Abstract
Depression is a common diagnosis throughout India. It is one of the major sequelae of modern lifestyle which is full of stress. Several drugs and therapies have been tried but a safe and effective treatment of depressive illness is yet not fully established. The main objective of this experimental study on animal models is to evaluate the antidepressant action of plant drug Vacha (Acorus calamus). The behavioral study was conducted and at the same time 5-HT receptor involvement was evaluated. The experimental study was done in rats to evaluate their Open Field Behavior (OFB), High Plus Maze (HPM) activity and 5-hydroxytryptamine (5-HT) receptor syndrome, before and after feeding Vacha. Concurrent Vacha administration in the depression model prevented the development of behavioral deficit in ambulation and rearing due to stress. Similarly, in High Plus Maze Test (HPMT), exploratory activity of rat was restored with Vacha administration. In adopted model of depression, when the animal was subjected to Vacha administration, the behavioural deficit was prevented very well as compared to stressed group. While eliciting the 5-HT syndrome, only two components out of five were influenced by Vacha, indicating that Vacha does not sensitize postsynaptic 5-HT1A receptors, which explains the behavioral deficit prevention in stressed rat group. Vacha definitely has antidepressant effects in animal model of depression.
Keywords: 5-hydroxytryptamine receptors, depression, Vacha
Introduction
Depression is one of the most common and most serious mental health problems that the people are facing. Earlier people were of the views that depression is not so common in India as stated by a British psychiatrist Venkaba R. A. appointed to the mental asylums in the country. The prevalence of various depressive disorders in the general population in different parts of the country varies from 1.5 to 32.9% and prevalence in outdoor and indoor sections of departments of general hospitals varies from 1.8 to 34.7%.[1] Although there are a number of studies on the socio-demographic and clinical variables of depression, the prevalence of various symptoms has not clearly emerged in India.[2] While it is only human to experience feelings of sadness, gloominess, or melancholy every now and then, clinical depression occurs when these feelings endure for long periods of time that can last for several weeks to several years, if left untreated. Depression can interfere with a person's ability to function effectively throughout the day or even the motivation to get out of bed in the morning.[3]
Biochemically, major depression is characterized by dysregulation of central biogenic amine neurotrans-mitter system (mainly serotonergic, noradrenergic and dopaminergic).[4,5]
The serotonin [5-hydroxytryptamine (5-HT)] hypothesis of depression has received special attention as 5-HT has been related to many of the major symptoms of depression, e.g. sleep, mood, activity and cognitive dysfunction.[6] Moreover, the preclinical studies on the action of different types of antidepressant treatment on the serotonin system revealed, as a common effect, an enhancement of the 5-HT neurotransmission.[7,8] Tricyclic antidepressants and electroconvulsive therapy enhance the sensitivity of the postsynaptic 5-HT2 receptors. 5-HT1 A receptor agonists (effective antidepressant) produce the tonic activation of postsynaptic 5-HT1 A receptors. Monoamine oxidase inhibitors enhance the availability of the releasable 5-HT. 5-HT uptake blockers increase the efficacy of 5-HT neurons by desensitizing the 5-HT autoreceptors located on 5-HT nerve terminals.[9]
Based on the above information, the present study was designed to investigate the effect of long-term Vacha as a prophylactic antidepressant drug, on a well-established animal model of depression, and secondly, to assess the functional sensitivity of the 5-HT1 A and 5-HT2 receptors following long-term Vacha administration to normal animals as well as models of depression.
Materials and Methods
Animals
Adult male albino rats (Charles-foster strain) weighing 150-200 g were used in the study. Initially, they were maintained on rat pellet diet and tap water (unless mentioned otherwise) ad libitum at a 12 hour light dark schedule. They were group housed (three to four per cage) at a temperature of 24 ± 1°C.
Time of experiment
All the behavioral testing was done between 1 and 2 hours in all groups so as to avoid variation of results due to circadian rhythm in biogenic amine.
Depression model
A slightly modified version of a stress-based model developed by Katz et al.[10] was used. The animals were subjected to a variety of different stressors over a 3-week period (6 days/week). Stressors were administered once per day, between the first and eighth hours of the light cycle, to maximize the unpredictability on the nature of the stressors and time of delivery.
