Abstract
Objectives
Systemic antibiotic treatment of Lyme borreliosis is effective during the early stages of the infection, while chronic manifestations of the disease may remain refractory and difficult to treat. This study was carried out in order to evaluate the potential of topically applied azithromycin to eliminate the spirochaetal organisms in the skin of the freshly bitten host and thereby prevent Lyme borreliosis.
Methods
Laboratory mice were challenged with Borrelia burgdorferi sensu stricto by needle inoculation or via infected ticks as vectors. Then, an azithromycin-containing formulation was applied once daily to the sites of exposure for three consecutive days. In the case of needle inoculation, a 5% azithromycin formulation was applied starting 1 h, 3 days and 5 days after infection. In the case of tick exposure, 4%, 10% and 20% azithromycin formulations were applied, starting directly after the detachment of the engorged ticks. Subsequently, the infection status of the mice was determined.
Results
Concentrations of azithromycin in murine skin were >3800-fold higher than the published minimal inhibitory concentration for B. burgdorferi as soon as 3 h after the first application. After needle inoculation, spirochaetes were not detectable in all infected mice after treatment, if the first application started 1 h or even after 3 days post-infection. Furthermore, no borrelial organisms were detected after topical treatment when ticks were used for spirochaete inoculation.
Conclusions
Our data indicate that topical treatment with a formulation containing azithromycin is a promising approach to prevent Lyme borreliosis shortly after a tick bite.
Keywords: Borrelia, preventive treatment, antibiotic treatment, spirochaetes, prophylaxis
Introduction
Lyme borreliosis is caused by infection with different species of the Borrelia burgdorferi sensu lato group that are transmitted by Ixodes ticks.1 Shortly after tick attachment, an increasing number of spirochaetes around the site of the tick bite may cause clinical signs of cutaneous inflammation and B. burgdorferi organisms disseminate to distant locations of the host's body during the following stages of infection. In various body sites, they can induce specific immune responses that may result in chronic lesions due to inflammatory reactions.2,3
Currently, the most recommended treatment regimen in the early phase of infection is oral therapy with doxycycline or amoxicillin for 10–20 days.4 Without adequate treatment, months after the onset of infection, ∼60% of patients experience prolonged clinical signs and symptoms, especially arthritic episodes in the large joints of the limbs and/or neurological abnormalities.5 Indeed, antibiotic intervention leads to an effective clearing of the infection in the early disease stages, but if treatment starts late (e.g. in the chronic phase of infection) ∼10% of patients may experience treatment-resistant symptoms of Lyme borreliosis.5
A principal cause that leads to this chronic, in some cases hardly manageable, disease is the difficult diagnosis of Lyme borreliosis in its early stages. Only 70%–80% of all human patients develop an erythema migrans at the site of tick attachment,6,7 the early hallmark of an infection with B. burgdorferi.5 Other non-specific clinical signs are generally fever, malaise, headache, myalgia and arthralgia. Additionally, serological tests are often too insensitive and non-specific during the first 1–2 weeks of infection.8–10
These problems, which many patients and physicians encounter during the early stage of infection, and the lack of a licensed vaccine for humans5 necessitate other means of disease prevention, e.g. a preventive therapeutic approach that clears the infection directly after removal of the feeding ticks. In fact, studies focusing on antimicrobial therapy for the prevention of Lyme borreliosis after exposure to Ixodes ticks have been performed in humans, but most of them were not effective in controlled treatment trials.11–15 Promising results were published by Nadelman et al.,16 who showed statistically significant prevention of Lyme disease after tick bites by a single oral dose of 200 mg of doxycycline, when the drug was applied within 72 h after removing attached Ixodes scapularis ticks. Based on this investigation, the recommendation of such a prophylactic approach was included in the Clinical Practice Guidelines of the Infectious Diseases Society of America under defined circumstances.4 The efficiency of doxycycline in preventing Lyme borreliosis by a single oral dose was also documented in a proof-of-concept study in mice.17 Furthermore, the available literature describing prophylactic approaches for preventing Lyme borreliosis were reviewed recently by Warshafsky et al.18 The summarized results of these reviewed clinical studies showed that the relative risk for acquiring Lyme disease was reduced by 91% in patients who had received antibiotic prophylactic therapy. Additionally, the authors postulated that the initiation of chemoprophylaxis soon after tick exposure is more important than the application of different types of antibiotic or varying treatment durations. However, all of these studies used prophylactic approaches that were based on systemic antibiotic therapies. The disadvantages of these strategies are the adverse effects documented for several patients, e.g. nausea and vomiting caused by doxycycline or rashes resulting from the use of amoxicillin and penicillin.4,16,18 Besides, doxycycline is contraindicated in pregnant woman and in children under the age of 8 years.4,16
Side effects due to the systemic application of antibiotics should be limited or even absent when drug concentrations are kept at a minimum. Experimental studies have shown that B. burgdorferi organisms remain localized for >2 days in the mammalian skin at the site of tick exposure, an area that is just a few centimetres in diameter and can even be removed by surgical means.19 In this context, Shih et al.20 showed that infection can be effectively terminated by the topical application of antibiotics (e.g. tetracycline hydrochlorides, penicillin G, amoxicillin, ceftriaxone and doxycycline) directly on areas of tick bites immediately after the detachment of I. scapularis nymphs carrying B. burgdorferi organisms. Although the results were promising, the authors recommended that this approach should not be further evaluated for human use, because the tested antibiotics had been dissolved in DMSO. The substance was used as a carrier and is potentially harmful for humans.20
The purpose of this study was to reconsider the feasibility of treating Lyme borreliosis by a topical application of an antibiotic on the area of tick exposure, and to evaluate the potential effects of an ethanol-based azithromycin-containing formulation for the prevention of infection and disease. Azithromycin was selected because of its lower MIC for B. burgdorferi (MIC ≤ 0.015 mg/L) and fewer adverse effects when compared with doxycycline (MIC ≤ 0.25 mg/L).4,21–23 In addition, azithromycin (i) shows excellent tissue penetration, (ii) accumulates in tissues, (iii) has a half-life of ∼5 days in tissues versus a few hours in serum and (iv) does not leak into the circulation when applied locally onto the skin. Consequently, mice were infected with B. burgdorferi organisms using infected ticks or via needle inoculation. Subsequently, animals were treated with the formulation containing various doses of azithromycin or placebo. The experiments demonstrated an efficient prevention of infection in all cases, if the treatment had started within 3 days post-tick infection. Therefore, the tested formulation is a promising candidate for the prevention of B. burgdorferi infection when applied topically and early on the site of tick exposure.
