Abstract
Viral entry inhibitors represent an emerging mode of therapy for human immunodeficiency virus type 1 (HIV-1) infection. PRO 542 (CD4-immunoglobulin G2) is a tetravalent CD4-immunoglobulin fusion protein that broadly neutralizes primary HIV-1 isolates. PRO 542 binds to the viral surface glycoprotein gp120 and blocks attachment and entry of virus into CD4+ cells. Previously, PRO 542 demonstrated antiviral activity without significant toxicity when tested at single doses ranging to 10 mg/kg. In this study, 12 HIV-infected individuals were treated with 25-mg/kg single-dose PRO 542 and then monitored for safety, antiviral effects, and PRO 542 pharmacokinetics for 6 weeks. The study examined two treatment cohorts that differed in the extent of HIV-1 disease progression. PRO 542 at 25 mg/kg was well tolerated and demonstrated a serum half-life of 3 days. Statistically significant acute reductions in HIV-1 RNA levels were observed across all study patients, and greater antiviral effects were observed in the cohort of patients with more advanced HIV-1 disease. In advanced disease (HIV-1 RNA > 100,000 copies/ml; CD4 lymphocytes < 200 cells/mm3), PRO 542 mediated an 80% response rate and statistically significant ≈0.5 log10 mean reductions in viral load for 4 to 6 weeks posttreatment. Similar findings were obtained in an analysis of all (n = 11) advanced disease patients treated to date with single doses of PRO 542 ranging from 1 to 25 mg/kg. In addition, a significant correlation was observed between antiviral effects observed in vivo and viral susceptibility to PRO 542 in vitro. The findings support continued development of PRO 542 for salvage therapy of advanced HIV-1 disease.
Current guidelines (36) for human immunodeficiency virus type 1 (HIV-1) therapy recommend combination use of three or more antiretroviral agents selected from the four available treatment classes, and these regimens have significantly decreased HIV-related morbidity and mortality in developed countries (21, 24, 25). However, current HIV-1 therapies are limited by viral resistance, drug toxicity, and complex dosing regimens, and most patients eventually exhaust their therapeutic options as the available drugs become ineffective due to viral resistance and/or intolerable due to toxic side effects. Consequently, there is an urgent need for new modes of HIV-1 therapy.
Entry of HIV-1 into target cells proceeds via a cascade of events that provide opportunities for therapy. HIV-1 entry is mediated by the viral envelope glycoproteins gp120 and gp41, and the process initiates when gp120 binds CD4, the primary receptor for HIV-1. Attachment triggers conformational changes that enable gp120 to bind coreceptor molecules, such as the chemokine receptors CCR5 and CXCR4. Coreceptor binding triggers conformational changes in gp41 that drive membrane fusion (7). Inhibitors of viral attachment, coreceptor interactions, and membrane fusion represent distinct treatment classes and are collectively referred to as HIV-1 entry inhibitors. Each treatment class has shown clinical promise [reviewed in references 4 and 23], and the fusion inhibitor enfuvirtide (T-20) was recently approved for HIV-1 therapy following favorable outcomes in each of two Phase 3 trials (19, 20), validating viral entry as a therapeutic target.
PRO 542 is a novel inhibitor of HIV-1 attachment and entry. PRO 542 is a tetravalent CD4-immunoglobulin fusion protein that comprises the D1 and D2 domains of human CD4 genetically fused to the heavy and light chain constant regions of human IgG2,κ (1). PRO 542 broadly and potently neutralizes primary HIV-1 isolates in a variety of in vitro, ex vivo, and in vivo preclinical settings (14, 15, 18, 22, 31, 33). Compared to prior-generation CD4-based proteins, PRO 542 possesses greater valency, size, and conformational flexibility, and these structural features may contribute to its enhanced antiviral activity (38). PRO 542 has demonstrated antiviral activity without appreciable toxicity at doses ranging to 10 mg/kg in previous clinical trials in HIV-infected adults and children (17, 29).
In this study, 12 HIV-infected adults were treated with 25-mg/kg single-dose PRO 542. The study examined two treatment cohorts that differed in the extent of HIV-1 disease progression. Here we report the tolerability, pharmacokinetics, and antiviral effects of PRO 542 in this setting, and antiviral effects are correlated with baseline viral susceptibility to this new agent. The findings support further testing of attachment inhibitors in the setting of advanced HIV-1 disease.
MATERIALS AND METHODS
Study design.
This open-label, nonrandomized study examined the tolerability, pharmacokinetics, and antiviral effects of a single 25-mg/kg intravenous dose of PRO 542. The study protocol was approved by the Institutional Review Board of the Mount Sinai School of Medicine, and all study participants provided written informed consent. PRO 542 (Progenics Pharmaceuticals, Inc.) was supplied at a concentration of 5 mg/ml in phosphate-buffered saline.
Patients received PRO 542 by infusion over ≈30 min and were followed for 6 weeks. The trial enrolled 12 adults (age ≥18 years) with confirmed diagnosis of HIV-1 infection. Entry criteria included stable or no antiviral therapy for ≥4 weeks and normal hematology and serum chemistries prior to entry into the study. Patients had >5,000 copies of HIV-1 RNA per ml and >50 CD4 lymphocytes/mm3 (cohort 1, n = 7) or HIV-1 RNA > 100,000 copies/ml and CD4 lymphocytes < 200 cells/mm3 (cohort 2, n = 5). Cohort 2 patients were enrolled after all cohort 1 patients had been treated. Exclusion criteria included any active, significant infection other than HIV-1 not controlled by antibiotics, life expectancy of <3 months, and concomitant use of immunoglobulin or immunosuppressive therapy.
Safety assessment.
A physical examination was performed immediately prior to treatment, and vital signs and adverse events were recorded at frequent intervals up to 8 h posttreatment and on follow-up visits. Safety laboratory evaluations included complete blood counts and serum chemistries drawn at screening and at 2 and 42 days posttreatment. Lymphocyte subset analyses were performed immediately prior to treatment and at 2 and 6 weeks posttreatment.
