Abstract
OBJECTIVE
To evaluate the effect of BPA on endometrial PR expression in non-human primates and human cells. BPA is a xenoestrogen endocrine disruptor. Both BPA exposure and diminished progesterone action have been associated with pregnancy loss, endometriosis and endometrial hyperplasia/cancer.
DESIGN
Controlled trial in primates.
SETTING
University
Animals
African green monkeys
INTERVENTIONS
After oophorectomy, BPA (50μg/kg/day), estradiol, both or vehicle control were administered. . Estradiol and BPA were used in Ishikawa cells.
MAIN OUTCOME MEASURES
PR expression using IHC and qPCR.
RESULTS
PR expression was increased in estradiol treated primates compared to controls. Exposure to the combination of estradiol and BPA resulted in decreased PR expression compared to estradiol exposure alone (p<0.01). In Ishikawa cells treated with estradiol, PR expression increased 5.1 fold, however, when Ishikawa cells were simultaneously treated with estradiol and BPA, PR expression was decreased to 0.6 fold that of cells treated with estradiol alone (p<0.05).
CONCLUSION
BPA alone functions as a weak estrogen. However, when administered with estradiol, BPA diminishes estradiol induced PR expression. The estrogen-like effect of BPA reported in exposed humans may be mediated by PR blockade and a resultant decrease in the estrogen inhibition normally imparted by progesterone. Diminished PR expression may underlie previous reports linking BPA exposure to endometrial dysfunction in humans.
Keywords: Bisphenol A, BPA, estrogen, progesterone receptor (PR), endocrine disruptor, xenoestrogen, endometriosis, endometrial hyperplasia
Introduction
Humans are widely exposed to chemicals that mimic the actions of endogenous hormones, thereby affecting endocrine function. Low doses of these chemicals can affect endocrine function in many species (1,2). Bisphenol-A (BPA) is used in the manufacture of polycarbonate plastics, epoxy resins, and dental sealants and is an estrogenic endocrine disruptor affecting fertility and reproduction (3). Exposure to BPA occurs as it leaches from polycarbarnate bottles, epoxy resin coating the interior of food cans, and dental sealants (4). BPA is virtually ubiquitous in the environment and food supply; ninety five percent of human urine samples contain BPA (5). Perinatal exposure has been associated with female reproductive tract abnormalities (6,7,8,9,10). These reproductive effects are likely mediated by BPA’s estrogenic activity. BPA binds both estrogen receptor (ER) alpha and beta, resulting in uterotrophic activity, although only 10−4 that of an equivalent dose of estradiol (11).
BPA exposure has been associated with pregnancy loss in humans (12). BPA has adverse effects on oocytes, leading to increased rates of anuploidy in mouse embryos; this may in part explain the observed incidence of pregnancy loss in women exposed to BPA (13). However, BPA, a xenoestrogen, may also affect pregnancy by targeting estrogen responsive organs such as the uterus. BPA exposure has also been associated with other endometrial defects including, endometriosis and endometrial hyperplasia (14,15,16,17). Pregnancy loss, endometriosis and endometrial hyperplasia can each be driven by deficient or absent progesterone action. Here we hypothesized a role for BPA functioning as a selective estrogen receptor modulator and antagonizing estradiol’s ability to mediate progesterone action.
Progesterone is produced in the ovaries and placenta and is required for the maintenance of pregnancy, prevention of endometrial hyperplasia and is also useful in treatment of endometriosis. The progesterone receptor (PR) mediates the physiological effects of progesterone and has two isoforms, PRA and PRB, differentiated by the addition of 165 amino acids in the N terminus of PRB (18). An inadequate response to progesterone due to changes in PR gene expression, can to lead to infertility, pregnancy loss or endometrial hyperplasia. The excessive endometrial growth in endometriosis is also associated with resistance to the normal effects of progesterone (13,19,20, 21, 22, 23, 24, 25). Estradiol normally increases endometrial PR expression; xenoestrogen endocrine-disrupting compounds such as BPA typically mimic estradiol, acting as weak estrogen receptor agonists. Here we hypothesized that BPA acts as a selective endocrine disruptor with the ability to antagonize estrogen action on the expression of some genes.
