Abstract
Hypoxia inducible factor (HIF-1α) expressed in the nuclei of tumor cells under hypoxic conditions, and is regulated, in part, by cytoplasmic prolyl hydroxylases (PHDs). Since HIF-1α is selectively expressed in tumor cells, inhibitors are under development for cancer therapy. Although methods for detection of HIF-1α and PHDs are available, an immunohistochemical (IHC) double staining method for these markers in individual tumor cells is not available. For the method development, human squamous cell carcinoma (SCC) xenograft A253 was used as a known positive control tissue for HIF-1α in well differentiated areas without microvessels. This laboratory demonstrated that tumor cells in these areas are strongly positive for hypoxia markers. Another human, poorly differentiated SCC xenograft FaDu, without hypoxic areas, was used as a negative control. PHD2 and 3 immunostaining was optimized individually using human kidney. To optimize HIF-1α detection the pressure cooker time for antigen retrieval, concentration of the primary antibody, amplification reagent, and DAB development time were decreased. Casein blocking further decreased background. The double staining resulted in brown nuclei for HIF-1α (DAB), and pink cytoplasmic staining for PHD2, 3 (fast red). The isotype matched controls were negative. Normal human tissues had no detectable HIF-1α, but expressed PHD2, 3. Potential utility of this new and improved method was confirmed by analyzing fifteen surgical biopsies of oropharyngeal SCC of which 6 were positive for HIF-1α. This new method defined optimal conditions for detection of HIF-1α and PHDs in individual tumor cells, and could have diagnostic and therapeutic potential.
Keywords: hypoxia, HIF-1α/PHDs, double immunostaining, immunohistochemistry
Introduction
Many solid cancers contain regions of hypoxia. Rapid cancer cell proliferation is faster than the proliferation of the endothelial cells forming very irregular and chaotic neovasculature, which results in the development of regional hypoxia in the tumor. Intratumoral hypoxia activates the key transcriptional factor, hypoxia – inducible factor 1 (HIF-1α). This mediates the activation of more than one hundred genes in tumor cells to adapt to a low oxygen environment, and promote continued tumor growth, resistance to chemo/radiotherapy. HIF-1α is expressed in a wide range of human solid tumors and its expression correlates with increased angiogenesis, chemo/radio resistance, and poor patient prognosis. Current efforts are underway to develop HIF-1α inhibitors, and to test their efficacy as potential anticancer agents. 1–3
As with other proteins, the level of HIF-1α cellular accumulation is determined by the rate of protein synthesis and degradation. Under normoxic conditions, oxygen dependent hydroxylation of prolin in HIF-1α by two enzymes, prolyl hydroxylase 2 and 3 (PHD2, 3), is the key step which leads to the recognition of HIF-1α by von Hippel – Lindau (VHL) protein, and degraded through the ubiquitin-proteosome pathway. Therefore under normoxic conditions, like in normal organs, HIF-1α is rapidly degraded and thus undetectable. Under hypoxic conditions, however, prolin hydroxylation and the level of PHD2 and 3 decreases, and VHL cannot bind to HIF-1α, resulting in a decreased rate of HIF-1α degradation, thus HIF-1α is expressed under hypoxia.1, 3 Although the main oxygen dependent regulators of HIF-1α are PHD 2 and 3, other oxygen independent mechanisms such as HSP90 and RACK1, have recently been described. 4, 5
Currently there are no double staining procedures for HIF-1α and PHD2 or PHD3 in the literature and there are no commercial kits available. HIF-1α and PHD2/3 are not soluble and therefore not released from the cell. Thus, the PHD2 and/or PHD3 level in the cytoplasm regulates nuclear HIF-1α expression in the same cell. HIF-1α is generally expressed focally. In serial sections, it is difficult to identify tumor cells that preferentially express HIF-1α and/or PHDs with individual immunostaining procedures. For simultaneous detection of HIF-1α and PHDs in individual cells, a double staining method was developed and validated using primary human surgical specimens.
Materials and Methods
Primary Antibodies
Antibody optimization of each of the three procedures was done alone: for HIF-1α (mouse anti-human monoclonal, clone:H1 alpha 67 from Novus Biologicals, Littleton, CO), for PHD2 (rabbit anti-human polyclonal also from Novus), and for PHD3 (rabbit anti-human polyclonal from Abcam, Cambridge, MA) before combining HIF-1α with PHD2, and HIF-1α with PHD3.
