Abstract
Ghee, also known as clarified butter, has been utilized for thousands of years in Ayurveda as a therapeutic agent. In ancient India, ghee was the preferred cooking oil. In the last several decades, ghee has been implicated in the increased prevalence of coronary artery disease (CAD) in Asian Indians due to its content of saturated fatty acids and cholesterol and, in heated ghee, cholesterol oxidation products. Our previous research on Sprague-Dawley outbred rats, which serve as a model for the general population, showed no effect of 5 and 10% ghee-supplemented diets on serum cholesterol and triglycerides. However, in Fischer inbred rats, which serve as a model for genetic predisposition to diseases, results of our previous research showed an increase in serum total cholesterol and triglyceride levels when fed a 10% ghee-supplemented diet. In the present study, we investigated the effect of 10% dietary ghee on microsomal lipid peroxidation, as well as serum lipid levels in Fischer inbred rats to assess the effect of ghee on free radical mediated processes that are implicated in many chronic diseases including cardiovascular disease. Results showed that 10% dietary ghee fed for 4 weeks did not have any significant effect on levels of serum total cholesterol, but did increase triglyceride levels in Fischer inbred rats. Ghee at a level of 10% in the diet did not increase liver microsomal lipid peroxidation or liver microsomal lipid peroxide levels. Animal studies have demonstrated many beneficial effects of ghee, including dose-dependent decreases in serum total cholesterol, low density lipoprotein (LDL), very low density lipoprotein (VLDL), and triglycerides; decreased liver total cholesterol, triglycerides, and cholesterol esters; and a lower level of nonenzymatic-induced lipid peroxidation in liver homogenate. Similar results were seen with heated (oxidized) ghee which contains cholesterol oxidation products. A preliminary clinical study showed that high doses of medicated ghee decreased serum cholesterol, triglycerides, phospholipids, and cholesterol esters in psoriasis patients. A study on a rural population in India revealed a significantly lower prevalence of coronary heart disease in men who consumed higher amounts of ghee. Research on Maharishi Amrit Kalash-4 (MAK-4), an Ayurvedic herbal mixture containing ghee, showed no effect on levels of serum cholesterol, high density lipoprotein (HDL), LDL, or triglycerides in hyperlipidemic patients who ingested MAK-4 for 18 weeks. MAK-4 inhibited the oxidation of LDL in these patients. The data available in the literature do not support a conclusion of harmful effects of the moderate consumption of ghee in the general population. Factors that may be involved in the rise of CAD in Asian Indians include the increased use of vanaspati (vegetable ghee) which contains 40% trans fatty acids, psychosocial stress, insulin resistance, and altered dietary patterns. Research findings in the literature support the beneficial effects of ghee outlined in the ancient Ayurvedic texts and the therapeutic use of ghee for thousands of years in the Ayurvedic system of medicine.
Keywords: Anhydrous milk fat, cholesterol, clarified butter, coronary artery disease, ghee, lipid peroxidation, vanaspati, vegetable ghee.
Introduction
Ghee, also known as clarified butter or anhydrous milk fat, is prepared by heating butter or cream to just over 100°C to remove water content by boiling and evaporation, then filtering out the precipitated milk solids. Ghee is known as ghrta[1] (commonly spelled ghrita) in Sanskrit. Ayurveda has traditionally considered ghee to be the healthiest source of edible fat, with many beneficial properties. According to Ayurveda, ghee promotes longevity and protects the body from various diseases.[2] It increases the digestive fire (agni) and improves absorption and assimilation. It nourishes ojas, the subtle essence of all the body's tissues (dhatus). It improves memory and strengthens the brain and nervous system. It lubricates the connective tissues, thereby rendering the body more flexible. With regard to the three doshas (organizing principles that govern the physiology), ghee pacifies Vata and Pitta and is acceptable for Kapha in moderation.[3]
Ghee is heavily utilized in Ayurveda for numerous medical applications, including the treatment of allergy, skin, and respiratory diseases. Many Ayurvedic preparations are made by cooking herbs into ghee. Ghee carries the therapeutic properties of herbs to all the body's tissues. It is an excellent anupana (vehicle) for transporting herbs to the deeper tissue layers of the body.[3] Proper digestion, absorption, and delivery to a target organ system are crucial in obtaining the maximum benefit from any therapeutic formulation; the lipophilic action of ghee facilitates transportation to a target organ and final delivery inside the cell since the cell membrane also contains lipid.[4] A study that compared different forms of herbs and herb extracts found that the efficacy increased when they were used with ghee, compared to usage in powder or tablet form.[5]
Ghee is considered sacred and used in religious rituals as well as in the diet in India.[6] In ancient India, ghee was the preferred cooking oil. It was considered pure and was felt to confer purity to foods cooked with it.[1] Ghee and other similar products such as samn (variant of the Arabic term samn) are used in many parts of the world.[7]
Our previous fatty acid analysis of ghee indicated it contains 47.8% saturated fat,[8] which is similar to data reported in the literature.[1,7] There has been concern about the possibility of ghee contributing to an increased risk of cardiovascular disease since it contains a high percentage of saturated fatty acids, leading to increased synthesis of cholesterol. The American Heart Association recommends limiting the consumption of saturated fats to less than 7% of energy to reduce the risk of cardiovascular disease.[9] Previous results from our laboratory indicated that 5 and 10% ghee-supplemented diets fed for 2 weeks to 2 months did not have any significant effect on serum total cholesterol and triglyceride levels in Sprague-Dawley rats, an outbred strain of rats used as a general experimental model. However, a 10% ghee-supplemented diet fed for 2 months increased serum total cholesterol and triglyceride levels in Fischer rats, an inbred strain of rats genetically predisposed to disease processes.[8]
Free radicals and reactive oxygen species have been linked to many chronic diseases, as well as the aging process.[10–12] Lipid peroxidation, a free radical-mediated reaction, has been implicated in various disorders such as post-ischemic conditions,[13] inflammation,[14] head injury,[15] stroke,[16] carcinogenesis,[17] cardiovascular disease,[18] and aging.[19] In the present study, we investigated the effects of 10% dietary ghee on microsomal lipid peroxidation, as well as serum lipids, in Fischer inbred rats to elucidate the effect of ghee on the risk of cardiovascular and other free radical-induced diseases.
