Abstract
Objective
To determine the association of family history of peripheral artery disease (PAD) with PAD prevalence and severity.
Background
PAD is a significant public health problem. Shared genetic and environmental factors may play an important role in the development of PAD. However, family history of PAD has not been adequately investigated.
Methods
The San Diego Population Study (SDPS) enrolled 2404 ethnically diverse men and women aged 29–91 who attended a baseline visit from 1994–98 to assess PAD and venous disease. Ankle brachial index (ABI) measurement was performed at the baseline clinic examination and family history of PAD was obtained via questionnaire. Family history of PAD was primarily defined as having any 1st degree relative with PAD. Prevalent PAD was defined as ABI ≤ 0.90 and severe prevalent PAD as ABI ≤ 0.70, with both definitions also including any previous leg revascularization. Logistic regression was used to evaluate the association of family history of PAD with prevalent PAD.
Results
The mean (SD) age was 59 (11) years, 66% were women, and 58% were Caucasian with 42% comprising other racial/ethnic groups. Prevalence of PAD was 3.6%, and severe prevalent PAD was 1.9%. In fully adjusted models, family history of PAD was associated with a 1.83-fold higher odds of PAD (95% CI (1.03, 3.26), p=0.04), an association which was stronger for severe prevalent PAD (OR 2.42, 95% CI (1.13, 5.23), p=0.02).
Conclusions
Family history of PAD is independently strongly associated with PAD prevalence and severity. This indicates a role for genetic factors and/or other shared environmental factors contributing to PAD.
Keywords: family history, peripheral artery disease, ankle brachial index
Introduction
Peripheral artery disease (PAD) is a significant public health issue, with approximately 8.5 million Americans currently affected (1). PAD is associated with increased risk of cardiovascular (CV) events and mortality (2–5). Morbidities resulting from PAD including functional decline, intermittent claudication, critical leg ischemia, and amputation, severely affect quality of life (6–9). PAD prevalence differs by racial/ethnic group with African-Americans having the highest prevalence (10). Several studies have evaluated potential explanatory factors for this higher prevalence in African-Americans, and have consistently concluded that it cannot be entirely explained by differences in traditional or novel cardiovascular disease (CVD) risk factors (1, 11–14). This result could indicate that genetic factors are important in the development of PAD.
Genes, environment, and the interaction of these factors likely play a role in the development of PAD. However, few genetic variants have been consistently associated with PAD (15). Familial aggregation is also likely an important factor in determining PAD susceptibility (16); however, the association of family history of PAD with prevalent PAD is largely unknown. To our knowledge, only two small studies have previously examined this association, but only evaluated premature onset PAD (≤ 49 years) and both were conducted in samples consisting almost exclusively of individuals of European descent (17, 18).
Thus, we examined the association of family history of PAD with prevalent PAD in a large ethnically diverse cohort of men and women ages 29–91 years who were participants in the San Diego Population Study (SDPS). Additionally, we examined the association of family history of PAD with prevalent CVD, and family history of CVD with prevalent PAD in this cohort.
Methods
Study Participants
The San Diego Population Study (SDPS) enrolled an ethnically diverse group of men and women who were current or former employees of the University of California, San Diego, and their significant others who were invited to participate between 1994 and 1998 in a study of PAD and venous diseases. Briefly, participants were randomly chosen within age, sex and ethnicity strata. Age strata were 29–49 years (primarily 40–49), 50–59, 60–69 and 70–91 (primarily 70–79). Women and ethnic minorities (African-American, Hispanic, Asian) were over-sampled in order to have adequate power for hypotheses involving these groups. Additionally, a small number of volunteers and their significant others (n=193) heard about the study, asked to participate, and were enrolled. The final sample included persons of all levels of education and occupation, and included working, unemployed, and retired persons. Further details of the study have been published elsewhere (13, 19, 20).
For all study procedures, participants provided signed informed consent after a detailed introduction and description of the study. The study received approval from the Committee on Investigations Involving Human Subjects at UCSD.