We tested the ability of BPA to alter PR expression using a primate model as well as an endometrial adenocarcinoma cell line. We demonstrate that treatment with BPA or estradiol individually each led to increased expression of PR. However, when BPA and estradiol are used in combination, PR expression is decreased compared to treatment with estradiol alone. BPA inhibits PR expression by antagonizing the action of estradiol.
Methods
Primate Treatment
Primate surgery and treatment was preformed as previously described (26). Briefly, we used ten adult female African green monkeys (Chlorocebus aethiops sabaeus) of reproductive age (body weight, 4–5 kg). All monkeys were anesthetized (20 mg/kg ketamine, i.m, followed by 20 mg/kg pentobarbital i.v.), intubated, and ovariectomized through a median laparatomy under sterile conditions.
One vehicle-treated control received a cholesterol-filled 4-cm long Silastic capsule (Dow Corning; 3.5 mm i.d., 4.65 mm o.d.) and a vehicle-filled minipump (Alzet 2ML4 osmotic pump, delivering fluid at a rate of 2.5 μl/h for 4 weeks). Three undergoing estradiol benzoate (EB) treatment received a Silastic capsule containing crystalline EB and an Alzet minipump loaded with vehicle. The three in the BPA-treatment group were given a cholesterol-filled Silastic capsule and a BPA-filled Alzet minipump. The three receiving a combination of EB+BP received a Silastic capsule containing crystalline EB and an Alzet minipump loaded with BPA. The Alzet minipumps supplied BPA at a rate of 50 μg/kg/day. The Silastic capsules and minipumps were implanted below the skin of the back.
Twenty-eight days later, the animals were deeply anesthetized (ketamine 20 mg/kg i.m, followed by pentobarbital, 100 mg/kg i.v.) and then euthanized by transcardial perfusion of heparinized saline (1.0 liter), followed by a fixative [1.5 liters, containing 4% paraformaldehyde and 0.1% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4)] with the descending aorta clamped. The uteri were dissected and postfixed overnight in the same, but glutaraldehyde-free, fixative. The tissues were stored and transported in phosphate buffer containing 0.1% sodium azide. Specimens were embedded in paraffin and serial 5-mm sections were obtained.
Animals and sterile surgical facilities were provided by the Saint Kitts Biomedical Research Foundation (SKBRF). The SKBRF traps or breeds its animals and has a complete 24-h veterinary service. The facility operates in full compliance with all applicable U.S. regulations and has provided an assurance of compliance (#A3005) to the Office of Laboratory Animal Welfare. All animal protocols used in this study were in compliance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and were approved by SKBRF’s Institutional Animal Care and Use Committee.
Immunohistochemistry
Slides were deparaffinized and hydrated through three 10 minute xylene washes followed by three 10 minute ethanol washes. The slides then underwent permeabilization in cold 95% ethanol and were rinsed with distilled water. Antigen retrieval was then performed for 20 minutes, by steaming the slides at 90°C in 0.01M sodium citrate. After cooling the slides were rinsed in PBS for 3 minutes and washed in PBS containing 0.1% Tween (PBST) for 5 minutes followed by a 3% hydrogen peroxide rinse for 3 minutes. The slides were then incubated at room temperature with 1.5% normal horse serum diluted in PBST for 1 hour and then with a PR monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C, followed by a biotinylated secondary antibody, anti-goat horse IgG (Vector Laboratories, Burlingame, CA) for 1 hour. Then the slides were incubated for 15 minutes in VECTASTAIN® Elite ABC (Vector Laboratories) and then incubated for 8 minutes in 3,3′-diaminobenzidine (DAB) (Vector Laboratories). The tissue was then exposed to hematoxylin for 20 seconds and mounted with Permount.
An analysis of PR expression was performed by two evaluators blinded to the treatment group and quantified using the H-Score. The H-Score represents glandular and stromal cell staining intensity and was calculated using the formula: H-Score = Σ Pi (i + 1), where the Pi represents the percentage of stained cells ranging from 0–100% and stain intensity (i) is assigned a value of 1, 2, or 3 indicating weak, moderate, or strong, respectively (27,28). Statistical analysis was performed using ANOVA on ranks and statistical significance was defined as p< 0.05.
Cell Culture
Ishikawa cells are a well-differentiated endometrial adenocarcinoma cell line that has been used to model human endometrial epithelial cells (29,30,31,32,33). Ishikawa cells express ER and demonstrate estrogen responsive PR expression. Ishikawa cells were a generous gift from Richard Hochberg (Yale University).
