Abstract
The evolution of orthologous genes coding for β-defensin 2 (BD2) in primates has been subject to positive selection during the divergence of the platyrrhines from the catarrhines and of the Cercopithecidae from the Hylobatidae, great apes, and humans. Three peptides have been selected for a functional analysis of the effects of sequence variations on the direct antimicrobial activity: human BD2 (hBD2), Macaca fascicularis BD2 (mfaBD2), and a variant of the human peptide lacking Asp4, (−D)hBD2, which is characteristic only of the human/great ape peptides. hBD2 and mfaBD2 showed a significant difference in specificity, the former being more active towards Escherichia coli and the later towards Staphylococcus aureus and Candida albicans. Asp4 in the human peptide appears to be important, as (−D)hBD2 was less structured and had a markedly lower antimicrobial activity. The evolution of β-defensin 2 in primates may thus have been driven, at least in part, by different environmental pressures so as to modulate antimicrobial activity.
Human β-defensin 2 (hBD2) is a host defense peptide originally isolated from the skin of psoriasis patients (6). The hBD2 gene is constitutively expressed in skin, lung, and trachea, being markedly upregulated by inflammatory stimuli, and its induced expression can also be detected in other tissues (5, 7, 11, 13). The peptide has a relatively broad antimicrobial activity spectrum, ranging from bacteria to yeasts (6), and it has been hypothesized that this depends on the interaction of an oligomeric form with the negatively charged microbial surface via an electrostatic charge-based mechanism (10). Apart from this direct antimicrobial activity, hBD2 also plays other roles in host defense, as it is a chemoattractant for immature dendritic cells and memory T cells (15).
Recently, the molecular evolution of the β-defensin 2 gene (originally DEFB2, now DEFB4) has been investigated in humans and 16 primate species (2) to obtain insights into how its evolution might have affected the structure of the protein and thus its function. Evidence of positive selection was indeed found during the divergence of the platyrrhines (New World monkeys) from the catarrhines (Old World monkeys) and during the divergence of the Cercopithecidae from the Hylobatidae, great apes, and humans. In contrast, the evolution of the DEFB1 gene orthologues in primates (coding for β-defensin 1 congeners) shows a random variation consistent with the neutral theory of molecular evolution (3), while DEFB103 gene orthologues (coding for β-defensin 3 congeners) are instead markedly conserved (1).
At the amino acid level, the human and great ape BD2 peptides are effectively identical, while those from Hylobates vary only in a short N-terminal stretch (2). The positively selected variations in the Cercopithecidae and New World monkeys concern a more marked variation in the C-terminal region of the mature peptides. On the basis of these variation patterns, three peptides have been selected for chemical synthesis and functional analysis to determine the effect on biological activity and whether particular aspects of this activity might have contributed to the genetic mechanisms responsible for variation. The amino acid sequences of the three β-defensins are shown in Fig. 1.
FIG. 1.
β-Defensin amino acid sequences.
The human peptide hBD2 and Macaca fascicularis peptide (mfaBD2) represent the two most different groups in this congener, among the investigated primates, while a variant of the human molecule lacking Asp4, (−D)hBD2, probes the role of the characteristic N terminus in the human/great ape peptides.
The peptides were prepared by the optimized solid-phase peptide synthesis protocol described previously (1). After reduction and reversed-phase high-pressure liquid chromatography (RP-HPLC) purification, 10 to 20 μM peptide was used directly for the oxidative folding reaction, which was carried out in N2-saturated aqueous buffer (0.1 M ammonium acetate, 2 mM EDTA, pH 7.8, containing 1 M guanidine HCl), in which cysteine (1 to 2 mM) and cystine (0.1 to 0.2 mM) were dissolved immediately prior to use. The mixture was left at room temperature for 24 to 48 h, and oxidation was monitored by analytical RP-HPLC (Waters Symmetry C18, 3.5 μm, 100 Å, 4.6 mm by 50 mm) and electrospray ionization-mass spectometry until completion prior to final desalting and purification on a preparative RP-HPLC column (Waters Delta-Pak C18, 15 μm, 300 Å, 25 mm by 100 mm; oxidized peptide molecular weights are 4329.0, 4213.1, and 4442.0 for hBD2, (−D)hBD2, and mfaBD2, compared to the calculated molecular weights, which are 4328.2, 4213.2, and 4441.4, respectively). The secondary structure was then studied by circular dichroism spectroscopy on 20 μM peptide solutions in 5 mM sodium phosphate buffer, pH 7.0, in the presence or absence of 50% (vol/vol) trifluoroethanol or 10 mM sodium dodecyl sulfate.