Family History of Peripheral Artery Disease
Family history of PAD was obtained via interviewer-administered questionnaire prior to the ABI measurements. The family history questionnaire was ascertained by study personnel who were not performing the ABI measurement, and who were blinded to the vascular examination results. Participants were specifically asked to exclude foster or adoptive family members, and asked about PAD history of all first degree relatives including mother, father, brothers, sisters, sons and daughters. The exact sequence of questions is as follows for parental history: “Has your father or mother ever had any of the following?”, and then, “Trouble with arterial circulation in the legs (peripheral arterial disease) including bypass surgery in the legs and/or amputation?” These questions are repeated for brothers, sisters, sons and daughters. This questionnaire has not been previously validated for family history of PAD, which would involve contact of parents, brothers, sisters, sons, or daughters, and/or obtaining medical records of these family members with regard to PAD. All questions regarding family history of PAD can be found in supplemental material. Family history of CVD was also assessed similarly via questionnaire, and included questions regarding history of heart attack, stroke, angina, percutaneous transluminal coronary angioplasty (PTCA), coronary artery bypass graft (CABG), and carotid endarterectomy of first degree relatives.
Ankle Brachial Index (ABI) measurement
Systolic blood pressure was measured in both arms with the participant in a supine position. Continuous wave Doppler ultrasound was used to measure systolic blood pressure two times in the posterior tibial artery; in the rare event that there was no obtainable signal in the posterior tibial artery, the dorsalis pedis was used. The ABI was calculated as average systolic blood pressure in the posterior tibial artery (or dorsalis pedis) divided by the higher of the SBP in the two arms. For these analyses, the “worst” ABI was used and was defined as the minimum ABI value of the left and right leg. The higher arm SBP was used due to previous studies showing a strong association between PAD and subclavian stenosis(21).
Covariates
Age, sex and race/ethnicity were determined via questionnaire. Diabetes was defined as self-report or use of anti-diabetic medications or insulin. Current and past cigarette smoking habits were ascertained via questionnaire, and pack-years of smoking were calculated as the average number of cigarettes smoked per day over all the years cigarettes were smoked divided by 20, multiplied by the total number of years cigarettes were smoked. Smoking was also defined as ever having smoked versus never smoked. Hypertension was defined as a systolic pressure ≥ 140 mm Hg or a diastolic pressure ≥ 90 mm Hg, or use of anti-hypertensive medications. Height (in centimeters) and weight (in kilograms) were measured, and the body mass index (BMI) was calculated as kg/m2. A blood sample was drawn, and total and HDL cholesterol were measured with standardized laboratory assays (Beckman Coulter analyzer). Dyslipidemia was defined as a ratio of total cholesterol to HDL cholesterol (TC/HDL) > 5.0 or use of hyper-lipidemic medications (22).
Statistical Analysis
Univariate associations were assessed using t-test, chi-square test or Wilcoxon test. Since pack years of smoking was a skewed variable, the difference between the PAD and non-PAD groups was assessed using a Wilcoxon test. All other continuous variables (i.e. HDL cholesterol, systolic blood pressure) were normally distributed and thus differences between the PAD and non-PAD groups for these variables were assessed using a t-test.
To examine the multivariate association of family history of PAD with prevalent PAD, staged logistic regression models were used, adding in adjustment variables for potential confounders and mediators. Family history of PAD was defined three ways: 1) any family history of PAD (including any first degree relative), 2) parental history of PAD (either parent), and 3) number of first degree relatives with PAD as a continuous variable. Prevalent PAD was defined as ABI ≤ 0.90 or leg revascularization, and severe prevalent PAD was defined as ABI ≤ 0.70 or leg revascularization.
In order to compare associations of family history of PAD and family history CVD with prevalent PAD and prevalent CVD outcomes, we defined family history of CVD or PAD separately as any parental history. Prevalent CVD was defined as any previous myocardial infarction, stroke, PTCA, or CABG. Staged logistic regression models were also used for this analysis.
SAS Version 9.1.3 (Cary, NC) was used for all analyses, and p<0.05 was considered statistically significant.
Results
Participant Characteristics
A total of 2376 participants had complete ABI and family history data, and had an ABI ≤ 1.4 in both legs. Overall the mean (SD) age was 59 (11) years, and 66% were women, 58% were Caucasian, 14% African-American, 15% Hispanic, and 14% were other race/ethnicities. There were 87 cases of prevalent PAD, defined as ABI ≤ 0.90 or leg revascularization, and 44 cases of severe prevalent PAD, defined as ABI ≤ 0.70 or leg revascularization. PAD prevalence differed significantly by sex and ethnic group, and was significantly higher in men and African-Americans (Table 1). Participants with prevalent PAD had significantly higher systolic blood pressure, total cholesterol, and pack years of smoking and were more likely to be ever smokers. Those with prevalent PAD were also more likely to have dyslipidemia, hypertension, diabetes, and CVD. Participants with PAD were somewhat more likely to have any family history of PAD (p=0.06), but did not differ significantly on parental history of PAD or parental history of CVD, or on the distribution of number of first degree relatives with PAD.