Ishikawa cells were maintained in 25-cm2 flasks using Dulbecco’s Modified Eagles Medium (DMEM, Sigma, St. Louis, MO, USA) supplemented with 10% charcoal-striped calf serum, 1% penicillin/streptomycin, and 1% sodium pyruvate. Cells were maintained at 37°C in a humidified atmosphere (5% CO2 in air) and allowed to reach 80% confluency. The cells were treated with BPA, estradiol, or a combination of BPA and estradiol, all at concentrations of 1μM. Control cells were treated with equivalent concentrations of methanol, ethanol, and methanol and ethanol, respectively, as found in the diluents of each active comparator.
RNA isolation, quantitative real-time RT-PCR
Total RNA from all cell cultures was purified using the Qiagen RNeasy Plus Mini kit (Qiagen, Valencia, CA), according to the manufacturer’s instructions. For real-time RT-PCR analysis, 200ng of RNA was converted to cDNA using the iScript cDNA Synthesis kit (Bio-Rad Laboratories, Hercules, CA, USA). RT-PCR was performed using the LightCycler SYBR Green RT-PCR kit (Roche, Stockholm, Sweden). PCR for PR was performed for 1 cycle at 50°C for 2 minutes; 1 cycle at 60°C for 30 minutes; 1 cycle at 95°C for 5 minutes; and 40 cycles at 95°C for 20 seconds followed by 62°C for 1 minute. Each experiment was performed in triplicate and repeated three times. Comparisons of PR and PR-B gene expression in all four treatment groups were analyzed by ANOVA on ranks.
Total PR (forward primer 5′-CGCCCTATCTCAACTACCTG -3′, reverse primer 5′-CCTGACAGCACTTTCTAAGG - 3′);
PR-B (forward primer 5′-CCCAGTTTGAGGAGATGAGG -3′, reverse primer 5′-ATCCCTGCCAATATCTTGGG- 3′);
B-Actin (forward primer 5′-GTGTTGGCGTACAGGTCTTTG -3′, reverse primer 5′-CGTACCACTGGCATCGTGAT- 3′);
Results
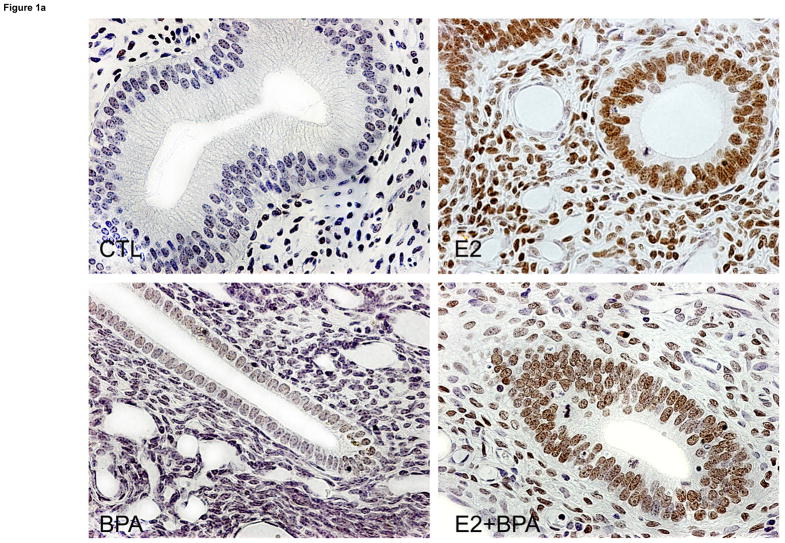
To determine the effects of Bisphenol-A on endometrial PR gene expression, we treated primates with BPA, estradiol, or a combination of the two. A total of ten African green monkeys (Cercopithecus aethiops sabaeus) were ovariectomized. Three were treated with BPA (50 microgram/kg/day), three with estradiol benzoate and three received combined treatment with BPA and estradiol, via Alzet pumps. The control was treated with insertion of an Alzet pump containing vehicle alone. Each animal was treated for 29 days. Uterine tissue was examined for PR expression using IHC. In the control primate, there was no detectable expression of PR in the endometrial stroma and glands (Figure 1). As expected, estradiol treatment increased the expression of PR in both the strma and glands. BPA treatment increased PR expression minimally compared to the control. When BPA and estradiol were co-administered, PR expression was decreased in the endometrial stroma and glands compared to those exposed to estradiol alone.