Materials and methods
Spirochaetes
Low-passage (passage 4) B. burgdorferi sensu stricto N40 (B. burgdorferi) organisms that are an isolate from a skin punch biopsy of an experimentally infected dog and that are infective for mice were used for the needle inoculation.24,25 Spirochaetes were cultured in modified Barbour–Stoenner–Kelly (BSK II) medium at 33°C.
Mice
The female C3H/HeN (C3H) mice used for the infection experiments were bred by and purchased from Sociéte Janvier, France. The mice were kept under specific-pathogen-free conditions in individually ventilated cages (Ehret, Berlin, Germany) at the animal facility of the Max Planck Institute for Evolutionary Anthropology (Leipzig, Germany). For the assessment of azithromycin concentrations in murine skin, 6-week-old female C3H mice were bred by and purchased from Harlan Winkelmann GmbH, Borchen, Germany. These mice were kept in ventilated cages at the animal facility of the Institute of Infectious Diseases and Zoonoses, LMU Munich (Munich, Germany). The animal experiments were carried out in accordance with the guidelines approved by the Animal Care and Usage Committee of the Regierungspräsidium Leipzig and the Sachgebiet 54-Tierschutz, Regierung von Oberbayern, Germany.
Infection by needle inoculation
Spirochaetes were grown to the late exponential phase. Forty C3H mice were inoculated intradermally into their shaven backs with B. burgdorferi (5 × 104 spirochaetes per mouse in a volume of 30 μL of BSK II medium).
Exposure to infected ticks
B. burgdorferi-infected ticks were bred by and purchased from M. Matuscka and D. Richter (Charité Berlin). Ticks were placed onto the shaven backs of 38 mice (four to five ticks per mouse), protected by a plastic capsule, for 96 h. During the complete period of tick attachment, mice were caged individually. In cases that the capsule came off, the cage litter was searched for removed ticks. Subsequently, in order to measure the infection rate of ticks, all detached ticks were tested with a quantitative real-time PCR (qPCR) for B. burgdorferi.
Topical treatment with the azithromycin-containing formulations
The azithromycin formulations (containing 4%, 5%, 10% or 20% azithromycin, polyacrylate, klucel and mygliol; 83.5%, 82.5%, 77.5% and 67.5% of the volume was 94% ethanol, respectively) as well as the placebo formulation (containing polyacrylate, klucel and mygliol; 87.5% of the volume was 94% ethanol) were kindly provided by Ixodes AG (Zürich, Switzerland). In the case of needle inoculation, three groups of 10 mice were treated with a formulation containing 5% azithromycin starting 1 h, 3 days and 5 days post-B. burgdorferi inoculation. Additionally, 10 mice received the placebo formulation (0% azithromycin) starting 1 h post-inoculation. In the case of tick-borne inoculation, seven mice received no formulation, nine mice were treated with placebo, nine mice with a 4% azithromycin formulation, eight mice with a 10% azithromycin formulation and five mice with a 20% azithromycin formulation. Treatment started directly after tick detachment (96 h post-tick attachment). In all cases, 200 ± 3 mg of formulation were applied directly onto the areas defined by needle inoculation or tick attachment for three consecutive days. This equates to a daily topical azithromycin dose of 3.00 ± 0.05 mg (4% formulation), 3.75 ± 0.06 mg (5%), 7.50 ± 0.11 mg (10%) and 15.00 ± 0.23 mg (20%). During the treatment period, the mice were caged individually.
Sera
Blood from all mice was obtained at day 56 post-tick detachment by cardiac puncture. Serum was prepared in serum separators (BD Microtainer; Becton Dickinson) by centrifugation (5000 g for 5 min, room temperature). Sera were stored frozen at −80°C. For the calculation of ELISA cut-offs, sera from four non-infected C3H/HeN mice served as negative controls.
Tissue samples for cultivation and PCR
Skin biopsies from mice were collected aseptically 7, 14 and 28 days post-needle inoculation or tick detachment from the same areas of previous spirochaetal exposure and were frozen at −80°C. In the case of needle inoculation, an additional skin biopsy sample was collected on day 28 post-inoculation for spirochaete detection in culture, as described below. Mice were sacrificed on day 56 post-inoculation or tick detachment. Tissues from the heart, skin from the right ear, right tarsal joint and bladder were collected under sterile conditions. Tissue samples were washed in 70% ethanol and in PBS. Whole bladder and joint, and parts of ear and heart were squashed in 200 μL of BSK II. These tissue mixtures were each individually transferred into 6 mL of sterile BSK II. Tissue cultures were kept at 33°C for 6 weeks and observed weekly for the presence of viable spirochaetes by dark-field microscopy.
Kinetic ELISA (KELA)
A computerized KELA was performed as described previously.26 Sonicated whole-cell lysate of B. burgdorferi served as the antigen. Murine sera were diluted 1 : 100 in PBS with 0.05% Tween® 20 and 2% milk powder (PBSTM). Borrelia-specific antibodies were detected with horseradish peroxidase-conjugated goat antimouse IgG (R&D Systems, Minneapolis, MN, USA) in a dilution of 1 : 1000 in PBSTM.