Table 1.
Baseline characteristics by Peripheral Arterial Disease (PAD) for San Diego Population Study Cohort*
| Characteristic | No PAD n=2289 |
PAD n=87 |
p-value |
|---|---|---|---|
| Age, years | 59 (11) | 69 (10) | <0.001 |
| Female Gender, n(%) | 1523 (67%) | 45 (52%) | 0.004 |
| Ethnicity, n(%) | |||
| Caucasian | 1328 (58%) | 56 (64%) | <0.001 |
| Hispanic | 341 (15%) | 6 (7%) | |
| African-American | 299 (13%) | 22 (25%) | |
| Other | 321 (14%) | 3 (3%) | |
| Ever Smoker, n(%) | 1113 (49%) | 60 (69%) | <0.001 |
| Pack Years of Smoking† | 0 (0, 10) | 15 (0, 45) | <0.001 |
| Body Mass Index, kg/m2 | 27 (5) | 27 (6) | 0.46 |
| Hypertension, n(%) | 1057 (46%) | 67 (77%) | <0.001 |
| Systolic BP mmHg | 131 (20) | 148 (28) | <0.001 |
| Diastolic BP mmHg | 77 (11) | 77 (12) | 0.95 |
| Dyslipidemia, n(%)‡ | 702 (31%) | 43 (49%) | <0.001 |
| Total cholesterol, mg/dL | 210 (41) | 226 (54) | 0.009 |
| HDL cholesterol, mg/dL | 54 (17) | 53 (18) | 0.36 |
| Diabetes, n(%) | 129 (6%) | 26 (30%) | <0.001 |
| Cardiovascular Disease, n(%) | 46 (2%) | 15 (17%) | <0.001 |
| Parental History of CVD, n(%)§ | 1366 (60%) | 57 (66%) | 0.28 |
| Any Family History PAD, n(%)∥ | 335 (15%) | 19 (22%) | 0.06 |
| Parental History of PAD, n(%)§ | 300 (13%) | 16 (18%) | 0.15 |
| No. of first relatives w/ PAD, n(%)∥ | |||
| 0 | 1954 (85%) | 68 (78%) | 0.14 |
| 1 | 303 (13%) | 18 (21%) | |
| ≥ 2 | 32 (1%) | 1 (1%) | |
Excluding those with ABI ≥ 1.4 in either leg, mean (SD) or n(%) presented for each characteristic
Median (Quartile 1, Quartile 3)
Total cholesterol/HDL cholesterol > 5.0
Includes mother and father only
Includes any first degree relative, i.e. mother, father, brothers, sisters, daughters, sons
Associations of family history of PAD with PAD prevalence and severity
In the fully adjusted analysis shown in Table 2, any family history of PAD, defined as any first degree relative with PAD, was significantly associated with higher odds of PAD (OR 1.83 (95% CI (1.03, 3.26), p=0.04). Parental history of PAD, defined as either mother or father with PAD, was similarly associated with higher odds of PAD (Table 2). Both any family history of PAD and parental history of PAD were strongly associated with severe prevalent PAD, with an approximately 2.4-fold higher odds of severe PAD for any family history, and 2.9-fold higher odds for parental history of PAD (Table 2). Defining smoking as pack years instead of ever smoking, or using HDL and total cholesterol instead of dyslipidemia did not change the results.
Table 2.