Human Tumor Xenografts
Human squamous cell carcinoma (SCC) xenograft (A253) was used as a known positive control tissue. This tumor contains well differentiated areas without microvessels and tumor cells in these areas are strongly positive for the hypoxia markers Hypoxyprobe© and CAIX as published by our lab.6 Therefore, tumor cells in these areas are expected to be hypoxic and positive for HIF-1α. Another human SCC xenograft (FaDu) was used as a known negative control tissue because this tumor is poorly differentiated and well vascularized, and does not contain hypoxic areas stained by hypoxia markers (hypoxyprobe, CAIX) as reported in the same paper.6
HIF-1α IHC Assay
Tissues were fixed in 10% buffered formalin and processed through traditional processes in the automatic tissue processor (Sakura VIP, Torrence, CA), embedded in paraffin, cut at 5μm and placed on charged glass slides. Antigen retrieval was done with Target Retrieval Solution (TRS – Dako, Carpentaria, CA) in a pressure cooker (Cell Marque, Inc. Rocklin, CA) for 3 min. according to the manufacturer’s protocol. Endogenous biotin was blocked with a biotin blocking kit (Dako). The primary mouse monoclonal antibody anti-human HIF-1α (Hi alpha 67 – Novus Biologicals, Littleton, CO) was applied at 0.4μg/ml overnight at 4°C. The primary antibody was diluted in 2.5% goat serum in PBS/T (phosphate buffered saline with 0.05% Tween 20). In the morning, the slides were rinsed in PBS/T, placed on an autostainer (Dako) and the following program was run. A biotinylated goat vs. mouse secondary antibody (Jackson Immuno Research Labs, West Grove PA) was applied for 15 min. and followed by Elite ABC reagent (Vector Labs, Burlingame, CA) for 20 min. The amplification reagent from the Catalyzed Signal Amplification (CSA) System (Dako) was used next, but it was diluted 1/35 (PBS/T) and applied to the slides for 10 min. Streptavidin conjugated to horseradish peroxidase (Zymed/Invitrogen, San Francisco, CA) was used for 20 min. as the last layer. The slide racks were then removed from the machine and the chromogen DAB (Dako) was applied off-line for only 1 min. to visualize the immunoreaction. All washes between the various steps were done with PBS/T. In addition to the washes, 0.03% casein (PBS/T) was applied before each reagent for 5 min. followed by a blow step before the next reagent was applied. This blocking step before each reagent reduced the background considerably. This proved to be a critical step in our previous IHC method development for P-glycoprotein.7 In place of the primary antibody, an isotype match (mouse IgG2b also at 0.4μg/ml) was placed on a duplicate slide as a negative control to verify the staining specifity.
Individual PHD2 and PHD3 IHC Assays
The same antigen retrieval was used as was developed for HIF -1α: TRS (Dako) in a pressure cooker for 3 minutes. The slides were rinsed in PBS/T, placed on the autostainer and the following program was run: 0.03% casein (in PBS/T) for 30 min. was blown off and followed by the primary polyclonal antibody for PHD2 (6μg/ml) or PHD3 (4μg/ml) for one hour. In place of the primary antibody, an isotype match (rabbit IgG at 6μg/ml or 4μg/ml respectively) was placed on a duplicate slide as the negative control. The slides were rinsed in PBS/T, and a rabbit alkaline phosphatase polymer (Leica, Bannockburn, IL) was applied for 30 min. A PBS/T rinse was followed by the chromogen fast red (Dako) for 5 min. Slides were removed from the autostainer after distilled water wash. Hematoxylin (Poly Scientific) was used as the counterstain. Slides were then washed in water and mounted with Faramount aqueous mounting medium (Dako).
HIF-1α/PHD2 double IHC Assay
The HIF -1α procedure described above was used first, and immediately following the DAB chromogen, the slides were washed with distilled water and then the racks/slides were placed back on the autostainer and the same program was run as for the individual PHD2 detection. In place of the primary antibodies, an isotype match (mouse IgG2b at 0.4μg/ml for HIF-1α and rabbit IgG at 6μg/ml for PHD2) was placed on a duplicate slide as the negative control for both antibodies.
HIF-1α/PHD3 double IHC Assay
The same procedure was followed as for HIF -1α/PHD2, except the concentration of the PHD3 primary antibody was 4μg/ml applied for one hour. In place of the primary antibody, an isotype match (mouse IgG2b at 0.4μg/ml for HIF-1α and rabbit IgG at 4μg/ml for PHD3) was placed on a duplicate slide as negative controls for the two antibodies.
