Abstract
Ertapenem is a new once-a-day parenteral carbapenem antimicrobial agent. The pharmacokinetics of unbound and total concentrations of ertapenem in plasma were investigated in elderly subjects and compared with historical data from young adults. In a single- and multiple-dose study, healthy elderly males and females (n = 14) 65 years old or older were given a 1-g intravenous (i.v.) dose once daily for 7 days. Plasma and urine samples collected for 24 h on days 1 and 7 following administration of the 1-g doses were analyzed by reversed-phase high-performance liquid chromatography. Areas under the concentration-time curve from 0 h to infinity (AUC0-∞) for elderly females and males were similar following administration of 1-g single i.v. doses, and thus, the genders were pooled in subsequent analyses. Concentrations in plasma and the half-life of ertapenem were generally higher and longer, respectively, in elderly subjects than in young adults. The mean AUC0-∞ of total ertapenem in the elderly was 39% higher than that in young subjects following administration of a 1-g dose. The differences were slightly greater for the mean AUC0-∞ of unbound ertapenem (71%). The unbound fraction of ertapenem in elderly subjects (∼5 to 11%) was generally greater than that in young adults (∼5 to 8%). As in young adults, ertapenem did not accumulate upon multiple dosing in the elderly. The pharmacokinetics of ertapenem in elderly subjects, while slightly different from those in young adults, do not require a dosage adjustment for elderly patients.
Ertapenem (INVANZ; Merck & Co., Inc.) is a parenteral β-lactam. It has excellent in vitro activity against gram-positive and gram-negative, aerobic and anaerobic bacteria generally associated with community-acquired and mixed infections (2). The antibiotic is approved for the treatment of complicated intra-abdominal infections, complicated skin and skin structure infections, community-acquired pneumonia, acute pelvic infections, and complicated urinary tract infections. It is a carbapenem with an anionic side chain and a 1-β-methyl group that provides stability against human renal dehydropeptidase I. Its major metabolite is the open-lactam form via dehydropeptidase I. Its protein binding in rat, monkey, and human plasma (about 96%) is nonlinear with increasing concentrations (7).
The objectives of this study were to investigate the pharmacokinetics of single and multiple intravenous (i.v.) doses of ertapenem in healthy elderly subjects and compare and contrast them with previously determined values from healthy young adults (4). The elderly respond differently than the young to some drugs and are often more susceptible to adverse effects. The greater sensitivity of the elderly may be due to altered disposition of the drug and result in higher concentrations (1). The pharmacokinetics of this study was assessed with the concentrations of total and unbound ertapenem in plasma. The plasma protein binding of ertapenem in the elderly and differences between female and male elderly subjects were also evaluated. Except for age and clinical site, the clinical, analytical, and pharmacokinetic aspects of the investigation in elderly subjects were nearly identical to those in the young.
On the basis of both total and unbound ertapenem, the pharmacokinetics of ertapenem in healthy young subjects (4) are nearly dose proportional over a 0.5- to 2-g dose range. The area under the concentration-time curve (AUC) of total ertapenem increases slightly less than dose proportionally, consistent with the plasma protein binding properties of ertapenem (7). The plasma clearance (CLP) of the total drug varies from 27.6 to 36.3 ml/min with doses of 0.5 to 3 g, and the mean apparent half-life (t1/2) is about 3.5 to 4 h. The fraction unbound is ∼5 to 6% at a total concentration of <50 μg/ml of plasma, ∼8% at a total concentration of approximately 150 μg/ml of plasma, and ∼15 to 18% at a total concentration of approximately 300 μg/ml of plasma. Following multiple dosing, the mean plasma profiles on day 8 are comparable to those on day 1, indicating little accumulation in young adults.
MATERIALS AND METHODS
Study design.
