6xHis tagged-ALN (His-ALN)
The aln gene, without the signal sequence, was amplified from A. haemolyticum ATCC9345 genomic DNA by PCR with His-ALNF (5'-CCCGGCGTTGCGGATCCAGTTGACGC-3') and ALN5 (5'-GGACCTTCTCGAGTATGTATCACTC-3') encoding BamHI and XhoI sites (underlined in the primer sequence), respectively. These primers amplified a 1,669 bp product. The PCR fragment was digested with BamHI-XhoI and cloned into pTrcHisB (Invitrogen), to generate pBJ51, which encoded the 63.7 kDa His-ALN. The final His-ALN translational fusion protein thus has the MWVGSQKHYFFYQDRGKIMTRRFLATVAGTALLAGAFAPGVAFG signal sequence removed and replaced with the sequence from the vector that leads to MGGSHHHHHHGMASMTGGQQMGRDLYDDDDKDP (6 His underlined). No other ALN native amino acids were removed. Cultures for purification of His-ALN were grown and lysed as described [9]. His-ALN was purified from the soluble cell fraction using TALON Metal Affinity Resin, as described (Clontech). His-ALN was eluted from the resin with 50 mM imidazole, 20 mM Tris-HCl, 100 mM NaCl, pH 8.0 (elution buffer). Total protein concentration was determined using Bradford Protein Assay Reagent (Bio-Rad).
For some experiments ALN was amplified from ATCC 9345 DNA using the primers ALN26-F (GCCGCCGCTAGCGTTGACGCTTCAACACAAACCGATCC) and ALN-R (GCCGCCCTCGAGTCACTCGCTATGAACGATGTTCTTG), cloned into expression vector pET28a (Novagen) using NheI and XhoI sites (underlined), and confirmed by sequencing. The plo gene encoding PLO was amplified from T. pyogenes ATCC 49698 DNA using the primers PYO28-F (GCCGCCCATATGGCCGGATTGGGAAACAGTTCG) and PYO-R (GCCGCCCTCGAGCTAGGATTTGACATTTTCCTC), cloned into pET28a using NdeI and XhoI sites (underlined), and confirmed by sequencing. The ily gene encoding ILY was amplified from Streptococcus intermedius and cloned into pET28a as described [22]. Purification of the His-tagged CDCs was as previously described [22,23].
SDS-PAGE and Western blotting
Proteins were separated by electrophoresis in 10% (w/v) SDS-polyacrylamide gels and transferred to nitrocellulose [15]. Western blots were immunostained using rabbit anti-His-ALN (prepared by immunization of a rabbit with His-ALN, Antibodies Inc., Davis, CA) and rabbit anti-goat IgG(H+L)-peroxidase conjugate (KPL), as the primary and secondary antibodies, respectively. Rabbit antiserum against PFO was kindly provided by Rodney K. Tweten, University of Oklahoma Health Sciences Center, OK.
Hemolytic assays
The hemolytic titers of His-ALN preparations were determined by incubation of two-fold serial dilutions of protein with an equal volume of 0.5% blood (Cleveland Scientific, Bath, OH) at 37°C for 1 h [14]. The hemolytic titer was the reciprocal of the highest dilution which resulted in 50% cell lysis, expressed as hemolytic units (HU) [14]. The specific activity of purified His-ALN was determined as HU/μg protein. Thiol activation was assessed by incubation of 5 HU His-ALN with 2% β-mercaptoethanol for 10 min at room temperature, prior to performing a hemolytic assay with human blood (Cleveland Scientific). Cholesterol inhibition was assessed by incubation of 5 HU His-ALN with 0.01-1 μM cholesterol for 30 min at room temperature with shaking, prior to performing a hemolytic assay with human blood. Cholesterol was diluted in absolute ethanol and an equal volume of ethanol was used as the cholesterol-free control. His-tagged perfringolysin O (PFO) [24] and His-tagged PLO [14] were used as controls in the various hemolytic assays. For some experiments hemolysis assays were performed as described [22,23].
Epithelial cell cytotoxicity
The epithelial cell cytotoxicity of His-ALN was determined using the CellTiter 96® Aqueous One Solution Cell Proliferation Assay (Promega). A549 (human lung, CCL-185), CHO (hamster ovary, CCL-61), HCT-8 (human colon, CCL-244), J774A.1 (mouse macrophage, TIB-67), MDBK (bovine kidney, CCL-22), MDCK (canine kidney, CCL-34) and RK-13 (rabbit kidney, CCL-37) cells were cultured in Iscove's Modified Dulbecco's Medium or RMPI 1640 with 10% fetal bovine serum and 10 μg/ml gentamicin in a humidified, 5% CO2 atmosphere at 37°C. Cells were seeded into 96-well plates at 2 × 104 cells/well and incubated for 18 h to achieve 80% confluence. Triplicate wells were incubated with doubling dilutions of His-ALN (0-2000 ng) and incubated for 2 h, prior to addition of substrate for 3 h. Determination of cell viability was performed using the appropriate control values (Promega).
