Abstract
Titanium (Ti) is commonly utilized in many cardiovascular devices, e.g. as a component of Nitinol stents, intra- and extracorporeal mechanical circulatory assist devices, but is associated with the risk of thromboemboli formation. We propose to solve this problem by lining the Ti blood-contacting surfaces with autologous peripheral blood-derived late outgrowth endothelial progenitor cells (EPCs) after having previously demonstrated that these EPCs adhere to and grow on Ti under physiological shear stresses and functionally adapt to their environment under flow conditions ex vivo. Autologous fluorescently-labeled porcine EPCs were seeded at the point-of-care in the operating room onto Ti tubes for 30 minutes and implanted into the pro-thrombotic environment of the inferior vena cava of swine (n = 8). After 3 days, Ti tubes were explanted, disassembled, and the blood-contacting surface was imaged. A blinded analysis found all 4 cell-seeded implants to be free of clot, whereas 4 controls without EPCs were either entirely occluded or partially thrombosed. Pre-labeled EPCs had spread and were present on all 4 cell-seeded implants while no endothelial cells were observed on control implants. These results suggest that late outgrowth autologous EPCs represent a promising source of lining Ti implants to reduce thrombosis in vivo.
1. Introduction
Titanium (Ti) is commonly utilized as a component in the engineering of cardiovascular devices. Examples include mechanical circulatory assist devices for the treatment of heart failure and Nitinol vascular stents, which are coated with a layer of Ti on their blood-contacting surface and used in the treatment of Peripheral Vascular Disease. Whereas mechanical circulatory assist devices are associated with the risk of distal thromboemboli causing strokes [1], Nitinol stents carry a high risk of in-stent restenosis and thrombosis. 40% - 50% of bare metal Nitinol stents in the femoropopliteal region are occluded within 2 years, and about 20% of patients who receive Nitinol carotid artery stents will suffer from re-occluded stented neck vessels by > 50% after only 2 years [2, 3]. Further, Nitinol stents occlude in > 30% of patients with superficial femoral artery disease lesions after only 1 year [4]. Drug-eluting stents may provide for better outcomes. However, no significant difference was found in a head-to head comparison between bare metal stents and Sirolimus drug-eluting stents implanted into the infrainguinal vasculature [5]. Even in the coronary vasculature, drug-eluting stents that once held the promise of mitigating in-stent restenosis have been hampered by an increasing risk of late-stent thrombosis, likely due to incomplete coverage with healthy endothelium secondary to the drugs used [6].
We propose to solve these problems by lining the titanium blood-contacting surfaces of intravascular devices with autologous peripheral blood-derived endothelial progenitor cells (EPCs) within minutes of implantation. Since these EPCs are derived from the same patient who would receive the cell-coated implant, there is no risk for rejection by the immune system. The anti-thrombotic endothelial coating would also reduce the necessity of anti-coagulative drugs for patients treated with intravascular devices, e.g. stents and circulatory assist devices, and reduce the bleeding complications associated with such medications.
We have recently demonstrated that it is feasible to grow blood-derived EPCs on Ti surfaces, that EPCs grow to a confluent monolayer on Ti under static conditions and physiological flow after only a few minutes prior adhesion time, and that EPCs adhere extremely well to such surfaces, even under supraphysiological shear stresses [7]. We have further shown that EPCs on smooth Ti surfaces functionally adapt to their environment under flow, produce nitric oxide (dependent on the magnitude of shear stress stimulation), and dramatically reduce platelet adhesion [7].
In order to provide the initial feasibility and benefit of such EPC-lined surfaces in a large animal model, we lined Ti tubes with porcine blood-derived EPCs and implanted these tubes into the inferior vena cava (IVC) of the pigs from which the cells were derived. Uncoated ‘bare’ Ti tubes implanted under the exact same conditions served as controls. We chose swine for this study because the pig is one of the most accepted animal models for studying coagulation biology, inflammation, and specifically vascular prostheses [8-11]. Since our null-hypothesis is that EPC-linings will not be able to reduce the incidence of thrombosis, we implanted the Ti tubes into the pro-thrombotic environment of the inferior vena cava, as opposed to the aorta, to increase the likelihood of thrombosis.
2. Materials and methods
2.1. Titanium tube assembly
Ti tubes (commercially pure, grade 2) were purchased with dimensions 12.7 mm OD and 9.4 mm ID (Tico Titanium) and cut to a length of 45 mm. Tubes were sectioned into 3 equal segments longitudinally with a high speed steel saw (Fig. S1). The inner surfaces were polished with a bench grinder and metalworking wheel (Scotch-Brite), followed by manual polishing with emery cloth (3M) to further even out the surface and remove any visible pits. Ti sections were then cleaned with Alconox soap solution and aqua regia (1:3 concentrated nitric acid to concentrated hydrochloric acid) as previously described for Ti surfaces [12]. Sets of three Ti sections were reassembled into tubes with Polyvinyl chloride (PVC) heat-shrink tubing (Insultab, expanded ID 15.88 mm, recovered ID 7.95 mm, Fig. S1). For the assembly of our seeding chamber, silicone tubing (16.64 mm OD, 9.52 mm ID, 3 cm length) was added to both ends of the Ti tube and a ‘cut-off’ syringe head with luer was added to one end (Fig. 1A). All components were gas sterilized prior to use (18 h, 55 °C).
Fig. 1.