In an open-label, single- and multiple-dose study, healthy elderly male and female subjects (n = 14) 65 years old or older (Table 1) were given a 1-g i.v. infusion over 30 min daily for 7 days. The 1-g dose was administered in the fasted state between 8 and 10 a.m. on days 1 to 7. Plasma and urine samples were collected for 24 h on days 1 and 7 following administration of the 1-g dose. Aliquots of the urine samples were collected to measure concentrations of creatinine to determine creatinine clearance. Plasma samples were stored at −70°C and later analyzed for total and unbound drug. Urine samples collected for ertapenem assay were stabilized at the study site with 0.1 M 2-(N-morpholino)ethanesulfonic acid salt (MES) buffer (pH 6.5), stored at −70°C, and later analyzed for total drug.
TABLE 1.
Subject characteristics
| Group | No. of:
|
Mean (SD)
|
|||
|---|---|---|---|---|---|
| Males | Females | Age (yr) | Wt (kg) | Creatinine clearance (ml/min) | |
| Elderly subjects | 8 | 6 | 73.1 (4.8) | 72.7 (9.1) | 81.8 (13.4) |
| Young adultsa | 8 | 8 | 32.8 (6.0) | 69.6 (13.2) | 112.7 (21.6) |
Data are from reference 4.
The study protocol and consent forms were reviewed and approved by the North Staffordshire Research Ethics Committee. All subjects gave written informed consent for participation in the study.
Bioanalytical procedures.
Plasma and urine samples were analyzed by reversed-phase high-performance liquid chromatography with UV absorbance detection (300 nm) (5). The plasma and urine assays for total drug involved online extraction with column switching of stabilized sample aliquots with lower limits of quantitation of 0.125 and 1.25 μg/ml, respectively. For unbound drug in plasma, samples were filtered first with a Centrifree device and the filtrate was analyzed by high-performance liquid chromatography with a lower limit of quantitation of 0.25 μg/ml (6). Nonspecific binding of ertapenem to the Centrifree device after filtration was <3%. The intraday accuracy and precision of the plasma and urine assays did not deviate more than 15% of nominal or exceed a 10% coefficient of variation (n = 5) at all standard concentrations, respectively. During batch analysis, quality control concentrations did not deviate by more than 20% of nominal.
Pharmacokinetic procedures.
The AUC was estimated from 0 h to the last measurable concentration (AUC0-t) by the linear-log trapezoidal rule. The apparent terminal elimination rate constant (β) was determined with a weighted (1/y), monoexponential curve-fitting program, Sigma Plot. The apparent t1/2 was calculated as ln 2/β. The AUC from 0 h to infinity (AUC0-∞) was calculated as the sum of AUC0-t and the last measurable concentration divided by β. The CLP was obtained by dividing the actual dose administered by the corresponding AUC0-∞. The renal clearance (CLR) was estimated from the urinary recovery of drug (fe) and corresponding increments of AUC. Creatinine clearance values were measured for the elderly subjects and based on the Cockcroft-Gault estimate for young adult subjects.
Statistical methods.
The pharmacokinetic data (except t1/2) were (natural) log transformed prior to statistical analysis.
The pharmacokinetics of ertapenem of elderly males and females were compared initially to determine if it is appropriate to pool the data of males and females. The geometric mean ratios (female/male) for unbound and total AUC0-∞ and associated 90% CIs were calculated on the basis of an analysis of covariance model that contained the gender and covariate weight.
To compare the AUC0-∞ values of unbound and total ertapenem following administration of a single 1-g dose in elderly and young adults, geometric mean ratios (elderly/young) and the corresponding 90% CIs were calculated with an analysis-of-covariance model including the factors age and gender and a covariate, body weight. The demographics of the elderly and young subjects compared in this study are listed in Table 1.
To compare the AUCs of unbound and total ertapenem following administration of 1-g doses on days 7 and 1, geometric mean ratios (day 7/day 1) and associated 90% CIs were calculated by analysis of variance. The comparison of interest was the geometric mean ratios of AUC0-24 following administration of the last dose on day 7 relative to the AUC0-24 following administration of the first dose on day 1.
To compare the nonrenal clearance (CLNR) values of unbound and total ertapenem following administration of a single 1-g dose in elderly and young adults, geometric mean ratios (elderly/young) and the corresponding 90% CIs were calculated by analysis of variance.