Membrane binding assay
The membrane binding assay was performed using erythrocytes as previously described [25]. His-ALN was diluted to 12.5 μg ml-1 in PBS, 40 μl was added to an equal volume of 50% (v/v) blood and the mixture was incubated on ice for 20 min. Cells were harvested by centrifugation at 14,000 g for 5 min at 4°C, resuspended in SDS-PAGE sample buffer and subjected to SDS-PAGE and Western blotting with antiserum against His-ALN.
Results
Cloning and nucleotide sequence determination of aln
A draft genome sequence of A. haemolyticum ATCC 9345 was determined and consists of 46 contigs that encompass ~1.945 Mb in size (D. J. McGee, S. J. Billington, and B. J. Jost, unpublished). 1,639 ORFs were preliminarily identified using the Rapid Annotation using Subsystem Technology (RAST) Server [26]. Within this sequence, we identified ORF Arch_1062, the translation of which displayed similarity to other CDCs. The 1,710 bp gene was designated aln, for arcanolysin (ALN). Upstream of aln are a phosphoglycerate mutase gene (pgm; Arch_1063) (EC 5.4.2.1) and an alanine tRNAGGC (Figure 1). In the 426 bp intergenic region are regulatory signals predicted to be involved in aln transcription, including a putative σ70 promoter and 3 direct repeats (ATTTT(G/C)(G/T)T) which are similar to those found immediately upstream of plo, encoding PLO, the CDC of T. pyogenes [27]. 6 bp downstream of aln is a transcriptional terminator with a ΔG = -18.05 kcal/mol. Downstream of aln and divergently transcribed is Arch_1061. The Arch_1061 protein displays amino acid similarity to hypothetical proteins from a number of genome sequences, including Corynebacterium jeikeium (GenBank YP_249820.1), and features a signal sequence. Further downstream is an additional alanine tRNACGC, which is 91% identical at the nucleotide level to the alanine tRNAGGC upstream of aln. Further downstream of the 2nd alanine tRNA is Arch_1060, a gene that is predicted to encode a conserved hypothetical protein related to Corynebacterium diphtheriae (DIP0761), and a gene, Arch_1059 (ubiE), with similarity to type II or SAM-dependent methyltransferases (EC 2.1.1.-).
Figure 1.
Map of the A. haemolyticum aln region and presence of aln in clinical isolates. (a) Map of the aln gene region of strain ATCC 9345 (= DSM20595 = 11018). The open arrows indicate the gene and the direction of transcription. Gene names are given and the number indicates the %G+C of the gene. A bar indicating 1 kb is shown on the right. (b) DNA dot hybridization of genomic DNA from A. haemolyticum strains with an aln-specific probe. Genomic DNA from 52 A. haemolyticum isolates and T. pyogenes BBR1, as a negative control (~500 ng each), was spotted onto a nylon membrane and hybridized with aln-specific probe under high stringency conditions. A. haemolyticum ATCC9345 DNA is in the second from last spot. T. pyogenes BBR1 DNA is in the last spot.
The %G+C for aln is 46.7% (Figure 1) compared with 49.7-60.3% for the surrounding genes and 53.1% for the entire genome. Given the lower %G+C of the aln gene and the presence of flanking tRNA genes, which can act as sites of foreign gene insertion [28], it is possible that the A. haemolyticum aln gene was acquired by horizontal gene transfer.
aln is widely distributed in A. haemolyticum isolates
The prevalence of the aln gene was determined by DNA hybridization. A DIG-labeled probe spanning bases 492-1,052 of the aln ORF was hybridized to genomic DNA from A. haemolyticum ATCC9345, 51 A. haemolyticum clinical isolates (Table 1) and T. pyogenes BBR1, as a negative control. The aln probe hybridized at high stringency to all A. haemolyticum isolates (n = 52), but not T. pyogenes genomic DNA (Figure 1b), indicating that this gene appears to be highly prevalent in A. haemolyticum. The region of aln from which the probe was derived has 62.8% identity to the corresponding nucleotide region in plo of T. pyogenes. Under high stringency hybridization conditions, DNA sequences which are less than 70% identical do not hybridize.
Analysis of the primary structure of ALN
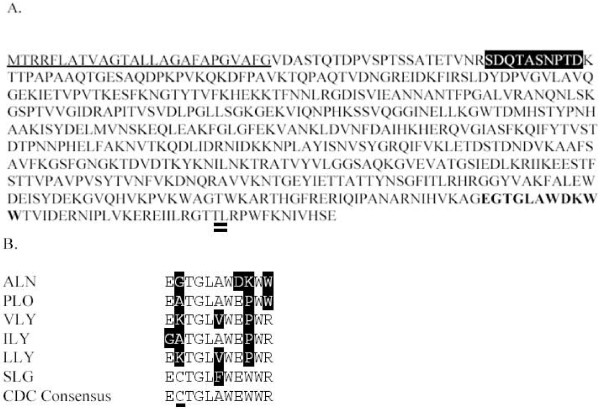
The predicted ALN protein is 569 amino acids in length, including a 26 amino acid signal sequence predicted by SignalP. The mature protein lacking the signal sequence has a predicted molecular mass of 60.1 kDa. ALN is most similar to PLO with 59.4% and 71.5% amino acid identity and similarity (Figure 2) and has ~50% similarity to other CDC family members. Within the ALN N-terminus, the pestfind algorithm identified a putative PEST sequence not present in PLO or most other CDC sequences (Figure 3a). Listeriolysin O (LLO), which contains a bona fide PEST sequence [29], returned a pestfind score of 4.71, while ALN had a score of 7.58, indicating a higher probability of containing a functional PEST sequence. Given that A. haemolyticum invades host cells [9], it is possible that the PEST sequence allows for a similar compartmentalization of ALN activity within the host cell. Like PLO, the predicted amino acid sequence of ALN has a variant undecapeptide in domain 4 and both lack the conserved cysteine residue (Figure 3b). The tryptophan spacing of ALN and PLO (WxxWW) also differs from the consensus sequence (WxWW) (Figure 3b).