Selection of drug
The Vacha plant (Acorus calamus) roots were procured from the market after proper identification and selection. Drugs were dried and ground to small pieces at the BHU Pharmacy and were taken to Department of Medicinal Chemistry, Banaras Hindu University, for alcoholic extraction.
Procedure of extraction
Vacha (1 kg) was taken and extraction was done with a soxlet apparatus. After complete extraction, the alcohol was evaporated on a water bath. Finally, 60 g extract was obtained.
Dosage schedule
The dosage of Vacha extract was 18 mg/kg. Vacha dose for rat was fixed as 8 times that of human adult dose, also considering the percentage yield of Vacha extract after the process of extraction. Vacha extract was dissolved in distilled water and the suspension was prepared with the addition of 2% gum acacia powder. In the case of Vacha, 720 mg extract was dissolved in 100 ml distilled water.
Plan of study
The animals from central animal house, B.H.U, Varanasi, were transferred to the Neuro-physiology Laboratory animal room, in the Department of Physiology, Institute of Medical Science, for acclimatization (48 hour) to the laboratory conditions. Before starting the drug feeding, all the rats were randomly grouped and individually tested in the Open Field Test (OFT) and High Plus Maze (HPM) for studying their initial emotional reactivity and exploratory behavior.
We had selected the plant drug, Vacha (A. calamus), for experimental study. Therefore, all the animals were assigned to one of the following groups:
normal controls
control + Vacha group (n = 5)
depression group (stressed) (n = 5)
depression + Vacha group (n = 5)
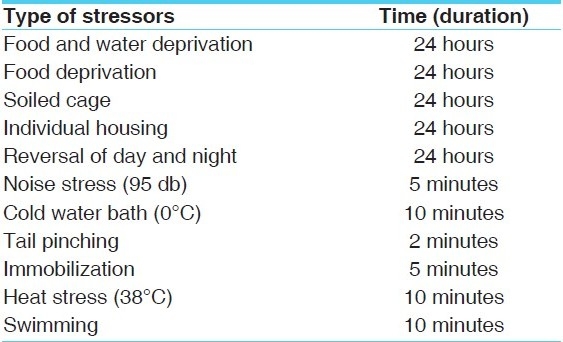
The types of stressors are given in Table 1.
Table 1.
Types of stressors and their duration
Methods and parameters of behavioral study
All the experimental animals were studied behaviorally once before and again after administration of drug and stressors. The evaluation of the 5-HT receptor sensitivity was done before and after the administration of drug and stressors.
Open field test
Principle
The OFT was done to study the locomotor activity, exploratory behavior and the emotional stability of the animal when placed in a new situation (open field). An “emotional animal” is the one which freezes, shows reduced ambulation, exhibits abnormal behavior of rearing and grooming, and shows augmented autonomic activity characterized by increased defecation.
Procedure
The OFT apparatus is made of a large circular arena of 70 cm diameter with 28 cm high opaque wall. The floor is marked off into 24 segments to allow quantification of locomotor activity. The segments are divided by two circles into outer, inner and a central region. A 40-W frosted bulb is suspended above the arena. The active motor behavior, exploratory behavior and emotional defecation were measured in 3 minutes test time. Each rat was placed at the center and was observed for number of square crossed (ambulation), number of standing on the hind legs (rearing) with or without support of wall, the grooming behavior and the number of fecal boluses (pellets) passed by the animals. Each animal was tested before starting the experiment and again 48 hours after stopping the treatment.
High plus maze test
Principle
This test was done on the rats to assess their anxiety level and is based on the principle that exposure of an animal to an elevated and open arm leads to an approach conflict which is substantially stronger than that evoked by exposure to an enclosed maze arm.[11] The devices have two open arms and two closed arms crossed in the form of plus sign. The open arm is of 50 × 10 cm and enclosed arms are of 50 ×10 × 40 cm with an open roof. The two entrances of the open arm are opposite to each other. The device is elevated from the floor to a height of 50 cm. Two lamps (25 W) are mounted 50 cm above the two open arms. Test was done in a peaceful condition.
Procedure
The testing was done as per the method described by Jonathan.[12] The rat was placed in the center of the maze, facing a closed arm. The following parameters were recorded in a 5 minute session.
The time spent in an open arm (the animal was considered to be in open arm only when all four paws were in that arm).
The number of times the animal crossed over to an open arm.