RESULTS
Fifteen subjects were included in the study and received 1-g i.v. doses once a day for 7 days. No serious clinical adverse experiences were observed. The most common adverse experiences were headache, dizziness, and somnolence, which were mostly judged by the investigator to be not drug related. A few episodes of diarrhea and nausea were also reported and considered in 50% of the cases to be drug related by the investigator. There were few adverse laboratory experiences and no clinically significant changes in the physical examinations. One subject was eliminated because of two adverse experiences after receiving a single 1-g i.v. dose. This 74-year-old Caucasian female did not continue in the study because of moderate nausea and dizziness that were determined by the investigator to be drug related.
The pattern of adverse clinical experiences found for young healthy subjects receiving single 1-g doses and multiple 1-g doses (4) was comparable to that observed for the elderly subjects.
The mean profiles of total ertapenem concentrations in the plasma of elderly men and women are comparable: the end-of-infusion concentrations appear to be slightly higher in elderly females than in elderly males, and the terminal t1/2 appears to be slightly less in elderly females than in elderly males. Similar trends were observed between young male and female adults (4). The mean AUC0-∞ values for total and unbound ertapenem (Table 2) following administration of 1-g doses in elderly males and females are comparable, with geometric mean ratios (males/females) of 0.93 and 1.02 (90% CIs, 0.71 to 1.22 and 0.76 to 1.37, respectively). As a result, the genders were pooled in subsequent pharmacokinetic analyses.
TABLE 2.
Pharmacokinetic parameters for unbound and total ertapenem following administration of a 1-g single dose in elderly and young adults
| Parameter and age group | n | Mean ± SD
|
|
|---|---|---|---|
| Total drug | Unbound drug | ||
| AUC0-∞ (μg · h/ml) | |||
| Elderly females | 6 | 733.5 ± 52.1 (743.1)a | 58.1 ± 6.3 (55.5)a |
| Elderly males | 8 | 787.6 ± 122.7 (800.9)a | 53.3 ± 6.3 (54.5)a |
| Elderly males and females | 4 | 781.5 ± 95.9 (779.7)a | 55.4 ± 7.6 (55.7)a |
| Young males and females | 6 | 572.1 ± 68.6 (562.1)a | 33.2 ± 5.5 (32.6)a |
| AUC0-24 (μg · h/ml) | |||
| Elderly (day 1) | 14 | 746.1 ± 79.4 (742.0)a | 52.4 ± 7.3 (52.2)a |
| Elderly (day 7)b | 14 | 681.9 ± 60.0 (679.3)a | 47.5 ± 7.0 (47.2)a |
| t1/2 (h) | |||
| Elderly | 14 | 5.2c | 4.7c |
| Young | 16 | 3.8 ± 0.5 | |
| CLR (ml/min) | |||
| Elderly | 14 | 8.6 ± 2.2 | 122.4 ± 28.6 |
| Young | 16 | 12.9 ± 4.3 | 223.3 ± 67.8 |
| CLNR (ml/min) | |||
| Elderly | 14 | 13.0 ± 2.9 | 184.2 ± 50.9 |
| Young | 16 | 16.1 ± 5.4 | 289.8 ± 117.8 |
| fe (% of dose) | |||
| Elderly | 14 | 37.6 ± 9.6 | |
| Young | 16 | 44.4 ± 14.8 | |
Geometric mean.
Day 7 AUC0-24 after daily administration of a 1-g i.v. dose for 7 days.
Harmonic mean.