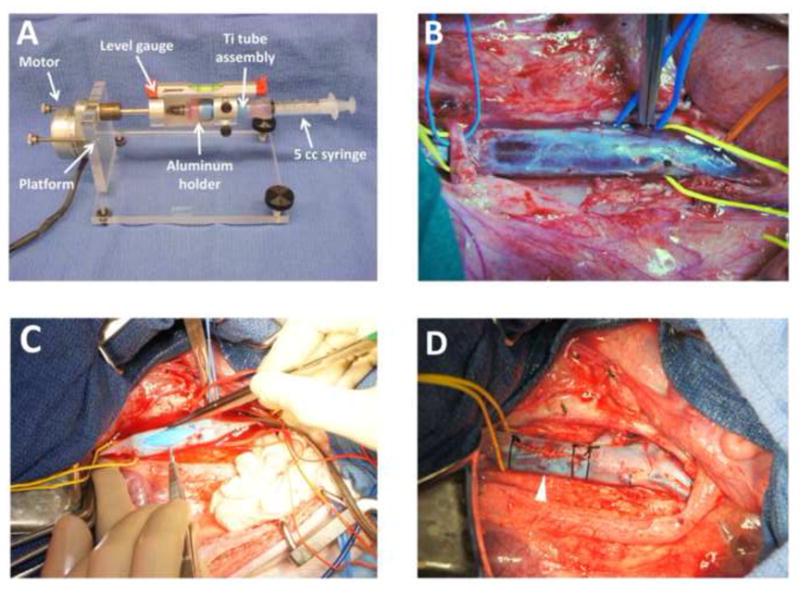
(A) Ti tube seeding device and Ti tube seeding chamber. The Ti tube is placed inside the aluminum holder. To assemble the seeding chamber, a ‘cut-off’ syringe head with luer is attached via silicone tubing to the Ti tube (left end of Ti tube) and a 5 cc syringe attached via silicone tubing (right end of Ti tube). The level gauge ensures equal distribution of EPCs during rotation of the seeding chamber. A sterile sheath surrounding the seeding chamber has been removed in order to facilitate viewing of the seeding chamber inside the aluminum holder. (B) Intraoperative view of skeletonized infrarenal IVC, prior to venotomy. The proximal end of the IVC is encircled with a yellow vessel loop (left). The overlying right renal artery is encircled with a blue vessel loop (left). A blue vessel loop encircles the left 7th lumbar vein. The distal IVC is encircled with a yellow vessel loop at its bifurcation into the common iliac veins (right) and the right external iliac artery is encircled with an orange vessel loop as it crosses the right common iliac vein (far right). (C) Ti tube insertion into IVC. Both ends of the IVC are clamped. The blue PVC heat shrink tubing is recognizable inside the IVC. (D) Post-insertion view of the infrarenal IVC. The yellow vessel loop encircles the proximal IVC. Note the 6-0 polypropylene ‘stay suture’ that was placed through the adventitia and PVC heat shrink tubing to prevent the device from migrating (white arrow).
2.2. X-ray photoelectron spectroscopy
The atomic composition of our Ti samples was determined using a Kratos Axis Ultra X-ray Photoelectron Spectroscope and compared to the surface of a Cordis Precise RX self-expanding Nitinol stent. Spectra were obtained at 2.0 × 10-8 Torr using a monochromated aluminum K-alpha X-ray source at a power of 15 kV and an emission current of 10 mA. Survey scans were performed over the range of 5 – 1,200 eV with a step eV of 1, a dwell time of 200 ms, a resolution of 160 eV, and a slot aperture measuring an area of 300 μm × 700 μm. A regional scan for titanium was performed on both samples over the range of 450 - 470 eV with a step eV of 0.1, a dwell time of 298.5 ms, a resolution of 160 eV, and the same slot aperture. In addition, a survey scan of the stent was performed using a “small spot” field of view with a 2 mm aperture measuring a 110 μm diameter spot on the stent surface and the same scanning parameters used for the previous survey scans. The elemental peaks of the samples were ascertained from survey scans using CasaXPS software and utilizing a relative sensitivity function library specific to the Kratos Axis Ultra system.
2.3. Optical profilometry
Surface roughness was analyzed by optical profilometry (Zygo NewView 5000). The 3D surface profiler uses non-contacting white light interferometry to acquire high resolution z-images, from which surface roughness values (Ra) are reported according to equation (1) [13], after subtraction of a “cylinder” shape in the system software (MetroPro, Zygo) to account for and remove the curvature of the tube itself.
| (1) |
where yi represents the differences between the surface profile and the surface mean line for the ith data point of n equally spaced data points along a trace. Three fields (0.72 × 0.5 mm each) were measured on each of 3 Ti tube sections, and all Ra values from a single Ti tube section were averaged to obtain n = 1 for a total sample size of n = 3.
2.4. EPC isolation
All experiments with swine were approved by the Duke University Institutional Animal Care and Use Committee (IACUC) and were conducted in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals. Yorkshire swine (2 male, 6 female, at an initial weight of 46.6 ± 0.89 kg) underwent a blood draw 30 days prior to Ti tube implantation to allow sufficient time for EPC isolation and amplification in culture (only 4 pigs actually received cell-seeded implants, the other 4 received bare metal control implants). The pigs were sedated with Acepromazine (1.1 mg/kg) and Ketamine (22 mg/kg) intramuscular. Intubation was achieved with an endotracheal tube (30 cm length, 8 mm ID), and the pigs were anesthetized with Isofluorane by mask. The pigs’ groins were cleaned and sterilized with DuraPrep and femoral vein access was obtained with a 5 F micro-introducer kit (Galt Medical) using the Seldinger technique [14]. After discarding the first 5 ml of blood, 45 ml of blood were collected into syringes containing 10 ml anticoagulant citrate dextrose solution (Pall Corporation). This was followed by 1:1 dilution with Hank’s buffered salt solution (without CaCl2, MgCl2, MgSO4) and layered on equal volumes of Histopaque to create well-defined layers. The mononuclear cell layer was collected as described previously [7] and plated into two 12-well plates in full EPC growth medium (MCDB-131 medium with 2 mM L-glutamine, Cellgro, 2% porcine serum, Gemini Bio-Products, and EGM-2 SingleQuots, Lonza) at 37 °C, 5% CO2.
2.5. Flow cytometry
For each cell isolation, EPCs were tested with flow cytometry for presence or absence of surface markers CD31 (Platelet Endothelial Cell Adhesion Molecule 1, PECAM-1), CD14 and CD45, as described for these cells previously [7]. Further, we tested for CD 106 (Vascular Cell Adhesion Molecule-1, VCAM-1) with directly fluorescein isothiocyanate-conjugated mouse anti-porcine VCAM-1 antibody (Antigenix America, APG106F). Mouse IgG1 served as isotype control.
