Abstract
Human papillomavirus (HPV) 16 is present in up to 60% of patients with head and neck squamous cell carcinoma (HNSCC) and confers a favorable prognosis in terms of recurrence and mortality. Previous reports demonstrated that HPV-16 DNA can be detected in the initial salivary rinses from these patients. In this study, we assessed the feasibility of post-treatment HPV DNA shed from the oral mucosa as a prognostic marker for persistent/recurrent head and neck cancer. Fresh tumor samples and pre- and post-treatment salivary rinses were collected from 59 patients with HNSCC. HPV-16 E6 and E7 DNA copy number in these samples were quantified by real time PCR. Twenty of 59 patients (33.9%) were HPV-16 positive in their tumors before treatment. Four of 20 HPV tumor positive patients ultimately developed recurrence, and 2 of these 4 patients were HPV-16 positive in surveillance salivary rinses (sensitivity = 50%). Of the 39 (66.1%) HPV-16 negative patients on initial clinical presentation and the 16 HPV-16 positive patients who did not recur, none were HPV-16 positive in salivary rinses after treatment (specificity = 100%). HPV-16 presence in follow-up salivary rinses preceded clinical detection of disease recurrence by an average of 3.5 months. Patients with presence of HPV-16 DNA in surveillance salivary rinses are at significant risk for recurrence. Quantitative measurement of salivary HPV-16 DNA has promise for surveillance and early detection of recurrence.
Keywords: Human Papilloma Virus (HPV), Head and Neck Squamous Cell Carcinoma (HNSCC), quantitative PCR
INTRODUCTION
Each year, head and neck cancer represents 650,000 new cases and 350,000 deaths worldwide.1 Approximately 90% of these cancers are squamous cell carcinomas. Both genetic and environmental factors contribute to the development of head and neck cancers. Known environmental risk factors leading to HNSCC include smoking, alcohol consumption2 and viral pathogens, including HPV-16.3,4 Using molecular biomarkers that provide early detection of disease recurrence or metastasis may afford more potential treatment options with better functional outcomes.
HPVs are a group of 7.9 kb double-stranded circular DNA viruses that are classified into high risk (e.g., types 16, 18, and 31) or low risk (e.g., types 11, 36, and 42) groups.5 High risk HPVs have been demonstrated to be involved in epithelial carcinogenesis. HPV is found in both premalignant and malignant tumors.6 Substantial molecular evidence has suggested that HPV type-16 is a high risk genotype that plays an important role in the pathogenesis of a subset of HNSCC.7,8 Malignant transformation caused by HPV-16 is mediated through the expression of E6 and E7, and to a less extent, E5 proteins. E6 and E7 proteins alter tumor suppressor pathways by inactivating p53 and Rb respectively9,10 while E5 protein modulates proliferation.11,12 Previous studies have shown that HPV-16 is identified in 90% of HPV associated HNSCC3 and in 50% of oropharyngeal SCC.3,13,14 We, therefore, selected HPV-16 E6 and E7 for our study for these reasons.
The existing body of literature has confirmed an independent, favorable outcome for HNSCC patients who have tumor positive HPV-16 status on initial evaluation.3,15 However, the role of HPV-16 detection in saliva obtained in follow-up visits remains unknown. The goal of our study was to determine the significance of HPV-16 E6 and E7 DNA presence in post-treatment salivary rinses. We hypothesized that patients who have detectable levels of HPV-16 DNA in post-treatment salivary rinses may have an elevated risk of recurrence.
MATERIALS AND METHODS
Study Patients
This study was nested within a longitudinal cohort study of 59 patients who presented with histopathologically confirmed HSNCC (this includes patients who presented for treatment of a recurrence after primary treatment at an outside hospital) to the outpatient clinic of the Johns Hopkins Hospital in Baltimore, Maryland from 1999 to 2005. Patients were included if they had at least one post-treatment salivary sample and consented for the study. All patients had undergone treatment with curative intent. The study protocol was approved by the institutional review board of the Johns Hopkins Hospital. Written informed consent was obtained from all patients.