Association of family history of peripheral arterial disease (PAD) with prevalent PAD
| Any Family History* OR (95% CI) |
p-value | Parents Only* OR (95% CI) |
p-value | |
|---|---|---|---|---|
| Prevalent PAD† | ||||
| Unadjusted | 1.63 (0.97, 2.75) | 0.07 | 1.50 (0.86, 2.61) | 0.16 |
| + Demographics‡ | 1.90 (1.10, 3.26) | 0.02 | 1.88 (1.05, 3.35) | 0.03 |
| + Lifestyle/Comorbidities§ | 1.77 (1.00, 3.11) | 0.05 | 1.80 (0.99, 3.28) | 0.06 |
| + SBP, DBP, Dyslipidemia∥ | 1.83 (1.03, 3.26) | 0.04 | 1.83 (1.00, 3.41) | 0.05 |
| Severe Prevalent PAD† | ||||
| Unadjusted | 2.18 (1.11, 4.28) | 0.02 | 2.22 (1.11, 4.43) | 0.02 |
| + Demographics‡ | 2.60 (1.29, 5.21) | 0.008 | 2.92 (1.42, 6.01) | 0.004 |
| + Lifestyle/Comorbidities§ | 2.39 (1.14, 5.01) | 0.02 | 2.82 (1.32, 6.05) | 0.008 |
| + SBP, DBP, Dyslipidemia∥ | 2.42 (1.13, 5.23) | 0.02 | 2.91 (1.33, 6.40) | 0.008 |
Any family history is any first degree relative with PAD (mother, father, brothers, sisters, sons, daughters); parents only is either mother or father having PAD
PAD is ABI≤0.90 or leg revascularization; severe prevalent PAD is ABI≤0.70 or leg revascularization
Age, gender, and race/ethnicity
Adding body mass index, ever smoker, diabetes, hypertension
SBP=Systolic blood pressure, DBP=Diastolic blood pressure
There were no statistically significant interactions of sex (p=0.15), race/ethnicity (p=0.86), BMI (p=0.20), pack years (p=0.41) or ever smoking (p=0.95) with any family history of PAD for prevalent PAD. There was, however, a marginally significant interaction of family history with diabetes (p=0.06) such that among those with no diabetes, any family history of PAD was significantly associated with a 6.42-fold higher odds of prevalent PAD (95% CI (3.35, 12.35), p<0.001), whereas among those with diabetes, any family history of PAD was not significantly associated with prevalent PAD (OR 1.67 (95% CI (0.46, 6.03), p=0.44).
Since participants with prevalent PAD who have undergone a leg revascularization procedure would have been aware of this at the time of the exam interview, which could have caused recall bias, we performed a sensitivity analysis excluding the n=10 participants with prevalent PAD who had undergone revascularization procedures. Results were very similar – for example, any family history of PAD was associated with 1.91-fold higher odds of prevalent PAD (95% CI (1.04, 3.52), p=0.04), and a 2.64-fold higher odds of severe prevalent PAD (95% CI (1.14, 6.10), p=0.02).
Number of relatives with PAD was only marginally associated with prevalent PAD. Each increase in the number of relatives with PAD (i.e. 0 to 1, 1 to 2, 2 to 3) was associated with an 1.51-fold greater odds of prevalent PAD (95% CI (0.95, 2.42), p=0.08). Number of relatives with PAD was, however, significantly associated with severe prevalent PAD (OR 1.91 (95% CI (1.07, 3.40), p=0.04) (Table 3).
Table 3.
Association of number of first degree relatives with peripheral arterial disease (PAD) and prevalent PAD*
| Prevalent PAD† OR (95% CI) |
p-value | Severe Prevalent PAD† OR (95% CI) |
p-value | |
|---|---|---|---|---|
| Unadjusted | 1.37 (0.90, 2.09) | 0.15 | 1.70 (1.02, 2.82) | 0.04 |
| + Demographics‡ | 1.47 (0.96, 1.12) | 0.08 | 1.84 (1.10, 3.06) | 0.02 |
| + Lifestyle/Comorbidities§ | 1.48 (0.93, 2.36) | 0.10 | 1.91 (1.08, 3.40) | 0.03 |