The MICs were determined against selected reference gram-positive and -negative bacteria and one yeast (Table 1) by a version of the microdilution susceptibility test (4, 14) suitable for β-defensins (1). These assays were carried out in a final volume of 100 μl of Mueller Hinton (MH) or Sabouraud/dextrose (SAB) medium [5% (vol/vol) in 10 mM sodium phosphate buffer (SPB), pH 7.4] by using 105 CFU of microorganisms per ml at logarithmic phase, and values are the means of results from of at least three experiments. For Enterococcus faecalis, which does not grow well in these conditions, MICs were extrapolated from time-killing experiments in SPB at different peptide concentrations.
TABLE 1.
Antimicrobial activity of β-defensin 2 variantsa
| Variant | MIC (μM)
|
||||||
|---|---|---|---|---|---|---|---|
| E. coli ML-35 | P. aeruginosa ATCC 27853 |
B. cepacia
|
S. aureus 710A | E. faecalis A16b | C. albicans | ||
| 6981 | 14273 | ||||||
| mfaBD2 | 4-8 | 8 | >32 | >32 | 4 | 2 | 4 |
| hBD2 | 4 | 4 | >32 | >32 | 8-16 | 4 | 16 |
| (−D)hBD2 | 4 | 16-32 | >32 | >32 | 16-32 | 4 | 32 |
MICs were determined in 5% MH broth for bacteria or in 5% SAB medium for the yeast in 10 mM SPB with 105 CFU of microorganisms at logarithmic phase per ml and are the means of at least three experiments.
This bacterium does not grow in 5% medium, so these values were extrapolated from killing experiments in SPB at different peptide concentrations.
The kinetics of bacterial killing for all three peptides were determined against E. coli ML-35, S. aureus 710A, and Enterococcus faecalis A16 at 107 CFU/ml with 8 μM peptide and against Enterococcus faecalis at 105 CFU/ml with 2 μM peptide, in the exponential phase in SPB incubated at 37°C. At different times, 50 μl of the bacterium-peptide suspension was diluted severalfold in ice-cold SPB, and the solution was plated on nutrient agar and incubated overnight to allow colony counts.
Permeabilization of the cytoplasmic membranes of E. coli ML-35/pYC and S. aureus 710A was evaluated by following the unmasking of cytoplasmic β-galactosidase to the extracellular chromogenic substrates o-nitrophenyl-β-d-galactopyranoside (ONPG) and ONPG-6-phosphate (ONPG-6P) (8), respectively (1.5 mM in 600 μl of SPB), by using 107 CFU of bacteria per ml exposed to 5 μM peptide, as described previously (1, 4, 14), following the increase of absorption at 405 nm with a Pharmacia Ultrospec 2000 spectrophotometer, and values are the means of results from at least three experiments.