Western blotting
Western blot analysis of selected mice sera was performed with membrane strips coated with Borrelia afzelii lysate antigen and recombinant VlsE (B. afzelii + VlsE Eco Blot IgG Western Blot; Genzyme Virotech GmbH, Rüsselsheim, Germany). The test procedure was carried out according to the manufacturer's instructions. Alkaline phosphatase-conjugated affinity-purified goat antimouse IgG (Rockland Inc., Gilbertsville, PA, USA) at a dilution of 1 : 4000 was used as the secondary antibody. The substrate reaction was stopped after 10 min. The interpretation of the test results was performed according to included kit-specific protein band templates and based on published criteria.25,27,28
Quantification of B. burgdorferi in ticks and skin biopsy samples with a quantitative real-time PCR
The DNA of ticks and skin biopsies was extracted using a commercial kit (DNeasy Blood & Tissue Kit; Qiagen, Hilden, Germany). The test procedure was carried out according to the instructions supplied with the kit. Extracted DNA from ticks was eluted in 50 μL of H2O and DNA from skin biopsy samples was eluted in 200 μL of H2O. Amplification of the 104 bp ospA DNA target was performed with 2.5 μL of template DNA in a 25 μL final volume containing 1× reaction buffer (Invitrogen, Karlsruhe, Germany), 20 U/mL Platinum Taq Polymerase (Invitrogen), 3.5 mM MgCl2 (Invitrogen), 900 nM of each oligonucleotide, 200 nM fluorogenic probe and 0.2 mM dNTPs. The oligonucleotide primers and probe were synthesized by TibMolBiol GmbH (Berlin, Germany). The sequences used to detect B. burgdorferi ospA were: ospA-N40.seq-17F, 5′-AATGTTAGCAGCCTTGACAGAA-3′; ospA-N40.seq-119R, 5′-GATCGTACTTGCCGTCTTTGTTT-3′; and ospA-N40.seq-41T, 5′-FAM-AACAGCGTTTCAGTAGATTTGCCTGGTGA-TAMRA-3′.29 All reactions were tested in triplicate and the PCR was performed with a LightCycler® 480 (Roche Diagnostics GmbH, Penzberg, Germany). The PCR was run for 2 min at 95°C, and 50 cycles of 15 s at 95°C and 30 s at 60°C. A standard curve for the quantification of B. burgdorferi DNA was generated by the addition of 107 spirochaetes to a skin biopsy sample of a naive mouse. After DNA extraction, a dilution series was created from 107 to 100 spirochaete copies per biopsy.
For skin biopsies, the concentration of murine β-actin was determined with the PCR conditions as described above. The sequences used to detect murine β-actin were: β-actin-F, 5′-TCACCCACACTGTGCCCATCTACGA-3′; β-actin-R, 5′-GGATGCCACAGGATTCCATACCCA-3′; and β-actin-probe, 5′-Cy5-TATGCTCTCCCTCACGCCATCCTGCGT-BBQ-3′.30
A titration series of extracted DNA derived from skin biopsies of naive mice was used as a standard curve for murine β-actin. Based on the β-actin quantification, the detected copy numbers of B. burgdorferi were normalized to 100 μg of mouse DNA.
Preparation of azithromycin standard dilutions for the measurement of growth inhibition areas
A standard dilution series was prepared to assess the bacterial growth inhibition induced by azithromycin. Micrococcus luteus (strain ATCC 4698; DSMZ, Braunschweig, Germany) was grown in liquid Mueller–Hinton II bouillon for 24 h at 37°C on a rotating plate incubator. Bacterial growth in the liquid media was measured spectrophotometrically (McFarland standard 0.5). An aliquot of 100 μL of M. luteus suspension was streaked out on agar plates containing solid antibiotic medium no. 11 (AM 11; Difco, Becton Dickinson GmbH, Heidelberg, Germany). Azithromycin-containing dilutions (Fluka, Sigma–Aldrich, Munich, Germany) were prepared in PBS ranging from 750 to 0.34 mg/L. Filter paper discs (Mast Diagnostica GmbH, Rheinfeld, Germany) were soaked individually with 20 μL of the azithromycin dilutions. Then, the discs were placed on AM 11 plates in quadruplicate and incubated for 24 h at 37°C. In addition, a commercial standard positive-control disc (15 μg of azithromycin; Mast Diagnostica GmbH, Rheinfeld, Germany) was also included in the assay. The growth inhibition areas (diameters in mm) were measured after 24 h.
Measurement of azithromycin concentrations in murine skin
Azithromycin concentrations in murine skin biopsy samples after the application of a single dose of 5% azithromycin were determined with a modified agar diffusion bioassay, as described by Bennett et al.31 The backs of all experimental mice were shaved (∼2.25 cm2) and 200 mg of a 5% azithromycin gel formulation was applied on this area. During the next 24 h, the mice were kept individually. The mice were weighed before and after the application of the formulation. Groups of three mice were sacrificed 3, 6, 12 and 24 h after treatment. Two 4 mm skin biopsy punch samples were collected from the shaven areas and weighted. Then, skin samples were placed into 40 μL of sterile PBS. Biopsies were squeezed strongly with sterile pestles to release tissue fluids. Twenty microlitres of this solution was placed on filter paper discs and growth inhibition areas were measured. Standard azithromycin dilutions were prepared for each timepoint.
Statistics
Statistical analysis and graphic visualization of the results were performed using the SigmaStat/SigmaPlot software (Systat Software GmbH, Erkrath, Germany). When applicable, treatment groups were compared using χ2 tests.