| + SBP, DBP, Dyslipidemia∥ | 1.51 (0.95, 2.42) | 0.08 | 1.91 (1.07, 3.40) | 0.04 |
Number of first degree relatives modeled as a continuous variable
Prevalent PAD is ABI≤0.90 or leg revascularization; severe prevalent PAD is ABI≤0.70 or leg revascularization
Age, gender, and race/ethnicity
Adding body mass index, ever smoker, diabetes, hypertension
SBP=Systolic blood pressure, DBP=Diastolic blood pressure
Associations of family history of PAD with prevalent CVD and family history of CVD with prevalent PAD
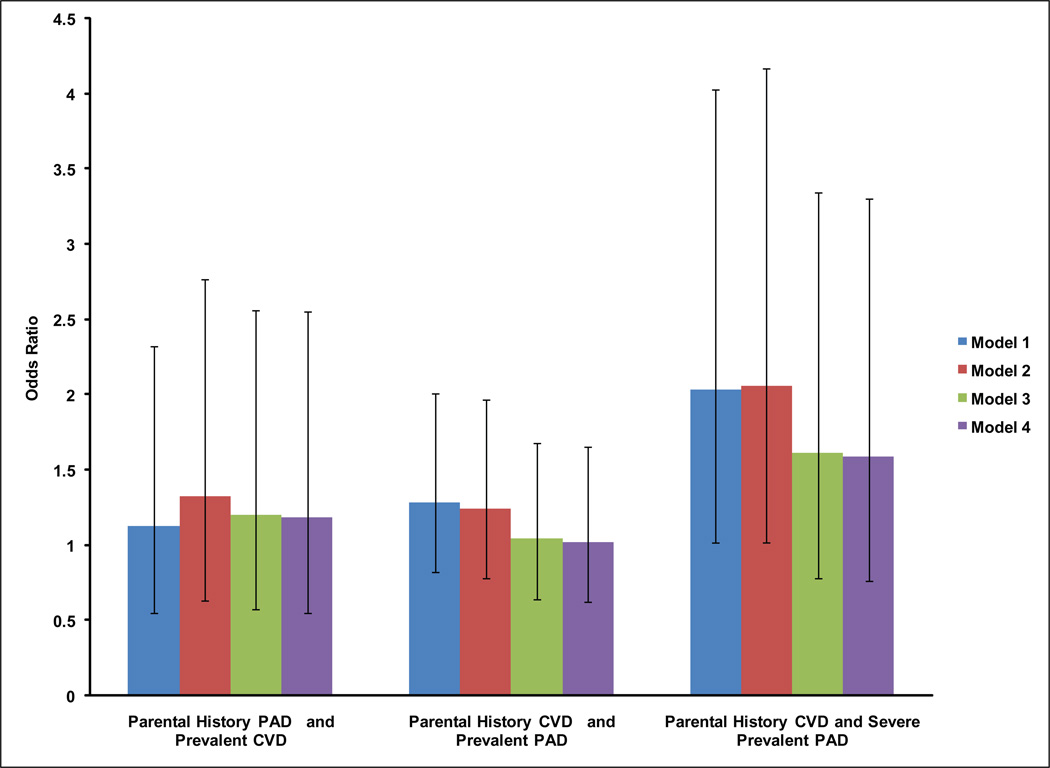
Figure 1 shows associations of parental history of PAD with prevalent CVD, and parental history of CVD with prevalent PAD, and with severe prevalent PAD. Associations were not statistically significant for parental history of PAD and prevalent CVD, or parental history of CVD and prevalent PAD (Figure 1). The association of parental history of CVD and severe prevalent PAD was significant in unadjusted (OR 2.03 (95% CI (1.02, 4.04), p=0.04) and demographic adjusted models (OR 2.06 (95% CI (1.02, 4.17), p=0.04); however, this association was attenuated in models further adjusted for comorbidities, lifestyle factors, blood pressure and dyslipidemia (Figure 1).
Figure 1. Association of Parental History of CVD with Prevalent PAD and Parental History of PAD with Prevalent CVD.
Model 1 is in blue and represents unadjusted associations. Model 2 is in red and adjusts for age, sex and ethnicity. Model 3 is in green and adjusts for Model 2 plus body mass index, diabetes, and ever smoking. Model 4 is in purple and adjusts for Model 3 plus systolic and diastolic blood pressures, and dyslipidemia.
Discussion
Using a large, ethnically diverse, well-characterized cohort, we report that having any first degree relative with PAD and parental history of PAD are both significantly associated with prevalent PAD; these familial associations are stronger for severe prevalent PAD. The number of first degree relatives with PAD is marginally associated with higher odds of prevalent PAD, and significantly associated with higher odds of severe prevalent PAD. Moreover, this study demonstrates that family history of PAD is more strongly associated with prevalent PAD than with prevalent CVD, and similarly, that family history of CVD is more strongly associated with prevalent CVD than PAD, suggesting some specificity of the type of family history.
Results of the present study indicate a significant genetic component exists for PAD. Studies have shown that ABI, a major criterion for PAD diagnosis, is moderately heritable, in the range of 0.20–0.50 (23–25), but to date results of candidate gene studies and GWAS for ABI or PAD have been mixed and/or not well-replicated (15, 26). Preliminary evidence suggests that genes in the inflammatory pathways, the renin-angiotensin pathway, and genes important in nicotine metabolism and dependence are important determinants of PAD or ABI (27–30) , although it is unclear to the extent which these associations may be mediated through smoking status or pack years. Previous studies have also suggested interactions of inflammatory or nicotine dependence genes with smoking and diabetes, two of the major risk factors for PAD (27, 29). In addition to association of the 9p21 locus with PAD (31), 9q33 has also recently been implicated, with higher odds for both abdominal aortic aneurysm and PAD (32).