2.6. Titanium tube seeding
The custom device for seeding Ti tubes was built by mounting a synchronous timing motor (Herbach & Rademan) on a plexiglass machined stand with variable height feet to allow adjustment of rotating device with a water leveling gauge (Swanson). The motor was connected via a stainless steel axle to a machined aluminum holder with a mold fitting the Ti tube seeding chamber of Section 2.1. (Fig. 1A). For in vitro seeding experiments, adherent EPCs were labeled with the fluorescent dye Cell Tracker Orange CMRA (Invitrogen) in serum-free medium (15 min, 37 °C). For in vivo experiments, adherent EPCs were labeled with the long-term fluorescent dye PKH26 (Sigma-Aldrich) at a concentration of 4 μM (4 min, room temperature), and the reaction was stopped with porcine serum (Gemini Bio-Products). Following labeling, cells were dissociated with Trypsin (0.25%, Lonza, at 37 °C for 3 min) and counted, then re-suspended to a final concentration of 2.0 × 106 cells/ml (see Section 3.5. for optimization of cell suspension density) in serum-free medium EBM-2 (Lonza) with EGM-2 SingleQuots (Lonza). The EPC suspension was introduced into the Ti tube by a syringe attached with silicone tubing (Fig. 1A), and a luer cap added to the end of the ‘cut-off’ syringe head after purging all air bubbles from the Ti tube. The Ti tube was placed inside a sterile sheath to maintain sterility of the system and secured into the machined aluminum holder (Fig. 1A). Next, the seeding device with cell suspension was rotated for 30 min at 37 °C in ambient air at 10 rotations per hour, as determined empirically in previous studies [15]. To evaluate adherent EPCs after the seeding period, Ti tube sections were rinsed twice in phosphate-buffered saline (with CaCl2 and MgCl2, PBS) after seeding and fixed in 10% formalin for 15 min, followed by 2 more rinses with PBS. Cell areas were measured in 100 - 120 cells per Ti tube section using ImageJ software immediately after 30 min seeding by rotation (designated time zero) and after a 24 hour static culture time following the seeding by rotation period (designated as time 24 hours). Values within each Ti tube section were averaged for a single sample; data from four samples were obtained at time zero and data from three samples after 24 hour static culture. To evaluate the surface density of adherent cells after the seeding period, the total number of cells was determined in 20 random fields per Ti tube section (Image J). Cell numbers were divided by the area of each field to obtain a density. Values from all fields within each experiment were averaged to obtain each n = 1, and a total of n = 3 Ti tubes were used at each of three different EPC suspension concentrations. The overall percent of cells adherent after seeding was calculated using the cell suspension concentration, volume and surface area for each Ti tube.
2.7. Shear stress approximation in porcine inferior vena cava
Vessel dimensions and flow rates were measured in an exploratory surgery in a pig (50 kg, representative of the size of pigs at device implantation) with an ultrasonic perivascular flow probe (Transonic), allowing the wall shear stress τ (dyn/cm2) to be estimated according to [16]
| (2) |
where, μ is the blood viscosity at 37 °C (0.0465 gcm-1s-1) [17], Q is the measured volumetric flow rate (8.33 cm3/s), and R is the measured vessel radius (0.7 cm). Therefore
| (2.1) |
Since this shear stress is approximately ten-fold lower than the 15 dyn/cm2 commonly used to model arterial flow [18], the IVC provides a pro-thrombotic environment in which to challenge our EPC-seeded devices.
2.8. Ti tube implantation in swine
Eight Yorkshire swine (2 male, 6 female, at a weight of 52 ± 1.6 kg) underwent consecutive surgeries with either EPC-seeded or bare metal device implantations (see Supplementary Data, Swine preparation for surgery and Swine laparotomy). After the IVC had been identified we used sharp and blunt dissection to free the vessel from the surrounding tissue and skeletonize it circumferentially from the level of the right renal vein proximally to its iliac bifurcation distally (Fig. 1B). Several lumbar veins were ligated with silk sutures and divided to prevent any retrograde bleeding around the implanted Ti tube once venous circulation was restored. Before proceeding any further, the Ti tube seeding was initiated in the operating room with fluorescently-labeled EPCs with our seeding device as described in Section 2.6. Unseeded bare metal Ti tubes were implanted as controls.
100 USP/kg of heparin was administered intravenously 5 min prior to clamping the IVC (and completion of the seeding process). A profunda clamp was placed, first on the distal end of the IVC at the iliac bifurcation, then proximally just distal to the right renal vein using a 45 degree straight vascular clamp. A # 11 scalpel blade was used to create a small venotomy in the middle of the exposed segment. This was extended with Potts scissors to fashion a longitudinal venotomy measuring 4 cm in length (Fig. 1C). The Ti tube was implanted (see Supplementary Data, Ti tube insertion) and two 2-O silk ties were placed around the IVC containing the device (at the proximal and distal end of the Ti tube) to prevent the development of a false lumen (Fig. 1D). Moreover, one 6-O polypropylene ‘stay suture’ was placed through the IVC’s adventitia into and through the heat-shrink PVC tubing to prevent migration of the device inside the IVC (Fig. 1D).
The abdomen was then closed and the pig recovered (see Supplementary Data, Swine abdominal closure). All surgeries were accomplished without any unforeseen complications. The blood loss was minimal in all procedures. The duration of IVC clamp time was similar between the groups (Fig. 2A). Furthermore, there was no significant difference in the total anesthesia time or the time pigs were immobilized for surgery between the cell-seeded treatment group and control group (Fig. 2B). All eight pigs in the study mobilized and fully recovered before device explantation 3 days later.
Fig. 2.
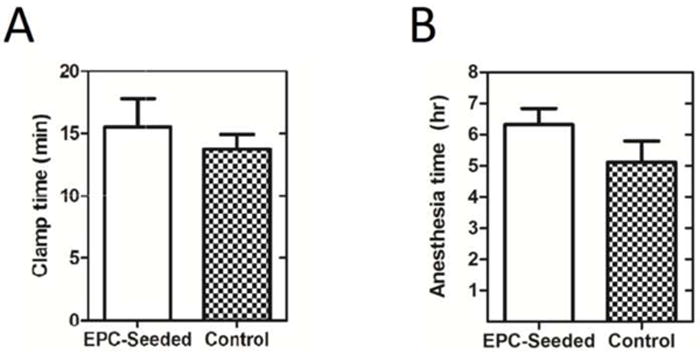
(A) Intraoperative clamp times of IVC during device insertion. Clamp times were not significantly different between EPC-seeded and control implant groups (15.5 ± 2.3 min and 13.8 ± 1.2 min, respectively, p = 0.517, n = 4 for each group, two-tailed t-test). (B) Total time of anesthesia during surgery (in hours). Duration of anesthesia was not significantly different between EPC-seeded and control implant groups (6 hours 19 minutes ± 31 minutes vs. 5 hours 7 minutes ± 41 minutes, p = 0.21, n = 4 for each group, two-tailed t-test).