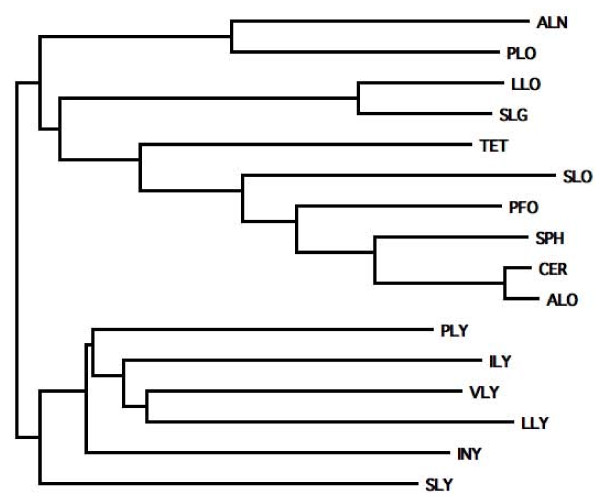
Figure 2.
Neighbor joining tree of amino acid sequences showing the relationship of ALN to other selected CDC family members. Abbreviations and gi ascession numbers from the NCBI protein database: ALN, arcanolysin (259156857); PLO, pyolysin (2252800); LLO, listeriolysin O (16802248); SLG seeligeriolysin (40889013); TET, tetanolysin (28211522); SLO, streptolysin O (15674372); PFO, perfringolysin O (18309145); SPH, sphaericolysin (146455206); CER, cereolysin (62550724); ALO, anthrolysin O (49186114); PLY, pneumolysin (15901747); ILY, intermedilysin (6729344); VLY, vaginolysin (187940699); LLY, lectinolysin (190576835); INY, inerolysin (259167149); SLY, suilysin (253752120).
Figure 3.
Salient features of the ALN predicted amino acid sequence. (a) ALN sequence with predicted signal sequence (underlined), putative PEST motif (inverse), undecapeptide (bold), and cholesterol-interacting TL motif (double underlined). (b) Undecapeptide sequences of ALN, other CDC undecapeptides known to differ from consensus, and the consensus CDC undecapeptide. The cysteine conserved in thiol-activated CDCs (but absent from ALN) is underlined in the consensus sequence. Differences from consensus depicted as inverse letters. Abbreviations as in Figure 2.
Cloning and expression of His-ALN
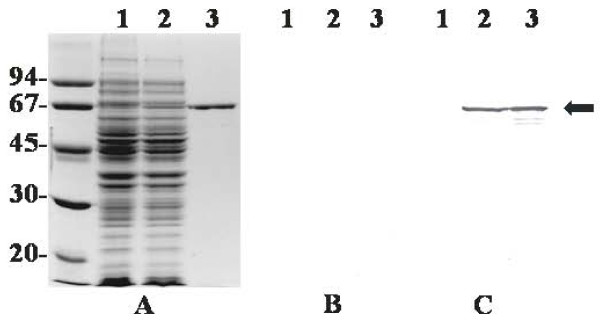
SDS-PAGE and Coomassie Brilliant Blue staining of IPTG-induced cultures of pBJ51-containing E. coli indicated the presence of an over-expressed protein of ~64 kDa (Figure 4a). His-ALN was purified to > 95% homogeneity using TALON resin (Figure 4a), and the size of this protein (~64 kDa) corresponded to its predicted molecular mass. Antiserum against ALN, but not pre-immune antiserum, reacted specifically with His-ALN and some possible HIS-ALN degradation products (Figure 4b and 4c).
Figure 4.
Overexpression and purification of His-ALN. Whole-cell lysates of IPTG-induced cultures of DH5αMCR(pTrcHis B) (lane 1) and DH5αMCR (pBJ51) (lane 2) and 500 ng purified His-ALN (lane 3) were subjected to SDS-PAGE. Separated proteins were stained with Coomassie brilliant blue (a) or were transferred to nitrocellulose by Western blotting and immunostained with 1/5000 rabbit pre-immune serum (b) or rabbit anti-His-ALN (c). The position of the ~64 kDa His-ALN band is indicated by the arrow. Molecular mass markers (kDa) are indicated on the left.