Total number of arm crossing (both close and open).
Number of fecal pellets released.
Number of rearing.
Evaluation of serotonin syndrome
The modification of postsynaptic 5-HT receptor (serotonin receptor) was assessed. The serotonin syndrome was measured before and 48 hours after the last feeding. The syndrome was elicited by administering 0.75 mg/kg of 5-HTlA selective agonist, 8-OH-2-(di-n-proplyl amino)-tetraline (8-OH-DPAT) (Sigma-St.Louise, MO 63103, USA, Yellowspring Co., Dayton, Oh, USA) intraperitoneally.
For testing the 5-HT syndrome, rats were placed in an observation arena for habituation, 5-6 minutes before drug administration. Drug was injected 2 minutes before the first observation was made. The following components of the syndrome were scored: flat posture, forepaw treading, tremor, straub tail and head waving. Scoring was done on a 4-point ranked intensity scale as follows:
0 = absent,
1 = equivocal,
2 = present and
3 = extreme.
Scoring was done in the observation period of 45 seconds per rat, after every 5 minutes for 45 minutes. Scores for each component were summed up over all the observation periods. Minimum four components out of the five had to be present, in order to accept the serotonin syndrome as present.
Hypothermia
The temperature of the rats was recorded before and after 30 minutes of drug administration, according to the method of Hjorth.[13] Temperature was recorded in an individual rat by a thermistor probe (Yellow Spring Co., USA) inserted 5 cm into the rectum. The probe was connected to a six-channel Telethermometer Apparatus (APLAB, Thane, MH, India). Hypothermia should be present in serotonin syndrome positive rat.
Results
Behavioral and neurochemical effects of vacha
Open field test
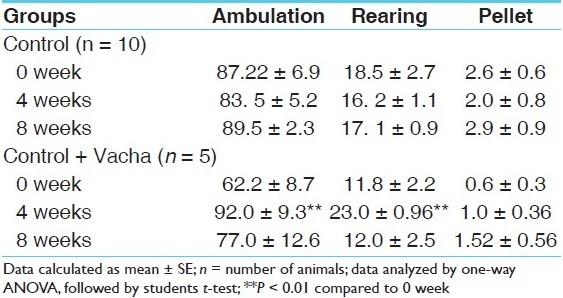
It can be seen in Table 2 that in normal rats, 4 weeks of chronic Vacha administration (18 mg/kg given orally) enhanced both ambulation (P < 0.01) and rearing (P < 0.01) compared to the pre-drug level as well as that of separate control. However, after 8 weeks of administration, the open field activity in terms of ambulation, rearing and pellets became almost similar to the preceding value.
Table 2.
OFT behavior of normal rats during 8 weeks Vacha administration (18 mg/kg given orally) compared to untreated rats
In Table 3, by one-way analysis of variance (ANOVA) it has been shown that the initial open field activity of stress and stress + Vacha groups were similar in terms of ambulation, rearing and pellets. However, following 8 weeks of chronic stressor application (depression model), ambulation and rearing decreased drastically (P < 0.001 and P < 0.05, respectively). The deficit was apparent within 4 weeks of stressor application.
Table 3.
OFT of behaviorally depressed rats and following concurrent administration of Vacha (18 mg/kg given orally)
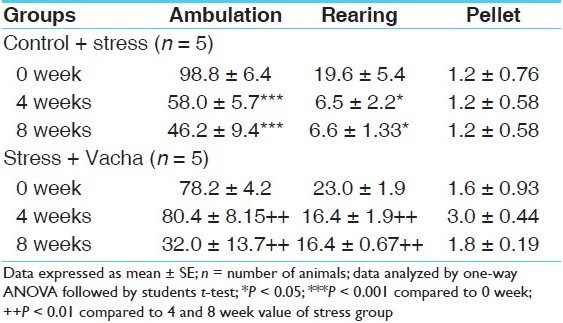
Concurrent Vacha administration in the depression model prevented the development of behavioral deficit in ambulation and rearing. At the end of 4 weeks in stress + Vacha group, both ambulation and rearing were significantly higher (P < 0.01 and P < 0.01, respectively) than ambulation and rearing after 4 and 8 weeks in stress group.
High plus maze test
Table 4 shows that control animals (without drug) maintained similar HPM activity during repeated testing at 4 and 8 weeks.