2.9. Ti tube explantation
Three days after implantation, pigs were sedated, intubated and anesthetized as described above. The laparotomy and dissection were conducted as outlined previously and the Ti tube was explanted en bloc after clamping the IVC proximal and distal and transecting the IVC proximal and distal with heavy scissors. The pig was euthanized after the device had been explanted with Euthasol euthanasia solution (390 mg/ml Pentobarbital Sodium and 50 mg/ml Phenytoin Sodium at 1 ml/10 lbs) according to our IACUC protocol. The Ti tube was immediately rinsed with PBS × 3 and its lumen photographed from both ends with a high resolution digital camera (Nex-3, Sony). The implant with clot (if present) was then submerged in 3.7% paraformaldehyde for fixation for 4 hours. After fixation, the Ti tube was opened by incising the PVC heat-shrink tubing with a # 15 scalpel blade and its inner surface photographed as well. None of the pigs received any form of anticoagulation other than the initial heparin bolus 5 min prior to implantation of the device (treatment and control alike).
2.10. Thrombosis scoring
Thrombosis outcomes of explanted Ti tubes were classified into three categories: ‘Fully clotted’, ‘partially clotted’, or ‘no clot’. Clots in the ‘fully clotted’ category extended throughout the entire length and cross-section of the tube lumen, whereas ‘partial clots’ did not fill the tube, either in length or in cross-section. Tubes, which were clean with no significant thrombosis, were classified as ‘no clot’. Outcomes were scored by presenting the gross photographs of the device lumina and their opened sections to three separate reviewers, who were blinded as to whether the device had been seeded with EPCs or was a control implant. Where the reviewers disagreed in their classification, we biased their assessment toward the null-hypothesis, that there is no difference in outcome between cell-seeded and bare Ti tubes, e.g. if a bare metal tube were rated ‘partially clotted’ vs. ‘fully clotted’, we would classify it as ‘partially clotted’.
2.11. Immunohistochemistry/ microscopy
In addition to the pre-implantation label PKH26 (Sigma-Aldrich), cell nuclei of fixed Ti tube segments were stained with Hoechst 34580 nucleic acid stain (Invitrogen, diluted 1:1000 in PBS × 15 min). To stain Ti tube sections for PECAM-1 (cell-cell junctions), samples were incubated with mouse anti-porcine CD31 (Antigenix America #APG311, diluted 1:100, for 1h at 37 °C) after blocking with 10% normal goat serum (Gibco, for 30 min at RT). Samples were then rinsed × 3 with PBS and incubated with the secondary antibody goat anti-mouse AlexaFluor488 (Invitrogen #A-11001, diluted 1:500, for 45 min at 37 °C). Appropriate positive and negative control experiments were performed to rule out non-specific antibody binding to Ti surfaces. Following 3 more washes with PBS, samples were visualized with an upright Leica DMRB fluorescent microscope with a Qimaging Qicam monochrome digital camera and Image Pro Plus software (Leica). Cell surface area was determined with Image J software, after seeding × 30 min vs. subsequent in vivo experiments × 3 days, as described in 2.6. The confluent density was calculated by counting the number of cells per field as described in Section 2.7. Additionally, samples were imaged with a Zeiss 780 confocal upright fixed stage microscope and 10x objective (Zeiss), and images were captured with Zeiss software.
2.12. Statistics
To evaluate cell spreading, a one-way ANOVA was performed comparing the area of cells at time point zero after seeding, after 1 day of static culture, and after 3 days in vivo (on the Ti tube implanted surface), followed by a post hoc two-tailed t-test. A one-way ANOVA was also used to test for differences in cell adhesion percentage with cell suspension density. Assuming a linear relationship between adhesion density and cell suspension [19, 20], correlation of these variables was performed with a linear regression analysis, followed by an F-test to confirm that the slope was significantly different from zero. A two-tailed t-test was used to compare IVC clamp time between treatment and control groups and total time of anesthesia between both groups, respectively. To compare thrombosis between control and cell-treated implants after 3 days in vivo, a 2 × 3 contingency table was used with the Fisher’s exact test [21]. The significance level was assumed to be 0.05 for all tests. Results were reported as mean ± standard error.
3. Results
3.1. Ti surface composition
X-ray photoelectron spectroscopy indicated that the chemical composition of the Nitinol self-expanding stent (Cordis) was very similar to our manufactured Ti tube surfaces. Regional scans of Ti 2p ranges showed that both, our Ti tube and Nitinol stent, were composed of Ti primarily as TiO2, with Ti 2p3/2 peaks matching an expected peak at 458.8 eV [22] (Fig. 3A,B). Of particular interest, XPS revealed the absence of nickel on the stent surface in the survey scan, confirming that polishing of the Nitinol stents by the manufacturer results in a titanium oxide outer layer on the alloy surface.
Fig. 3.
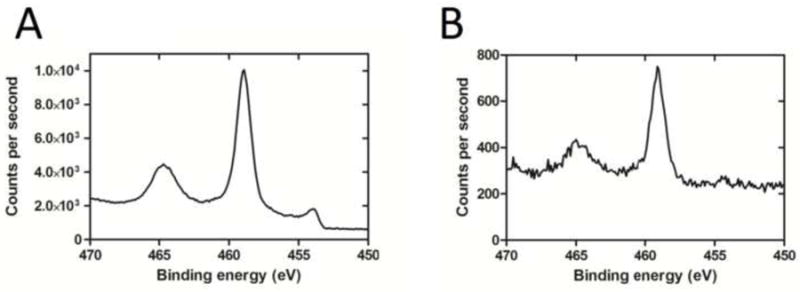
(A) XPS spectrum of Ti tube with binding energy peaking at 458.9 eV, corresponding to the Ti 2p3/2 electron configuration. (B) XPS spectrum of the Nitinol stent with binding energy peaking at 459.1 eV, indicating comparable TiO2 composition. Note that the Nitinol stent was imaged in its expanded state with a small field of view focusing on a stent strut only, whereas the titanium tube was imaged with a larger field of view, which is reflected by the difference in signal intensity (measured in counts per second) between the Ti tube and Nitinol stent.
3.2. Ti surface topography
The surface topography of our Ti tubes was evaluated with optical profilometry. The roughness average (Ra) for the titanium tubes was found to be in the sub-micrometer range (0.59 ± 0.02 μm, n = 3), similar to roughness values reported for Nitinol alloys used in biomedical applications [23].