Recombinant ALN has cytotoxic activity
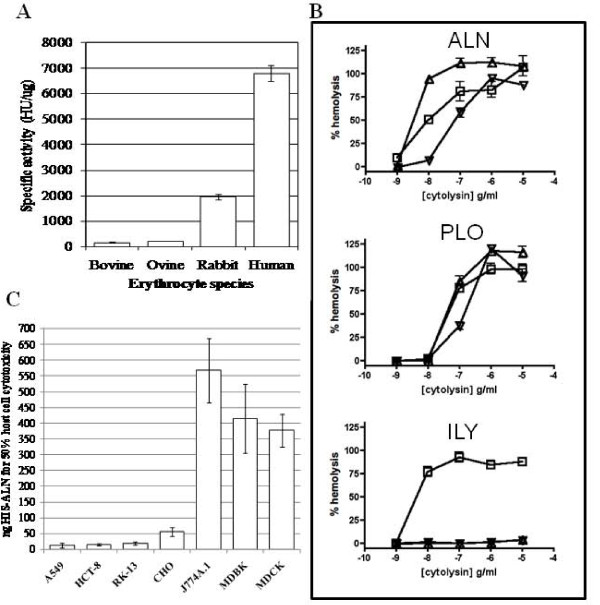
A. haemolyticum is not strongly hemolytic when grown on ovine (sheep) blood agar [10]. Likewise, the E. coli strain expressing His-ALN did not display hemolysis when grown on bovine blood agar (data not shown). Similarly, His-ALN displays low hemolysis with bovine or ovine erythrocytes (Figure 5a). In contrast, His-ALN had ~4- and 10-fold increased hemolytic activity on rabbit and human erythrocytes, respectively (Figure 5a). This is in contrast to PFO or PLO, which show little difference in specific activity on erythrocytes from different hosts. Consistent with these findings, hemolysis assays demonstrated that ALN has a preference for horse or human cells over porcine cells but lyses all of these at high toxin concentrations (Figure 5b). This is in contrast to intermedilysin (ILY) from Streptococcus intermedius, which retains human-specific tropism over a wide concentration range, and PLO, which is less selective than ALN (Figure 5b).
Figure 5.
ALN has differential activity on cells from various mammalian species. (a) The specific activities of ALN were determined by incubation of dilutions of His-ALN with erythrocytes from different host species. Results are an average of at least three independent experiments conducted in duplicated and error bars represent standard deviation. (b) The species selectivity of ALN was compared to ILY and PLO in hemolysis assays using human (square), horse (triangle), and pig (inverted triangle) erythrocytes. Representative of two experiments conducted in triplicate and error bars represent standard error of the mean. (c) Dilutions of His-ALN were added to cultured host cells and the amount of ALN required to reduce the cell viability by 50% was determined using the CellTiter 96® Aqueous One Solution Cell Proliferation Assay (Promega). Error bars indicate one standard deviation from the mean calculated from the averages of at least three independent experiments conducted in triplicate.
The highly-conserved Cys residue in the undecapeptide of CDCs is responsible for Thiol activation of this group of toxins [30]. ALN lacks the Cys residue in the undecapeptide (Figure 3a), and like PLO [14], its activity was unaffected by treatment with β-mercaptoethanol (data not shown).
We also determined the effect of recombinant ALN on cultured mammalian cells. His-ALN was applied to human, bovine, canine, hamster, mouse and rabbit cell lines and was highly active on human and rabbit cells (Figure 5c), with low activity on bovine, mouse and canine cells. This toxin had intermediate activity on hamster cells (Figure 5c). This finding mirrors the activity of ALN on blood from different host species (Figure 5a), and is less species-specific than intermedilysin (ILY) or vaginolysin (VLY) [23,31]. ILY, VLY, and lectinolysin (LLY) use human CD59 (hCD59) as a membrane receptor [23,32,33], leading to host-specificity. Unlike these other CDC toxins ALN hemolysis was not blocked with a monoclonal antibody against hCD59 (data not shown). Consistent with this finding, the predicted ALN amino acid sequence lacks the Tyr-X-Tyr-X14-Ser-Arg signature motif common to all known hCD59-dependent CDCs [33].
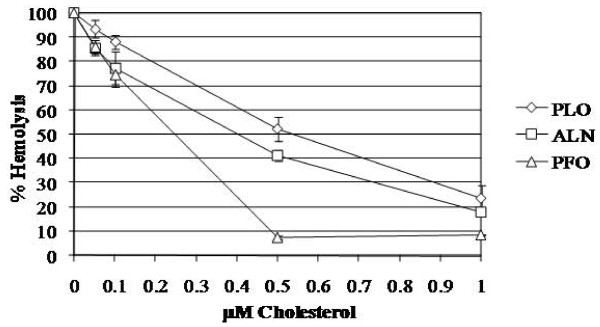
The activity of ALN is less sensitive to cholesterol inhibition than PFO
Given the more restrictive host species preference of ALN over that of PFO, along with the variant undecapeptide sequence in ALN, we hypothesized that ALN might be less sensitive to inhibition by free cholesterol. As expected, PFO activity was almost completely inhibited by exogenous 0.5 μM cholesterol (7.6%; Figure 6). In contrast, PLO and ALN retained 52.5% and 41.4% activity, respectively, when incubated with 0.5 μM cholesterol and retained ~20% of hemolytic activity at 1 μM cholesterol (Figure 6). These data indicate that ALN and PLO have intermediate sensitivity to cholesterol compared to a CDC (PFO) with the conserved undecapeptide sequence.