FIGURE 1.
A) Endometrial PR expression using immunohistochemcial analysis in the non-human primate. PR immunostaining was nearly absent either the glands or stroma of control animals. BPA treatment had minimal effect on endometrial PR expression. As expected, estradiol treatment led to a large increase in both glandular and stromal PR expression. Treatment with the combination of estradiol and BPA led to decreased glandular and stromal expression compared to treatment with estradiol alone. Representative photomicrographs taken at 400X magnification are shown.
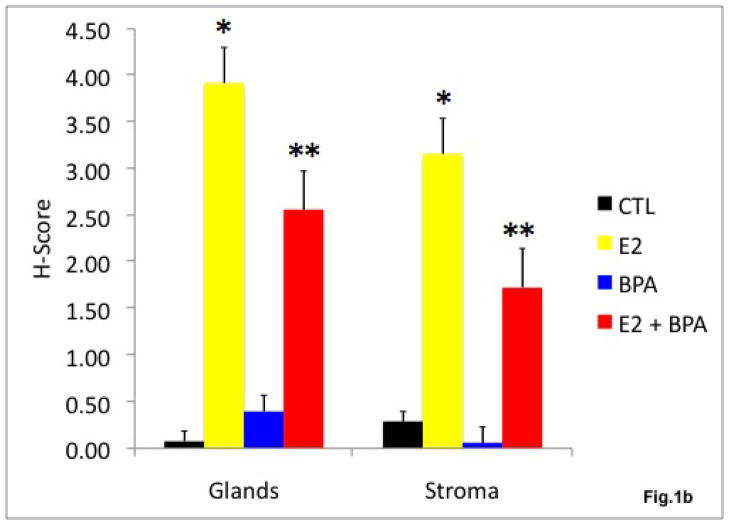
B) The effect of estradiol and BPA on endometrial PR protein expression in the non-human primate. H score was used as a semi-quantitative measure of PR expression. Estradiol exposure led to a 49 fold increase in glandular PR expression and an 11 fold increase in stromal PR expression compared to vehicle treated control (*p<0.01). Exposure to a combination of estradiol and BPA decreased glandular PR expression to 62% and decreased stromal PR expression to 50% of the H-score in obtained in endometrium of animals treated with estradiol alone (**p<0.01).
An H-score was used to obtain a semi-quantitative measure of expression. Estradiol exposure led to a 49 fold increase in glandular PR expression (p<0.01) and an 11 fold increase in stromal PR expression (p<0.01) compared to vehicle treated control. BPA exposure led to a 5 fold increase in glandular PR expression and a 5 fold decrease in stromal PR expression compared to control, however, the difference was not statistically significant. Exposure to a combination of estradiol and BPA decreased glandular PR expression to 62% (p<0.01) and decreased stromal PR expression to 50%, (p<0.01) of that seen in the endometrium of primates treatment with estradiol alone.
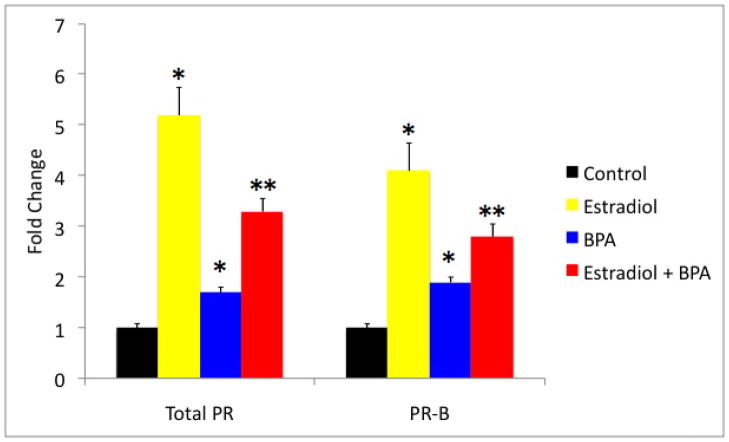
In order to determine if BPA affected endometrial PR gene expression directly, Ishikawa cells were exposed to estradiol, BPA, the combination of the two or vehicle control for 24 hours. Quantitative real time RT-PCR was performed on mRNA isolated from Ishikawa cells after treatment. Exposure to estradiol alone led to a 5.2 fold increase in PR expression compared to vehicle control (p<0.01). BPA acted as a weak estrogen; exposure to BPA increased the expression of PR 1.7 fold (p <0.05). However, when estradiol and BPA were used in combination, PR expression was increased compared to control (p <0.01), however PR expression was decreased to 61% of that seen in cells treated with estradiol alone (p<0.01). Evaluation of the PR-B isoform showed similar results. The expression of PR-B in the estradiol treated group was increased by 4.1 fold (p <0.01). The expressions of PR-B in the BPA treated group were increased by 1.9 fold (p<0.05). Yet, when estradiol and BPA were in used combination, the expression of PR-B was increased by only 2.8 fold (p<0.05) compared to control, and decreased to 70% of that seen in cells treated with estradiol alone (p< 0.05).