Human Tissue Specimens
A tissue microarray (TMA) block containing 15 normal human tissues (kidney, lung, liver, spleen, ovary, testis, colon, adrenal gland, thyroid, pancreas, lymph node, stomach, brain, endometrium, myometrium) and conventional surgical blocks from 15 de-identified human oropharyngeal SCC, were used for validation of the double IHC methods. In the TMA block, each organ was represented by 3–4 cores (0.6 mm in diameter) from different cases. The IHC for the single markers was used first, to assure accuracy of the staining patterns before combining antibodies for double IHC. All cases of the human SCC were double stained as well, to confirm the accuracy of the double staining procedures. All slides were reviewed by K. T. a board certified, experienced pathologist.
Results
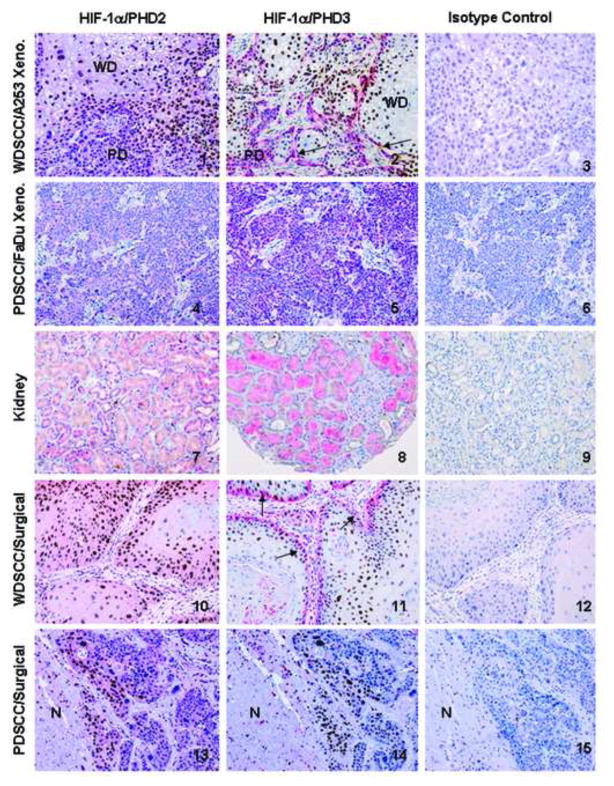
Figure 1 shows the double-staining method for detection of HIF-1α and PHDs in individual cells in several representative photomicrographs that demonstrate the efficacy of the method. HIF-1α expression is confined to the nuclei (brown) of the tumor cells and PHD2/3 is expressed in the cytoplasm as expected and demonstrated by the panels. Panels 1, 2 show that in our model xenograft tumors, HIF-1α was expressed predominately in the well differentiated (WD) part of the tumors associated with low or no expression of PHD2/3. Panels 2, 11 show that WD areas express HIF-1α in nuclei (brown) without PHD3 expression in the cytoplasm. Arrows show one layer of poorly differentiated (PD) tumor cells at the rim of WD areas, where the majority of nuclei are HIF-1α negative, with strong PHD3 positive cytoplasm (strong pink) indicating that there is a difference in PHD3 expression in HIF-1α positive and negative tumor cells. To test the specificity of the double immunostaining, a duplicate slide was treated with an isotype match for both antibodies, mouse IgG2b for HIF-1α, and rabbit IgG for PHD2 and 3 in the same concentrations as the primary antibodies. Panels 3, 6, 9, 12, and 15 are the corresponding isotype controls, and did not show any nuclear or cytoplasmic reactivity in the tumor or normal cells, convincingly demonstrating the specificity of the immunostaining procedures. Panels 1, 2, 4, and 5 demonstrate that the PD tumor cells in A253 and the PD tumor FaDu did not express HIF-1α, and the majority of tumor cells expressed PHD2/3. Panels 7 and 8 show that normal human kidney has no HIF-1α expression along with all 15 normal, non-hypoxic human tissues (not shown). The tubular epithelium expressed PHD2/3 in various intensities in the cytoplasm. The lack of HIF-1α expression in the other normal organs was also linked with various levels of PHD2/3 expression (not shown) confirming previous findings.8 There were individual differences in expression among the same type of samples. Panels 10 and 11 illustrate that one of the human WD SCC has a very similar expression and distribution pattern for both HIF-1α and PHD2/3 that is seen in WD SCC xenograft model A253, namely that HIF-1α positivity is present mainly in the WD tumor cells.