3.3. Flow cytometry results
All EPCs exhibited typical cobblestone morphology by phase-contrast microscopy and tested positive for presence of PECAM-1 and negative for surface markers CD14 and CD45. Further, we confirmed absence of VCAM-1 for cells cultured in vitro, which, if present, would indicate an undesirable pro-inflammatory activated phenotype of our endothelial progenitor cells [24, 25].
3.4. EPC adherence and spreading on Ti tube surface ex vivo
EPCs seeded onto Ti tubes with our seeding device (Fig. 1A) and cultured under static conditions for 24 hours adhered to and spread on the Ti surface and formed a confluent layer. Cell areas were significantly greater after 24 hours of static culture than immediately following cell seeding (852.0 ± 23.2 μm2 vs. 184.5 ± 6.1 μm2, p < 0.0001, Fig. 4A). We found in these seeding experiments with static culture that seeding concentrations of 1 - 2 × 106 cells/ml resulted in confluent cell coverage after 24 hours.
Fig. 4.
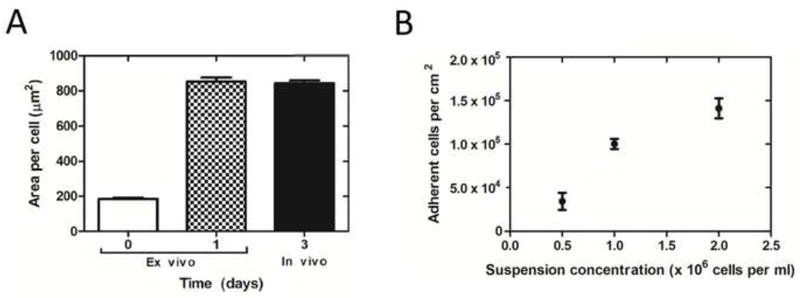
(A) EPC Spreading on Ti tubes at time zero after seeding, after 1 day of static culture ex vivo, and after three days in vivo. EPCs’ area was significantly greater at 1 and 3 days than at time zero (p < 0.0001, n = 3 at 1 day, n = 4 at 0 and 3 days, 1-way ANOVA and post hoc t-test). (B) EPC surface coverage with suspension density. The number of adherent cells per area significantly increased as suspension concentration increased (p < 0.001, r2 = 0.82, n = 3 for each concentration, linear regression analysis).
3.5. EPC suspension density
The number of adherent cells inside the Ti tubes immediately after the 30 min seeding process increases with the cell suspension concentration (Fig. 4B). To compensate for any possible loss of EPCs during handling in the surgical implantation, we determined the optimal seeding density of 2 × 106 cells/ml. This resulted in a greater number of cells adhering to the surface than required for a confluent layer of EPCs inside the Ti tubes (1.41 ± 0.11 × 105 cells/cm2 adherent vs. 1.04 ± 0.03 × 105 cells/cm2 at confluence after 24 hours of static culture, n = 3 for each concentration tested). The optimal suspension density was used for all in vivo experiments. Only 34% ± 9% (n = 9) of the total number of cells in suspension adhered, and the percentage of adherent cells was not significantly different for different suspension densities (p = 0.27).
3.6. Thrombosis results
Thrombosis results, as scored by blinded reviewers, are as follows: In the bare metal control group, 2 of 4 implants were fully clotted (Fig. 5 A,B) and 2 of 4 implants were partially clotted (Fig. 5 C,D). All EPC-seeded Ti tubes (4 of 4) were free of clot after 3 days implantation (Fig. 5E,F). By Fisher’s exact test [21], the treatment significantly protected against thrombosis (p < 0.03).
Fig. 5.
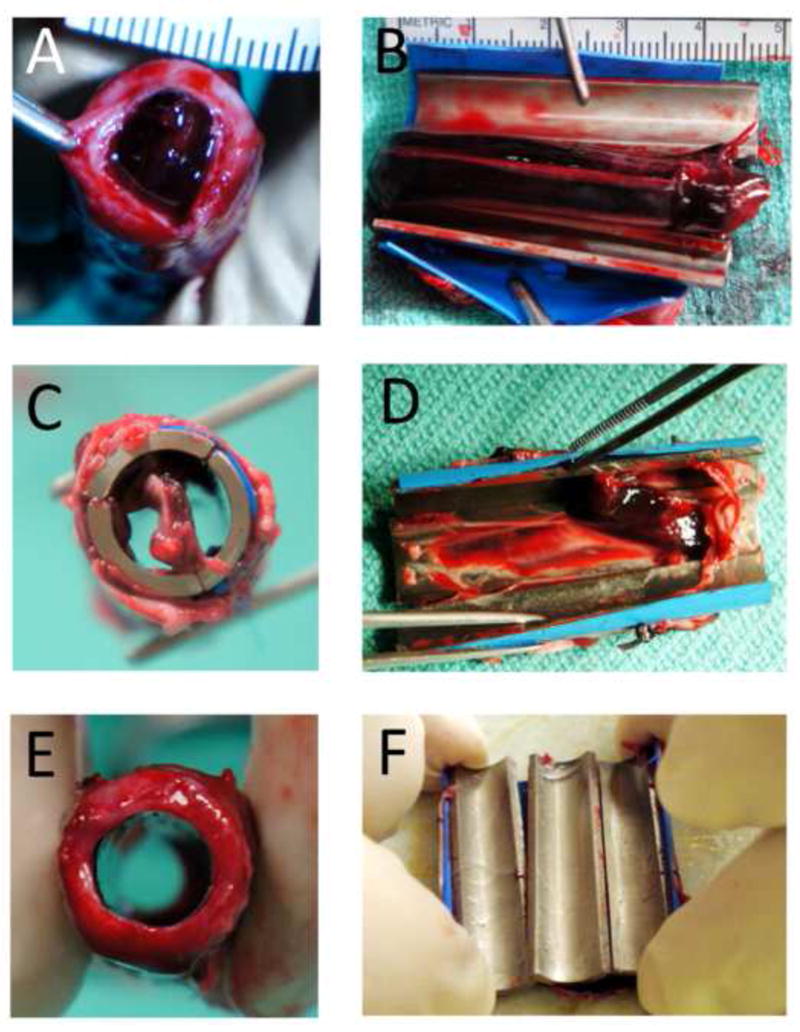
Categorization of thrombosis outcomes. Representative pictures of Ti tubes (A, B) fully clotted, (C, D) partially clotted, and (E, F) not clotted. Tubes are shown in two orientations - a transverse view with tubes intact (left column) - and in a longitudinal view of the inner surface after tube dissection (right column).