Figure 6.
ALN, a cholesterol-dependent cytolysin, has hemolytic activity that is less sensitive to cholesterol inhibition than PFO. His-tagged CDCs were preincubated with dilutions of cholesterol for 30 min at room temperature prior to hemolytic assay. Abbreviations as in Figure 2. Error bars indicate one standard deviation from the mean calculated from the averages of three independent experiments conducted in triplicate.
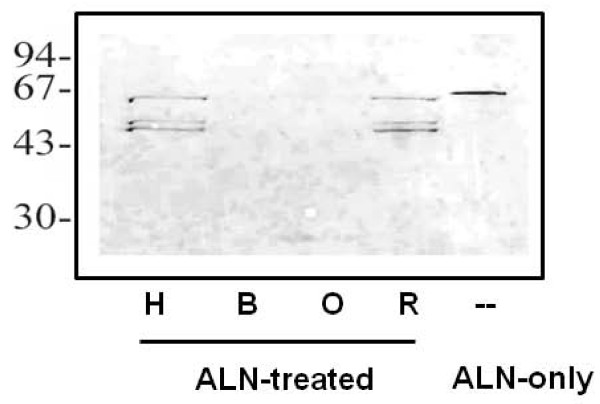
ALN binds differentially to host cell membranes
Hemolytic assays measure the full spectrum of CDC binding, oligomerization and pore formation leading to cell lysis. However, initial toxin binding to membranes can be determined by incubation of CDCs with host cells at 4°C, which prevents subsequent oligomerization and pore formation [34]. Using this approach, His-ALN bound to human and rabbit erythrocytes as determined by Western blotting (Figure 7). Probable ALN degradation products were also detected. His-ALN did not exhibit detectable binding to bovine or ovine erythrocyte membranes under these conditions. As a control, His-PFO was incubated with human, bovine, ovine or rabbit erythrocytes, and bound toxin was detected with anti-PFO antiserum. His-PFO bound to all cell types at approximately equivalent amounts (data not shown). These data suggest that ALN host preference may occur at the initial contact of the toxin with the host cell membrane.
Figure 7.
ALN has a differential ability to bind to erythrocyte cell membranes from different host species. His-ALN (500 ng) or buffer (negative control) was added to erythrocytes, and the mixture was incubated on ice for 20 min. Untreated (no reactivity, data not shown) or ALN-treated erythrocyte membrane fractions from human (H), bovine (B), ovine (O) or rabbit (R) blood were separated by SDS-PAGE, transferred to nitrocellulose, and immunostained with 1/1000 rabbit anti-His-ALN. His-Aln (500 ng) in absence of erythrocyte membrane fractions (ALN) serves as the positive control. Molecular mass markers (kDa) are indicated on the left.
Discussion
The CDCs are a family of bacterial toxins produced by diverse Gram-positive bacteria and are generally important in pathogenesis [35-37]. CDCs have a four-domain structure and a conserved C-terminal undecapeptide sequence in domain 4 that is important for toxin function. Soluble CDC monomers bind to host membrane targets, oligomerize into a large homomeric structure known as the prepore complex, and transition to a true pore, leading to cytolysis of target cells [38]. CDCs interact with membrane cholesterol through a conserved threonine-leucine pair in domain 4, and this interaction is crucial to the formation of functional pores [39]. Some CDCs, including ILY, VLY, and LLY, require the presence of hCD59 as a membrane receptor, conferring human-specific activity [23,33,40]. Among the CDCs, PLO is unusual, as it contains a variant of the highly conserved domain 4 undecapeptide, and this divergent sequence is essential for full PLO activity [41]. The ALN undecapeptide is most similar to that of PLO (Figure 3B), in that it retains the three tryptophan residues of the consensus undecapeptide but employs an alternate spacing (i.e. WxxWW rather than WxWW). The tryptophan residues of the undecapeptide are known to be important for insertion of domain 4 into host cell membranes [42]. Like the human-specific CDCs (VLY, ILY, and LLY), ALN contains a proline in its undecapeptide sequence. However, the hemolytic activity of ALN was not blocked by antibodies to human CD59, which acts as a receptor for the human-specific CDCs [23,32,33], suggesting that ALN may interact with a distinct membrane receptor, perhaps in addition to cholesterol. The nature of the ALN receptor is currently unknown and is under investigation. Although the cysteine residue in the consensus undecapeptide confers the property of thiol activation to CDCs, the cysteine is not essential for streptolysin O and pneumolysin toxin function [43,44]. The human-specific CDCs (VLY, ILY, LLY), PLO, and ALN all lack this conserved cysteine residue, but the contribution of this sequence variation to toxin function is not yet known for these toxins.