It is also likely true that a complex interplay of genetic and environmental factors, including smoking, diabetes, obesity, diet, sex, and racial/ethnic group, lead to the development of PAD, although we did not find any indication of interaction of any family history of PAD and ever smoking, pack years of smoking, sex, or race/ethnicity for prevalent PAD in the current study. This is likely due to the limited power to detect interactions with the relatively modest number of prevalent PAD cases within each subgroup in this study. We did find a marginally significant interaction between any family history of PAD and diabetes such that family history was associated with much higher odds of prevalent PAD among those without diabetes as compared with those with diabetes. This could indicate that the set of genetic variants involved in diabetes could be distinct from those involved in PAD; however, these results should be interpreted with caution given the wide confidence intervals.
Only two studies have previously examined the association of family history of PAD with PAD or symptomatic CVD (17, 18). One study found participants with a sibling with premature PAD had a 3-fold higher odds of PAD (17). A second study found first degree relatives of individuals with premature PAD had higher odds of early onset CVD events than relatives of healthy participants (18). However, these studies were in small, family-based samples almost exclusively of European descent, only studied premature onset PAD (≤ 49 years), or concentrated on CVD events as an outcome. Results of the current study are consistent with Valentine et al (17). Our study extends the literature by showing the association of family history of PAD with both prevalent PAD and CVD concurrently, confirming the relationship in a multi-ethnic and much larger community-living sample.
Our study suggests that having a parental history of CVD does not significantly increase the odds of PAD, nor does having a parental history of PAD significantly increase the odds of CVD. These results are not consistent with the Valentine et al study (18) which found first degree relatives of individuals with premature PAD had higher odds of early onset CVD events than relatives of healthy participants; however, the present study did not restrict to premature PAD cases. Our differing results could be due to a lack of specificity of family history of CVD for PAD or vice versa. They could also be due to heterogeneity inherent in complex chronic diseases such as CVD and PAD, or the genetic heterogeneity in these diseases. In this regard, traditional risk factors do not entirely overlap for PAD and CVD; some traditional risk factors such as smoking and diabetes are stronger for PAD than CVD (2). Genetic risk factors also do not appear to entirely overlap or be consistent findings in all studies of PAD and CVD, suggesting that some genetic factors exclusive to PAD may exist. One study did find 9p21, a well-replicated locus for CHD and myocardial infarction (MI)(33), significantly associated with PAD and ABI after accounting for MI(31). However, in another study 9p21 was only marginally associated with clinical PAD(34), and after accounting for CHD, the association was no longer marginally significant.
Our study has several strengths. The SDPS is a population-based cohort of ethnically diverse men and women with a large age range, and was designed specifically to study PAD. Careful ABI measurement was performed by trained vascular technicians and ascertainment of other traditional risk factors was standard and complete. The current study also did not restrict the definition of PAD to premature PAD only, but sought to examine whether family history of PAD was important factors despite age at onset, and despite severity of PAD. The study also has important limitations. Family history of PAD and CVD was obtained via interview-administered questionnaire which can result in misclassification, but this would likely result in bias toward the null hypothesis. However, information on family history was obtained before the ABI measurement to avoid recall bias. Results are from an actively employed and retired population so may not generalize fully to the overall population.
In summary, we found that family history of PAD was significantly associated with PAD, but not with CVD. To date, significant genetic risk factors for PAD are odds ratios generally in the range of 1.1 to 1.3. Currently familial aggregation appears to be the most strongly associated genetic risk factor for PAD, with an odds ratio similar in magnitude to those of smoking, hypertension and diabetes which all range from 2–4 fold greater odds for PAD(2). This finding also suggests that asking about family history of PAD specifically (rather than CVD) may be more useful to identify individuals at risk for PAD. Based on these findings, future studies are warranted to identify genetic loci and/or gene/environment interactions that may contribute to development of PAD.
Acknowledgements
This research was supported by National Institutes of Health–National Heart, Lung, and Blood Institute grant 53487 and National Institutes of Health General Clinical Research Center Program grant MO1 RR0827. The authors would like to thank the participants of the San Diego Population Study for their participation.
Abbreviations list
- CVD
cardiovascular disease
- PAD
peripheral artery disease
- ABI
ankle brachial index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no disclosures to report.