Tissue and Saliva
Tumor samples and salivary rinses from 59 HNSCC patients were obtained. The oral rinses were performed with 20 ml of normal saline gargled twice, for 20 and 10 seconds respectively. Pre-operative tumor and saliva specimens were collected prior to commencement of any therapy at the Johns Hopkins Hospital. In 24 cases where fresh frozen tumor DNA was not available, archival paraffin embedded tissue sections were obtained and in situ hybridization was performed as described previously16 to assess the initial HPV-16 status. In total, we analyzed 53 cases with matched pairs of tumor and pre-treatment saliva DNA and 6 cases with tumor DNA only as pre-treatment saliva DNA was not available. Surveillance salivary rinses were obtained in the follow-up clinic visits from all the patients. Our protocol states that the first post-treatment rinse was collected 3 months after the completion of all treatment and then every 3 months for two years total. However we were not able to obtain follow-up salivary rinses every three months for all patients so we included patients with at least one post-treatment salivary sample in this analysis.
DNA Extraction
Microdissected tissues and saliva were extracted using techniques described previously.17 Briefly, microdissected fresh tissues and saliva were extracted with phenol-chloroform, precipitated in 100% ethanol, centrifuged at 5100 rpm for 45 minutes, washed in 70% ethanol twice, dissolved DNA in LoTE buffer, and stored at −20°C. The samples were diluted to 50 ng in each reaction and then analyzed by quantitative PCR (Taqman HT 7900, Applied Biosystem).
Quantitative PCR
The 7900HT real time PCR system was used to perform quantitative PCR for HPV-16 E6 and E7 and B-actin. Specific primers and probes have been designed to amplify the E6 and E7 regions of HPV type 16: HPV-16 E6 forward primer, 5’-TCAGGACCCACAGGAGCG-3’; HPV-16 E6 reverse primer, 5’-CCTCACGTCGCAGTAACTGTTG-3’, HPV-16 E6 TaqMan probe, 5’-(FAM)-CCCAGAAAGTTACCACAGTTATGCACAGAGCT-(TAMRA)-3’, HPV-16 E7 forward primer, 5’-CCGGACAGAGCCCATTACAA-3’, HPV-16 E7 reverse primer, 5’-CGAATGTCTACGTGTGTGCTTTG-3’, HPV-16 E7 TaqMan probe, 5’-(FAM)-CGCACAACCGAAGCGTAGAGTCACACT-(TAMRA)-3’. A housekeeping gene (B-globin) were run in parallel with HPV-16 E6 and E7 to standardize the input DNA: B-actin forward primer, 5’-TCACCCACACTGTGCCCATCTACGA-3’, B-actin reverse primer, 5’-CAGCGGAACCGCTCATTGCCAATGG-3’, B-actin TaqMan probe, 5’-(FAM)-ATGCCCTCCCCCATGCCATCCTGCGT-(TAMRA)-3’. All samples were run in triplicate.
Data Analysis
The CaSki (American Type Culture Collection, Manassas, VA) cell line was used to develop standard curves for the HPV viral copy number as it is known to have 600 copies/genome equivalent. Standard curves for HPV-16 E6 and E7 were developed by using DNA extracted from CaSki cells, serially diluted into 50 ng, 5 ng, 0.5 ng, 0.05 ng, and 0.005 ng. A standard curve was also developed for the housekeeping gene B-actin (2 copies/genome), using the same serial dilutions of CaSki DNA, as described previously.6 Tumor samples with ≥ 0.1 copy/genome and salivary samples with > 0 copy/genome were considered as HPV positive. Simple sensitivity and specificity analyses were performed on the cases with local recurrence. No statistical correlation was attempted due to modest sample size.