Materials and Methods
Two groups of Fischer rats were used; there were five rats in each group. One group served as the control and received rodent chow, and the other group was placed on a diet supplemented with 10% ghee. The rats were permitted to have food and water ad libitum. At the end of 4 weeks, blood was obtained from the rats after a 14-hour fast, by cardiac puncture under ether anesthesia. Serum was prepared from the blood and analyzed for triglyceride and total cholesterol levels using Sigma diagnostic Kits 405 B and 402, respectively (Sigma Chemical Company, St. Louis, MO, USA), as described by Dwivedi et al.[8] Liver microsomes were prepared by differential centrifugation as described by Dwivedi et al.[20] Microsomal lipid peroxidation was assayed using the method described by Engineer et al.[21] and Dwivedi et al.[22] Microsomal lipid peroxide levels were assayed by the method described by Sridhar et al.[23]
Statistical analysis
The software INSTAT (GraphPad, San Diego, CA, USA) was used to analyze the data. Student's t-test was used to compare the effects of ghee on different parameters. Significance in all cases was considered as P < 0.05.
Results
A 10% ghee-supplemented diet fed for a period of 4 weeks did not have any significant effect on the serum total cholesterol level of Fischer inbred rats [Figure 1].However, 10% dietary ghee significantly (P < 0.05) increased the serum triglyceride level [Figure 2]. There was no significant effect of 10% dietary ghee on liver microsomal lipid peroxidation [Figure 3]. Similarly, 10% dietary ghee fed for 4 weeks did not have any effect on liver microsomal lipid peroxide levels [Figure 4].
Figure 1.
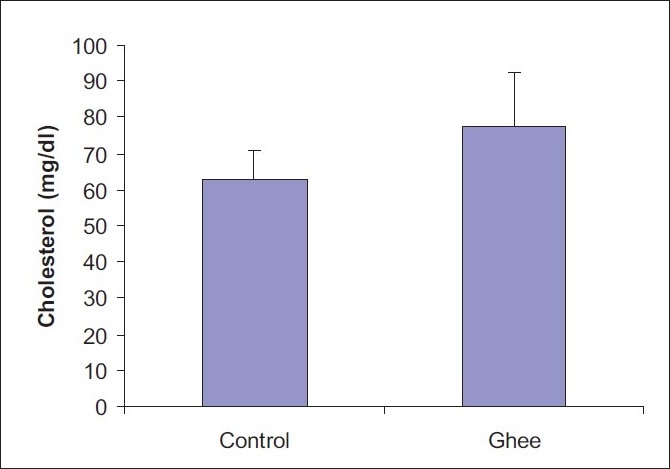
Effect of 10% ghee on serum total cholesterol level of Fischer inbred rats; values represent mean ± SD derived from five rats
Figure 2.
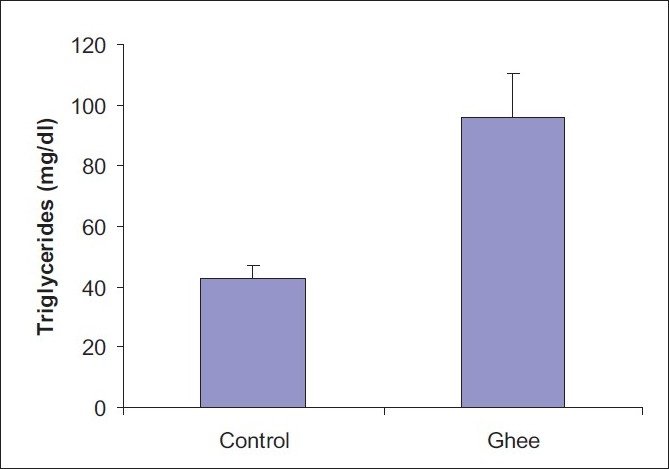
Effect of 10% ghee on serum triglyceride level of Fischer inbred rats; values represent mean ± SD derived from five rats; *significant difference (P < 0.05)
Figure 3.