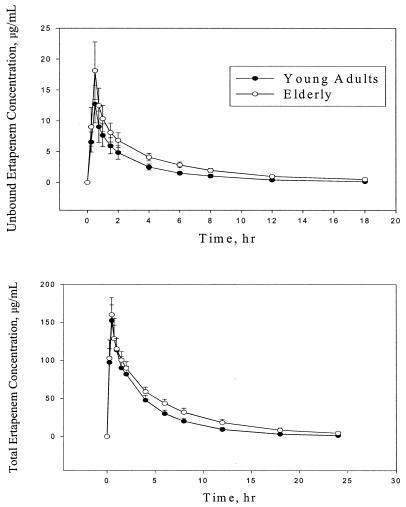
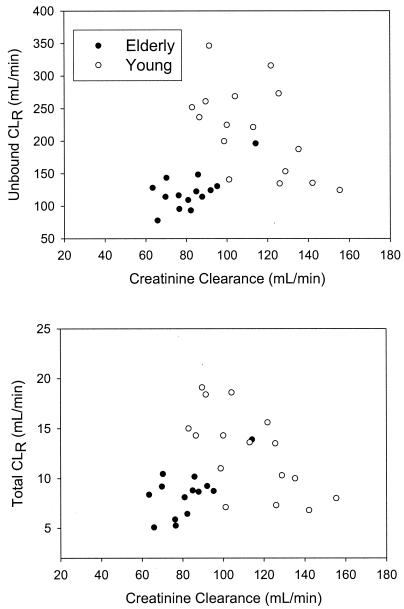
Concentrations of total and unbound ertapenem in plasma were generally higher in elderly subjects than in young adults. The mean plasma concentration-time profiles for elderly and young adults are show in Fig. 1. The mean AUC0-∞ value of total ertapenem in the elderly following administration of a 1-g dose was 39% higher than that in young subjects (P < 0.0001) (Table 2). The difference was somewhat greater for the mean AUC0-∞ of unbound ertapenem, at 71% higher (P < 0.0001). The higher concentrations and AUC values for the elderly were associated with a longer terminal t1/2 and slower CLR. The mean terminal t1/2 of ertapenem was longer for the elderly (5.1 h) than for young adults (3.8 h; Table 2). The mean CLR of the unbound and total drug was slower in the elderly than in young adults, about 30% slower for the total drug and about 45% slower for the unbound drug at a dose of 1 g. This is supported by the lower measured fe of ertapenem of the elderly (about 37%) compared with that of young adults (about 44%). The slower CLRs of the intact drug in the elderly may be related to their diminished kidney function compared with that of young adults. A plot (Fig. 2) of ertapenem CLR as a function of creatinine clearance for elderly persons and young adults demonstrates a relationship and supports the above comparisons.
FIG. 1.
Mean concentrations in plasma of unbound and total ertapenem following administration of a single 1-g dose in elderly persons (n = 14) and young adults (n = 16). Error bars show standard deviations.
FIG. 2.
AUC0-∞ of unbound and total ertapenem following administration of a single 1-g i.v. dose as a function of creatinine clearance in elderly and young adults.
The CLNR of ertapenem, i.e., the difference between CLP and CLR, was also slower in the elderly by 17% for the total drug and 34% for the unbound drug (Table 2). The increases in the AUC for the elderly may be explained by a slower CLR and a slower CLNR and thus a slower CLP (mean value, 21.6 ± 2.6 ml/min) for the total drug at a 1-g i.v. dose; for young adults, the mean value was 29.5 ± 3.4 ml/min.
The profiles of mean unbound and total ertapenem concentrations in plasma on days 1 and 7 following administration of a once-daily i.v. 1-g dose for 7 days are very similar, suggesting that the drug did not accumulate upon administration of multiple once-daily doses to the elderly. The geometric mean total and unbound AUC0-24 values on day 7 are slightly less than the corresponding value observed on day 1 (Table 2): 742 and 52.2 μg · h/ml for day 1 and 679 and 47 μg · h/ml for day 2, respectively.
The unbound fraction of ertapenem in the plasma of elderly persons was generally greater than that in the plasma of young adults. The unbound percentage was consistently greater in the elderly, at about 5 to 11%, than in the young adults, at about 5 to 8%. At the end of the 30-min infusion of the 1-g dose, the mean unbound fraction was significantly higher in the elderly (about 11%) than in the young adults (about 8%) (P < 0.0001). The differences between the elderly and young adults were smaller at the lower concentrations observed at later time points. Similar results have been observed for ceftriaxone with higher levels of unbound drug in the elderly (15 to 22%, unbound/total) than in the young (11 to 17%) after administration of a 1-g i.v. infusion for 30 min (3).