Discussion
BPA is a xenoestrogen endocrine disruptor that is thought to act as a weak estrogen by binding to the estrogen receptor with low affinity. It is a common ingredient in the manufacture of polycarbonate plastics and production has grown rapidly, from 16 million pounds in 1991 to 2.3 billion pounds in 2004 in the United States alone (34). Several harmful health effects have been associated with BPA exposure including prostate cancer, breast cancer, obesity, early sexual maturation in females, and altered reproductive function (35). The effects of BPA are dependent on time of exposure and dose. Low dose neonatal exposure to BPA in mice causes abnormalities in the female reproductive tract including early vaginal opening and ovarian and reproductive tract abnormalities in females (36,37). Low levels of BPA exposure in-utero have also led to uterine disruption in rat offspring and altered vaginal morphology in postpubertal offspring (7,9) BPA leads to epigenetic alterations and an increase in HOXA10 mRNA expression; an essential gene associated with uterine development and fertility (38,39).
In humans there is an association between recurrent miscarriage and BPA exposure (12). This correlation is interesting given that mice exposed to low doses of BPA have high rates of meiotic failure, specifically an increase in aneuploid eggs and embryos (40,41). While the increased aneuploidy rates are expected contribute to adverse pregnancy outcomes and pregnancy loss, uterine effects of BPA are likely to have an impact on pregnancy as well.
Uterine proliferation and differentiation during the preimplantation period and in pregnancy have been well characterized and these processes are dependent on estrogen and progesterone. These two hormones are both necessary and sufficient to achieve endometrial development and to support pregnancy. Estrogen induces expression of progesterone receptor (PR). A balance between estrogen and progesterone is necessary for the normal and cyclic function of the endometrium, and disruption of this balance can lead to pathologies such as infertility, abnormal bleeding, endometriosis, pregnancy loss, and cancer (42). Rhesus monkeys treated with CDR0I-85/287, a non-steroidal estrogen antagonist known for its anti-implantation effects, show markedly decreased progesterone receptor expression (43). Similarly, in humans an inadequate response to progesterone or an insufficient serum progesterone concentration may lead to pregnancy loss and infertility (40). The alteration of PR expression by BPA could provide an explanation for how BPA exerts its estrogenic endocrine disrupting effects in early pregnancy.
BPA exposure has also been associated with disorders characterized by unrestrained endometrial growth such as endometriosis and endometrial hyperplasia (14,15,16,17). Estrogens typically cause endometrial growth and proliferation, while progestins cause endometrial differentiation. Estrogen exposure unopposed by progesterone leads to excessive endometrial growth hyperplasia and an increased risk of endometrial epithelial cancer. Similarly endometriosis is characterized by a loss of the usual inhibitory effects of progesterone and is considered progesterone resistant (13,19,20,21, 22,23,24,25,). Diminished PR expression and therefore diminished progesterone action would be expected to result in increased endometrial proliferation, seen in both of these conditions. The association of BPA with each of the previously described clinical conditions may be due to diminished PR expression, progesterone resistance, diminished opposition of estrogen and therefore, indirectly, a functional increase in estrogen action.
In summary BPA exposure results in antagonism of estradiol’s effect on PR. By decreasing PR expression BPA decreases the ability of progesterone to inhibit estradiol action. BPA exposure in women may affect the balance between estrogen and progesterone action and in turn lead to a heightened estrogen response. Therefore, BPA indirectly leads to increased estrogen action. The effect of BPA on PR may explain the association of BPA exposure with diseases associated with increased endometrial proliferation and diminished differentiation, including spontaneous abortion, endometriosis, endometrial hyperplasia and cancer.