Results
The infection status of the animals was determined by culturing B. burgdorferi organisms from skin and tissue samples, by detecting B. burgdorferi-specific DNA in tissue with qPCR, and by demonstrating specific antibodies against the infectious agent. Azithromycin concentrations in skin after the application of the antibiotic were assessed in a separate experimental approach.
Assessing azithromycin concentrations of skin biopsy samples from needle-inoculated mice
Using a modified agar diffusion assay,31 azithromycin concentrations in skin biopsy samples were measured 3, 6, 12 and 24 h after a single application of the supplied 5% azithromycin formulation. Overall, the azithromycin concentrations in biopsy samples ranged from 29.0 to >750 mg/L (Table S1; available as Supplementary data at JAC Online). The published MIC of azithromycin for B. burgdorferi is 0.015 mg/L.22 Therefore, azithromycin concentrations in skin biopsy samples taken from the application site were up to >100 000 times the MIC after just a single treatment.
Measurement of B. burgdorferi DNA concentrations in skin biopsy samples from needle-inoculated mice
In skin biopsy samples from all mice that had received the mock treatment (0% azithromycin), ospA was detectable 7 days post-inoculation. On day 14 post-inoculation, ospA was detected in five biopsy samples of the placebo group and on day 28 post-inoculation, biopsy samples from two mice were positive for ospA in the placebo group (Table 1).
Table 1.
Overall infection status of experimental mice that had received topical treatment with formulations containing 5% azithromycin starting at varying timepoints after needle inoculation of B. burgdorferi by combining all available data: ospA real-time PCR, culture and serological investigations
| Concentration of azithromycin/treatment start (%/time) |
ospA PCR (skin biopsies) |
Culture | ELISA | Western blot | Infection status | ||
|---|---|---|---|---|---|---|---|
| 7 days post-inoculation | 14 days post-inoculation | 28 days post-inoculation | |||||
| 0/1 h post-inoculation | 10/10a | 5/10 | 2/10 | 1/10 | 9/10 | 9/10 | 9/10 |
| 5/1 h post-inoculation | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 |
| 5/3 days post-inoculation | 0/10 | 0/10 | 0/10 | 0/10 | 6/10 | 0/10 | 0/10 |
| 5/5 days post-inoculation | 2/10b | 0/10 | 1/10 | 0/10 | 7/10 | 0/10 | 0/10 |
Differences between placebo and treatment groups were statistically highly significant (P = 0.000; χ2 test).
aNumber positive/number tested.
bBiopsies were collected on day 8 instead of day 7 post-inoculation.
In contrast to the placebo group, no ospA was detectable in biopsy samples collected from mice that had received the first application 1 h or 3 days post-inoculation, independent of the timepoint of biopsy sampling, suggesting an efficient elimination of the spirochaetes in these groups. In mice that were treated 5 days post-inoculation, two biopsy skin samples collected on day 8 post-inoculation were positive for ospA and one sample collected on day 28 was ospA-positive (Table 1). Detailed results of the PCR analyses are summarized in Table S2 (available as Supplementary data at JAC Online).
Reisolation of spirochaetes from tissues of needle-inoculated mice
Viable spirochaetes were reisolated from the inoculation site, heart and urinary bladder of one mouse in the placebo group (0% azithromycin). All tissues derived from the other animals of this group as well as from the animals of the treatment groups were culture-negative for B. burgdorferi organisms (Table 1). Detailed results of the reisolation experiments are summarized in Table S3 (available as Supplementary data at JAC Online).
IgG antibody responses against B. burgdorferi after needle inoculation
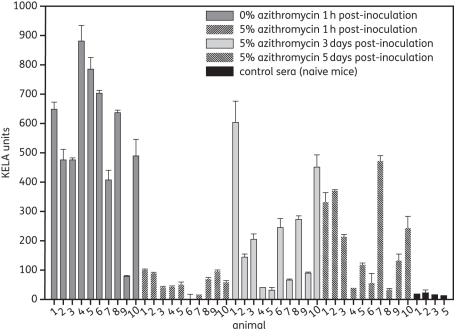
Antibody levels of treated and untreated mice are shown in Figure 1. High antibody levels were detected in 9 out of 10 mice that had received the placebo formulation (0% azithromycin). In contrast, no B. burgdorferi-specific antibodies were detectable in mice that had received the azithromycin formulation also starting 1 h after inoculation. Groups of mice that received a delayed treatment (treatment started 3 or 5 days post-needle inoculation) reacted heterogeneously. Elevated antibody levels were present in sera from 6 out of 10 mice that had received the treatment starting 3 days post-inoculation and in 7 out of 10 mice that received the treatment starting 5 days post-inoculation. It is noteworthy that the antibody levels in 10 seropositive animals, which were treated starting days 3 or 5 post-infection, were clearly lower than those found in animals that had received the placebo treatment.
Figure 1.
IgG antibody levels in C3H mice 56 days post-needle inoculation of B. burgdorferi. Mice were inoculated intradermally with 5 × 104 B. burgdorferi organisms and treated 1 h, 3 days or 5 days after the inoculation. Error bars represent standard deviations of duplicates.