Bioanalytical analyses.
Sera were collected at frequent intervals up to 42 days posttreatment, cryopreserved, and tested in batch for levels of PRO 542 and anti-PRO 542 antibodies with validated enzyme-linked immunosorbent assays (17). The lower limits of quantification of the assays are 40 ng/ml (PRO 542) and 12.5 ng/ml (anti-PRO 542), and the coefficient of variation for test samples was <25%. Terminal serum half-life and area under the concentration-time curve from time zero to infinity (AUC0→∞) were calculated as described previously with WinNonlin software (PharSight Corporation, Mountain View, Calif.) (17). The linear trapezoidal rule was used for all AUC calculations (16).
Antiviral analyses.
Plasma HIV-1 RNA levels were measured with the Amplicor v1.5 HIV Monitor assay (Roche Diagnostic Systems, Branchburg, N.J.), which has a threshold of 400 copies/ml. Baseline HIV-1 RNA levels were measured immediately prior to treatment. Viral AUC was calculated as a measure of the cumulative antiviral effect of single-dose PRO 542 therapy. Viral AUC was calculated by integrating the area under the viral load-time curve with WinNonlin software. For this analysis, viral loads were log-transformed and normalized to the baseline value (e.g., as in Fig. 3). Viral AUC was expressed in days, such that a 1-log reduction in viral load sustained for 28 days would yield a 4-week viral AUC of −28 days.
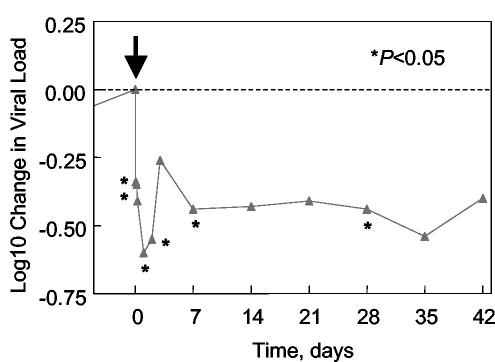
FIG. 3.
Antiviral effects of PRO 542 in advanced disease. Mean log10 change in viral load is plotted for cohort 2 patients with PRO 542-susceptible virus (R5 and X4 relative IC50 values < 5; patients 8 to 11). PRO 542 was administered at time zero, as indicated by an arrow. Asterisks indicate reductions that were significantly different from zero in two-sided t tests. In addition, the day 3, day 14, and day 21 reductions were significant (P < 0.05) in one-sided tests.
Drug susceptibility.
Viral susceptibility to PRO 542 and existing antiretrovirals was determined with the PhenoSense Entry (27) and GeneSeq assays, respectively. The PhenoSense Entry assay utilizes vectors designed to accept and express envelope sequences derived by amplification of patient virus sequences. Virus particles expressing patient virus envelopes were produced by transfecting HEK 293 cells with the envelope expression vector plus an HIV-1 genomic vector containing a firefly luciferase indicator gene. These “pseudotyped” viruses were used to infect U87 cells that express either CD4 plus CCR5 (R5 assay) or CD4 plus CXCR4 (X4 assay). Drug susceptibility was reported as 50% inhibitory concentration (IC50) values, which represent the concentration required for 50% inhibition of viral infectivity.
To control for day-to-day assay variation, the dual-tropic (R5X4) reference virus HIV-192HT594 was tested in parallel. Data are reported as relative IC50 values (also called fold change) that equal the IC50 of the test isolate divided by the IC50 of HIV-192HT594. The relative IC50 thus is a dimensionless parameter. The actual IC50 of PRO 542 for HIV-192HT594 averaged ≈2 μg/ml in both the R5 and X4 assays. HIV-192HT594 was isolated from a seropositive individual in Haiti and was obtained from the National Institutes of Health AIDS Research and Reference Reagent Program.
Pretreatment susceptibilities were determined for all 12 study patients from plasma samples taken immediately prior to treatment. In addition, posttreatment analyses were performed for cohort 2 patients with plasma samples collected on the last day of follow-up, either day 42 (n = 4) or day 28 (n = 1).
Viral tropism.
Viruses were also examined for their ability to utilize CCR5 and CXCR4 as entry coreceptors. Coreceptor usage was examined with both PhenoSense Entry and whole-virus assays. For the PhenoSense assay, parallel analyses were performed to measure infectivity in the absence of drug and in the presence of CCR5 and CXCR4 inhibitors. Coreceptor tropism was defined by assessing the ability of the pseudotyped viruses to infect CD4-CCR5 cells and CD4-CXCR4 cells and whether or not infection was specifically inhibited by a CCR5 or CXCR4 inhibitor. For whole-virus assays, replication-competent viruses were isolated from peripheral blood mononuclear cells as described previously (17) and tested for tropism by culture on fresh peripheral blood mononuclear cells in the presence of the CCR5 antibody PRO 140 (32) or the CXCR4 antagonist AMD3100 (8).
Virion release.
PRO 542's effects on virion release were examined with the chronically infected cell lines 8E5/LAV (13), ACH-2 (11), OM 10.1 (5), and U1/HIV-1 (12), which were obtained from the National Institutes of Health AIDS Research and Reference Reagent Program. Cells were passaged in RPMI medium supplemented with 10% fetal bovine serum, 2 mM glutamine, and penicillin-streptomycin. PRO 542 was added at serial concentrations ranging to 5 mg/ml, and cells were stimulated with 10 mM phorbol myristate acetate to induce virus production. Supernatants were harvested daily for three days and analyzed for p24 levels by enzyme-linked immunosorbent assay.
Statistical analyses.