3.7. EPC retention and spreading inside Ti tubes in vivo
The autologous fluorescent EPCs, which were seeded onto Ti tubes before surgery, were visualized on every one of the 4 cell-seeded implants after 3 days in vivo and found to have spread on the surface to form a confluent monolayer (Fig. 6A,B). The area per cell was significantly greater after 3 days exposed to blood flow than immediately following cell seeding (842.5 ± 16.0 μm2 vs. 184.5 ± 6.1 μm2, p < 0.0001), but not significantly different than the area of confluent cells cultured under static conditions ex vivo (842.5 ± 16.0 μm2 vs. 852.0 ± 23.2 μm2, p = 0.74, Fig. 4A). All endothelial cells visualized on the EPC-seeded Ti tubes contained PKH26 (red), suggesting that they were of pre-seeded EPC origin. Immunohistochemical counterstaining of the pre-labeled fluorescent EPCs on the explanted Ti device surface with anti-PECAM-1 antibodies confirmed that all endothelial-like cells on the Ti surface had maintained their endothelial-like morphology in vivo (Fig. 6A,B). Since there were no endothelial cells observed on the surface that did not exhibit the pre-implantation florescent EPC label, we conclude that no ‘fall-out’ endothelial cells from blood had adhered to and populated the implant surface. Anti-PECAM-1 staining of thrombus-free areas in the partially occluded Ti tubes failed to reveal any endothelial cells. However, we did find clusters of leukocytes (based on nuclear morphology) in those areas of bare metal implants (Fig. 6C).
Fig. 6.
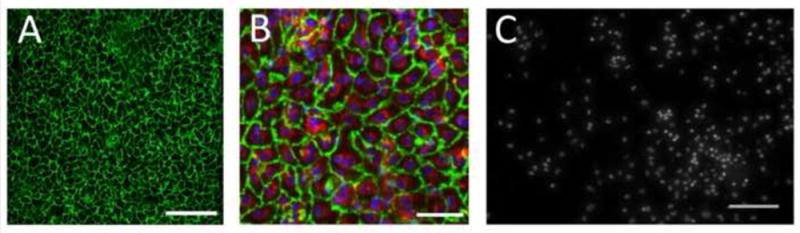
EPCs on Ti surface were stained for PECAM-1 (green) after 3 days in porcine IVC. (A) Scale bar = 200 μm. (B) EPCs additionally showing pre-implantation stain PKH26 (red) and nuclei stained with Hoechst 34580 (blue). Scale bar = 50 μm. (C) Cell nuclei on bare metal control implant surface stained with Hoechst 34580, showing presence of macrophages, T-cells and granulocytes in a non-uniform distribution. Scale bar = 100 μm.
4. Discussion
Our results demonstrate that blood-derived EPCs can protect against thrombosis in a short-term large animal model. We chose to use pigs as the animal model for this study because their anatomy and physiology closely resembles that of humans [26]. Additionally, the vessel size of our 50 kg pigs is very similar to those of children or small adults.
Since our ex vivo studies had previously demonstrated that late outgrowth EPCs on Ti surfaces can withstand more than 5 to 10-fold higher shear stresses than they would encounter in the arterial circulation [7], we elected to evaluate the EPCs’ functional benefit in the low shear stress environment of the inferior vena cava, which biases our outcomes towards thrombosis, rather than implanting them into the higher shear stress environment of the aorta. Furthermore, porcine blood is hypercoagulable as compared to human blood [8]. Still, EPC treatment was able to prevent clot formation in all 4 of 4 implants. Moreover, in our earlier studies we had shown that EPC treatment blocks platelet adhesion to the titanium surface [7]. Therefore, we believe that EPC treatment will even more so prevent thrombosis in humans, especially when utilized to line intravascular devices in the higher shear stress environment of the arterial system. In order to prevent the introduction of bias in evaluating our surgical outcomes – device thrombosis or patency – we had 3 different investigators, who were blinded to the treatment of Ti tubes, and had them score the degree of thrombosis in high resolution photographs of the implants. Whereas everyone had judged the cell-treated devices to be free of clot, there was some disagreement among investigators in 2 control implants as to whether they should be classified as fully thrombosed or partially thrombosed. According to our scoring system, these were automatically biased towards the null-hypothesis of no difference between the groups and ranked as only partially thrombosed. Still, we were able to find a statistically significant difference in the outcomes of our 8 consecutive surgeries with p < 0.03.
It is well established that the duration of surgery and anesthesia time is associated with the risk for venous thrombosis [27-29]. Therefore, we compared the total time each pig was immobilized for the procedure (total time of anesthesia) between the groups. We did not find any significant difference – in fact, the anesthesia time was slightly longer in the EPC-treated device implantations as compared to that of bare metal devices. Furthermore, the cross clamp time is directly related to venous thromboembolism [30]. Therefore we compared the average clamp times between treatment group and control group and also failed to find a significant difference.
Since all technical aspects of the surgeries were identical and bias was avoided in the scoring of surgical outcomes, we conclude that our results are a real representation of the protective effect of autologous blood-derived EPCs in our large animal model. We believe that this conclusion is confirmed by the presence of our fluorescently pre-labeled EPCs in all 4 implants after 3 days in vivo.
However, cells other than EPCs were also observed on some of the Ti blood-contacting surfaces. These cells were present overlying confluent areas of EPCs (as confirmed by PKH26 and PECAM-1 stains,Fig. S2 A,B). Based on the cells’ nuclear morphology, they appeared to be cells of the innate immune system. The distribution of these leukocytes was non-uniform with some fields of view revealing large aggregations while others showed very few, if any at all. We hypothesize that the presence of leukocytes relates to either the low shear environment ‘activating’ EPCs, or to the short-term nature of the study and the fact that Ti tubes were explanted 3 days after the initial surgical insult. Testing of these hypotheses will be possible in future longer-term implantation studies.