Table 4.
HPM activity of rats during 8 weeks Vacha administration to normal rats (18 mg/kg body weight given orally)
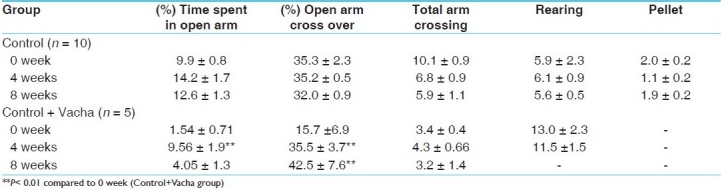
Chronic Vacha administration exerted an anxiolytic effect in normal rats after 4 weeks. The percentage time spent in open arm as well as the percentage open arm crossing enhanced significantly (P < 0.01 in both cases) after 4 weeks drug administration.
Table 5 shows that in the animal model of depression following 4 weeks of stressor application, the time spent in open arm as well as the percentage open arm crossing decreased significantly (P < 0.01 in both cases), while rearing and pellet release were unchanged. After 8 weeks of stress, there was a complete inhibition of open arm crossing and exploration.
Table 5.
HPM activity of behaviorally depressed (stress group) rats compared to stress + Vacha group
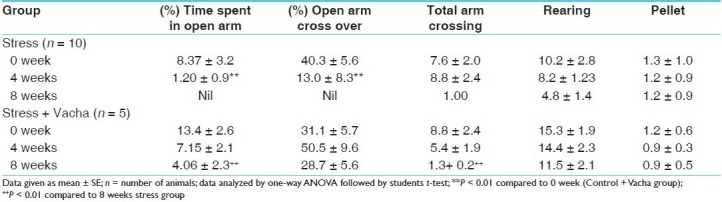
Chronic concurrent administration of Vacha was unable to prevent the deficit in open arm activity by either 4 or 8 weeks treatment; thus, Vacha was apparently not effective as an anxiolytic drug in this model of depression.
5-HT receptor mediated behavior syndrome
5-HT syndrome and hypothermia
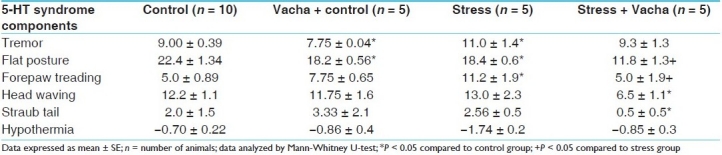
5-HT1A receptor agonist, 8-OH-DPAT, administered intraperitoneally, produced a series of behavioral symptoms which are elicited by activation of postsynaptic 5-HT1A receptor. Following 8 weeks of chronic Vacha administration (orally), two components of the 5-HT syndrome, viz. tremor and flat posture, were significantly diminished (P < 0.05 for both components) compared to control group. However, hypothalamic receptor 5-HT1A mediated hypothermia was not manifested by Vacha. In the depression model, flat posture was significantly diminished (P < 0.05) and tremor was enhanced (P < 0.05). Other components of the syndrome were unaltered. Table 6 shows that Vacha given prophylactically along with stressors for 8 weeks, reduced the intensity of flat posture (P < 0.05), forepaw treading (P < 0.05) and head waving (P < 0.05) compared to the stressed group. Results of an experimental study showed that Vacha possesses an antidepressant property but its anxiolytic effect was not marked. Vacha was reported to have tranquillizers and Mono amine oxidase (MAO) -inhibitor like effect. A recent study described Vacha as a potent antidepressant drug.[14] However, in our study, Vacha did not produce any significant change in 5-HT1A receptor sensitivity [Table 6].
Table 6.