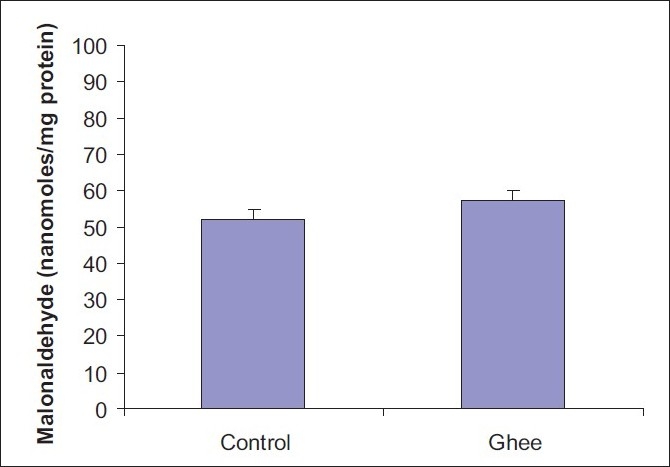
Effect of 10% ghee on liver microsomal lipid peroxidation in Fischer inbred rats; values represent mean ± SD derived from five rats
Figure 4.
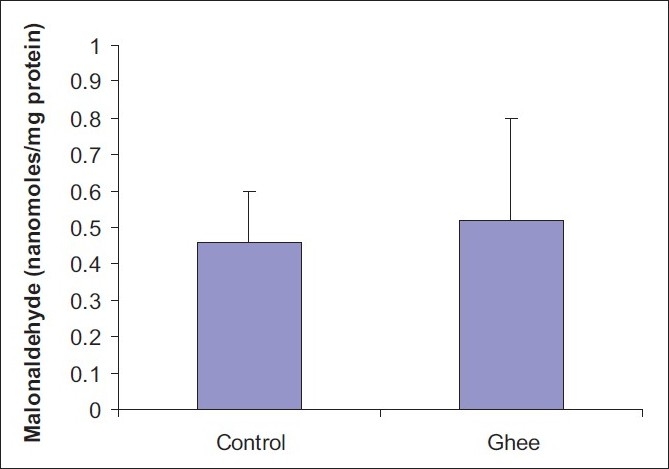
Effect of 10% ghee on liver microsomal lipid peroxide levels in Fischer inbred rats; values represent mean ± SD derived from five rats
Discussion
Kumar and colleagues have shown that the consumption of up to 10% ghee in the diet had a positive effect on serum lipid profiles in Wistar rats (an outbred strain).[24] There was a dose-dependent decrease in total cholesterol, LDL, very low density lipoprotein (VLDL), and triglycerides when ghee was given at levels greater than 2.5% in the diet. Liver cholesterol and triglycerides were also decreased, and when ghee was the sole source of fat at a 10% level, polyunsaturated fatty acids (PUFA) in the serum and liver lipids were significantly reduced. In light of previous concern over cholesterol oxidation products generated in heated ghee,[25] the investigators also fed the animals ghee that had been heated to 120°C. Similar results were seen with the heated ghee as with the “native” ghee (fresh ghee that was not subjected to any further heating). These results (for both the heated and native ghee) included a significant decrease in serum total cholesterol levels, a decrease of 20–25% in serum triglycerides, a 14–16% decrease in liver total cholesterol levels, a 14–29% decrease in liver triglyceride levels, and a lower level of nonenzymatic-induced lipid peroxidation in liver homogenate, compared to the control animals. Levels of cholesterol esters, which are important constituents of serum lipoproteins and are implicated in the process of atherogenesis, were decreased significantly in the liver. Oleic acid, which enables low density lipoprotein (LDL) to resist oxidation, was increased by 36–40% in serum lipids when ghee was used as the sole source of fat at a 10% level. Arachidonic acid, a key inflammatory intermediate in the process of atherosclerosis, was decreased by 65% in serum lipids when ghee was used as the sole source of fat at a 10% level, compared to controls.