Some CDCs have a number of functions beyond simple pore formation. Streptococcus pyogenes uses streptolysin O to introduce a bacterial effector into host cells via a novel mechanism termed cytolysin-mediated translocation (CMT) [45]. At sublytic concentrations, CDCs may act as ligands for toll-like receptors [46,47] and may induce a cycle of p38 mitogen-activated protein kinase (MAPK) phosphorylation and dephosphorylation [48,49]. LLO allows Listeria monocytogenes to escape from the vacuole into the cytoplasm where the organism can rapidly multiply [50]. The site-specific nature of LLO is controlled by cytosolic down-regulation of LLO function due to an N-terminal PEST-like sequence, which usually targets eukaryotic proteins for cytosolic degradation. The PEST sequence results in a substantially reduced half-life of LLO in the cytoplasm of the host cell [29].
Conclusions
ALN has several unique features among the CDC family. ALN has a variant undecapeptide and possesses an unusual N-terminal extension, with a putative PEST sequence. Moreover, ALN lacks the conserved cysteine of thiol-activated CDCs, explaining why β-mercaptothanol had no effect on ALN function. The unique sequences and predicted structural features of ALN will make it an interesting toxin to conduct future structure-function analyses to identify additional unique properties of this toxin. ALN displays an unusual pattern of target cell species selectivity, with high activity against human, horse, and rabbit cells and lesser activity against cells derived from other species. This selectivity appears to function at the level of membrane binding and may contribute to the host range of A. haemolyticum. Further work will focus on understanding the role of ALN in A. haemolyticum pathogenesis.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
BHJ, EAL and AJR designed and conducted the experiments and analyzed data, BHJ drafted the manuscript, AJR, SJB and DJM revised the manuscript and figures. All authors read and approved the final manuscript.
Contributor Information
B Helen Jost, Email: bhjost@cox.net.
Erynn A Lucas, Email: lucase@yosemite.edu.
Stephen J Billington, Email: stephen.billington@ventana.roche.com.
Adam J Ratner, Email: ar127@columbia.edu.
David J McGee, Email: dmcgee@lsuhsc.edu.
Acknowledgements
The authors thank Petteri Carlson, University of Helsinki for providing the A. haemolyticum isolates, and Maricela V. Pier and Andrew E. Clark, University of Arizona for technical assistance. Support for this work was provided by USDA Hatch ARZT-136828-H-02-129, the College of Agriculture and Life Sciences, University of Arizona to BHJ, National Institutes of Health R01-AI092743 to AJR, and start-up funds from LSU Health Sciences Center-Shreveport to DJM.
References
- Linder R. Rhodococcus equi and Arcanobacterium haemolyticum: two "coryneform" bacteria increasingly recognized as agents of human infection. Emerging Infectious Diseases. 1997;3:145–153. doi: 10.3201/eid0302.970207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banck G, Nyman M. Tonsillitis and rash associated with Corynebacterium haemolyticum. J Infect Dis. 1986;154:1037–1040. doi: 10.1093/infdis/154.6.1037. [DOI] [PubMed] [Google Scholar]
- Mackenzie A, Fuite LA, Chan FT, King J, Allen U, MacDonald N, Diaz-Mitoma F. Incidence and pathogenicity of Arcanobacterium haemolyticum during a 2-year study in Ottawa. Clin Infect Dis. 1995;21:177–181. doi: 10.1093/clinids/21.1.177. [DOI] [PubMed] [Google Scholar]
- Miller RA, Brancato F, Holmes KK. Corynebacterium haemolyticum as a cause of pharyngitis and scarlatiniform rash in young adults. Ann Intern Med. 1986;105:867–872. doi: 10.7326/0003-4819-105-6-867. [DOI] [PubMed] [Google Scholar]
- Collins MD, Jones D, Schofield GM. Reclassification of 'Corynebacterium haemolyticum' (MacLean, Liebow & Rosenberg) in the genus Arcanobacterium gen. nov. as Arcanobacterium haemolyticum nom. rev., comb. nov. J Gen Microbiol. 1982;128:1279–1281. doi: 10.1099/00221287-128-6-1279. [DOI] [PubMed] [Google Scholar]
- Jost BH, Billington SJ. Arcanobacterium pyogenes: molecular pathogenesis of an animal opportunist. Antonie van Leeuwenhoek. 2005;88:87–102. doi: 10.1007/s10482-005-2316-5. [DOI] [PubMed] [Google Scholar]
- Cuevas WA, Songer JG. Arcanobacterium haemolyticum phospholipase D is genetically and functionally similar to Corynebacterium pseudotuberculosis phospholipase D. Infect Immun. 1993;61:4310–4316. doi: 10.1128/iai.61.10.4310-4316.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucek A, Souckova A. Toxicity of bacterial sphingomyelinases D. J Hyg Epidemiol Microbiol Immunol. 1974;18:327–335. [PubMed] [Google Scholar]