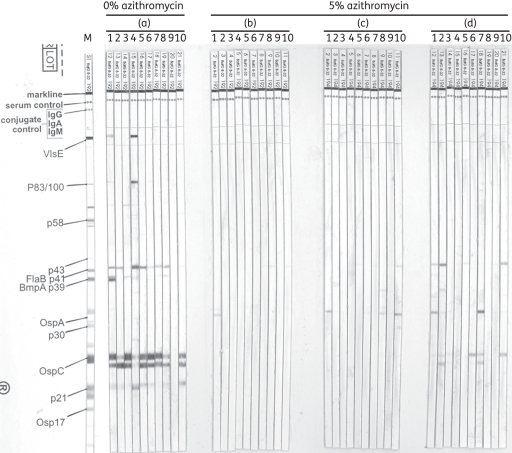
The results of the western blot analysis are shown in Figure 2. Sera were considered positive for infection if signals were visible for recombinant VlsE or at least two of the following bands: p83/100; p58; p43; p39; p30; p23 (OspC); p21; and p17 (Osp17).25,27,28 Nine out of 10 mice that received the placebo formulation were considered positive for an infection-specific contact with borrelial antigen (Figure 2a). In contrast, mice that had received treatment starting 1 h post-inoculation (Figure 2b), 3 days post-inoculation (Figure 2c) and 5 days post-inoculation (Figure 2d) were considered negative for infection. Solely weak signals against OspA and OspC, which may have been induced by antigen contact during the inoculation of culture-adapted spirochaetes,32 as well as weak non-specific signals at 41 kDa (FlaB, p41) were detectable in these groups.
Figure 2.
Western blot analysis of murine serum samples 56 days post-needle inoculation of B. burgdorferi. Placebo or topical azithromycin were applied 1 h (a and b), 3 days (c) or 5 days (d) post-inoculation. M, positive-control marker for protein bands.
Infection with B. burgdorferi via ticks with subsequent topical azithromycin treatment
Demonstration of B. burgdorferi DNA in murine skin biopsy samples and ticks
Results of the PCR analyses are summarized in Table 2. Detailed results of ospA detection in the skin and ticks are listed in Tables S2 and S4 (available as Supplementary data at JAC Online). Copies of the ospA gene were present in skin biopsies collected from four out of seven mice that had received no treatment at all and from three out of nine mice that had received the placebo formulation. In general, skin biopsy samples were positive throughout the complete observation period (7, 14 and 28 days post-tick detachment), with the exception of one mouse in which ospA was detectable only on days 7 and 14 post-tick detachment. No ospA genes were detectable in mice that had received azithromycin-containing formulations, independent of the azithromycin dose used. Notably, PCR analysis of the ticks that were detached from the control mice that remained negative for B. burgdorferi infection revealed that these vectors contained very low ospA copy numbers (≤1.0 × 102 ospA copies). Such low bacterial burdens are considered insufficient for host infection.
Table 2.
Overall infection status of experimental mice that had received topical treatment with formulations containing varying concentrations of azithromycin starting immediately after tick detachment by combining all available data: ospA real-time PCR, culture and serological investigations
| Concentration of azithromycin |
ospA PCR (skin biopsy samples) |
Culture | ELISA | Infection status | ||
|---|---|---|---|---|---|---|
| 7 days post-tick detachment | 14 days post-tick detachment | 28 days post-tick detachment | ||||
| No treatment | 4/7a | 4/7 | 4/7 | 4/7 | 4/7 | 4/7 |
| 0% (placebo) | 3/9 | 3/9 | 2/9 | 2/9 | 2/9 | 2/9 |
| 4% | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 |
| 10% | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 |
| 20% | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
Differences between all groups were significant (P = 0.009; χ2 test). Comparison of the two control groups (no treatment versus placebo) revealed no significant differences (P = 0.152; χ2 test). Control groups (no treatment combined with placebo) versus all treatment groups differed significantly (P = 0.001; χ2 test).
aNumber positive/number tested.
Reisolation of spirochaetes after tick exposure
The results are summarized in Table 2 and listed completely in Table S5 (available as Supplementary data at JAC Online).
Viable spirochaetes were reisolated only from mice that had positive ospA skin biopsy samples throughout the complete observation period (7, 14 and 28 days post-tick detachment). All four PCR-positive animals that had received no treatment carried viable B. burgdorferi organisms in all types of tested tissues. The two animals that had received the placebo formulation and were ospA-positive (PCR) on day 28 post-tick detachment carried viable B. burgdorferi organisms in their ear tissues.
IgG antibody response to B. burgdorferi after tick exposure
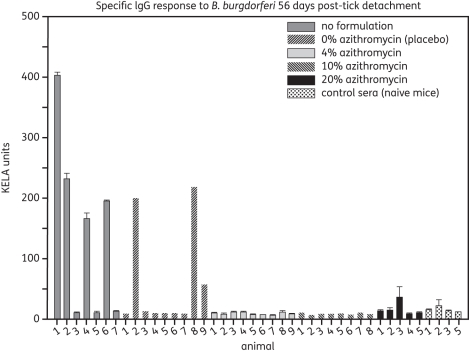
The antibody responses of mice against borrelial antigens were assessed on day 56 post-tick detachment using a KELA, as described above. The antibody levels are shown in Figure 3. All animals that were positive in tissue cultures contained specific antibodies against B. burgdorferi. However, specific antibodies were absent in animals that were not treated or had received the placebo formulation and concurrently showed no ospA signals in their biopsy samples (PCR) on day 28 post-tick detachment. All animals that had received the azithromycin-containing formulations failed to produce specific antibodies.
Figure 3.
IgG antibody levels in C3H mice 56 days post-detachment of B. burgdorferi infected ticks. Error bars represent standard deviations of duplicates.
Discussion
The aim of this study was to evaluate the efficacy of an azithromycin-containing formulation for the prevention of infection with B. burgdorferi and probably other species belonging to the B. burgdorferi sensu lato complex.
This hypothesis of preventing B. burgdorferi infection and, consequently, disease by applying topical azithromycin was tested in a murine model based on two different routes of bacterial inoculation: (i) needle inoculation of a defined dose of B. burgdorferi with subsequent topical application of 5% azithromycin or placebo starting 1 h, 3 days or 5 days post-inoculation; and (ii) spirochaete infection induced with experimentally infected ticks as vectors with subsequent topical application of 0%, 4%, 10% and 20% azithromycin formulation starting immediately after tick detachment.