The authors discussed several theories that may account for the observed results of their investigation. 1) The hypocholesterolemic effect of dairy products may be mediated through the inhibition of cholesterol biosynthesis which enhances the fecal excretion of sterols and bile acids. 2) Ghee contains conjugated linoleic acid which has been shown to decrease serum LDL and atherogenesis in a rabbit model. 3) Serum oleic acid levels that increased when the animals were fed ghee-supplemented diets may enable LDL to resist oxidation, which in turn may prevent plaque formation. In a follow-up study to determine the mechanism of action for the hypocholesterolemic effect of ghee, diets supplemented with 2.5 and 5% ghee, both native and “oxidized” (heated) were fed to Wistar rats.[26] The diets were made isocaloric with groundnut oil. Dietary ghee did not affect the 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase activity in liver microsomes, indicating that it did not affect cholesterol biosynthesis, but significantly increased the excretion of bile constituents and lowered serum cholesterol levels. Liver is the primary site for the biosynthesis of cholesterol, which is regulated by HMG CoA reductase. This enzyme is downregulated by cholesterol levels in the diet and is also inhibited by oxysterols. Even though the heated ghee contained cholesterol oxides, it did not affect HMG CoA reductase activity. Dietary ghee significantly decreased total cholesterol levels by 10–25% in the serum, and by 7–14% in the intestinal mucosal cells, compared to control animals fed groundnut oil. There was a corresponding decrease in cholesterol ester fractions in the serum and intestinal mucosa, with an indication that the esterification process in the intestine was inhibited by ghee lipids. Cholesterol excretion in the bile of these animals was significantly increased by 18–30%. Bile is an important mode of transport for the excretion of cholesterol and its metabolites. There was also a significant increase in the excretion of total bile acids, uronic acid, and phospholipids. The authors concluded that ghee exhibited hypocholesterolemic effects by enhancing the secretion of biliary constituents.
A preliminary clinical study on the effect of medicated ghee on serum lipid levels in psoriasis patients demonstrated hypolipidemic effects of ghee when given at high doses.[27] The patients were given daily incremental doses of 60 ml medicated ghee over a 7-day period. There was an 8.3% decrease in serum total cholesterol, a 26.6% decrease in serum triglycerides, a 17.8% decrease in serum phospholipids, and a 15.8% decrease in serum cholesterol esters. The patients experienced a significant reduction in scaling, erythema, pruritis, and itching, and a marked improvement in the overall appearance of the skin.
The effectiveness of medicated ghee in the treatment of psoriasis may be due in part to ghee's ability to lower prostaglandin levels and decrease secretion of leukotrienes, which are inflammatory mediators derived from the arachidonic acid cascade. Arachidonic acid is an essential fatty acid present in the phospholipids of cell membranes. In the arachidonic acid cascade, an elaborate signaling system, arachidonic acid is cleaved from phospholipids by the enzyme phospholipase A2, then serves as a substrate for the production of proinflammatory lipid mediators known as prostanoids and leukotrienes.[28] Prostanoids include prostaglandins and thromboxanes and are synthesized from arachidonic acid by cyclooxygenase enzymes.[29] Leukotrienes are synthesized from arachidonic acid by lipoxygenase enzymes.[30] A study on Wistar rats fed native and oxidized ghee showed that a 10% ghee-supplemented diet decreased arachidonic acid levels in macrophage phospholipids in a dose-dependent manner, ranging from 8 to 61% decrease. Serum thromboxane levels were significantly decreased by 27–35% and 6-keto-prostaglandin F1alpha decreased by 23–37%.[31] When the diets were supplemented with 2.5–10% ghee, there was a dose-dependent decrease in serum prostaglandin E2 levels ranging from 17 to 52%. When ghee was fed at levels greater than 2.5%, there was a significant decrease in the secretion of leukotrienes B4 (LTB4), C4 (LTC4), and D4 (LTD 4) by peritoneal macrophages activated with calcium ionophore. The secretion of large amounts of leukotrienes such as LTB4, LTC4, and LTD4 is seen in patients with psoriasis and asthma, and suppression of leukotriene formation is used in the treatment of these disorders. Ghee contains conjugated linoleic acid which has been shown to reduce the formation of inflammatory mediators such as leukotrienes, prostaglandins, and interleukins.[31] The ability of ghee to lower the levels of arachidonic acid metabolites such as thromboxane and prostaglandin and decrease secretion of leukotrienes is also beneficial in preventing cardiovascular disease.[29–32]
These studies provide evidence that dietary ghee up to 10% does not have any adverse effect on serum lipids and may in fact be protective for diseases in outbred rats. Our previous results on Sprague-Dawley outbred rats[8] are consistent with these findings. Our studies in Fischer rats, an inbred strain that serves as a model for genetic predisposition to diseases, indicate that 10% dietary ghee does not have significant effects on serum total cholesterol levels when fed for 4 weeks but raises total cholesterol levels when fed for 2 months. There was an increase in serum triglyceride levels in Fischer rats fed 10% dietary ghee for 4 weeks and for 2 months. However, 10% dietary ghee did not have any significant effect on liver microsomal lipid peroxidation and liver microsomal lipid peroxide levels; thus, it is not likely to increase the risk of free radical induced diseases such as cardiovascular disease, neurodegenerative diseases, and cancer.