- Lucas EA, Billington SJ, Carlson P, McGee DJ, Jost BH. Phospholipase D promotes Arcanobacterium haemolyticum adhesion via lipid raft remodeling and host cell death following bacterial invasion. BMC Microbiology. 2010;10:270. doi: 10.1186/1471-2180-10-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke G, von Graevenitz A, Clarridge III JE, Bernard KA. Clinical microbiology of coryneform bacteria. Clin Microbiol Rev. 1997;10:125–159. doi: 10.1128/cmr.10.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan AA, Ulbegi-Mohyla H, Kanbar T, Alber J, Lammler C, Abdulmawjood A, Zschock M, Weiss R. Phenotypic and genotypic characterization of Arcanobacterium haemolyticum isolates from infections of horses. Journal of Clinical Microbiology. 2009;47(1):124–128. doi: 10.1128/JCM.01933-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean PD, Liebow AA, Rosenberg AA. A haemolytic bacterium resembling Corynebacterium ovis and Corynebacterium pyogenes in man. J Infect Dis. 1946;79:69–90. doi: 10.1093/infdis/79.1.69. [DOI] [PubMed] [Google Scholar]
- Linder R, Bernheimer AW. Enzymatic oxidation of membrane cholesterol in relation to lysis of sheep erythrocytes by corynebacterial enzymes. Arch Biochem Biophys. 1982;213:395–404. doi: 10.1016/0003-9861(82)90565-3. [DOI] [PubMed] [Google Scholar]
- Billington SJ, Jost BH, Cuevas WA, Bright KR, Songer JG. The Arcanobacterium (Actinomyces) pyogenes hemolysin, pyolysin, is a novel member of the thiol-activated cytolysin family. J Bacteriol. 1997;179:6100–6106. doi: 10.1128/jb.179.19.6100-6106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. Vol. 1. New York, NY: Greene Publishing Associates and John Wiley and Sons, Inc.; 1994. [Google Scholar]
- Yasawong M, Teshima H, Lapidus A, Nolan M, Lucas S, Glavina Del Rio T, Tice H, Cheng JF, Bruce D, Detter C. et al. Complete genome sequence of Arcanobacterium haemolyticum type strain (11018) Stand Genomic Sci. 2010;3(2):126–135. doi: 10.4056/sigs.1123072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucl Acids Res. 1997;25:955–964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- Zucker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucl Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampersaud R, Planet PJ, Randis TM, Kulkarni R, Aguilar JL, Lehrer RI, Ratner AJ. Inerolysin, a cholesterol-dependent cytolysin produced by Lactobacillus iners. Journal of Bacteriology. 2011;193(5):1034–1041. doi: 10.1128/JB.00694-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelber SE, Aguilar JL, Lewis KL, Ratner AJ. Functional and phylogenetic characterization of Vaginolysin, the human-specific cytolysin from Gardnerella vaginalis. Journal of Bacteriology. 2008;190(11):3896–3903. doi: 10.1128/JB.01965-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Miyakawa ME, Jost BH, Billington SJ, Uzal FA. Lethal effects of Clostridium perfringens epsilon toxin are potentiated by alpha and perfringolysin-O toxins in a mouse model. Vet Microbiol. 2007;127:379–385. doi: 10.1016/j.vetmic.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost BH, Trinh HT, Songer JG, Billington SJ. Immunization with genetic toxoids of the Arcanobacterium pyogenes cholesterol-dependent cytolysin, pyolysin, protects mice against infection. Infect Immun. 2003;71:2966–2969. doi: 10.1128/IAI.71.5.2966-2969.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer F, Paarmann D, D'Souza M, Olson RD, Glass EM, Kubal M, Paczian T, Rodriguez A, Stevens R, Wilke A. et al. The metagenomics RAST server - a public resource for the automatic phylogenetic and functional analysis of metagenomes. BMC Bioinformatics. 2008;9:386. doi: 10.1186/1471-2105-9-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnick ST, Jost BH, Songer JG, Billington SJ. The gene encoding pyolysin, the pore-forming toxin of Arcanobacterium pyogenes, resides within a genomic islet flanked by essential genes. FEMS Microbiol Lett. 2003;225:241–247. doi: 10.1016/S0378-1097(03)00527-5. [DOI] [PubMed] [Google Scholar]
- Williams KP. Integration sites for genetic elements in prokaryotic tRNA and tmRNA genes: sublocation preference of integrase subfamilies. Nucl Acids Res. 2002;30:866–875. doi: 10.1093/nar/30.4.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decatur AL, Portnoy DA. A PEST-like sequence in listeriolysin O essential for Listeria monocytogenes pathogenicity. Science. 2000;290:992–995. doi: 10.1126/science.290.5493.992. [DOI] [PubMed] [Google Scholar]
- Alouf JE, Billington SJ, Jost BH. In: The comprehensive sourcebook of bacterial protein toxins. 3. Alouf JE, Popoff MR, editor. London: Academic Press; 2006. Repertoire and general features of the family of cholesterol-dependent cytolysins; pp. 643–658. [Google Scholar]
- Nagamune H. Streptococcal cytolysins. Seikagaku. 1997;69:343–348. [PubMed] [Google Scholar]