RESULTS
We examined HNSCC tumors and paired pre- and post-treatment salivary rinse samples from 59 patients for HPV-16 using quantitative PCR. Table 1 summarizes the demographic data and risk factors of our HNSCC patients by HPV-16 status. There were 20/59 patients who were determined to be HPV-16 positive on initial presentation (6 were determined by in situ hybridization and 14 were determined by quantitative PCR). As reported in prior studies, the HPV-16 positive HNSCC were located in the oropharynx (p < 0.001). Most of the HPV-16 positive patients presented with advanced disease (p = 0.003), and there was a higher rate of lymph node metastasis in the HPV-16 positive patients (p = 0.001), which was in agreement with prior published studies.18,19 There was no difference in the size of the primary tumor between HPV-16 positive and negative groups (p = 0.644), suggesting that the high stage was due to early lymph node metastasis. Other risk factors for HNSCC such as smoking and alcohol consumption did not show statistically significant differences between the HPV-16 positive and negative patients.
Table 1.
Demographic and Risk Factors By HPV-16 Status
| Variables | HPV-16negative | HPV-16positive | p-value |
|---|---|---|---|
| Gender | |||
| Male | 30 (76.9) | 18 (90.0) | 0.222 |
| Female | 9 (23.1) | 2 (10.0) | |
| Age | |||
| <50yr | 10 (25.6) | 7 (35.0) | 0.621 |
| 50–64yr | 22 (56.4) | 11 (55.0) | |
| ≥65yr | 7 (18.0) | 2 (10.0) | |
| Race | |||
| Caucasian | 27 (69.2) | 20 (100.0) | 0.021 |
| African American | 10 (25.7) | 0 (0.0) | |
| Other | 2 (5.1) | 0 (0.0) | |
| Smoking status | |||
| Never | 12 (30.8) | 7 (35.0) | 0.257 |
| Former | 6 (15.4) | 7 (35.0) | |
| Current | 17 (43.6) | 5 (25.0) | |
| Unknown | 4 (10.2) | 1 (5.0) | |
| Drinking status | |||
| Never | 8 (20.5) | 2 (10.0) | 0.585 |
| Former | 4 (10.3) | 1 (5.0) | |
| Current | 19 (48.7) | 13 (65.0) | |
| Unknown | 8 (20.5) | 4 (20.0) | |
| Stage | |||
| Early (I–II) | 17 (44.7) | 1 (5.26) | 0.003 |
| Advanced (III–IV) | 21 (55.3) | 18 (94.74) | |
| TNM | |||
| Tumor | |||
| 1 | 16 (42.1) | 7 (36.84) | 0.574 |
| 2 | 11 (28.9) | 7 (36.84) | |
| 3 | 8 (21.1) | 5 (26.32) | |
| 4 | 3 (7.9) | 0 (0.0) | |
| Lymph Node | |||
| N− | 22 (56.4) | 2 (10.0) | 0.001 |
| N+ | 17 (43.6) | 18 (90.0) | |
| Tumor Site | |||
| Oropharynx | 4 (10.2) | 19 (95.0) | <0.001 |
| Oral cavity | 23 (59.0) | 0 | |
| Larynx | 9 (23.1) | 0 | |
| Hypopharynx | 2 (5.1) | 0 | |
| Unknown primary | 1 (2.6) | 1 (5.0) | |
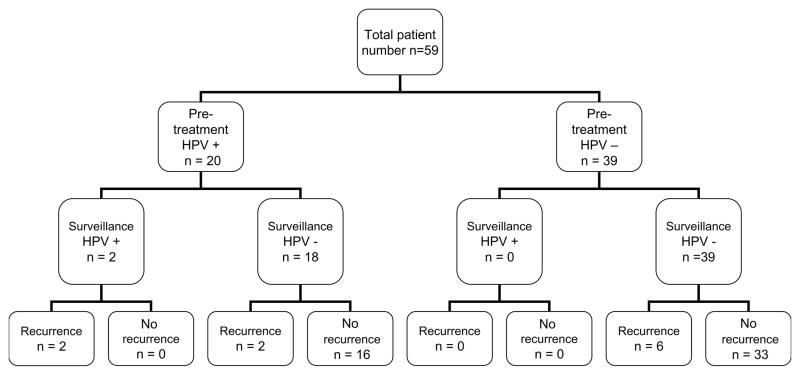
Treatment outcomes were evaluated and correlated with the HPV-16 status. Figure 1 shows the breakdown of patient population by pre-treatment and surveillance HPV-16 status and disease recurrence. Overall, 10/59 (16.95%) of patients developed a recurrence after completion of their treatment, with 4 of these 10 patients demonstrating HPV-16 positivity in their initial tumors prior to treatment. Four of 20 (20.0%) patients with HPV-16 positive tumors developed loco-regional with or without distant metastasis, and 2 of these patients were HPV-16 positive in surveillance salivary rinses (sensitivity = 50%). The other 2 patients, however, had no detectable HPV-16 in their surveillance saliva despite disease recurrence. One of these two patients did have positive HPV-16 in-situ hybridization in the biopsy of recurrent tumor. Of note, there were no false negative results, all of the patients who did not recur remained HPV negative in their surveillance saliva to date (specificity = 100%). Table 2 details the statistical analysis of these patients.