In general, borrelial inoculation in mice by either needle inoculation or tick exposure was successful, since infected mice were present in both groups. Single tick-exposed control mice (receiving no treatment or placebo treatment) were considered negative for infection when tested at the end of the observation period. However, PCR testing of ticks, which had been detached from these animals, demonstrated spirochaetal burden (Table S4 available as Supplementary data at JAC Online). The numbers of organisms per tested tick, which we determined using PCR, were lower than the doses considered to be necessary for the successful infection of mice.33,34 Nevertheless, the other placebo-treated control mice became infected when they were exposed to ticks carrying sufficient bacteria. Topical application of the placebo formulation had no impact on the survival of the Borrelia organisms and on the following immune response of the host. In contrast to the control animals, none of the azithromycin-treated mice that were exposed to the ticks, which contained sufficient bacteria for infection, showed evidence of infection with B. burgdorferi even after an observation time of 56 days. Furthermore, no evidence of infection was found in needle-inoculated and subsequently azithromycin-treated animals when topical treatment was delayed for 3 days post-inoculation. Therefore, we speculate that the applied azithromycin formulation is highly efficient in reducing local B. burgdorferi burden, even if the treatment starts late (3 days post-infection). Specific antibodies that were detected with western blots in several animals, which had received the delayed treatment, were predominately directed against the outer surface proteins OspA and OspC. OspA is commonly up-regulated by B. burgdorferi in questing ticks or when the spirochaetes are cultured in medium.35 Therefore, it is likely that the signals seen on our blots were remnant signals of inoculated, culture-derived spirochaetes, as described previously.32 When B. burgdorferi organisms are transmitted by ticks, delivery of the bacteria into the skin of the host starts ∼24–48 h after tick attachment.36 In our experimental setting, spirochaetes transmitted via tick exposure should have been present in the mice's skin for several days, especially at the time when the antibiotic was applied topically 3 days after tick attachment. In contrast to needle inoculation, the failure to produce specific antibodies by these animals could be the result of an immune suppressive effect induced by the tick saliva. Consequently, the production of detectable levels of antibodies, which in the course of B. burgdorferi infection occurs weeks after the start of infection,25 probably was suppressed initially. In the further course of our experiment, spirochaetes were killed as expected by azithromycin, were removed and the adaptive immune system failed to initiate an appropriate response.
The infection rates of mice that had received treatment starting 5 days after tick detachment require careful consideration. Serological data as well as our culture results suggest that initial infections were cleared. Positive PCR signals in two mice 7 days after inoculation and, even more importantly, in one animal 28 days post-inoculation imply the presence of non-culturable spirochaetes in these cases.37–39 It was shown that B. burgdorferi remains localized for >2 days at the site of infection before the organisms start to disseminate.19 Therefore, it is plausible that a treatment starting 5 days after inoculation is too late for clearing the early infection. Nevertheless, topical azithromycin treatment starting immediately or up to 3 days post-Borrelia inoculation seems to be able to abort the infection. Our results are in accordance with those produced by Shih et al.,20 who tested the topical application of penicillin G, amoxicillin, ceftriaxone and doxycycline after tick detachment. These authors confirmed efficient clearing of B. burgdorferi organisms when treatment started up to 2 days post-tick detachment.20
In contrast to Shih et al.,20 we used an antibiotic from the macrolide group (azithromycin) to fend off B. burgdorferi organisms. Azithromycin was chosen because of its high efficacy against B. burgdorferi22,40,41 and its known repository effect. This assumption was confirmed when we measured the azithromycin concentrations in murine skin, which were >3800 times higher than the published MIC. This suggests that the killing of spirochaetes localized in the skin is possible. Moreover, ethanol was used instead of DMSO as a solvent ingredient in the formulation used for this study. This is a prerequisite for this formulation to find its way into clinical practice. Blood levels of azithromycin in the mice were not determined, because the aim of the current study was to test the proof of concept. Likewise, the efficacy of the azithromycin-containing formulation against other pathogenic Borrelia species, such as B. afzelii or Borrelia garinii, was not tested in our model. However, several publications suggest that these species are susceptible to azithromycin as well.22,40
In summary, our data suggest that topical treatment with an azithromycin-containing formulation shortly after tick exposure and attachment can stop and effectively clear a B. burgdorferi infection. Considering common side effects linked to antibiotics, this approach seems to be beneficial when compared with standard antibiotic therapies, which are applied systemically. Drug concentrations are kept extremely low, so side effects are avoided or minimized. Treatment starts at a timepoint when the infection is very treatable,4,42 and spirochaetal dissemination and, consequently, persistent infection can be prevented. However, clinical studies in humans using the azithromycin-containing formulation are necessary to adapt the antibiotic concentration to the conditions necessary for human skin and clarify the pharmacokinetics of azithromycin after topical application.
Funding
This work was supported by Ixodes AG, Zurich, Switzerland.
Transparency declarations
All authors are associated with German research institutions and none of them owns stocks or options of Ixodes AG.
Supplementary data
Tables S1 to S5 are available as Supplementary data at JAC Online (http://jac.oxfordjournals.org/).
Acknowledgements
We are very grateful to the members of the animal facility of the Max-Planck-Institute for Evolutionary Anthropology (Leipzig, Germany), involved in breeding and maintaining the animals, and Martina Conrad as well as Elisabeth Aschinger-Kirch and Christine Geiger for technical assistance.