- Giddings KS, Zhao J, Sims PJ, Tweten RK. Human CD59 is a receptor for the cholesterol-dependent cytolysin intermedilysin. Nat Struct Mol Biol. 2004;11:1173–1178. doi: 10.1038/nsmb862. [DOI] [PubMed] [Google Scholar]
- Wickham SE, Hotze EM, Farrand AJ, Polekhina G, Nero TL, Tomlinson S, Parker MW, Tweten RK. Mapping the Intermedilysin-Human CD59 Receptor Interface Reveals a Deep Correspondence with the Binding Site on CD59 for Complement Binding Proteins C8{alpha} and C9. J Biol Chem. 2011;286(23):20952–20962. doi: 10.1074/jbc.M111.237446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de los Toyos JR, Mendez FJ, Aparicio JF, Vázquez F, del Mar García Suárez M, Fleites A, Hardisson C, Morgan PJ, Andrew PW, Mitchell TJ. Functional analysis of pneumolysin by use of monoclonal antibodies. Infect Immun. 1996;64:480–484. doi: 10.1128/iai.64.2.480-484.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert RJ. Cholesterol-dependent cytolysins. Advances in Experimental Medicine & Biology. 2010;677:56–66. doi: 10.1007/978-1-4419-6327-7_5. [DOI] [PubMed] [Google Scholar]
- Heuck AP, Moe PC, Johnson BB. The cholesterol-dependent cytolysin family of gram-positive bacterial toxins. Sub-Cellular Biochemistry. 2010;51:551–577. doi: 10.1007/978-90-481-8622-8_20. [DOI] [PubMed] [Google Scholar]
- Tweten R. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect Immun. 2005;73:6199–6209. doi: 10.1128/IAI.73.10.6199-6209.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuck AP, Tweten RK, Johnson AE. Assembly and topography of the prepore complex in cholesterol-dependent cytolysins. J Biol Chem. 2003;278:31218–31225. doi: 10.1074/jbc.M303151200. [DOI] [PubMed] [Google Scholar]
- Farrand AJ, LaChapelle S, Hotze EM, Johnson AE, Tweten RK. Only two amino acids are essential for cytolytic toxin recognition of cholesterol at the membrane surface. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(9):4341–4346. doi: 10.1073/pnas.0911581107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giddings KS, Johnson AE, Tweten RK. Redefining cholesterol's role in the mechanism of the cholesterol-dependent cytolysins. Proc Natl Acad Sci USA. 2003;100:11315–11320. doi: 10.1073/pnas.2033520100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billington SJ, Songer JG, Jost BH. The variant undecapeptide sequence of the Arcanobacterium pyogenes haemolysin, pyolysin, is required for full cytolytic activity. Microbiology. 2002;148:3947–3954. doi: 10.1099/00221287-148-12-3947. [DOI] [PubMed] [Google Scholar]
- Soltani CE, Hotze EM, Johnson AE, Tweten RK. Specific protein-membrane contacts are required for prepore and pore assembly by a cholesterol-dependent cytolysin. Journal of Biological Chemistry. 2007;282(21):15709–15716. doi: 10.1074/jbc.M701173200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkney M, Beachey E, Kehoe M. The thiol-activated toxin streptolysin O does not require a thiol group for cytolytic activity. Infect Immun. 1989;57:2553–2558. doi: 10.1128/iai.57.8.2553-2558.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders FK, Mitchell TJ, Walker JA, Andrew PW, Boulnois GJ. Pneumolysin, the thiol-activated toxin of Streptococcus pneumoniae, does not require a thiol group for in vitro activity. Infect Immun. 1989;57:2547–2552. doi: 10.1128/iai.57.8.2547-2552.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden JC, Ruiz N, Caparon M. Cytolysin-mediated translocation (CMT): a functional equivalent of type III secretion in Gram-positive bacteria. Cell. 2001;104:143–152. doi: 10.1016/S0092-8674(01)00198-2. [DOI] [PubMed] [Google Scholar]
- Malley R, Henneke P, Morse SC, Cieslewicz MJ, Lipsitch M, Thompson CM, Kurt-Jones E, Paton JC, Wessels MR, Golenbock DT. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(4):1966–1971. doi: 10.1073/pnas.0435928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JM, Ng VH, Maeda S, Rest RF, Karin M. Anthrolysin O and other gram-positive cytolysins are toll-like receptor 4 agonists. J Exp Med. 2004;200:1647–1655. doi: 10.1084/jem.20041215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar JL, Kulkarni R, Randis TM, Soman S, Kikuchi A, Yin Y, Ratner AJ. Phosphatase-dependent regulation of epithelial mitogen-activated protein kinase responses to toxin-induced membrane pores. PLoS ONE [Electronic Resource] 2009;4(11):e8076. doi: 10.1371/journal.pone.0008076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner AJ, Hippe KR, Aguilar JL, Bender MH, Nelson AL, Weiser JN. Epithelial cells are sensitive detectors of bacterial pore-forming toxins. Journal of Biological Chemistry. 2006;281(18):12994–12998. doi: 10.1074/jbc.M511431200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Dominguez-Bernal G, Goebel W, Gonzalez-Zorn B, Wehland J, Kreft J. Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev. 2001;14:584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]