HIV-1 RNA levels were log-transformed and determined to follow a normal distribution with the Shapiro-Wilk method (2). Longitudinal changes were evaluated for significance with parametric t tests and SAS software (SAS Institute, Cary, N.C.). Similar methods were used to compare differences between study groups. Correlations between viral AUC and baseline parameters were assessed by linear regression and evaluated for statistical significance by analysis of variance with SAS software. Two-sided statistical tests were used unless otherwise indicated.
RESULTS
Patient characteristics.
Cohort 1 (n = 7) comprised three blacks and four Hispanics with a median age of 49 years (range, 43 to 59 years). Cohort 2 (n = 5) comprised three blacks and two Hispanics with a median age of 33 years (range, 28 to 52 years). Two female patients were included in each cohort. At baseline, the mean viral loads and CD4 counts were 67,847 copies/ml and 333 cells/mm3, respectively, for cohort 1 patients. The corresponding values for cohort 2 were 376,579 copies/ml and 82 cells/mm3, respectively. The differences between the cohorts were statistically significant for both viral load (P = 0.014) and CD4 counts (P = 0.016). The number of patients receiving concurrent antiretroviral therapy was four of seven in cohort 1 and zero of five in cohort 2. GeneSeq analysis indicated median resistance to four antiretroviral agents from 1.5 treatment classes for cohort 1 and six agents from two classes for cohort 2.
The viral load and CD4 entry criteria for cohort 2 were selected on the findings of a pooled analysis of data from the seven cohort 1 patients from this study and 15 patients from a previous study that examined single doses ranging to 10 mg/kg (17). When 1-week viral AUCs were plotted versus baseline parameters and analyzed with linear regression, significant trends were observed for both viral load (P = 0.024) and CD4 counts (P = 0.029). The greatest antiviral effects were observed for patients with high viral loads and low CD4 counts. The trends were similar for 2-week viral AUCs (P = 0.043 for viral load and P = 0.087 for CD4 counts) but absent at 4 weeks (P ≥ 0.15), consistent with a progressive loss of antiviral effect over time following single-dose therapy.
Safety.
All patients (n = 12) received a full 25-mg/kg dose and completed 6 weeks of follow-up except for one patient who was available for 4 weeks only. No significant adverse events were observed.
Pharmacokinetics and immunogenicity.
Peak serum concentrations averaged 590 μg/ml (range, 299 to 814 μg/ml) and typically were observed within 2 h of treatment. The mean AUC0→∞ was 1,100 days × μg/ml, and the mean serum half-life was 2.9 days. These findings are consistent with those observed previously for doses ranging to 10 mg/kg, which also exhibited a terminal serum half-life of ≈3 days (17). For most patients, serum concentrations exceeded the in vitro antiviral IC50 value for the patient isolate until approximately 2 weeks posttreatment. There was no significant difference between cohorts 1 and 2 in either PRO 542 serum half-life or AUC0→∞ (P > 0.41). No patient developed measurable levels of antibodies to PRO 542.
Antiviral effects.
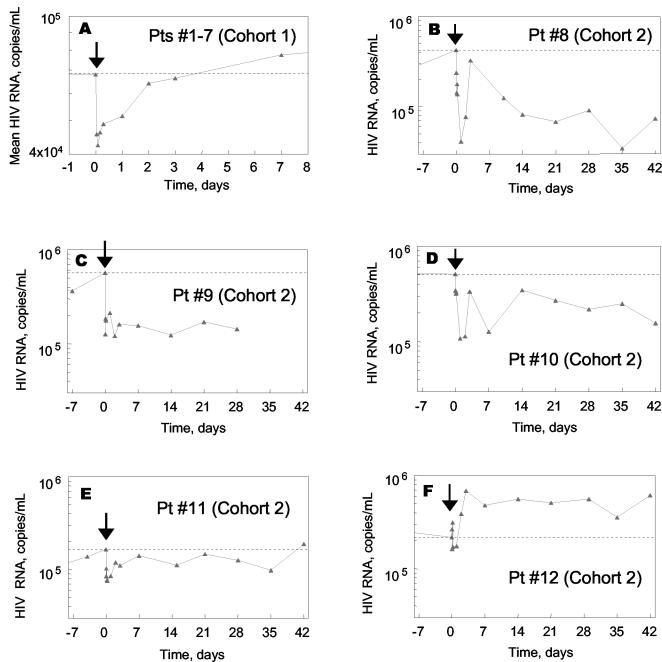
HIV-1 RNA levels are plotted in Fig. 1. Mean viral load data are plotted for the seven cohort 1 patients (Fig. 1A), and individual data are plotted for the five cohort 2 patients (Fig. 1B to 1F). An acute drop in HIV-1 RNA was observed for both groups of patients. Within the first 24 h posttreatment, the HIV-1 RNA nadirs averaged 0.24 log10 for cohort 1, 0.56 log10 for cohort 2, and 0.37 log10 for cohorts 1 and 2 combined. The mean viral load reductions were statistically significant for cohort 1 patients (P = 0.0022, n = 7), cohort 2 patients (P = 0.016, n = 5), and for cohorts 1 and 2 combined (P = 0.0002, n = 12). These results are similar to those reported previously for 10-mg/kg single-dose PRO 542, which mediated a statistically significant 0.4 log10 reduction in HIV-1 RNA within 24 h of treatment (17).
FIG. 1.
Effects of PRO 542 on HIV-1 RNA. (A) Mean change in HIV-1 RNA levels for cohort 1 patients (Pts, n = 7) (B to F) HIV-1 RNA levels of individual cohort 2 patients (patients 8 to 12). Viral load data for cohort 2 patients are plotted on an identical scale to facilitate interpatient comparisons. PRO 542 was administered at time zero, as indicated by an arrow. The dotted line indicates the predose viral load.