DISCUSSION
The mean AUC0-∞ values for total and unbound drug were elevated in the elderly relative to those of young adults. The higher AUC0-∞ values for elderly subjects are reflected in their slower drug clearances from plasma. CLRs at the 1-g dose were moderately slower in the elderly than in the young, as observed with the declines in renal function in the elderly. The fraction excreted unchanged in urine decreased only slightly, from a mean of 44.4% for a 1-g dose in young adults to 36% in the elderly, and this change did not equal the AUC increase observed. The higher AUC values for the elderly appear to be a result of both slower CLRs and CLNRs, giving an overall slower clearance from plasma, 26% for the total drug and 40% for the unbound drug. The lower CLNR in the elderly most likely is due to slowed metabolism of ertapenem to its major metabolite, the open-lactam form of ertapenem.
The mean AUC0-24 values for the unbound drug and the total drug on day 7 after administration of multiple daily 1-g i.v. doses are no higher than those determined on day 1 in these elderly subjects, indicating no accumulation of ertapenem. The ratios of day 7 to day 1 AUCs for the total and unbound drug were 0.92 and 0.91 with 90% CIs of 0.88 to 095 and 0.86 to 0.96, respectively. The slight deviation from a ratio of 1.0 is not considered to be clinically significant. The same comparisons were shown for young adults in a previous study (4).
In summary, since the systemic exposure to total ertapenem in the elderly differs by only about 39% from that of young adults and multiple 1-g doses are well tolerated, a dosage adjustment away from that used in younger patients is not recommended for the elderly.
Acknowledgments
This study was funded by Merck & Co., Inc.
REFERENCES
- 1.Dawling, S., and P. Crome. 1989. Clinical pharmacokinetic considerations in the elderly, an update. Clin. Pharmacokinet. 17(4):236-263. [DOI] [PubMed] [Google Scholar]
- 2.Friedland, I., L. A. Mixson, A. Majumdar, M. Motyl, and G. L. Woods. 2002. In vitro activity of ertapenem against common clinical isolates in relation to human pharmacokinetics. J. Chemother. 14(5):483-491. [DOI] [PubMed] [Google Scholar]
- 3.Laderer, J. R., I. H. Patel, J. Durkin, and D. W. Schneck. 1984. Age and ceftriaxone kinetics. Clin. Pharmacol. Ther. 35:19-25. [DOI] [PubMed] [Google Scholar]
- 4.Majumdar, A. K., D. G. Musson, K. L. Birk, C. J. Kitchen, S. Holland, J. McCrea, G. Mistry, M. Hesney, L. Xi, S. X. Li, R. Haesen, R. A. Blum, R. L. Lins, H. Greenberg, S. Waldman, P. Deutsch, and J. D. Rogers. 2002. Pharmacokinetics of ertapenem in healthy young volunteers. Antimicrob. Agents Chemother. 46:3506-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Musson, D. G., K. L. Birk, A. M. Cairns, A. K. Majumdar, and J. D. Rogers. 1998. High-performance liquid chromatographic methods for the determination of a new carbapenem antibiotic, L-749345, in human plasma and urine. J. Chromatogr. B 720:99-106. [DOI] [PubMed] [Google Scholar]
- 6.Musson, D. G., K. L. Birk, A. Majumdar, and J. D. Rogers. 2003. Assay methodology for the quantitation of unbound L-749345, a new carbapenem antibiotic in human plasma. J. Chromatogr. B 783:1-9. [DOI] [PubMed] [Google Scholar]
- 7.Wong, B. K., P. J. Bruhn, and J. H. Lin. 1999. Dose-dependent plasma clearance of MK-826, a carbapenem antibiotic, arising from concentration-dependent plasma protein binding in rats and monkeys. J. Pharm. Sci. 88(2):277-280. [DOI] [PubMed] [Google Scholar]