References
- 1.Burgdorfer W, Barbour AG, Hayes SF, et al. Lyme disease—a tick-borne spirochetosis? Science. 1982;216:1317–9. doi: 10.1126/science.7043737. doi:10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 2.Steere AC, Schoen RT, Taylor E. The clinical evolution of Lyme arthritis. Ann Intern Med. 1987;107:725–31. doi: 10.7326/0003-4819-107-5-725. [DOI] [PubMed] [Google Scholar]
- 3.Steere AC. Lyme disease. N Engl J Med. 1989;321:586–96. doi: 10.1056/NEJM198908313210906. doi:10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 4.Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–134. doi: 10.1086/508667. doi:10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 5.Steere AC, Coburn J, Glickstein L. The emergence of Lyme disease. J Clin Invest. 2004;113:1093–101. doi: 10.1172/JCI21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith RP, Schoen RT, Rahn DW, et al. Clinical characteristics and treatment outcome of early Lyme disease in patients with microbiologically confirmed erythema migrans. Ann Intern Med. 2002;136:421–8. doi: 10.7326/0003-4819-136-6-200203190-00005. [DOI] [PubMed] [Google Scholar]
- 7.Steere AC, Sikand VK. The presenting manifestations of Lyme disease and the outcomes of treatment. N Engl J Med. 2003;348:2472–4. doi: 10.1056/NEJM200306123482423. doi:10.1056/NEJM200306123482423. [DOI] [PubMed] [Google Scholar]
- 8.Guero-Rosenfeld ME, Wang G, Schwartz I, et al. Diagnosis of lyme borreliosis. Clin Microbiol Rev. 2005;18:484–509. doi: 10.1128/CMR.18.3.484-509.2005. doi:10.1128/CMR.18.3.484-509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nowakowski J, Schwartz I, Liveris D, et al. Laboratory diagnostic techniques for patients with early Lyme disease associated with erythema migrans: a comparison of different techniques. Clin Infect Dis. 2001;33:2023–7. doi: 10.1086/324490. doi:10.1086/324490. [DOI] [PubMed] [Google Scholar]
- 10.Tugwell P, Dennis DT, Weinstein A, et al. Laboratory evaluation in the diagnosis of Lyme disease. Ann Intern Med. 1997;127:1109–23. doi: 10.7326/0003-4819-127-12-199712150-00011. [DOI] [PubMed] [Google Scholar]
- 11.Agre F, Schwartz R. The value of early treatment of deer tick bites for the prevention of Lyme disease. Am J Dis Child. 1993;147:945–7. doi: 10.1001/archpedi.1993.02160330035013. [DOI] [PubMed] [Google Scholar]
- 12.Costello CM, Steere AC, Pinkerton RE, et al. A prospective study of tick bites in an endemic area for Lyme disease. Conn Med. 1989;53:338–40. [PubMed] [Google Scholar]
- 13.Shapiro ED, Gerber MA, Holabird NB, et al. A controlled trial of antimicrobial prophylaxis for Lyme disease after deer-tick bites. N Engl J Med. 1992;327:1769–73. doi: 10.1056/NEJM199212173272501. doi:10.1056/NEJM199212173272501. [DOI] [PubMed] [Google Scholar]
- 14.Warshafsky S, Nowakowski J, Nadelman RB, et al. Efficacy of antibiotic prophylaxis for prevention of Lyme disease. J Gen Intern Med. 1996;11:329–33. doi: 10.1007/BF02600042. doi:10.1007/BF02600042. [DOI] [PubMed] [Google Scholar]
- 15.Wormser GP, Nadelman RB, Dattwyler RJ, et al. Practice guidelines for the treatment of Lyme disease. The Infectious Diseases Society of America. Clin Infect Dis. 2000;31(Suppl 1):1–14. doi: 10.1086/314053. doi:10.1086/314053. [DOI] [PubMed] [Google Scholar]
- 16.Nadelman RB, Nowakowski J, Fish D, et al. Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. N Engl J Med. 2001;345:79–84. doi: 10.1056/NEJM200107123450201. doi:10.1056/NEJM200107123450201. [DOI] [PubMed] [Google Scholar]
- 17.Zeidner NS, Brandt KS, Dadey E, et al. Sustained-release formulation of doxycycline hyclate for prophylaxis of tick bite infection in a murine model of Lyme borreliosis. Antimicrob Agents Chemother. 2004;48:2697–9. doi: 10.1128/AAC.48.7.2697-2699.2004. doi:10.1128/AAC.48.7.2697-2699.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warshafsky S, Lee DH, Francois LK, et al. Efficacy of antibiotic prophylaxis for the prevention of Lyme disease: an updated systematic review and meta-analysis. J Antimicrob Chemother. 2010;65:1137–44. doi: 10.1093/jac/dkq097. doi:10.1093/jac/dkq097. [DOI] [PubMed] [Google Scholar]
- 19.Shih CM, Pollack RJ, Telford SR, III, et al. Delayed dissemination of Lyme disease spirochetes from the site of deposition in the skin of mice. J Infect Dis. 1992;166:827–31. doi: 10.1093/infdis/166.4.827. doi:10.1093/infdis/166.4.827. [DOI] [PubMed] [Google Scholar]
- 20.Shih CM, Spielman A. Topical prophylaxis for Lyme disease after tick bite in a rodent model. J Infect Dis. 1993;168:1042–5. doi: 10.1093/infdis/168.4.1042. doi:10.1093/infdis/168.4.1042. [DOI] [PubMed] [Google Scholar]
- 21.Dever LL, Torigian CV, Barbour AG. In vitro activities of the everninomicin SCH 27899 and other newer antimicrobial agents against Borrelia burgdorferi. Antimicrob Agents Chemother. 1999;43:1773–5. doi: 10.1128/aac.43.7.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunfeld KP, Kraiczy P, Wichelhaus TA, et al. Colorimetric in vitro susceptibility testing of penicillins, cephalosporins, macrolides, streptogramins, tetracyclines, and aminoglycosides against Borrelia burgdorferi isolates. Int J Antimicrob Agents. 2000;15:11–7. doi: 10.1016/s0924-8579(00)00116-3. doi:10.1016/S0924-8579(00)00116-3. [DOI] [PubMed] [Google Scholar]