Figure 1.
Demonstration the colocalization of HIF-1α/PHD2,3 by double immunohistochemical staining method (HIF-1α: brown nuclei; PHD2,3: pink cytoplasm) in formalin/paraffin sections of human well differentiated (WD) squamous cell carcinoma (SCC) xenograft A253 (Panels 1–3); poorly differentiated (PD) SCC xenograft FaDu (Panels 4–6); kidney as normal human tissue (Panels 7–9); and representative surgical samples of oropharyngeal SCC: WD (Panels 10–12) and PD (Panels 13–15). All sections were counterstained with hematoxylin. Original magnification of each photomicrograph is the same (200X). 1: The WD part of the tumor is hypoxic (because of the lack of blood vessels) therefore, the cancer cell nuclei are positive for HIF-1α (brown) and PHD2 expression is not detectable or low in the cytoplasm. 2: Same pattern of immunostaining is seen for HIF-1α and PHD3, as in the previous panel demonstrating the inverse correlation between them. 2 and 11: arrows show one layer of PD tumor cells at the rim of WD areas, where the majority of nuclei are HIF-1α negative, with strong PHD3 positive cytoplasm (strong pink) indicating that there is a difference in PHD3 expression in HIF-1α positive and negative tumor cells. 3 and 6, 9, 12, 15: are all the corresponding isotype matched negative controls, showing no immunoreactivity in the nuclei or the cytoplasm of tumor or normal cells. These photomicrographs are representative isotype matched controls for both HIF-1α/PHD2 and HIF-1α/PHD3 since none of them showed nonspecific staining. 4: There is no immunoreaction in the nuclei, indicating the lack of HIF-1α expression, but there is expression of PHD2 in the same tumor cells visualized by pink cytoplasmic staining. 5: It demonstrates the same correlation, as panel 4, but for HIF-1α and PHD3. 7: Nuclei of the tubules are unreactive, showing no detectable HIF-1α, while the cytoplasm of the majority of the tubules is pink, indicative for PHD2 expression. 8: Same pattern of expression and distribution is seen for HIF-1α/PHD3 like in panel 7 for PHD2. 10-11: The WDSCC surgical sample shows a very similar expression profile for HIF-1α/PHD2, 3 that is seen in the corresponding histological type of xenografts tumor A253 in panels 1 and 2. 13-14: show that another human PDSCC expressed HIF-1α at the characteristic perinecrotic region of the tumor (N: necrosis). In this tumor HIF-1α positive (brown nuclei) and HIF-1α negative (blue nuclei) tumor cells do not differ either in PHD2 expression (pink) in panel 13, nor in the PHD3 expression (undetectable) in panel 14.
Panels 13 and 14 show that another human PDSCC expressed HIF-1α at the characteristic perinecrotic region of the tumor (N: necrosis). In this tumor HIF-1α positive (brown nuclei) and HIF-1α negative (blue nuclei) tumor cells do not differ either in PHD2 expression (pink) in Panel 13, nor in the PHD3 expression (undetectable) in Panel 14.
Table 1 summarizes the semiquantitative assessment of HIF-1α, PHD2, 3 expressions studied by our double immunostaining methods in formalin/paraffin sections of surgical biopsies from oropharyngeal cancer cases (n=15). Background and/or nonspecific staining was not a limiting factor, so all 15 cases could be evaluated. A tumor was considered negative for HIF-1α, if none of the tumor cells expressed HIF-1α. To evaluate whether a relationship or interaction exists between HIF-1α and PHDs in the same cell, evaluation of fifteen tumors revealed that six tumors were positive for HIF-1α with various overall levels of PHD2, 3. Since in cancer tissue the individual tumor cell borders are not seen clearly, the cytoplasm immediately around the HIF-1α positive (brown) nuclei was evaluated. The data in Table 1 shows that in three of six HIF-1α positive tumors, the cytoplasmic PHD3 expression was lower or undetectable in HIF-1α positive cells than in HIF-1α negative tumor cells, suggesting its regulatory role in these cells. However, in the other three HIF-1α positive tumors, differences in neither PHD2, nor PHD3 cytoplasmic expression between HIF-1α positive and negative tumor cells were detected. These data suggest the possibility that HIF-1α may be regulated by other mechanisms. Among HIF-1α negative surgical samples (Table 1), four PD cancer showed strong expression of PHD2 in the tumor cell cytoplasm (similar to staining pattern seen in PD FaDu xenografts), as compared to the weaker expression seen in WD tumors. Collectively, the data in Table 1 indicates that tumor cells which are positive for HIF-1α show a tendency of expressing lower levels of PHD 3 only. In contrast, cells which are negative for HIF-1α are generally expressing higher levels of PHDs. Further, the expression of PHD2 was uniformly distributed throughout the tumor but with various intensity.