FIGURE 2.
Total PR and PR-B mRNA expression in Ishikawa cell line after treatment with estradiol and /or BPA. Real-time PCR results show that total PR and PR-B expression increased 5.2 fold and 4.1 fold, respectively, in cells treated with estradiol compared to the control (*p<0.01). PR and PR-B expression increased 1.7 fold and 1.9 fold, respectively, in cells treated with BPA when compared to the control (*p<0.05). PR and PR-B expression decreased to 0.6 fold and 0.7 fold respectively, in cells treated with a combination estradiol and BPA when compared to those treated with Estradiol alone (** p<0.01 total PR and p<0.05 PR B).
Acknowledgments
Grant Support: NIH R01 ES010510 and U54 HD052668
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tyler CR, Jobling S, Sumpter JP. Endocrine disruption in wildlife: a critical review of the evidence. Crit Rev Toxicol. 1998:319–61. doi: 10.1080/10408449891344236. [DOI] [PubMed] [Google Scholar]
- 2.Foster WG. Environmental estrogens and endocrine disruption: importance of comparative endocrinology. Endocrinology. 2008:4267–8. doi: 10.1210/en.2008-0736. [DOI] [PubMed] [Google Scholar]
- 3.Maffini MV, Rubin BS, Sonnenschein C, Soto AM. Endocrine disruptors and reproductive health: the case of bisphenol-A. Mol Cell Endocrinol. 2006:179–86. doi: 10.1016/j.mce.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 4.Mountfort KA, Kelly J, Jickells SM, Castle L. Investigations into the potential degradation of polycarbonate baby bottles during sterilization with consequent release of bisphenol A. Food Addit Contam. 1997:737–740. doi: 10.1080/02652039709374584. [DOI] [PubMed] [Google Scholar]
- 5.Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect. 2005:391–395. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honma S, Suzuki A, Buchanan DL, Katsu Y, Watanabe H, Iguchi T. Low dose effect of in utero exposure to bisphenol A and diethylstilbestrol on female mouse reproduction. Reprod Toxicol. 2002:117–22. doi: 10.1016/s0890-6238(02)00006-0. [DOI] [PubMed] [Google Scholar]
- 7.Schönfelder G, Flick B, Mayr E, Talsness C, Paul M, Chahoud I. In utero exposure to low doses of bisphenol A lead to long-term deleterious effects in the vagina. Neoplasia. 2002:98–102. doi: 10.1038/sj.neo.7900212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nikaido Y, Yoshizawa K, Danbara N, Tsujita-Kyutoku M, Yuri T, Uehara N, et al. Effects of maternal xenoestrogen exposure on development of the reproductive tract and mammary gland in female CD-1 mouse offspring. Reprod Toxicol. 2004:803–11. doi: 10.1016/j.reprotox.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Schönfelder G, Friedrich K, Paul M, Chahoud I. Developmental effects of prenatal exposure to bisphenol a on the uterus of rat offspring. Neoplasia. 2004:584–94. doi: 10.1593/neo.04217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markey CM, Wadia PR, Rubin BS, Sonnenschein C, Soto AM. Long-term effects of fetal exposure to low doses of the xenoestrogen bisphenol-A in the female mouse genital tract. Biol Reprod. 2005:1344–51. doi: 10.1095/biolreprod.104.036301. [DOI] [PubMed] [Google Scholar]
- 11.Sheehan DM. Activity of environmentally relevant low doses of endocrine disruptors and the bisphenol A controversy: initial results confirmed. Proc Soc Exp Biol Med. 2000:57–60. doi: 10.1046/j.1525-1373.2000.22401.x. [DOI] [PubMed] [Google Scholar]
- 12.Sugiura-Ogasawara M, Ozaki Y, Sonta S, Makino T, Suzumori K. Exposure to bisphenol A is associated with recurrent miscarriage. Hum Reprod. 2005:2325–9. doi: 10.1093/humrep/deh888. [DOI] [PubMed] [Google Scholar]