Spiteller has proposed a theory on atherogenesis that implicates cell membrane alteration reactions in the induction of lipid peroxidation processes involving PUFAs, in lieu of implicating foods containing cholesterol and saturated fatty acids.[19,33] The cell membrane structure can be altered by various factors including inflammation, pressure such as hypertension, attack by microorganisms, organic and inorganic compounds, etc. This alteration apparently influences the channels crossing the cell wall, causing an influx of Ca2+ ions which induce activation of phospholipases. The phospholipases cleave phospholipids, generating free PUFAs that serve as substrates for cyclooxygenases and lipoxygenases. Lipoxygenases transform PUFAs into lipid hydroperoxides. If the impact on the cell is severe enough, the enzymatic lipid peroxidation processes switch over to nonenzymatic lipid peroxidation reactions in which peroxyl radicals are produced. Peroxyl radicals are much more reactive than lipid hydroperoxides and attack nearly all types of biological molecules including lipids, sugars, and proteins.[34]
Spiteller points out that saturated fatty acids do not undergo lipid peroxidation processes and therefore atherosclerosis is not induced by the consumption of fats containing saturated fatty acids. PUFAs are readily oxidized however and Spiteller implicates cholesterol-PUFA esters in the process of atherogenesis. Due to the PUFA content, the cholesterol esters become oxidized and are then incorporated into LDL and transferred to endothelial cells where they cause damage that induces structural changes, ultimately resulting in the lipid peroxidation processes described above.[19] Ghee contains antioxidants, including vitamin E, vitamin A, and carotenoids,[7] which may be helpful in preventing lipid peroxidation. Vitamin E is found in all cell membranes and functions aggressively in this lipid environment to quench free radicals and prevent the massive lipid peroxidation that results from a free radical chain reaction speeding along the membrane.[12] The herbs in ghee-based Ayurvedic formulations have high concentrations of antioxidants[12] which can prevent oxidation of LDL. A preliminary clinical study on an herbal mixture known as Maharishi Amrit Kalash-4 (MAK-4) showed an increased resistance of LDL to oxidation in hyperlipidemic patients who ingested MAK-4 for 18 weeks. There was no change in levels of serum total cholesterol, high density lipoprotein (HDL), LDL, or triglycerides in these patients.[35] A follow-up study showed similar results, i.e., inhibition of the oxidation of LDL in hyperlipidemic patients who ingested MAK-4, indicating it may be beneficial for preventing and treating atherosclerosis.[36]
Another category of fatty acids that has been linked to increased risk of cardiovascular disease is trans fatty acids. These are unsaturated fatty acids with at least one double bond in the trans configuration. They are formed during the partial hydrogenation of vegetable oils. Compared to the consumption of equal calories from saturated fats, trans fatty acids raise levels of LDL and reduce levels of HDL. They also increase blood levels of triglycerides.[37] In India, partially hydrogenated vegetable oil known as vanaspati was introduced in the 1960s and promoted as “vegetable ghee.” It contains up to 40% trans fatty acids and has gained wide usage in home-based cooking. It is also heavily utilized in the preparation of commercially fried, processed, bakery, ready-to-eat, and street foods.[38] Singh and colleagues studied the association of ghee and vegetable ghee intake with higher risk of coronary artery disease (CAD) in rural and urban populations in northern India. Increased prevalence of CAD was associated with the intake of ghee plus vegetable ghee, but the risk was lower with consumption of ghee alone.[39] A study on a rural population in Rajasthan, India, revealed a significantly lower prevalence of CAD in men who consumed higher amounts of ghee.[40]
Ghee has been heavily utilized in Ayurveda for thousands of years for its health-promoting properties. It is administered alone and is used in conjunction with herbs to treat various disorders. There are 55–60 types of medicated ghee described in the Ayurvedic texts.[5] Positive results have been reported in research conducted on several mixtures containing ghee. As previously discussed, medicated ghee demonstrated hypolipidemic effects in psoriasis patients and significantly improved psoriasis-related symptoms.[27] The herbal mixture MAK-4 inhibited the oxidation of LDL in hyperlipidemic patients.[35,36] Mixtures containing ghee have also shown hepatoprotective effects,[41] anticonvulsant activity,[42] enhancement of memory, and enhancement of wound healing.[43]
In the last two to three decades, ghee has been implicated in the increasing prevalence of CAD in Asian Indians living outside India, as well as upper socioeconomic classes living in large towns and cities in India.[25,44,45] Data available in the literature do not support a conclusion of harmful effects of the moderate consumption of ghee in the general population. Raheja points out that Asian Indians previously had a low incidence of coronary heart disease and for generations had been using ghee in their cooking, which is low in PUFAs such as linoleic acid and arachidonic acid. The epidemic of coronary heart disease in India began two to three decades ago when traditional fats were replaced by oils rich in linoleic and arachidonic acid,[44,45] as well as trans fatty acids which comprise 40% of vanaspati.[38] Adulteration of commercially prepared ghee with vanaspati is also prevalent in India.[46] In light of this, researchers investigating ghee should ensure that the ghee used in their experiments is not adulterated with vanaspati which could yield spurious results.