Table 1.
Distribution of HIF-1α, PHD2/3 expressions studied by double immunostaining methods in formalin/parrafin sections of surgical biopsies from oropharyngeal SCC cases.
| HIF-1α positive cases | |||||
|---|---|---|---|---|---|
| Number of cases | Histology | HIF-1α | PHD2 | PHD3 | Difference in PHD2 and/or 3 expression between HIF-1α positive and negative tumor cells |
| 1 | 1 PD | 2 m-s/10 | s/100 | m/30 | no difference |
| 2 | WD | w-s/20 | s/100 | m/30 | PHD3 is lower |
| 3 | WD | s/20 | m/100 | m/30 | no difference |
| 4 | PD | s/10 | s/100 | m-s/90 | PHD3 is lower |
| 5 | WD | m-s/80 | w/100 | s/10 | PHD3 is lower |
| 6 | PD | s/30 | w/100 | m-s/80 | no difference |
|
| |||||
| HIF-1α negative cases | |||||
|
| |||||
| 7 | WD | nd | w/100 | m-s/40 | |
| 8 | WD | nd | w-m/100 | m-s/40 | |
| 9 | WD | nd | w/100 | m-s/80 | |
| 10 | PD | nd | s/100 | m-s/10 | |
| 11 | PD | nd | s/100 | s/70 | |
| 12 | WD | nd | m-s/100 | m-s/50 | |
| 13 | PD | nd | s/100 | s/100 | |
| 14 | WD | nd | w-m/100 | m-s/30 | |
| 15 | PD | nd | m-s/100 | s/40 | |
PD: poorly differentiated WD: well differentiated
Immunostaining: s:strong, m:moderate, w:weak, nd:not detectable
Numbers after the symbols indicate the estimated percentage of immunostained tumor cells.
In our model HIF-1α was expressed predominantly in the WD part of the tumors and not in the PD portion. Table 2 shows that this type of correlation between HIF-1α expression and differentiation is not so characteristic in every surgical sample because it is present whether tumor cells are WD or PD.
Table 2.
Distribution of HIF-1α expression among well differentiated (WD) and poorly differentiated (PD) oropharyngeal squamous cell carcinoma surgical biopsies
| WD | PD | ||
|---|---|---|---|
| Number of cases | 15 | 8 | 7 |
| HIF-1α positive | 6 | 3 | 3 |
| HIF-1α negative | 9 | 5 | 4 |
Discussion
To develop double immunohistochemical procedures for HIF-1α and PHDs, both markers should be reliably and reproducibly detectable individually. For this reason, the method for specific detection of HIF-1α was first optimized. Triple staining for HIF-1α, PHD2 and PHD3 was not considered as a potentially successful option because both PHD2 and 3 are localized in the cytoplasm. There would therefore be a large overlap of those two markers. The immunohistochemical detection of HIF-1α was challenging due to the lack of methodological details in the literature, such as method development, optimization of conditions, and the absence of known positive and known negative controls.
Adapting a method developed by Zhang et al 9, revealed non-specific staining in the cytoplasm in both the slide with the primary antibody, and the isotype matched (IgG 2b) control slide. Several significant modifications were introduced to keep the specific strong nuclear reaction, and to eliminate the nonspecific “very disturbing” cytoplasmic granular staining.