References
- 1.Allison MA, Ho E, Denenberg JO, et al. Ethnic-specific prevalence of peripheral artery disease in the united states. Am J Prev Med. 2007 Apr;32:328–333. doi: 10.1016/j.amepre.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral artery disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the american association for vascular Surgery/Society for vascular surgery, society for cardiovascular angiography and interventions, society for vascular medicine and biology, society of interventional radiology, and the ACC/AHA task force on practice guidelines (writing committee to develop guidelines for the management of patients with peripheral artery disease): Endorsed by the american association of cardiovascular and pulmonary rehabilitation; national heart, lung, and blood institute; society for vascular nursing; TransAtlantic inter-society consensus; and vascular disease foundation. Circulation. 2006 Mar 21;113:e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 3.Golomb BA, Dang TT, Criqui MH. Peripheral artery disease: Morbidity and mortality implications. Circulation. 2006 Aug 15;114:688–699. doi: 10.1161/CIRCULATIONAHA.105.593442. [DOI] [PubMed] [Google Scholar]
- 4.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral artery disease. N Engl J Med. 1992 Feb 6;326:381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 5.Resnick HE, Lindsay RS, McDermott MM, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: The strong heart study. Circulation. 2004 Feb 17;109:733–739. doi: 10.1161/01.CIR.0000112642.63927.54. [DOI] [PubMed] [Google Scholar]
- 6.McDermott MM, Guralnik JM, Ferrucci L, et al. Functional decline in lower-extremity peripheral artery disease: Associations with comorbidity, gender, and race. J Vasc Surg. 2005 Dec;42:1131–1137. doi: 10.1016/j.jvs.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 7.McDermott MM, Guralnik JM, Ferrucci L, et al. Asymptomatic peripheral artery disease is associated with more adverse lower extremity characteristics than intermittent claudication. Circulation. 2008 May 13;117:2484–2491. doi: 10.1161/CIRCULATIONAHA.107.736108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDermott MM, Guralnik JM, Tian L, et al. Associations of borderline and low normal ankle-brachial index values with functional decline at 5-year follow-up: The WALCS (walking and leg circulation study) J Am Coll Cardiol. 2009 Mar 24;53:1056–1062. doi: 10.1016/j.jacc.2008.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herman SD, Liu K, Tian L, et al. Baseline lower extremity strength and subsequent decline in functional performance at 6-year follow-up in persons with lower extremity peripheral artery disease. J Am Geriatr Soc. 2009 Dec;57:2246–2252. doi: 10.1111/j.1532-5415.2009.02562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral artery disease in the united states: Results from the national health and nutrition examination survey, 1999–2000. Circulation. 2004 Aug 10;110:738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 11.Ix JH, Allison MA, Denenberg JO, Cushman M, Criqui MH. Novel cardiovascular risk factors do not completely explain the higher prevalence of peripheral artery disease among african americans. the san diego population study. J Am Coll Cardiol. 2008 Jun 17;51:2347–2354. doi: 10.1016/j.jacc.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 12.Allison MA, Criqui MH, McClelland RL, et al. The effect of novel cardiovascular risk factors on the ethnic-specific odds for peripheral artery disease in the multi-ethnic study of atherosclerosis (MESA) J Am Coll Cardiol. 2006 Sep 19;48:1190–1197. doi: 10.1016/j.jacc.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 13.Criqui MH, Vargas V, Denenberg JO, et al. Ethnicity and peripheral artery disease: The san diego population study. Circulation. 2005 Oct 25;112:2703–2707. doi: 10.1161/CIRCULATIONAHA.105.546507. [DOI] [PubMed] [Google Scholar]
- 14.Bennett PC, Silverman S, Gill PS, Lip GY. Ethnicity and peripheral artery disease. QJM. 2009 Jan;102:3–16. doi: 10.1093/qjmed/hcn140. [DOI] [PubMed] [Google Scholar]
- 15.Knowles JW, Assimes TL, Li J, Quertermous T, Cooke JP. Genetic susceptibility to peripheral artery disease: A dark corner in vascular biology. Arterioscler Thromb Vasc Biol. 2007 Oct;27:2068–2078. doi: 10.1161/01.ATV.0000282199.66398.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berg AO, Baird MA, Botkin JR, et al. National institutes of health state-of-the-science conference statement: Family history and improving health. Ann Intern Med. 2009 Dec 15;151:872–877. doi: 10.7326/0003-4819-151-12-200912150-00165. [DOI] [PubMed] [Google Scholar]