Other factors that may be involved in the increased prevalence of CAD include an increased level of stress associated with the lifestyles of Asian Indian immigrants and upper socioeconomic classes in India. Psychosocial stressors are now recognized as significant risk factors for cardiovascular disease.[47–50] According to Spiteller's theory, stress is a main factor in the induction of atherogenesis because it results in the release of adrenaline which induces narrowing of the arteries and subsequent lipid peroxidation reactions as discussed previously.[19]
Conclusion
For thousands of years Ayurveda has considered ghee to be the healthiest source of edible fat. In the last several decades, ghee has been implicated in the increasing prevalence of CAD in Asian Indians. Our previous research and data available in the literature do not support a conclusion of harmful effects of the moderate consumption of ghee in the general population. Our present study on Fischer inbred rats indicates that consumption of 10% ghee may increase triglyceride levels, but does not increase lipid peroxidation processes that are linked to a higher risk of cardiovascular disease. Many research studies have been published, which report beneficial properties of ghee and herbal mixtures containing ghee. In animal studies, there was a dose-dependent decrease in serum total cholesterol, LDL, VLDL, and triglycerides; decreased liver total cholesterol, triglycerides, and cholesterol esters; and a lower level of nonenzymatic-induced lipid peroxidation in liver homogenate, in Wistar outbred rats. Similar results were obtained with heated (oxidized) ghee. When ghee was used as the sole source of fat at a 10% level, there was a large increase in oleic acid levels and a large decrease in arachidonic acid levels in serum lipids.[24] In rats fed ghee-supplemented diets, there was a significant increase in the biliary excretion of cholesterol with no effect on the HMG CoA reductase activity in liver microsomes.[26] A 10% ghee-supplemented diet decreased arachidonic acid levels in macrophage phospholipids in a dose-dependent manner. Serum thromboxane and prostaglandin levels were significantly decreased and secretion of leukotrienes by activated peritoneal macrophages was significantly decreased.[31]
A study on a rural population in India showed a significantly lower prevalence of coronary heart disease in men who consumed higher amounts of ghee.[40] High doses of medicated ghee decreased serum cholesterol, triglycerides, phospholipids, and cholesterol esters in psoriasis patients. There were significant improvements in the patients’ psoriasis symptoms as well.[27] MAK-4, a herbal mixture containing ghee, increased the resistance of LDL to oxidation in hyperlipidemic patients and had no effect on levels of serum total cholesterol, HDL, LDL, or triglycerides in these patients.[35,36] Other mixtures containing ghee have shown hepatoprotective effects,[41] anticonvulsant activity,[42] effects on enhancement of memory, and enhancement of wound healing.[43]
These positive research findings support the beneficial effects of ghee outlined in the ancient Ayurvedic texts and the therapeutic use of ghee for thousands of years in the Ayurvedic system of medicine.
Acknowledgments
The authors wish to thank Ellen Kauffman for her assistance in preparation of the manuscript. The study was supported in part by funds provided by Lancaster Foundation, New Bethesda, MD, USA.
References
- 1.Acharya KT. Ghee, vanaspati, and special fats in India. In: Gunstone FD, Padley FB, editors. Lipid Technologies and Applications. New York: Marcel Dekker Inc; 1997. pp. 369–90. [Google Scholar]
- 2.Tirtha SS. Bayville, NY: Ayurveda Holistic Center Press; 1998. The Ayurveda Encyclopedia. [Google Scholar]
- 3.Lad V. New York: Harmony Books; 1998. The Complete Book of Ayurvedic Home Remedies. [Google Scholar]
- 4.Sharma HM. Butter oil (ghee) – Myths and facts. Indian J Clin Pract. 1990;1:31–2. [Google Scholar]
- 5.Illingworth D, Patil GR, Tamime AY. Anhydrous milk fat manufacture and fractionation. In: Tamime AY, editor. Dairy Fats and Related Products. Chichester, West Sussex: Wiley-Blackwell; 2009. [Google Scholar]
- 6.Rajorhia GS. Ghee. In: Macrae R, Robinson RK, Sadler MJ, editors. Encyclopedia of Food Science. Vol. 4. London: Academic Press; 1993. pp. 2186–99. [Google Scholar]
- 7.Sserunjogi ML, Abrahamsen RK, Narvhus J. A review paper: Current knowledge of ghee and related products. Int Dairy J. 1998;8:677–88. [Google Scholar]
- 8.Dwivedi C, Crosser AE, Mistry VV, Sharma HM. Effects of dietary ghee (clarified butter) on serum lipids in rats. J Appl Nutr. 2002;52:65–8. [Google Scholar]