One other limitation of our study is the observation that we did not find 100% of the Ti blood-contacting surface area covered with EPCs. We contribute this to the cells ‘peeling off’ as a sheet during the disassembly process of the Ti tube. In fact, we did see distinctive ‘peel line’ demarcations when examining our implants (Fig. S3). Since the implants had been fixed in paraformaldehyde prior to disassembly, we hypothesize that cells are more strongly linked to each other than they are to the underlying Ti surface - and invariably separate from the undersurface when the Ti tube sections are broken apart. In future evaluations of currently ongoing long-term implantations, we may be able to prevent this problem by either utilizing an endoscope to examine the device lumen without taking it apart after explantation, or a Cellvizio LAB imaging system (Visualsonics) that allows for insertion of a small diameter fiberoptic microprobe percutaneously into the IVC and device lumen to image the inside surface in live animals. We have already successfully conducted an exploratory trial with the minimally invasive Cellvizio system in 2 long-term implantations in vivo to ascertain presence of pre-labeled EPCs in patent implants (3 weeks, Fig. S4 A,B). Our X-ray photoelectron spectroscopy spectra and optical profilometry results show that our Ti tubes are similar to titanium-coated biomedical implants in atomic composition and surface roughness. Studies are currently underway to adapt our technology of point-of-care seeding to self-expanding Ti-coated Nitinol stents. The same principles, however, can be used to coat many other implantable devices, e.g. the inflow cannula of ventricular assist devices in an effort to engineer an anti-thrombotic universal inflow conduit for mechanical circulatory support systems.
Since EPCs used in this proof-of-concept study are derived from peripheral blood, invasive methods of harvesting endothelial cells are avoided. Especially in sick patients who are most likely to benefit from implantation of cell-coated devices, an additional surgery to harvest cells could be avoided. Unlike other types of stem cells, e.g. induced pluripotent stem cells [31], our autologous EPCs have the important advantage of not requiring immunosuppression of the recipient patient. This not only avoids the significant side effects of immunosuppressive drugs and but also their propensity to expose patients to opportunistic infections.
Finally, our technology avoids impractical ex vivo culture times of EPCs on the device surface. Whereas cell-seeding approaches have been hindered by the requirement to culture cells together with the device before implantation into a patient, we now have successfully demonstrated that blood-derived EPCs will spread on the three-dimensional Ti-blood-contacting surface after only a short seeding time with our rotating seeding device at the point-of-care and form a confluent cell layer in vivo.
Longer-term implantation studies are currently underway with the goal of translating this cell-seeding technology into clinical practice.
5. Conclusions
We have demonstrated the feasibility to utilize autologous blood-derived endothelial progenitor cells to seed the blood-contacting surface of vascular devices at the point-of-care. Further we have provided first proof-of-concept in a large animal model that such EPC-coatings of titanium surfaces protect against device thrombosis. This technology can be used to protect Ti blood-contacting surfaces, e.g. Nitinol vascular stents, mechanical circulatory assist devices, and blood-contacting extracorporeal devices against the devastating complications of device thrombosis and thromboemboli formation. Longer-term implantation studies are needed to pave the way for translating this technology into clinical practice.
Supplementary Material
Acknowledgments
We thank Gemini Bio-Products for providing the porcine serum used in this study. We would] also like to thank the NIH for supporting this work through Grant “Autologous EPC lining to improve biocompatibility of circulatory assist devices”, RC1HL099863-01, and the National Science Foundation Graduate Research Fellowship for its financial support of Alexandra Jantzen. We are indebted to Steven Owen for machining Ti tubes and assembling the seeding device; George Quick, Mike Lowe and Ianthia Parker for handling our research animals; Kevin Collins for assisting with EPC isolation; Lukas Keil and Cherry Liu for collecting blood samples; Sa Do Kang and Visakha Suresh for analyzing images, and Drs. Marcus Darrabie, Dean Troupes, Antonio Jose Arciniegas and Jose Mantilla for assisting with explant surgeries.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Achneck HE, Sileshi B, Parikh A, Milano CA, Welsby IJ, Lawson JH. Pathophysiology of bleeding and clotting in the cardiac surgery patient: from vascular endothelium to circulatory assist device surface. Circulation. 2010;122:2068–77. doi: 10.1161/CIRCULATIONAHA.110.936773. [DOI] [PubMed] [Google Scholar]
- 2.Schillinger M, Sabeti S, Dick P, Amighi J, Mlekusch W, Schlager O, et al. Sustained benefit at 2 years of primary femoropopliteal stenting compared with balloon angioplasty with optional stenting. Circulation. 2007;115:2745–9. doi: 10.1161/CIRCULATIONAHA.107.688341. [DOI] [PubMed] [Google Scholar]
- 3.Maleux G, Marrannes J, Heye S, Daenens K, Verhamme P, Thijs V. Outcome of carotid artery stenting at 2 years follow-up: comparison of nitinol open cell versus stainless steel closed cell stent design. J Cardiovasc Surg (Torino) 2009;50:669–75. [PubMed] [Google Scholar]
- 4.Dick P, Wallner H, Sabeti S, Loewe C, Mlekusch W, Lammer J, et al. Balloon angioplasty versus stenting with nitinol stents in intermediate length superficial femoral artery lesions. Catheter Cardiovasc Interv. 2009;74:1090–5. doi: 10.1002/ccd.22128. [DOI] [PubMed] [Google Scholar]
- 5.Duda SH, Bosiers M, Lammer J, Scheinert D, Zeller T, Oliva V, et al. Drug-eluting and bare nitinol stents for the treatment of atherosclerotic lesions in the superficial femoral artery: long-term results from the SIROCCO trial. J Endovasc Ther. 2006;13:701–10. doi: 10.1583/05-1704.1. [DOI] [PubMed] [Google Scholar]