Figure 1.
Layout of patient groups by HPV status pre-treatment and post-treatment and recurrence.
Table 2.
Sensitivity, specificity and predictive values of surveillance HPV-16 status
| Recurrence | Surveillance salivary HPV-16 status | ||
|---|---|---|---|
| positive | negative | ||
| Positive | 2 | 2 | Sensitivity = 2/4 = 50% |
| Negative | 0 | 16 | Specificity = 16/16 = 100% |
| PPV = 2/2 = 100% | NPV = 16/18 =88.89% | ||
PPV = positive predictive value; NPV = negative predictive value
Table 3 shows the relevant clinical data in the 4 HPV-16 positive patients who developed recurrences. All these patients had stage IV HNSCC with significant regional lymph node metastasis, and they all developed recurrence within 24 months after completion of treatment. We found an average of 3.5 months of lead time in detection of recurrence of HNSCC when compared to standard clinical evaluation. In this study, there did not appear to be any correlation between local, regional, or distant recurrence and our ability to detect HPV-16 in the surveillance saliva.
Table 3.
Clinical course on pre-treatment HPV-16 positive patients who developed recurrence.
| Post-treatment HPV-16 Status | Patient number | Initial Stage | T | N | Site of Tumor | Site of Recurrence | Disease Free Survival (months) | Lead Time to Clinical Diagnosis Using Saliva HPV DNA (months) |
|---|---|---|---|---|---|---|---|---|
| salivary rinse HPV-16 positive | 68 | 4 | 1 | 2b | OP | right clavicle, left thyroid, bilateral lungs, chest wall, and multiple tracheal, carinal, bronchiolar and hilar LN | 16.5 | 3.5 |
| 141 | 4 | 2 | 2c | OC | tongue base, R axilla | 12 | 3.5 | |
| salivary rinse HPV-16 negative | 66 | 4 | 2 | 2c | OP | contralateral neck, superior nasopharyngeal margin; lung | 21 | not applicable |
| 17 | 4 | 3 | 2c | OP | neck recurrence, with circumferentially involved carotid artery | 9 | not applicable |
OP = oropharynx; OC = oral cavity; LN = lymph node
DISCUSSION
HNSCC is commonly associated with smoking and alcohol use, although other factors such as HPV infection, diet, and poor oral hygiene may also play a role in tumorigenesis. HPV-16 is most frequently present in a subset of HNSCC patients who have lesions localized in the oropharynx. The aim of this study was to determine the correlation between post-therapy HPV-16 status in the saliva and recurrence in HNSCC patients by using quantitative PCR to investigate the feasibility of early detection of recurrence using this technique.
In this study, 20/59 (33.90%) patients were HPV-16 positive on presentation, similar to the prevalence rates reported previously.14,20–22 We also confirmed that HPV-16 was most commonly found in oropharyngeal cancers, and these patients tended to present with advanced stage disease.23 However we did not find an association of HPV status with alcohol use (p = 0.572) and smoking (p = 0.225), perhaps due to a relatively small sample size as compared to prior studies.