- 13.Cakmak H, Taylor HS. Molecular mechanisms of treatment resistance in endometriosis: the role of progesterone-hox gene interactions. Semin Reprod Med. 2010:69–74. doi: 10.1055/s-0029-1242996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Signorile PG, Spugnini EP, Mita L, Mellone P, D’Avino A, Bianco M, et al. Pre-natal exposure of mice to bisphenol A elicits an endometriosis-like phenotype in female offspring. Gen Comp Endocrinol. 2010:318–25. doi: 10.1016/j.ygcen.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 15.Cobellis L, Colacurci N, Trabucco E, Carpentiero C, Grumetto L. Measurement of bisphenol A and bisphenol B levels in human blood sera from healthy and endometriotic women. Biomed Chromatogr. 2009:1186–90. doi: 10.1002/bmc.1241. [DOI] [PubMed] [Google Scholar]
- 16.Newbold RR, Jefferson WN, Padilla-Banks E. Long-term adverse effects of neonatal exposure to bisphenol A on the murine female reproductive tract. Reprod Toxicol. 2007:253–8. doi: 10.1016/j.reprotox.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiroi H, Tsutsumi O, Takeuchi T, Momoeda M, Ikezuki Y, Okamura A, et al. Differences in serum bisphenol a concentrations in premenopausal normal women and women with endometrial hyperplasia. Endocr J. 2004:595–600. doi: 10.1507/endocrj.51.595. [DOI] [PubMed] [Google Scholar]
- 18.Gadkar-Sable S, Shah C, Rosario G, Sachdeva G, Puri C. Progesterone receptors: various forms and functions in reproductive tissues. Front Biosci. 2005:2118–30. doi: 10.2741/1685. [DOI] [PubMed] [Google Scholar]
- 19.Fazleabas AT. Progesterone resistance in a baboon model of endometriosis. Semin Reprod Med. 2010:75–80. doi: 10.1055/s-0029-1242997. [DOI] [PubMed] [Google Scholar]
- 20.Aghajanova L, Velarde MC, Giudice LC. Altered gene expression profiling in endometrium: evidence for progesterone resistance. Semin Reprod Med. 2010:51–8. doi: 10.1055/s-0029-1242994. [DOI] [PubMed] [Google Scholar]
- 21.Bruner-Tran KL, Ding T, Osteen KG. Dioxin and endometrial progesterone resistance. Semin Reprod Med. 2010:59–68. doi: 10.1055/s-0029-1242995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aghajanova L, Tatsumi K, Horcajadas JA, Zamah AM, Esteban FJ, Herndon CN, et al. Unique Transcriptome, Pathways, and Networks in the Human Endometrial Fibroblast Response to Progesterone in Endometriosis. Biol Reprod. 2010 Sep 23; doi: 10.1095/biolreprod.110.086181. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aghajanova L, Giudice LC. Reprod Sci Molecular Evidence for Differences in Endometrium in Severe Versus Mild Endometriosis. 2010 Nov 9; doi: 10.1177/1933719110386241. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osteen KG, Bruner-Tran KL, Eisenberg E. Reduced progesterone action during endometrial maturation: a potential risk factor for the development of endometriosis. Fertil Steril. 2005:529–37. doi: 10.1016/j.fertnstert.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 25.Bruner-Tran KL, Ding T, Osteen KG. Dioxin and endometrial progesterone resistance. Semin Reprod Med. 2010:59–68. doi: 10.1055/s-0029-1242995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leranth C, Hajszan T, Szigeti-Buck K, Bober J, MacLusky NJ. Bisphenol A prevents the synaptogenic response to estradiol in hippocampus and prefrontal cortex of ovariectomized nonhuman primates. Proc Natl Acad Sci U S A. 2008:14187–91. doi: 10.1073/pnas.0806139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lessey BA, Castelbaum AJ, Sawin SW, Buck CA, Schinnar R, Bilker W, et al. Aberrant integrin expression in the endometrium of women with endometriosis. J Clin Endocrinol Metab. 1994:643–9. doi: 10.1210/jcem.79.2.7519194. [DOI] [PubMed] [Google Scholar]