Whereas viral loads typically returned to baseline levels within a few days for cohort 1 patients (Fig. 1A), more durable antiviral effects were observed for cohort 2 patients (Fig. 1B to F). For most cohort 2 patients, viral loads had not returned to baseline levels at 6 weeks. Patient 8 experienced a ≈1 log10 reduction in HIV-1 RNA that was sustained throughout follow-up, while patient 9 experienced a 0.5 to 0.7 log10 reduction that remained relatively constant for 4 weeks. Patients 10 and 11 also experienced reductions in viral load at nearly all posttreatment time points, but the reductions were generally smaller in magnitude. Patient 12 was the only nonresponder in cohort 2. It should be noted that none of the patients in cohort 2 was receiving concurrent antiretroviral therapy, and thus the viral load changes do not reflect changes in adherence to existing regimens.
There was no significant change in CD4 lymphocyte counts posttreatment (P = 0.49). Increases in CD4 lymphocyte counts were not expected given the short duration of the study.
Drug susceptibility.
Relative IC50 values (IC50 of the patient virus divided by the IC50 of the reference virus) are indicated in Table 1. Pretreatment IC50s were obtained for all patients, and posttreatment IC50s were also obtained for patients in cohort 2.
TABLE 1.
Susceptibility of patient isolates to PRO 542 in vitro
| Cohort | Patient no. | Relative IC50
|
|||
|---|---|---|---|---|---|
| Pretreatment
|
Posttreatment
|
||||
| R5 assay | X4 assay | R5 assay | X4 assay | ||
| 1 | 1 | 0.4 | No entrya | ||
| 2 | 4.0 | No entry | |||
| 3 | 1.4 | No entry | |||
| 4 | 5.9 | No entry | |||
| 5 | 3.0 | No entry | |||
| 6 | 1.0 | No entry | |||
| 7 | 2.4 | 5.1 | |||
| 2 | 8 | 1.2 | No entry | 0.40 | No entry |
| 9 | 2.5 | 0.11 | 2.6 | 1.7 | |
| 10 | No entry | 1.9 | 3.0 | No entry | |
| 11 | 2.4 | No entry | 1.6 | No entry | |
| 12 | 6.5 | 3.2 | 12 | No entry | |
Pseudotyped virus did not mediate measurable levels of entry into the indicator cell line.
Baseline IC50s ranged from 0.4 to 6.5 for R5 viruses and 0.11 to 5.1 for X4 viruses (Table 1). The average relative IC50 value was 2.8 for R5 viruses and 2.6 for X4 viruses (P = 0.86). Thus, R5 and X4 viruses exhibited similar susceptibilities to PRO 542, as observed previously in whole-virus assays (31). In addition, the HIV-192HT594 reference virus is modestly more susceptible to PRO 542 than the patient isolates from this study. There was no significant difference in baseline drug susceptibility between cohorts 1 and 2 (P = 0.85), and larger studies will be needed to assess whether viral susceptibility to PRO 542 may vary subtly with disease progression.
Viral susceptibility to PRO 542 was also analyzed at the conclusion of follow-up for cohort 2 patients (Table 1). No significant difference was observed between pre- and posttreatment IC50s (P = 0.67), indicating no emergence of drug-resistant virus.
Correlation between antiviral effects in vivo and drug susceptibility in vitro.
Within cohort 2, patient 8 had the overall most susceptible virus (relative R5 IC50 = 1.2) and the largest acute and 6-week reductions in viral load (Fig. 1B). The second-largest reduction in viral load was observed for patient 9, whose virus exhibited normal susceptibility in the R5 assay (relative IC50 = 2.5) and increased susceptibility in the X4 assay (relative IC50 = 0.11). The nonresponder (patient 12) had the least susceptible virus (relative IC50 = 6.5 and 3.2 in R5 and X4 assays).
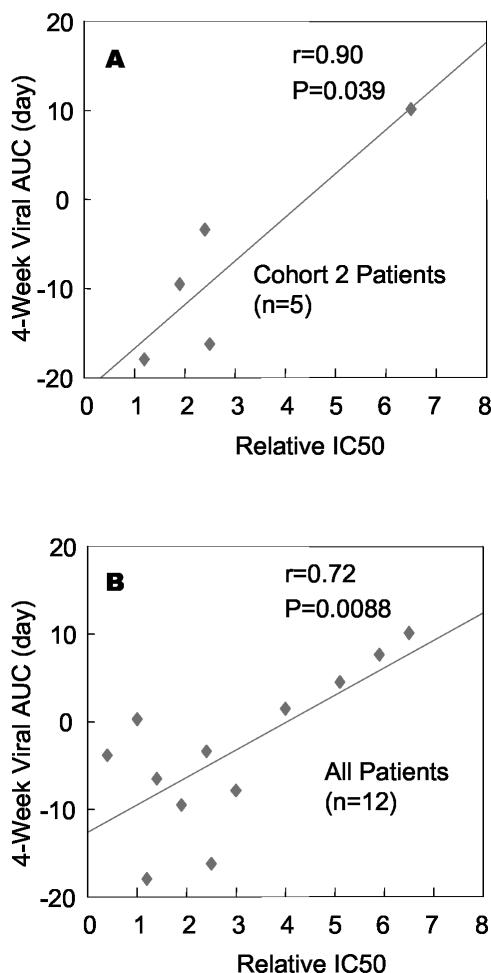
To further examine these trends, linear regression analysis was performed on the 4-week viral AUC and relative IC50 values. The higher of the R5 and X4 relative IC50 values was used for R5X4 viruses under the premise that drug resistance would be governed by the less-susceptible virus. A significant correlation (r = 0.9, P = 0.039) was observed between clinical outcome and in vitro susceptibility for cohort 2 patients (Fig. 2a), although the correlation is largely driven by the result for a single patient (patient 12, who had the least susceptible virus). However, a significant correlation (r = 0.72, P = 0.0088) was also observed across all 12 study patients (Fig. 2b). In each case, the largest antiviral effects (negative AUC values) correlated with increased drug susceptibility. Correlations for 1- and 2-week viral AUCs were similar and trended towards significance (P = 0.076 and P = 0.056, respectively).