5-HT syndrome induced by selective 5-HT1A against 8-OH-DPAT (0.75 mg/kg IP) in control, treated, behaviorally depressed and stress + Vacha groups
Discussion
In open field behavioral study of normal control animals, 4 weeks of Vacha administration (18 mg/kg given orally) enhanced both ambulation (P < 0.01) and rearing (P < 0.01) as compared to the pre-drug level. In Table 3, by one way ANOVA it has been shown that the initial open field activity of stress and stress + Vacha groups were similar in terms of ambulation, rearing and pellets. However, following 8 weeks of chronic stressor application (depression model), ambulation and rearing decreased drastically (P < 0.01 and P < 0.01, respectively). The deficit was apparent within 4 weeks of stressor application. However, concurrent Vacha administration in the depression model prevented the development of behavioral deficit in ambulation and rearing. In High Plus Maze Test (HPMT) study, chronic concurrent administration of Vacha was unable to prevent the deficit in open arm activity by either 4 or 8 weeks treatment; thus, Vacha was apparently not effective as an anxiolytic drug in this model of depression. Whereas, with 8 weeks of chronic Vacha administration (orally), only two components of the 5-HT syndrome, viz. tremor and flat posture, were significantly diminished (P < 0.05 for both components) compared to control group. Thus, hypothalamic receptor 5-HT1A mediated hypothermia was not attained by Vacha. It is inferred from OFT study that Vacha produces a marked antidepressant activity, and the anxiolytic activity of Vacha is not marked as seen from HPMT study. In our study, Vacha did not produce any significant change in 5-HT1A receptor sensitivity. Results of experimental study showed that Vacha possesses an antidepressant property but anxiolytic effect was not marked.
Conclusion
It can be concluded that Vacha possesses marked antidepressant effect but the anxiolytic effect of Vacha is not marked, and Vacha did not produce any significant change in 5-HT 1A receptor sensitivity.
Figure 1.
Open field (above) and high plus maze (below) testing
References
- 1.Gupta R. Endogenous depression: A report from Ludhiana. Indian J Psychol Med. 1988;11:13–6. [Google Scholar]
- 2.Kupfer D, Spencer R. Delta sleep ration—a biological correlate of early recurrence in unipolar affective disorder. Arch Gen Psychiatry. 1990;47:1100–5. doi: 10.1001/archpsyc.1990.01810240020004. [DOI] [PubMed] [Google Scholar]
- 3.Meyer E, Kimling L. Advantages of a behaviour-depressive approach to investigating stress in the depressive disorders. In: RA De Pue., editor. The psychology of the depressive disorders. New York: Academic Press; 1979. pp. 391–408. [Google Scholar]
- 4.Snaith RP. The concept of mild depression. Br J Psychiatry. 1987;150:387–93. doi: 10.1192/bjp.150.3.387. [DOI] [PubMed] [Google Scholar]
- 5.Sethi BB, Chaturvedi PK, Tiwari SC. Vol. 7. Bangalore: Indian Psychiatric Society; 1988. Biological basis of depressive disorders.Continuing medical education program booklet; pp. 22–36. [Google Scholar]
- 6.Horne JA. Human sleep, sleep loss and behaviour: Psychiatric disorder. Br J Psychiatry. 1993;162:413–9. doi: 10.1192/bjp.162.3.413. [DOI] [PubMed] [Google Scholar]
- 7.Dey S, Singh RH. Varanasi: Banaras Hindu University; 1993. Probable involvement of central serotonin neurotransmission in the anti depressant effect of physical exercise: A preclinical study. Ph.D. Thesis, Department of Kayachikitsa, IMS. [Google Scholar]
- 8.Pathak SR, Singh RH. Varanasi: Banaras Hinru University; 1990. A neurohumoral study on brain function in stress and anti-stress drug action (with special reference to the effect of some plant products). ph.D. Thesis, Department of Kayachikitsa, IMS. [Google Scholar]
- 9.Bebbington P, Der G, MacCarthy B, Wykes T, Brugha T, Sturt P, et al. Stress incubation and the onset of affective disorder. Br J Psychiatry. 1993;162:358–62. doi: 10.1192/bjp.162.3.358. [DOI] [PubMed] [Google Scholar]
- 10.Katz R. Nijhar T.Stress incubation in depression. Eur J Phycol. 1982;117:532–40. [Google Scholar]
- 11.Pellow M, Jennifer H. Pattern of aneiety response in rat testing. Exp Physiol. 1985;89:168–76. [Google Scholar]
- 12.Jonathan S. Updates in experimental neurophysiology. Am J Clin Neurophysiol. 1992;168:321–7. [Google Scholar]
- 13.Hjorth 5-HT receptor mediated hypothermia in experimental rats. Exp Physiol. 1985;89:224–9. [Google Scholar]
- 14.Schultes RE. The virgin field in psychoactive plant research. Ethnobotany. 1993;5:5–61. [Google Scholar]