Previous studies have shown the presence of HPV-16 in oral exfoliated cells of HNSCC patients, using either quantitative20 or nonquantitative PCR methods.24 Here we have confirmed the feasibility of correlating the HPV-16 DNA presence in surveillance salivary rinses and disease progression. We detected 2/4 HPV positive tumor recurrences through salivary rinses, and there were no cases of false positive HPV-16 detection in this cohort. Moreover, the presence of HPV-16 DNA in surveillance saliva was 100% specific for tumor recurrence. We also found that the presence of HPV-16 in surveillance saliva was a marker for locoregional recurrence or distant metastasis. The presence of detectable HPV-16 DNA in surveillance saliva could represent microscopic residual cancer or micro-metastasis that escaped detection by conventional pathology or radiological evaluations. Alternatively, the presence of HPV-16 DNA in salivary rinses may reflect an inability to eliminate HPV-16 DNA containing cells in local, regional and distant sites. Additional investigation, would, of course, be necessary to confirm this hypothesis.
In this limited cohort, we found that patients with HPV-16 positive surveillance salivary rinses are at high risk for development of recurrence and distant metastasis. This finding may represent an immunologic impairment that contributes to cancer development and subsequent recurrence and metastasis. Therefore the presence of HPV in saliva may be used as a screening/surveillance tool and as a prognostic biomarker for disease progression. However, a larger scale, prospective study specifically directed at this issue would be necessary to confirm the feasibility of early detection of recurrence using quantitative HPV-16 PCR in follow up salivary rinses.
Acknowledgments
Dr. Califano is supported by a Clinical Innovator Award from the Flight Attendant Medical Research Institute, the National Institute of Dental and Craniofacial Research (1R01DE015939-01) and the National Cancer Institute SPORE program P50 CA96784.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferlay J, Bray F, Pisani P, Parkin D. Globocan 2002: cancer incidence and mortality worldwide. , Vol. version 2.0. Lyon, France: IARC; 2004. [Google Scholar]
- 2.Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001;345(26):1890–900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 3.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92(9):709–20. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 4.D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case- control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–56. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 5.Campisi G, Panzarella V, Giuliani M, Lajolo C, Di Fede O, Falaschini S, et al. Human papillomavirus: its identity and controversial role in oral oncogenesis, premalignant and malignant lesions (review) Int J Oncol. 2007;30(4):813–23. [PubMed] [Google Scholar]
- 6.Ha PK, Pai SI, Westra WH, Gillison ML, Tong BC, Sidransky D, et al. Real-time quantitative PCR demonstrates low prevalence of human papillomavirus type 16 in premalignant and malignant lesions of the oral cavity. Clin Cancer Res. 2002;8(5):1203–09. [PubMed] [Google Scholar]
- 7.McKaig RG, Baric RS, Olshan AF. Human papillomavirus and head and neck cancer: epidemiology and molecular biology. Head Neck. 1998;20(3):250–65. doi: 10.1002/(sici)1097-0347(199805)20:3<250::aid-hed11>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 8.Mork J, Lie AK, Glattre E, Hallmans G, Jellum E, Koskela P. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344(15):1125–31. doi: 10.1056/NEJM200104123441503. [DOI] [PubMed] [Google Scholar]