- 23.Levin JM, Nelson JA, Segreti J, et al. In vitro susceptibility of Borrelia burgdorferi to 11 antimicrobial agents. Antimicrob Agents Chemother. 1993;37:1444–6. doi: 10.1128/aac.37.7.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barthold SW, Beck DS, Hansen GM, et al. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990;162:133–8. doi: 10.1093/infdis/162.1.133. doi:10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- 25.Krupka I, Knauer J, Lorentzen L, et al. Borrelia burgdorferi sensu lato species occurring in Europe induce diverse immune responses against C6 peptides in infected mice. Clin Vaccine Immunol. 2009;16:1546–62. doi: 10.1128/CVI.00201-09. doi:10.1128/CVI.00201-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Topfer KH, Straubinger RK. Characterization of the humoral immune response in dogs after vaccination against the Lyme borreliosis agent A study with five commercial vaccines using two different vaccination schedules. Vaccine. 2007;25:314–26. doi: 10.1016/j.vaccine.2006.07.031. doi:10.1016/j.vaccine.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 27.Hauser U, Lehnert G, Lobentanzer R, et al. Interpretation criteria for standardized western blots for three European species of Borrelia burgdorferi sensu lato. J Clin Microbiol. 1997;35:1433–44. doi: 10.1128/jcm.35.6.1433-1444.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hauser U, Lehnert G, Wilske B. Validity of interpretation criteria for standardized western blots (immunoblots) for serodiagnosis of Lyme borreliosis based on sera collected throughout Europe. J Clin Microbiol. 1999;37:2241–7. doi: 10.1128/jcm.37.7.2241-2247.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Straubinger RK. PCR-based quantification of Borrelia burgdorferi organisms in canine tissues over a 500-day postinfection period. J Clin Microbiol. 2000;38:2191–9. doi: 10.1128/jcm.38.6.2191-2199.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pahl A, Kuhlbrandt U, Brune K, et al. Quantitative detection of Borrelia burgdorferi by real-time PCR. J Clin Microbiol. 1999;37:1958–63. doi: 10.1128/jcm.37.6.1958-1963.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bennett JV, Brodie JL, Benner EJ, et al. Simplified, accurate method for antibiotic assay of clinical specimens. Appl Microbiol. 1966;14:170–7. doi: 10.1128/am.14.2.170-177.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Straubinger RK, Dharma RT, Davidson E, et al. Protection against tick-transmitted Lyme disease in dogs vaccinated with a multiantigenic vaccine. Vaccine. 2001;20:181–93. doi: 10.1016/s0264-410x(01)00251-1. doi:10.1016/S0264-410X(01)00251-1. [DOI] [PubMed] [Google Scholar]
- 33.Piesman J, Oliver JR, Sinsky RJ. Growth kinetics of the Lyme disease spirochete (Borrelia burgdorferi) in vector ticks (Ixodes dammini) Am J Trop Med Hyg. 1990;42:352–7. doi: 10.4269/ajtmh.1990.42.352. [DOI] [PubMed] [Google Scholar]
- 34.Wang G, Liveris D, Brei B, et al. Real-time PCR for simultaneous detection and quantification of Borrelia burgdorferi in field-collected Ixodes scapularis ticks from the Northeastern United States. Appl Environ Microbiol. 2003;69:4561–5. doi: 10.1128/AEM.69.8.4561-4565.2003. doi:10.1128/AEM.69.8.4561-4565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang X, Popova TG, Goldberg MS, et al. Influence of cultivation media on genetic regulatory patterns in Borrelia burgdorferi. Infect Immun. 2001;69:4159–63. doi: 10.1128/IAI.69.6.4159-4163.2001. doi:10.1128/IAI.69.6.4159-4163.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piesman J, Mather TN, Sinsky RJ, et al. Duration of tick attachment and Borrelia burgdorferi transmission. J Clin Microbiol. 1987;25:557–8. doi: 10.1128/jcm.25.3.557-558.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bockenstedt LK, Mao J, Hodzic E, et al. Detection of attenuated, noninfectious spirochetes in Borrelia burgdorferi-infected mice after antibiotic treatment. J Infect Dis. 2002;186:1430–7. doi: 10.1086/345284. doi:10.1086/345284. [DOI] [PubMed] [Google Scholar]
- 38.Hodzic E, Feng S, Freet KJ, et al. Borrelia burgdorferi population kinetics and selected gene expression at the host–vector interface. Infect Immun. 2002;70:3382–8. doi: 10.1128/IAI.70.7.3382-3388.2002. doi:10.1128/IAI.70.7.3382-3388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hodzic E, Feng S, Holden K, et al. Persistence of Borrelia burgdorferi following antibiotic treatment in mice. Antimicrob Agents Chemother. 2008;52:1728–36. doi: 10.1128/AAC.01050-07. doi:10.1128/AAC.01050-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hunfeld KP, Wichelhaus TA, Rodel R, et al. Comparison of in vitro activities of ketolides, macrolides, and an azalide against the spirochete Borrelia burgdorferi. Antimicrob Agents Chemother. 2004;48:344–7. doi: 10.1128/AAC.48.1.344-347.2004. doi:10.1128/AAC.48.1.344-347.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson RC, Kodner C, Russell M, et al. In-vitro and in-vivo susceptibility of Borrelia burgdorferi to azithromycin. J Antimicrob Chemother. 1990;25(Suppl A):33–8. doi: 10.1093/jac/25.suppl_a.33. [DOI] [PubMed] [Google Scholar]
- 42.Steere AC. Lyme disease. N Engl J Med. 2001;345:115–25. doi: 10.1056/NEJM200107123450207. doi:10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.