FIG. 2.
Correlations between antiviral response in vivo and viral susceptibility to PRO 542 in vitro. Linear regression analysis of the correlation between clinical antiviral effects (4-week viral AUC) and baseline in vitro viral susceptibility to PRO 542 (relative IC50) as measured in the PhenoSense Entry assay (Table 1). (A) Cohort 2 patients (n = 5). (B) All 12 study patients. r is the Pearson correlation coefficient of the best-fit line, and P reflects the significance that the slope of the line differs from zero. Viral AUC represents the area under the viral load-time curve. For this analysis, viral load was log-transformed and normalized to the pretreatment value, and time was expressed in days (e.g., as in Fig. 3). By way of example, a constant 0.5 log10 reduction in HIV-1 RNA for 28 days would translate into a 4-week viral AUC of −14 days, whereas a constant 1 log10 reduction would give a 4-week viral AUC of −28 days.
When R5 relative IC50 values were used exclusively, the trends were similar but less robust, in part due to the smaller sample size. Too few study patients (n = 4) harbored R5X4 or pure X4 viruses to examine correlations based on X4 IC50 values alone. Also, no difference (P = 0.65) in viral AUC was observed for patients with pure R5 viruses (n = 8) versus those with R5X4 or X4 viruses (n = 4) prior to treatment.
Similar trends were also observed between relative IC50 values and the posttreatment nadirs in HIV-1 RNA, but the results did not reach statistical significance (P = 0.090 for cohort 2 patients and P = 0.12 for all 12 study patients). These findings support the use of viral AUC to assess the overall antiviral effect of single-dose PRO 542.
Coreceptor usage.
Coreceptor usage patterns varied between cohort 1 and cohort 2 viruses (Table 1). In the PhenoSense Entry assay, pure R5 viruses were observed in six of seven (86%) of cohort 1 patients and two of five (40%) of cohort 2 patients prior to treatment. This distribution is consistent with the known broadening of coreceptor usage with disease progression.
Nine of twelve pretreatment viruses were available for whole-virus analyses of coreceptor usage, including viruses from cohort 2 patients 8, 9, 10, and 12. Overall there was good agreement in the coreceptor usage patterns observed in PhenoSense and whole-virus assays. Identical results were obtained for the five cohort 1 patients that were tested with both methods, as each assay detected four pure R5 viruses and one R5X4 virus. Identical results were also obtained for cohort 2 patients 8 (pure R5 virus) and 10 (pure X4 virus). However, a difference was observed for patients 9 and 12: whereas R5X4 viruses were observed with PhenoSense technology (Table 1), pure X4 viruses were observed in the whole-virus assay. The difference could reflect an overestimation of coreceptor utilization via use of coreceptor-transfected indicator cell lines (37) and/or differential selection of viral quasispecies following cultivation in vitro for whole-virus assays (34).
Intriguingly, PRO 542 therapy led to an apparent loss of X4 viruses in two of three cases (patients 10 and 12, Table 1). Whereas pure X4 or R5X4 viruses were detected pretreatment, only R5 viruses were detected posttreatment. PRO 542 therapy did not lead to the emergence of X4 virus in any patient examined.
Antiviral effects of PRO 542 in advanced HIV-1 disease.
Figure 3 depicts the mean HIV-1 RNA reductions for cohort 2 patients with PRO 542-susceptible virus (R5 and X4 relative IC50 values < 5, patients 8 to 11). Significant ≈0.5 log10 mean reductions in viral load were observed out to 4 weeks posttreatment. These results largely corroborate our retrospective analysis (described under patient characteristics) performed prior to treatment of cohort 2 patients.
Given the consistent outcomes between the retrospective and prospective analyses of patients with advanced disease, all advanced disease patients treated to date were considered in a pooled analysis. This group includes patients treated at 25-mg/kg in the present study and patients treated with doses ranging to 10 mg/kg in our previous study (17). To date, a total of 11 patients with HIV-1 RNA > 100,000 copies/ml and CD4 lymphocytes < 200/mm3 have been treated with single-dose PRO 542: two patients treated with 1 mg/kg, one with 5 mg/kg, two with 10 mg/kg, and six with 25 mg/kg (the five cohort 2 patients plus one patient [patient 6] in cohort 1). The analysis thus includes all advanced disease patients independent of dose or viral susceptibility. Significant and sustained antiviral effects were observed across all patients with advanced disease. For these 11 patients, a 0.4 log10 mean reduction in HIV-1 RNA was observed between days 7 and 28 posttreatment (P < 0.05).
A dose relationship cannot be established for antiviral effects in advanced disease given the small number of patients. However, our retrospective analysis argues against a linear dose relationship, suggesting that doses lower than 25 mg/kg may prove active in this setting.
Effects on virion release in vitro.
A rapid decline in plasma levels of HIV-1 RNA is characteristic of PRO 542 therapy (Fig. 1 and 3). Viral loads often nadir within 24 h, partially rebound, and then undergo a secondary decline over several days. The kinetics of the secondary decline are consistent with inhibiting viral entry, whereas the initial rapid decline may reflect other processes.
A rapid reduction in plasma HIV-1 RNA could reflect modulation of virion release from infected cells, and PRO 542 conceptually could influence this process by binding nascent envelope on infected cells. However, PRO 542 neither inhibited nor measurably delayed HIV-1 release from any of 4 chronically infected cells in vitro at concentrations ranging to 5 mg/ml (data not shown). Unless there are significant differences in the mechanics of viral budding from primary cells, the acute drop in HIV-1 RNA is unlikely to reflect PRO 542-mediated effects on virion release.
DISCUSSION
Single-dose PRO 542 demonstrated measurable antiviral activity without appreciable toxicity in advanced HIV-1 disease. Significant reductions in viral RNA were observed for 4 to 6 weeks following a single infusion of PRO 542. In addition, PRO 542 was well tolerated at 25 mg/kg, the highest dose tested to date. Thus, PRO 542 may hold promise for salvage therapy of HIV-1 infection.