- 28.Sharpe-Timms KL, Ricke EA, Piva M, Horowitz GM. Differential expression and localization of de-novo synthesized endometriotic haptoglobin in endometrium and endometriotic lesions. Hum Reprod. 200:2180–5. doi: 10.1093/humrep/15.10.2180. [DOI] [PubMed] [Google Scholar]
- 29.Martin R, Taylor MB, Krikun G, Lockwood C, Akbas GE, Taylor HS. Differential cell-specific modulation of HOXA10 by estrogen and specificity protein 1 response elements. J Clin Endocrinol Metab. 2007:1920–6. doi: 10.1210/jc.2006-1694. [DOI] [PubMed] [Google Scholar]
- 30.Akbas GE, Song J, Taylor HS. A HOXA10 estrogen response element (ERE) is differentially regulated by 17 beta-estradiol and diethylstilbestrol (DES) AJ Mol Biol. 2004:1013–23. doi: 10.1016/j.jmb.2004.05.052. [DOI] [PubMed] [Google Scholar]
- 31.Sarno JL, Kliman HJ, Taylor HS. HOXA10, Pbx2, and Meis1 protein expression in the human endometrium: formation of multimeric complexes on HOXA10 target genes. J Clin Endocrinol Metab. 2005:522–8. doi: 10.1210/jc.2004-0817. [DOI] [PubMed] [Google Scholar]
- 32.Fei X, Chung H, Taylor HS. Methoxychlor disrupts uterine Hoxa10 gene expression. Endocrinology. 2005:3445–51. doi: 10.1210/en.2005-0341. [DOI] [PubMed] [Google Scholar]
- 33.Taylor HS, Arici A, Olive D, Igarashi P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest. 1998:1379–84. doi: 10.1172/JCI1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Toxicology Program U.S. Department of Health and Human Services. NTP-CERHR Report on the Reproductive and Developmental Toxicity of Bisphenol A. Dec, 2006. [Google Scholar]
- 35.vom Saal FS, Akingbemi BT, Belcher SM, Birnbaum LS, Crain DA, Eriksen M, et al. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol. 2007:131–8. doi: 10.1016/j.reprotox.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Honma S, Suzuki A, Buchanan DL, Katsu Y, Watanabe H, Iguchi T. Low dose effect of in utero exposure to bisphenol A and diethylstilbestrol on female mouse reproduction. Reprod Toxicol. 2002:117–22. doi: 10.1016/s0890-6238(02)00006-0. [DOI] [PubMed] [Google Scholar]
- 37.Markey CM, Wadia PR, Rubin BS, Sonnenschein C, Soto AM. Long-term effects of fetal exposure to low doses of the xenoestrogen bisphenol-A in the female mouse genital tract. Biol Reprod. 2005:1344–51. doi: 10.1095/biolreprod.104.036301. [DOI] [PubMed] [Google Scholar]
- 38.Smith CC, Taylor HS. Xenoestrogen exposure imprints expression of genes (Hoxa10) required for normal uterine development. FASEB J. 2007:239–46. doi: 10.1096/fj.06-6635com. [DOI] [PubMed] [Google Scholar]
- 39.Bromer JG, Zhou Y, Taylor MB, Doherty L, Taylor HS. Bisphenol-A exposure in utero leads to epigenetic alterations in the developmental programming of uterine estrogen response. FASEB J. 2010:2273–80. doi: 10.1096/fj.09-140533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Can A, Semiz O, Cinar O. Bisphenol-A induces cell cycle delay and alters centrosome and spindle microtubular organization in oocytes during meiosis. Mol Hum Reprod. 2005:389–96. doi: 10.1093/molehr/gah179. [DOI] [PubMed] [Google Scholar]
- 41.Hunt P, Koehler K, Susiarjo M, Hodges CA, Ilagan A, Voigt RC, et al. Bisphenol A is a meiotic aneugen. Curr Biol. 2003:546–53. doi: 10.1016/s0960-9822(03)00189-1. [DOI] [PubMed] [Google Scholar]
- 42.Young SL, Lessey BA. Progesterone function in human endometrium: clinical perspectives. Semin Reprod Med. 2010:5–16. doi: 10.1055/s-0029-1242988. Review. [DOI] [PubMed] [Google Scholar]
- 43.Dwivedi A, Bansode FW, Setty BS, Dhar JD. Endometrial steroid receptors during decidualization in rhesus monkey (Macaca mulatta); their modulation by anti-oestrogen CDRI-85/287. Hum Reprod. 1999:1090–5. doi: 10.1093/humrep/14.4.1090. [DOI] [PubMed] [Google Scholar]