- 9.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, et al. Diet and lifestyle recommendations revision 2006: A scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 10.Sharma H. Toronto: Veda Publishing; 1993. Freedom from Disease: How to Control Free Radicals, a Major Cause of Aging and Disease. [Google Scholar]
- 11.Harman D. Free radical theory of aging: Role of free radicals in the origination and evolution of life, aging, and disease processes. In: Johnson JE Jr, Walford R, Harman D, Miquel J, editors. Free Radicals, Aging, and Degenerative Diseases. New York: Alan R. Liss; 1986. pp. 3–49. [Google Scholar]
- 12.Sharma HM. Free radicals and natural antioxidants in health and disease. J Appl Nutr. 2002;52:26–44. [Google Scholar]
- 13.Tekin IO, Sipahi EY, Comert M, Acikgoz S, Yurdakan G. Low-density lipoproteins oxidized after intestinal ischemia/reperfusion in rats. J Surg Res. 2009;157:e47–54. doi: 10.1016/j.jss.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Agha AM, Gad MZ. Lipid peroxidation and lysosomal integrity in different inflammatory models in rats: The effects of indomethacin and naftazone. Pharmacol Res. 1995;32:279–85. doi: 10.1016/s1043-6618(05)80015-5. [DOI] [PubMed] [Google Scholar]
- 15.Hall ED, Yonkers PA, Andrus PK, Cox JW, Anderson DK. Biochemistry and pharmacology of lipid antioxidants in acute brain and spinal cord injury. J Neurotrauma. 1992;9:S425–42. [PubMed] [Google Scholar]
- 16.Zeiger SL, Musiek ES, Zanoni G, Vidari G, Morrow JD, Milne GJ, et al. Neurotoxic lipid peroxidation species formed by ischemic stroke increase injury. Free Radic Biol Med. 2009;47:1422–31. doi: 10.1016/j.freeradbiomed.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasapović J, Pejić S, Todorović A, Stojiljković V, Pajović SB. Antioxidant status and lipid peroxidation in the blood of breast cancer patients of different ages. Cell Biochem Funct. 2008;26:723–30. doi: 10.1002/cbf.1499. [DOI] [PubMed] [Google Scholar]
- 18.Aviram M. Review of human studies on oxidative damage and antioxidant protection related to cardiovascular diseases. Free Radic Res. 2000;33:S85–97. [PubMed] [Google Scholar]
- 19.Spiteller G. The important role of lipid peroxidation processes in aging and age dependent diseases. Mol Biotechnol. 2007;37:5–12. doi: 10.1007/s12033-007-0057-6. [DOI] [PubMed] [Google Scholar]
- 20.Dwivedi C, Downie AA, Webb TE. Net glucuronidation in different rat strains: Importance of microsomal beta-glucuronidase. FASEB J. 1987;1:303–7. doi: 10.1096/fasebj.1.4.3115856. [DOI] [PubMed] [Google Scholar]
- 21.Engineer FN, Sridhar R. Attenuation of daunorubicin-augmented microsomal lipid peroxidation and oxygen consumption by calcium channel antagonists. Biochem Biophys Res Commun. 1991;179:1101–6. doi: 10.1016/0006-291x(91)91933-4. [DOI] [PubMed] [Google Scholar]
- 22.Dwivedi C, Sharma HM, Dobrowski S, Engineer FN. Inhibitory effects of Maharishi-4 and Maharishi-5 on microsomal lipid peroxidation. Pharmacol Biochem Behav. 1991;39:649–52. doi: 10.1016/0091-3057(91)90141-n. [DOI] [PubMed] [Google Scholar]
- 23.Sridhar R, Dwivedi C, Anderson J, Baker PB, Sharma HM, Desai P, et al. Effects of verapamil on the acute toxicity of doxorubicin in vivo. J Natl Cancer Inst. 1992;84:1653–60. doi: 10.1093/jnci/84.21.1653. [DOI] [PubMed] [Google Scholar]
- 24.Kumar MV, Sambaiah K, Lokesh BR. Effect of dietary ghee - the anhydrous milk fat, on blood and liver lipids in rats. J Nutr Biochem. 1999;10:96–104. doi: 10.1016/s0955-2863(98)00088-6. [DOI] [PubMed] [Google Scholar]
- 25.Jacobson MS. Cholesterol oxides in Indian ghee: Possible cause of unexplained high risk of atherosclerosis in Indian immigrant populations. Lancet. 1987;2:656–8. doi: 10.1016/s0140-6736(87)92443-3. [DOI] [PubMed] [Google Scholar]
- 26.Kumar MV, Sambaiah K, Lokesh BR. Hypocholesterolemic effect of anhydrous milk fat ghee is mediated by increasing the secretion of biliary lipids. J Nutr Biochem. 2000;11:69–75. doi: 10.1016/s0955-2863(99)00072-8. [DOI] [PubMed] [Google Scholar]
- 27.Kumar MV, Sambaiah K, Mangalgi SG, Murthy NA, Lokesh BR. Effect of medicated ghee on serum lipid levels in psoriasis patients. Indian J Dairy & Biosci. 1999;10:20–3. [Google Scholar]