- 6.Slottow TL, Waksman R. Drug-eluting stent safety. Am J Cardiol. 2007;100:10M–7M. doi: 10.1016/j.amjcard.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Achneck HE, Jamiolkowski RM, Jantzen AE, Haseltine JM, Lane WO, Huang JK, et al. The biocompatibility of titanium cardiovascular devices seeded with autologous blood-derived endothelial progenitor cells: EPC-seeded antithrombotic Ti Implants. Biomaterials. 2011;32:10–8. doi: 10.1016/j.biomaterials.2010.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velik-Salchner C, Schnurer C, Fries D, Mussigang PR, Moser PL, Streif W, et al. Normal values for thrombelastography (ROTEM) and selected coagulation parameters in porcine blood. Thromb Res. 2006;117:597–602. doi: 10.1016/j.thromres.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Kang C, Bonneau M, Brouland JP, Bal dit Sollier C, Drouet L. In vivo pig models of venous thrombosis mimicking human disease. Thromb Haemost. 2003;89:256–63. [PubMed] [Google Scholar]
- 10.Dal Nogare AR, Toews GB. Characteristics of alveolar macrophages in an animal model of resolving pulmonary inflammation. Am Rev Respir Dis. 1990;142:660–7. doi: 10.1164/ajrccm/142.3.660. [DOI] [PubMed] [Google Scholar]
- 11.Ueberrueck T, Tautenhahn J, Meyer L, Kaufmann O, Lippert H, Gastinger I, et al. Comparison of the ovine and porcine animal models for biocompatibility testing of vascular prostheses. J Surg Res. 2005;124:305–11. doi: 10.1016/j.jss.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 12.Achneck HE, Serpe MJ, Jamiolkowski RM, Eibest LM, Craig SL, Lawson JH. Regenerating titanium ventricular assist device surfaces after gold/palladium coating for scanning electron microscopy. Microsc Res Tech. 2010;73:71–6. doi: 10.1002/jemt.20757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeGarmo PE, Black JT, Kohser RA. Materials and Processes in Manufacturing. Materials and Processes in Manufacturing. 9. Wiley; 2003. p. 223. [Google Scholar]
- 14.Seldinger SI. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta radiol. 1953;39:368–76. doi: 10.3109/00016925309136722. [DOI] [PubMed] [Google Scholar]
- 15.Achneck HE, Jamiolkowski RM, Jantzen AE, Li M, Haseltine JM, Galinat LJ, et al. Blood-derived endothelial progenitor cells (epcs) create biocompatible lining for titanium blood-contacting surfaces; American Heart Association Scientific Sessions, Abstract Oral Sessions, Medical Aspects End Stage Heart Failure: Transplantation and Device Therapies; Chicago, Illinois. 2010. [Google Scholar]
- 16.Truskey GA, Yuan F, Katz DF. 2.7.3 Pressure-Driven Flow through a Cylindrical Tube. In: Horton MJ, editor. Transport Phenomena in Biological Systems. Upper Saddle River: Pearson Education; 2009. [Google Scholar]
- 17.Raymond MA, Smith ER, Liesegang J. The physical properties of blood--forensic considerations. Sci Justice. 1996;36:153–60. doi: 10.1016/S1355-0306(96)72590-X. [DOI] [PubMed] [Google Scholar]
- 18.Truskey GA, Yuan F, Katz DF. Rheology and Flow of Blood. In: Horton MJ, editor. Transport Phenomena in Biological Systems. Upper Saddle River, New Jersey 07458: Pearson Education, Inc.; 2004. pp. 103–5. [Google Scholar]
- 19.Wegener J, Janshoff A, Galla HJ. Cell adhesion monitoring using a quartz crystal microbalance: comparative analysis of different mammalian cell lines. Eur Biophys J. 1999;28:26–37. doi: 10.1007/s002490050180. [DOI] [PubMed] [Google Scholar]
- 20.Rameis MT, Cei S, Bernardi J, Watzek G, Gruber R. Development of an in vitro model on cellular adhesion on granular natural bone mineral under dynamic seeding conditions--a pilot study. J Biomed Mater Res B Appl Biomater. 2009;91:766–71. doi: 10.1002/jbm.b.31453. [DOI] [PubMed] [Google Scholar]
- 21.Fisher RA. Statistical Methods for Research Workers Collection of the University of Michigan Library. 1925. [Google Scholar]
- 22.Moulder JF, Stickle WF, Sobol PE. Handbook of X Ray Photoelectron Spectroscopy (P/N 624755): Perkin-Elmer. Physical Electronics Division. 1992 [Google Scholar]
- 23.Samaroo HD, Lu J, Webster TJ. Enhanced endothelial cell density on NiTi surfaces with sub-micron to nanometer roughness. Int J Nanomedicine. 2008;3:75–82. doi: 10.2147/ijn.s2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nappo F, Esposito K, Cioffi M, Giugliano G, Molinari AM, Paolisso G, et al. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: role of fat and carbohydrate meals. J Am Coll Cardiol. 2002;39:1145–50. doi: 10.1016/s0735-1097(02)01741-2. [DOI] [PubMed] [Google Scholar]
- 25.Li H, Cybulsky MI, Gimbrone MA, Jr, Libby P. An atherogenic diet rapidly induces VCAM-1, a cytokine-regulatable mononuclear leukocyte adhesion molecule, in rabbit aortic endothelium. Arterioscler Thromb. 1993;13:197–204. doi: 10.1161/01.atv.13.2.197. [DOI] [PubMed] [Google Scholar]
- 26.Rashid ST, Salacinski HJ, Hamilton G, Seifalian AM. The use of animal models in developing the discipline of cardiovascular tissue engineering: a review. Biomaterials. 2004;25:1627–37. doi: 10.1016/s0142-9612(03)00522-2. [DOI] [PubMed] [Google Scholar]
- 27.Achneck HE, Sileshi B, Lawson JH. Review of the biology of bleeding and clotting in the surgical patient. Vascular. 2008;16(Suppl 1):S6–13. [PubMed] [Google Scholar]
- 28.Jaffer AK, Barsoum WK, Krebs V, Hurbanek JG, Morra N, Brotman DJ. Duration of anesthesia and venous thromboembolism after hip and knee arthroplasty. Mayo Clin Proc. 2005;80:732–8. doi: 10.1016/S0025-6196(11)61526-7. [DOI] [PubMed] [Google Scholar]
- 29.Ellsworth WA, Basu CB, Iverson RE. Perioperative considerations for patient safety during cosmetic surgery - preventing complications. Can J Plast Surg. 2009;17:9–16. [PMC free article] [PubMed] [Google Scholar]
- 30.Forbes TJ, Rosenthal GL, Reul GR, Jr, Ott DA, Feltes TF. Risk factors for life-threatening cavopulmonary thrombosis in patients undergoing bidirectional superior cavopulmonary shunt: an exploratory study. Am Heart J. 1997;134:865–71. doi: 10.1016/s0002-8703(97)80009-9. [DOI] [PubMed] [Google Scholar]
- 31.Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011 doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