We assumed that the nonspecific background was because of the higher than optimal primary antibody concentration, harsh pretreatment and/or use of a strong amplification kit. Reducing the primary antibody concentration alone did not take care of the background problem. Harsh pretreatments can make the tissue more “sticky” and therefore more difficult to remove excess reagents by washing. Also, if an amplification kit is used, it will amplify the specific and nonspecific signal as well. This prompted us to dilute the amplification reagent (1:35) in order to further reduce the nonspecific signal. After testing all traditional antigen retrieval methods, only the pressure cooker with Target Retrieval Solution (TRS Dako) gave us the nuclear reaction product we knew to be correct. We did however reduce the time in the pressure cooker from 5 minutes to 3 minutes. This substantially reduced the cytoplasmic background staining. Now we had to adjust the primary antibody concentration so we still had signal, but no background. We tried a range of concentrations from 0.1μg/ml up to 15 μg/ml. Using a low concentration with some amplification was necessary to maintain specific signal and keep the non-specific background to a minimum so we reduced the primary antibody to 0.4μg/ml and applied it overnight at 4°C. This low concentration gave an excellent signal and little noise even with amplification. The avidin/biotin and casein blocking incorporation into the protocol further decreased the nonspecific background.
Incidence of HIF-1α expression studied with IHC methods in various locations in SCC of human head and neck varied: 94%, 10 58% in nasopharynx, 11 100%, 12 52%, 13, and 63%. 14 Using the newly developed method, 6 of 15 HIF-1α positive human oropharyngeal SCC cases were detected. This lower incidence of HIF-1α indicates that the high percentage (90–100%) quoted in the literature is most likely overestimated.
In A253 HIF-1α expression was associated with low or no PHD2 and PHD3 expression in the same tumor cells, mainly in the WD part of the tumor. The PD part, and the entire PD FaDu xenograft, was mostly negative for HIF-1α associated with higher levels of PHD2 and PHD3. In normal organs there was a very consistent pattern of immunostaining: none of the nuclei of normal tissues (N=15) expressed HIF-1α, and the cytoplasm showed various intensities of PHD2, 3 with PHD3 expression being less frequent.
The objective of studying the same type of surgical samples like A253 and FaDu xenografts was to generate data regarding the feasibility and efficacy of the new method and to analyze whether the expression based association between the three markers is similar to those found in our model system. Among the six HIF-1α positive cases, PHD2 was not decreased convincingly. As in our A253 xenografts, PHD3 expression decreased, or was not present in three of the surgical cases. Among the HIF-1α negative surgical samples, four PD tumors showed stronger PHD2 expression in the tumor cell cytoplasm than was seen in WD tumors (See Table 1). HIF-1α and PHD2 expression were studied individually in consecutive sections of oral and oropharyngeal cancers (n=44). It was found that most of the less differentiated tumor regions with high PHD2 expression showed downregulation of HIF-1α, 15 consistent with our findings.
In our model, HIF-1α was expressed predominantly in the WD part of the tumor, which was not so obvious in the study analyzing surgical samples (Table 2). In A253, the WD part is highly differentiated while in surgical samples the level of differentiation and the size of the tumor cell groups differ. These factors can influence the blood supply of tumor cells and the presence or absence of hypoxia, which may partially explain the abovementioned discrepancy. The presence of HIF-1α expression in PD surgical cancers is not surprising, because of their larger size than our FaDu tumor weighing 150–200mg without hypoxia. If the tumor was bigger (300–500mg), HIF-1α positive foci could be detected (data not shown).
Similar, but not identical association of HIF-1α, PHD2, 3 and the differentiation status of tumor cells were found in our A253 and FaDu SCC xenograft models as compared to surgical samples of oropharyngeal SCC cases. Our models can not represent all forms of oropharyngeal cancers, because it is well documented that individual human tumors generally show various heterogeneity in phenotypes and genotypes (stage, various level of differentiation, chaotic neovasculature, differences in expression of genes, etc). Tumor cell line xenografts are generally simpler systems. In addition, this was a limited study with low specimen numbers that did not allow a final conclusion, but it indicated the more complex regulation of these markers, and demonstrated the applicability of the method in a clinical setting.
Utilizing the well validated double staining method presented here, should provide a more reliable and reproducible method for simultaneous detection of HIF-1α expression co-localized with PHD2 and 3 expressions. This method could be used to develop drugs targeting HIF-1α and/or PHDs and to evaluate their therapeutic potential in cancer patients. Currently, efforts are underway to develop HIF-1α inhibitors and testing their efficacy as potential anticancer agents. 16 This method could be utilized for the characterization of the molecular pathogenesis of other diseases in which decreased blood supply causes ischemia and hypoxia (like in myocardium, brain, extremities, etc.).