In both a prospectively treated cohort of patients and our retrospective analysis of previously treated patients, greater reductions in HIV-1 RNA were observed in patients with advanced versus early-stage disease. The finding is novel, and we are not aware of another antiviral agent whose activity is modulated by disease progression independent of drug susceptibility.
Why would an HIV-1 attachment inhibitor exert greater antiviral effects in advanced disease? As described above, the pharmacokinetics of PRO 542 did not vary with disease progression, nor did viral susceptibility. Conceptually, however, antiviral activity could be influenced by the number of CD4+ target cells, whose depletion is a hallmark of HIV-1 disease progression. At the simplest level, one can consider the fate of a newly released virion. The fewer the number of target cells, the greater the distance and time the virus must travel prior to initiating infection, and consequently the longer the window of opportunity for PRO 542 to neutralize the virus. The situation undoubtedly is more complex in vivo, where target cell availability may additionally reflect the integrity of the lymphoid architecture and disease compartmentalization [reviewed in reference 10]. Also, PRO 542 may begin to neutralize virus prior to budding, perhaps contributing to its activity in all disease settings.
This study is the first to examine correlations between antiviral effects and baseline viral susceptibility to entry inhibitors. Despite the small data set, significant and positive correlations were observed with a phenotypic assay, and the study thus begins to define an in vitro susceptibility threshold for PRO 542 therapy. Further studies are warranted to assess the potential utility of resistance-testing technologies in designing salvage regimens that contain PRO 542 and other entry inhibitors.
Single-dose PRO 542 exhibits complex pharmacodynamics. Typically, viral loads drop within hours, partially rebound, undergo a secondary decline over a period of days, and then may remained reduced for up to 6 weeks. The secondary decline is consistent with inhibiting viral entry, while the initial decline may reflect other processes. A similar acute decline in viral RNA has been observed following passive immunotherapy of SIVmac251-infected macaques with immune globulin, where a viral nadir was observed 12 h posttreatment (3).
In principle, the acute reduction in HIV RNA could reflect PRO 542-mediated effects on virion clearance, virion release from infected cells, and/or lysis of virally infected cells. However, we observed no effect of PRO 542 on virion release from any of four chronically infected cell lines in vitro. In addition, lysis of virally infected cells appears unlikely given that PRO 542 possesses an IgG2 Fc region, which is poorly active in mediating immunologic effector functions such as complement-dependent cytotoxicity or antibody-dependent cellular cytotoxicity. Notably, PRO 542 does not measurably bind Fc receptors in vitro (1). Thus, the initial decline is more likely to reflect enhanced clearance of PRO 542-complexed virions through the reticuloendothelial system.
The durability of PRO 542's antiviral effect was not predicted by the pharmacokinetic and susceptibility analyses, since serum concentrations remained above the in vitro IC50 value for ≈2 weeks in most patients. Further comparisons of serum concentrations, IC50 values, and antiviral effects will be needed to define an inhibitory quotient for PRO 542. In addition, it is possible that this agent is preferentially taken up in lymphoid tissues or other sites of viral replication, resulting in higher local concentrations. Lastly, the sustained antiviral effect could reflect the selection of viruses with reduced replication capacity, which was not measured in this study. The transitory nature of single-dose studies may preclude a complete understanding of the PRO 542 pharmacodynamics, and further insight may require multidose studies that maintain steady-state drug levels. One such study is under way.
In some patients, PRO 542 resulted in an apparent loss of X4 viruses, which typically are more pathogenic, and their emergence often presages disease progression (6, 28, 35). As reported here and previously (31), X4 viruses are not more susceptible to PRO 542 in vitro. However, selective loss of X4 viruses has also been reported following conventional antiretroviral therapy (9, 26, 30) and may reflect a higher turnover rate of cells infected by X4 viruses. PRO 542 therapy did not lead to emergence of X4 viruses in any of the patients studied.
In summary, PRO 542 was well tolerated at 25 mg/kg, the highest dose tested to date. Single doses resulted in significant reductions in HIV-1 RNA for 4 to 6 weeks in treatment-experienced individuals with advanced HIV-1 disease, and a correlation was observed between antiviral effects in vivo and viral susceptibility in vitro. The findings support development of PRO 542 for salvage therapy of HIV-1 infection.
Acknowledgments
We thank the patients for their participation in the study and William Shearer for thoughtful review of the manuscript.
The research was supported by Public Health Service grants AI43084 and AI048990 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Allaway, G. P., K. L. Davis-Bruno, G. A. Beaudry, E. B. Garcia, E. L. Wong, A. M. Ryder, K. W. Hasel, M.-C. Gauduin, R. A. Koup, J. S. McDougal, and P. J. Maddon. 1995. Expression and characterization of CD4-IgG2, a novel heterotetramer which neutralizes primary HIV-1 isolates. AIDS Res. Hum. Retroviruses 11:533-539. [DOI] [PubMed] [Google Scholar]
- 2.Berry, D. A., and B. W. Lindgren. 1996. Statistics: theory and methods. Wadsworth Publishing Company, Belmont, Calif.