- 17.Valentine RJ, Guerra R, Stephan P, Scoggins E, Clagett GP, Cohen J. Family history is a major determinant of subclinical peripheral artery disease in young adults. J Vasc Surg. 2004 Feb;39:351–356. doi: 10.1016/j.jvs.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Valentine RJ, Verstraete R, Clagett GP, Cohen JC. Premature cardiovascular disease is common in relatives of patients with premature peripheral atherosclerosis. Arch Intern Med. 2000 May 8;160:1343–1348. doi: 10.1001/archinte.160.9.1343. [DOI] [PubMed] [Google Scholar]
- 19.Criqui MH, Jamosmos M, Fronek A, et al. Chronic venous disease in an ethnically diverse population: The san diego population study. Am J Epidemiol. 2003 Sep 1;158:448–456. doi: 10.1093/aje/kwg166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Criqui MH, Denenberg JO, Bergan J, Langer RD, Fronek A. Risk factors for chronic venous disease: The san diego population study. J Vasc Surg. 2007 Aug;46:331–337. doi: 10.1016/j.jvs.2007.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shadman R, Criqui MH, Bundens WP, et al. Subclavian artery stenosis: Prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004 Aug 4;44:618–623. doi: 10.1016/j.jacc.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 22.Natarajan S, Glick H, Criqui M, Horowitz D, Lipsitz SR, Kinosian B. Cholesterol measures to identify and treat individuals at risk for coronary heart disease. Am J Prev Med. 2003 Jul;25:50–57. doi: 10.1016/s0749-3797(03)00092-8. [DOI] [PubMed] [Google Scholar]
- 23.Carmelli D, Fabsitz RR, Swan GE, Reed T, Miller B, Wolf PA. Contribution of genetic and environmental influences to ankle-brachial blood pressure index in the NHLBI twin study. national heart, lung, and blood institute. Am J Epidemiol. 2000 Mar 1;151:452–458. doi: 10.1093/oxfordjournals.aje.a010230. [DOI] [PubMed] [Google Scholar]
- 24.Kullo IJ, Turner ST, Kardia SL, Mosley TH, Jr, Boerwinkle E, de Andrade M. A genome-wide linkage scan for ankle-brachial index in african american and non-hispanic white subjects participating in the GENOA study. Atherosclerosis. 2006 Aug;187:433–438. doi: 10.1016/j.atherosclerosis.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Murabito JM, Guo CY, Fox CS, D'Agostino RB. Heritability of the ankle-brachial index: The framingham offspring study. Am J Epidemiol. 2006 Nov 15;164:963–968. doi: 10.1093/aje/kwj295. [DOI] [PubMed] [Google Scholar]
- 26.McDermott MM, Lloyd-Jones DM. The role of biomarkers and genetics in peripheral artery disease. J Am Coll Cardiol. 2009 Sep 29;54:1228–1237. doi: 10.1016/j.jacc.2009.04.081. [DOI] [PubMed] [Google Scholar]
- 27.Kardia SL, Greene MT, Boerwinkle E, Turner ST, Kullo IJ. Investigating the complex genetic architecture of ankle-brachial index, a measure of peripheral artery disease, in non-hispanic whites. BMC Med Genomics. 2008 May 15;1:16. doi: 10.1186/1755-8794-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Donnell CJ, Cupples LA, D'Agostino RB, et al. Genome-wide association study for subclinical atherosclerosis in major arterial territories in the NHLBI's framingham heart study. BMC Med Genet. 2007 Sep 19;8 Suppl 1:S4. doi: 10.1186/1471-2350-8-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thorgeirsson TE, Geller F, Sulem P, et al. A variant associated with nicotine dependence, lung cancer and peripheral artery disease. Nature. 2008 Apr 3;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zintzaras E, Zdoukopoulos N. A field synopsis and meta-analysis of genetic association studies in peripheral artery disease: The CUMAGAS-PAD database. Am J Epidemiol. 2009 Jul 1;170:1–11. doi: 10.1093/aje/kwp094. [DOI] [PubMed] [Google Scholar]
- 31.Cluett C, McDermott MM, Guralnik J, et al. The 9p21 myocardial infarction risk allele increases risk of peripheral artery disease in older people. Circ Cardiovasc Genet. 2009 Aug;2:347–353. doi: 10.1161/CIRCGENETICS.108.825935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gretarsdottir S, Baas AF, Thorleifsson G, et al. Genome-wide association study identifies a sequence variant within the DAB2IP gene conferring susceptibility to abdominal aortic aneurysm. Nat Genet. 2010 Aug;42:692–697. doi: 10.1038/ng.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schunkert H, Gotz A, Braund P, et al. Repeated replication and a prospective meta-analysis of the association between chromosome 9p21.3 and coronary artery disease. Circulation. 2008 Apr 1;117:1675–1684. doi: 10.1161/CIRCULATIONAHA.107.730614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Helgadottir A, Thorleifsson G, Magnusson KP, et al. The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm. Nat Genet. 2008 Feb;40:217–224. doi: 10.1038/ng.72. [DOI] [PubMed] [Google Scholar]