Acknowledgments
Source of Support: This study was supported by a Comprehensive Cancer Center Support Grant CA16056 and by a grant CA133682-01A2 (S. C.) from National Cancer Institute, Bethesda, MD
The authors thank Dr. W. Bshara from the Department of Pathology for his assistance selecting the oropharyngeal cancer cases.
References
- 1.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 2.Mabjeesh NJ, Amir S. Hypoxia-inducible factor (HIF) in human tumorigenesis. Histol Histopathol. 2007;22:559–572. doi: 10.14670/HH-22.559. [DOI] [PubMed] [Google Scholar]
- 3.Hirota K, Semenza GL. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit Rev Oncol Hematol. 2006;59:15–26. doi: 10.1016/j.critrevonc.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Yee Koh M, Spivak-Kroizman TR, Powis G. HIF-1 regulation: Not so easy come, easy go. Trends Biochem Sci. 2008;33:526–534. doi: 10.1016/j.tibs.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Koh MY, Powis G. Haf. The new player in oxygen-independent HIF-1alpha degradation. Cell Cycle. 2009;8:1359–1366. doi: 10.4161/cc.8.9.8303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharya A, Toth K, Mazurchuk R, et al. Lack of microvessels in well-differentiated regions of human head and neck squamous cell carcinoma A253 associated with functional magnetic resonance imaging detectable hypoxia, limited drug delivery, and resistance to irinotecan therapy. Clin Cancer Res. 2004;10:8005–8017. doi: 10.1158/1078-0432.CCR-04-1306. [DOI] [PubMed] [Google Scholar]
- 7.Toth K, Vaughan MM, Slocum HK, et al. New immunohistochemical “Sandwich” Staining method for mdr1 p-glycoprotein detection with jsb-1 monoclonal antibody in formalin-fixed, paraffin-embedded human tissues. Am J Pathol. 1994;144:227–236. [PMC free article] [PubMed] [Google Scholar]
- 8.Soilleux EJ, Turley H, Tian YM, et al. Use of novel monoclonal antibodies to determine the expression and distribution of the hypoxia regulatory factors phd-1, phd-2, phd-3 and fih in normal and neoplastic human tissues. Histopathology. 2005;47:602–610. doi: 10.1111/j.1365-2559.2005.02280.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhong H, De Marzo AM, Laughner E, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5835. [PubMed] [Google Scholar]
- 10.Aebersold DM, Burri P, Beer KT, et al. Expression of hypoxia-inducible factor-1alpha: A novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Cancer Res. 2001;61:2911–2916. [PubMed] [Google Scholar]
- 11.Hui EP, Chan AT, Pezzella F, et al. Coexpression of hypoxia-inducible factors 1alpha and 2alpha, carbonic anhydrase ix, and vascular endothelial growth factor in nasopharyngeal carcinoma and relationship to survival. Clin Cancer Res. 2002;8:2595–2604. [PubMed] [Google Scholar]
- 12.Beasley NJ, Leek R, Alam M, et al. Hypoxia-inducible factors HIF-1alpha and HIF- 2alpha in head and neck cancer: Relationship to tumor biology and treatment outcome in surgically resected patients. Cancer Res. 2002;62:2493–2497. [PubMed] [Google Scholar]
- 13.Koukourakis MI, Giatromanolaki A, Sivridis E, et al. Hypoxia-inducible factor (HIF1a and HIF2a), angiogenesis, and chemoradiotherapy outcome of squamous cell head-and- neck cancer. Int J Radiat Oncol Biol Phys. 2002;53:1192–1202. doi: 10.1016/s0360-3016(02)02848-1. [DOI] [PubMed] [Google Scholar]
- 14.Fillies T, Werkmeister R, van Diest PJ, et al. HIF1-alpha overexpression indicates a good prognosis in early stage squamous cell carcinomas of the oral floor. BMC Cancer. 2005;5:84. doi: 10.1186/1471-2407-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jokilehto T, Rantanen K, Luukkaa M, et al. Overexpression and nuclear translocation of hypoxia-inducible factor prolyl hydroxylase phd2 in head and neck squamous cell carcinoma is associated with tumor aggressiveness. Clin Cancer Res. 2006;12:1080–1087. doi: 10.1158/1078-0432.CCR-05-2022. [DOI] [PubMed] [Google Scholar]
- 16.Semenza GL. Evaluation of HIF-1 inhibitors as anticancer agents. Drug Discov Today. 2007;12:853–859. doi: 10.1016/j.drudis.2007.08.006. [DOI] [PubMed] [Google Scholar]