- 3.Binley, J. M., B. Clas, A. Gettie, M. Vesanen, D. C. Montefiori, L. Sawyer, J. Booth, M. Lewis, P. A. Marx, S. Bonhoeffer, and J. P. Moore. 2000. Passive infusion of immune serum into simian immunodeficiency virus-infected rhesus macaques undergoing a rapid disease course has minimal effect on plasma viremia. Virology 270:237-249. [DOI] [PubMed] [Google Scholar]
- 4.Biscone, M. J., T. C. Pierson, and R. W. Doms. 2002. Opportunities and challenges in targeting HIV entry. Curr. Opin. Pharmacol. 2:529-533. [DOI] [PubMed] [Google Scholar]
- 5.Butera, S. T., V. L. Perez, B. Y. Wu, G. J. Nabel, and T. M. Folks. 1991. Oscillation of the human immunodeficiency virus surface receptor is regulated by the state of viral activation in a CD4+ cell model of chronic infection. J. Virol. 65:4645-4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connor, R., K. E. Sheridan, D. Ceradini, S. Choe, and N. R. Landau. 1997. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J. Exp. Med. 185:621-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doms, R. W., and J. P. Moore. 2000. HIV-1 membrane fusion: targets of opportunity. J. Cell Biol. 151:F9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donzella, G. A., D. Schols, S. W. Lin, J. A. Este, K. A. Nagashima, P. J. Maddon, G. P. Allaway, T. P. Sakmar, G. Henson, E. De Clercq, and J. P. Moore. 1998. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat. Med. 4:72-77. [DOI] [PubMed] [Google Scholar]
- 9.Equils, O., E. Garratty, L. S. Wei, S. Plaeger, M. Tapia, J. DeVille, P. Krogstad, M. S. Sim, K. Nielsen, and Y. J. Bryson. 2000. Recovery of replication-competent virus from CD4 T cell reservoirs and change in coreceptor use in human immunodeficiency virus type 1-infected children responding to highly active antiretroviral therapy. J. Infect. Dis. 182:751-757. [DOI] [PubMed] [Google Scholar]
- 10.Fauci, A. S., G. Pantaleo, S. Stanley, and D. Weissman. 1996. Immunopathogenic mechanisms of HIV infection. Ann. Intern. Med. 124:654-663. [DOI] [PubMed] [Google Scholar]
- 11.Folks, T. M., K. A. Clouse, J. Justement, A. Rabson, E. Duh, J. H. Kehrl, and A. S. Fauci. 1989. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc. Natl. Acad. Sci. 86:2365-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folks, T. M., J. Justement, A. Kinter, C. A. Dinarello, and A. S. Fauci. 1987. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science 238:800-802. [DOI] [PubMed] [Google Scholar]
- 13.Folks, T. M., D. Powell, M. Lightfoote, S. Koenig, A. S. Fauci, S. Benn, A. Rabson, D. Daugherty, H. E. Gendelman, and M. D. Hoggan. 1986. Biological and biochemical characterization of a cloned Leu-3-cell surviving infection with the acquired immune deficiency syndrome retrovirus. J. Exp. Med. 164:280-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gauduin, M.-C., G. P. Allaway, P. J. Maddon, C. F. Barbas, D. R. Burton, and R. A. Koup. 1996. Effective ex vivo neutralization of plasma HIV-1 by recombinant immunoglobulin molecules. J. Virol. 70:2586-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gauduin, M.-C., G. P. Allaway, W. C. Olson, R. Weir, P. J. Maddon, and R. A. Koup. 1998. CD4-immunoglobulin G2 protects Hu-PBL-SCID mice against challenge by primary human immunodeficiency virus type 1 isolates. J. Virol. 72:3475-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibaldi, M., and D. Perrier. 1982. Pharmacokinetics, 2nd ed. Marcel Dekker, Inc., New York, N.Y.
- 17.Jacobson, J. M., I. Lowy, C. V. Fletcher, T. J. O'Neill, D. N. H. Tran, T. J. Ketas, A. Trkola, M. E. Klotman, P. J. Maddon, W. C. Olson, and R. J. Israel. 2000. Single-dose safety, pharmacology and antiviral activity of the human immunodeficiency virus (HIV) type 1 entry inhibitor PRO 542 in HIV-infected adults. J. Infect. Dis. 182:326-329. [DOI] [PubMed] [Google Scholar]
- 18.Ketas, T. J., I. Frank, P. J. Klasse, B. M. Sullivan, J. P. Gardner, C. Spenlehauer, M. Nesin, W. C. Olson, J. P. Moore, and M. Pope. 2003. Hum. immunodeficiency virus type 1 attachment, coreceptor and fusion inhibitors are active against both direct and trans infection of primary cells. J. Virol. 77:2762-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lalezari, J. P., K. Henry, M. O'Hearn, J. S. G. Montaner, P. J. Piliero, B. Trottier, M. S. Walmsley, C. Cohen, D. R. Kuritzkes, J. J. Eron, J. Chung, R. DeMasi, P. L. Donatacci, C. Drobnes, J. Delehanty, M. Salgo, and the TORO 1 Study Group. 2003. Enfuvirtide, an HIV-1 fusion inhibitor, for drug-resistant HIV infection in North and South America. N. Engl. J. Med. 348:2175-2185. [DOI] [PubMed] [Google Scholar]
- 20.Lazzarin, A., B. Clotet, D. Cooper, J. Reynes, K. Arasteh, M. Nelson, C. Katlama, H. J. Stellbrink, J. F. Delfraissy, J. Lange, L. Huson, R. DeMasi, C. Wat, J. Delehanty, C. Drobnes, M. Salgo, and the TORO 2 Study Group. 2003. Efficacy of enfuvirtide in patients infected with drug-resistant HIV-1 in Europe and Australia. N. Engl. J. Med. 348:2186. [DOI] [PubMed] [Google Scholar]
- 21.Mouton, Y., S. Alfandari, M. Valette, F. Cartier, P. Dellamonica, G. Humbert, J. M. Lang, P. Massip, D. Mechali, P. Leclercq, J. Modai, and H. Portier. 1997. Impact of protease inhibitors on AIDS-defining events and hospitalizations in 10 French AIDS reference centres. Federation National des Centres de Lutte contre le SIDA. AIDS 11:F101-F105. [DOI] [PubMed] [Google Scholar]