- 9.Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248(4951):76–9. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- 10.Kessis TD, Slebos RJ, Nelson WG, Kastan MB, Plunkett BS, Han SM, et al. Human papillomavirus 16 E6 expression disrupts the p53-mediated cellular response to DNA damage. Proc Natl Acad Sci U S A. 1993;90(9):3988–92. doi: 10.1073/pnas.90.9.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Genther Williams SM, Disbrow GL, Schlegel R, Lee D, Threadgill DW, Lambert PF. Requirement of epidermal growth factor receptor for hyperplasia induced by E5, a high-risk human papillomavirus oncogene. Cancer Res. 2005;65(15):6534–42. doi: 10.1158/0008-5472.CAN-05-0083. [DOI] [PubMed] [Google Scholar]
- 12.Scheurer ME, Tortolero-Luna G, Adler-Storthz K. Human papillomavirus infection: biology, epidemiology, and prevention. Int J Gynecol Cancer. 2005;15(5):727–46. doi: 10.1111/j.1525-1438.2005.00246.x. [DOI] [PubMed] [Google Scholar]
- 13.Fouret P, Monceaux G, Temam S, Lacourreye L, St Guily JL. Human papillomavirus in head and neck squamous cell carcinomas in nonsmokers. Arch Otolaryngol Head Neck Surg. 1997;123(5):513–6. doi: 10.1001/archotol.1997.01900050063008. [DOI] [PubMed] [Google Scholar]
- 14.Paz IB, Cook N, Odom-Maryon T, Xie Y, Wilczynski SP. Human papillomavirus (HPV) in head and neck cancer. An association of HPV 16 with squamous cell carcinoma of Waldeyer's tonsillar ring. Cancer. 1997;79(3):595–604. doi: 10.1002/(sici)1097-0142(19970201)79:3<595::aid-cncr24>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 15.Sugiyama M, Bhawal UK, Kawamura M, Ishioka Y, Shigeishi H, Higashikawa K, et al. Human papillomavirus-16 in oral squamous cell carcinoma: clinical correlates and 5-year survival. Br J Oral Maxillofac Surg. 2007;45(2):116–22. doi: 10.1016/j.bjoms.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Begum S, Gillison ML, Nicol TL, Westra WH. Detection of human papillomavirus-16 in fine-needle aspirates to determine tumor origin in patients with metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13(4):1186–91. doi: 10.1158/1078-0432.CCR-06-1690. [DOI] [PubMed] [Google Scholar]
- 17.Califano J, van der Riet P, Westra W, Nawroz H, Clayman G, Piantadosi S, et al. Genetic progression model for head and neck cancer: implications for field cancerization. Cancer Res. 1996;56(11):2488–92. [PubMed] [Google Scholar]
- 18.Hoffmann M, Orlamunder A, Sucher J, Gottschlich S, Gorogh T, Fazel A, et al. HPV16 DNA in histologically confirmed tumour-free neck lymph nodes of head and neck cancers. Anticancer Res. 2006;26(1B):663–70. [PubMed] [Google Scholar]
- 19.Hoffmann M, Gottschlich S, Gorogh T, Lohrey C, Schwarz E, Ambrosch P, et al. Human papillomaviruses in lymph node neck metastases of head and neck cancers. Acta Otolaryngol. 2005;125(4):415–21. doi: 10.1080/00016480510028528. [DOI] [PubMed] [Google Scholar]
- 20.Zhao M, Rosenbaum E, Carvalho AL, Koch W, Jiang W, Sidransky D, et al. Feasibility of quantitative PCR-based saliva rinse screening of HPV for head and neck cancer. Int J Cancer. 2005;117(4):605–10. doi: 10.1002/ijc.21216. [DOI] [PubMed] [Google Scholar]
- 21.Koskinen WJ, Chen RW, Leivo I, Makitie A, Back L, Kontio R, et al. Prevalence and physical status of human papillomavirus in squamous cell carcinomas of the head and neck. Int J Cancer. 2003;107(3):401–6. doi: 10.1002/ijc.11381. [DOI] [PubMed] [Google Scholar]
- 22.Ringstrom E, Peters E, Hasegawa M, Posner M, Liu M, Kelsey KT. Human papillomavirus type 16 and squamous cell carcinoma of the head and neck. Clin Cancer Res. 2002;8(10):3187–92. [PubMed] [Google Scholar]
- 23.Dahlgren L, Mellin H, Wangsa D, Heselmeyer-Haddad K, Bjornestal L, Lindholm J, et al. Comparative genomic hybridization analysis of tonsillar cancer reveals a different pattern of genomic imbalances in human papillomavirus-positive and -negative tumors. Int J Cancer. 2003;107(2):244–9. doi: 10.1002/ijc.11371. [DOI] [PubMed] [Google Scholar]
- 24.Smith EM, Ritchie JM, Summersgill KF, Hoffman HT, Wang DH, Haugen TH, et al. Human papillomavirus in oral exfoliated cells and risk of head and neck cancer. J Natl Cancer Inst. 2004;96(6):449–55. doi: 10.1093/jnci/djh074. [DOI] [PubMed] [Google Scholar]