- 28.Bogatcheva NV, Sergeeva MG, Dudek SM, Verin AD. Arachidonic acid cascade in endothelial pathobiology. Microvasc Res. 2005;69:107–27. doi: 10.1016/j.mvr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Smyth EM, Grosser T, Wang M, Yu Y, FitzGerald GA. Prostanoids in health and disease. J Lipid Res. 2009;50:S423–8. doi: 10.1194/jlr.R800094-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bäck M. Leukotriene signaling in atherosclerosis and ischemia. Cardiovasc Drugs Ther. 2009;23:41–8. doi: 10.1007/s10557-008-6140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar MV, Sambaiah K, Lokesh BR. The anhydrous milk fat, ghee, lowers serum prostaglandins and secretion of leukotrienes by rat peritoneal macrophages. Prostaglandins Leukot Essent Fatty Acids. 1999;61:249–54. doi: 10.1054/plef.1999.0097. [DOI] [PubMed] [Google Scholar]
- 32.Riccioni G, Capra V, D’Orazio N, Bucciarelli T, Bazzano LA. Leukotriene modifiers in the treatment of cardiovascular diseases. J Leukoc Biol. 2008;84:1374–8. doi: 10.1189/jlb.0808476. [DOI] [PubMed] [Google Scholar]
- 33.Spiteller G. The relation of lipid peroxidation processes with atherogenesis: A new theory on atherogenesis. Mol Nutr Food Res. 2005;49:999–1013. doi: 10.1002/mnfr.200500055. [DOI] [PubMed] [Google Scholar]
- 34.Spiteller G. Peroxyl radicals: Inductors of neurodegenerative and other inflammatory diseases: Their origin and how they transform cholesterol, phospholipids, plasmalogens, polyunsaturated fatty acids, sugars, and proteins into deleterious products. Free Radic Biol Med. 2006;41:362–87. doi: 10.1016/j.freeradbiomed.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Hanna AN, Sundaram V, Falko JM, Stephens RE, Sharma HM. Effect of herbal mixtures MAK-4 and MAK-5 on susceptibility of human LDL to oxidation. Complement Med Int. 1996;3:28–36. [Google Scholar]
- 36.Sundaram V, Hanna AN, Lubow GP, Koneru L, Falko JM, Sharma HM. Inhibition of low-density lipoprotein oxidation by oral herbal mixtures Maharishi Amrit Kalash-4 and Maharishi Amrit Kalash-5 in hyperlipidemic patients. Am J Med Sci. 1997;314:303–10. doi: 10.1097/00000441-199711000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med. 2006;354:1601–13. doi: 10.1056/NEJMra054035. [DOI] [PubMed] [Google Scholar]
- 38.Ghafoorunissa G. Role of trans fatty acids in health and challenges to their reduction in Indian foods. Asia Pac J Clin Nutr. 2008;17:212–5. [PubMed] [Google Scholar]
- 39.Singh RB, Niaz MA, Ghosh S, Beegom R, Rastogi V, Sharma JP, et al. Association of trans fatty acids (vegetable ghee) and clarified butter (Indian ghee) intake with higher risk of coronary artery disease in rural and urban populations with low fat consumption. Int J Cardiol. 1996;56:289–98. doi: 10.1016/0167-5273(96)02760-x. [DOI] [PubMed] [Google Scholar]
- 40.Gupta R, Prakash H. Association of dietary ghee intake with coronary heart disease and risk factor prevalence in rural males. (83).J Indian Med Assoc. 1997;95:67–69. [PubMed] [Google Scholar]
- 41.Achliya GS, Wadodkar SG, Dorle AK. Evaluation of hepatoprotective effect of Amalkadi Ghrita against carbon tetrachloride-induced hepatic damage in rats. J Ethnopharmacol. 2004;90:229–32. doi: 10.1016/j.jep.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 42.Achliya GS, Wadodkar SG, Dorle AK. Evaluation of sedative and anticonvulsant activities of Unmadnashak Ghrita. J Ethnopharmacol. 2004;94:77–83. doi: 10.1016/j.jep.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 43.Prasad V, Dorle AK. Evaluation of ghee based formulation for wound healing activity. J Ethnopharmacol. 2006;107:38–47. doi: 10.1016/j.jep.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 44.Raheja BS. Dietary fats and habits and susceptibility of Asian Indians to NIDDM and atherosclerotic heart disease. J Diabet Assoc India. 1991;31:21–8. [Google Scholar]
- 45.Raheja BS. Obesity and coronary risk factors among South Asians. Lancet. 1991;337:971. [PubMed] [Google Scholar]
- 46.Ganguli NC, Jain MK. Ghee: Its chemistry, processing and technology. J Dairy Sci. 1973;56:19–25. [Google Scholar]
- 47.Figueredo VM. The time has come for physicians to take notice: The impact of psychosocial stressors on the heart. Am J Med. 2009;122:704–12. doi: 10.1016/j.amjmed.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Esler M. Heart and mind: Psychogenic cardiovascular disease. J Hypertens. 2009;27:692–5. doi: 10.1097/HJH.0b013e328324f72b. [DOI] [PubMed] [Google Scholar]
- 49.Gianaros PJ, Sheu LK. A review of neuroimaging studies of stressor-evoked blood pressure reactivity: Emerging evidence for a brain-body pathway to coronary heart disease risk. Neuroimage. 2009;47:922–36. doi: 10.1016/j.neuroimage.2009.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Das S, O’Keefe JH. Behavioral cardiology: Recognizing and addressing the profound impact of psychosocial stress on cardiovascular health. Curr Atheroscler Rep. 2006;8:111–8. doi: 10.1007/s11883-006-0048-2. [DOI] [PubMed] [Google Scholar]