- 22.Nagashima, K. A., D. A. D. Thompson, S. I. Rosenfield, P. J. Maddon, T. Dragic, and W. C. Olson. 2001. Human immunodeficiency virus type 1 entry inhibitors PRO 542 and T-20 are potently synergistic in blocking virus-cell and cell-cell fusion. J. Infect. Dis. 183:1121-1125. [DOI] [PubMed] [Google Scholar]
- 23.O'Hara, B. M., and W. C. Olson. 2002. HIV entry inhibitors in clinical development. Curr. Opin. Pharmacol. 2:523-528. [DOI] [PubMed] [Google Scholar]
- 24.Palella, F. J., K. M. Delaney, A. C. Moorman, M. O. Loveless, J. Fuhrer, G. A. Satten, D. J. Aschman, S. D. Holmberg, and The HIV Outpatient Study Investigators. 1998. Declining morbidity and mortality among patients with advanced Human Immunodeficiency Virus infection. N. Engl. J. Med. 338:853. [DOI] [PubMed] [Google Scholar]
- 25.Palella, F. J., M. Deloria-Knoll, J. S. Chmiel, A. C. Moorman, K. C. Wood, A. E. Greenberg, S. D. Holmberg, and HIV Outpatient Study Investigators. 2003. Survival benefit of initiating antiretroviral therapy in HIV-infected persons in different CD4+ cell strata. Ann. Intern. Med. 138:620-626. [DOI] [PubMed] [Google Scholar]
- 26.Philpott, S., B. Weiser, K. Anastos, C. M. Kitchen, E. Robison, W. A. Meyer, H. S. Sacks, U. Mathur-Wagh, C. Brunner, and H. Burger. 2001. Preferential suppression of CXCR4-specific strains of HIV-1 by antiviral therapy. J. Clin. Investig. 107:431-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richman, D. D., T. Wrin, S. J. Little, and C. J. Petropoulos. 2003. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. 100:4144-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scarlatti, G., E. Tresoldi, A. Bjorndal, R. Fredriksson, C. Colognesi, H. K. Deng, M. S. Malnati, A. Plebani, A. G. Siccardi, D. R. Littman, E. M. Fenyo, and P. Lusso. 1997. In vivo evolution of HIV-1 co-receptor usage and sensitivity to chemokine-mediated suppression. Nat. Med. 3:1259-1265. [DOI] [PubMed] [Google Scholar]
- 29.Shearer, W. T., R. J. Israel, S. Starr, C. V. Fletcher, D. Wara, M. Rathore, J. Church, J. DeVille, T. Fenton, B. Graham, P. Samson, S. Staprans, J. McNamara, J. Moye, P. J. Maddon, and W. C. Olson. 2000. Recombinant CD4-IgG2 in HIV-1 infected children: Phase I/II study. J. Infect. Dis. 182:1774-1779. [DOI] [PubMed] [Google Scholar]
- 30.Skrabal, K., V. Trouplin, B. Labrosse, V. Obry, F. Damond, A. J. Hance, F. Clavel, and F. Mammano. 2003. Impact of antiretroviral treatment on the tropism of HIV-1 plasma virus populations. AIDS 17:809-814. [DOI] [PubMed] [Google Scholar]
- 31.Trkola, A., T. Ketas, V. N. KewalRamani, F. Endorf, J. M. Binley, H. Katinger, J. Robinson, D. R. Littman, and J. P. Moore. 1998. Neutralization sensitivity of human immunodeficiency virus type 1 primary isolates to antibodies and CD4-based reagents is independent of coreceptor usage. J. Virol. 72:1876-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trkola, A., T. J. Ketas, K. A. Nagashima, L. Zhao, T. Cilliers, L. Morris, J. P. Moore, P. J. Maddon, and W. C. Olson. 2001. Potent, broad-spectrum inhibition of human immunodeficiency virus type 1 by the CCR5 monoclonal antibody PRO 140. J. Virol. 75:579-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trkola, A., A. P. Pomales, H. Yuan, B. Korber, P. J. Maddon, G. P. Allaway, H. Katinger, C. F. Barbas, D. R. Burton, D. D. Ho, and J. P. Moore. 1995. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-IgG2. J. Virol. 69:6609-6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Briesen, H., M. Grez, H. Ruppach, I. Raudonat, R. E. Unger, K. Becker, B. Panhans, U. Dietrich, and H. Rubsamen-Waigmann. 1999. Selection of HIV-1 genotypes by cultivation in different primary cells. AIDS 13:307-315. [DOI] [PubMed] [Google Scholar]
- 35.Xiao, L., D. L. Rudolph, S. M. Owen, T. J. Spira, and R. B. Lal. 1998. Adaptation to promiscuous usage of CC and CXC-chemokine coreceptors in vivo correlates with HIV-1 disease progression. AIDS 12:F137-F143. [DOI] [PubMed] [Google Scholar]
- 36.Yeni, P. G., S. M. Hammer, C. C. Carpenter, D. A. Cooper, M. A. Fischl, J. M. Gatell, B. G. Gazzard, M. S. Hirsch, D. M. Jacobsen, D. A. Katzenstein, J. S. Montaner, D. D. Richman, M. S. Saag, M. Schechter, R. T. Schooley, M. A. Thompson, S. Vella, and P. A. Volberding. 2002. Antiretroviral treatment for adult HIV infection in 2002: updated recommendations of the International AIDS Society-USA Panel. JAMA 288:222-235. [DOI] [PubMed] [Google Scholar]
- 37.Zhang, Y. J., T. Dragic, Y. Cao, L. Kostrikis, D. S. Kwon, D. R. Littman, V. N. KewalRamani, and J. P. Moore. 1998. Use of coreceptors other than CCR5 by non-syncytium-inducing adult and pediatric isolates of human immunodeficiency virus type 1 is rare in vitro. J. Virol. 72:9337-9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu, P., W. C. Olson, and K. H. Roux. 2001. Structural flexibility and functional valency of CD4-IgG2 (PRO 542): potential for crosslinking HIV-1 envelope spikes. J. Virol. 75:6682-6686. [DOI] [PMC free article] [PubMed] [Google Scholar]