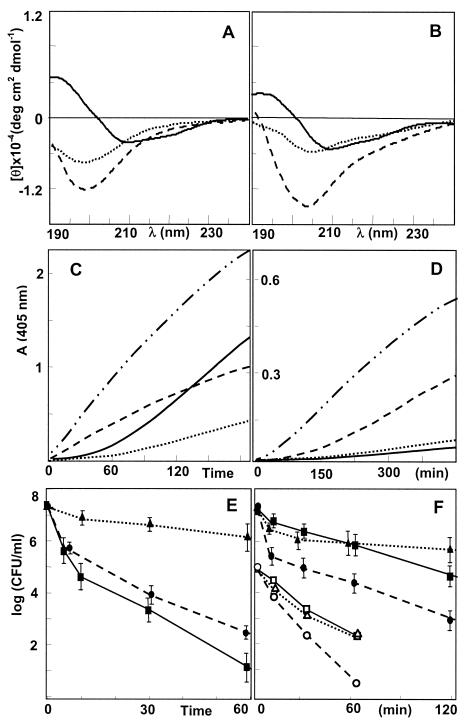
Circular dichroism spectroscopy of the synthesized and folded peptides (Fig. 2A and B) indicates that hBD2 is already structured in aqueous buffer (Fig. 2A) and is compatible with the presence of both the β-sheet core and the N-terminal α-helical stretch as observed in the crystal structure (10). Furthermore, the spectrum does not change substantially in the presence of membrane-mimicking sodium dodecyl sulfate micelles (Fig. 2B) or 50% (vol/vol) trifluoroethanol (not shown). The Asp4 residue seems to play an essential role in the formation of the α-helix, as the (−D)hBD2 variant shows little helical content in aqueous buffer (Fig. 2A), and the structure is also less stable in the presence of sodium dodecyl sulfate (Fig. 2B). In this respect, mfaBD2 seems to behave more like (−D)hBD2 than hBD2, so the presence of a stable N-terminal α-helical stretch may be characteristic of the great ape and human molecules.
FIG. 2.
Structure, bacterial membrane permeating capacity and bacterial killing kinetics of β-defensin 2 peptides hBD2 (solid line), mfaBD2 (dashed line), and (−D)hBD2 (dotted line) and a reference membranolytic α-helical peptide, P19(5/B) (dash-dotted line). (A and B) Circular dichroism spectra of 20 μM peptide in aqueous buffer (A) and in the presence of 10 mM sodium dodecyl sulfate micelles (B). Hydrolysis of ONPG in the presence of E. coli ML-35/pYC and D) and (C) of ONPG-6P in the presence of S. aureus 710A (107 CFU/ml and 5 μM peptide for both) (D). (E and F) Bacterial inactivation kinetics for E. coli ML-35 (107 CFU/ml, 8 μM peptide) (E), and for S. aureus 710A (107 CFU/ml, 8 μM peptide) with hBD2 (▪), mfaBD2 (•), and (−D)hBD2 (▴) and Enterococcus faecalis A16 (105 CFU/ml, 2 μM peptide) with hBD2 (□), mfaBD2 (○), and (−D)hBD2 (Δ) (F).
All three peptides showed a broad but different spectrum of antimicrobial activity on the bacterial and fungal microorganisms tested, at a 5% (vol/vol) medium concentration (Table 1), which markedly decreased at a higher medium concentration [50% (vol/vol) MH or SAB broth] or salt concentration (150 mM NaCl; data not shown), a behavior typical for β-defensins. Only Burkholderia cepacia was resistant, a feature of this bacterium which applies to antimicrobial peptides in general. The presence of Asp4 in the human peptide appears to be important, as (−D)hBD2 was generally less active in terms of MIC. mfaBD2 showed a significantly different behavior from hBD2, being more active towards gram-positive bacteria and the yeast.
Hydrolysis of the impermeant substrates ONPG and ONPG-6P by cytoplasmic β-galactosidase activity in E. coli ML-35/pYC and S. aureus 710A, respectively (Fig. 2C and D), indicated that all peptides are capable of permeabilizing the bacterial cytoplasmic membranes, albeit with a considerably slower kinetics than for a reference membranolytic α-helical peptide, P19(5/B) (4). The hydrolysis traces for the human and macaque defensins are quite different; hBD2 is most efficient in permeabilizing the gram-negative microorganism, after an initial lag period (Fig. 2C), while relatively ineffective towards the gram-positive microorganism. mfaBD2 conversely shows a significant permeabilization of the gram-positive bacterium (Fig. 2D). As (−D)hBD2 shows a slow permeabilization kinetics towards both microorganisms, it appears that both the structure of the N-terminal stretch and variation in the central and C-terminal portion of the peptides play a role in mediating interaction with the bacterial membrane leading to permeabilization. Furthermore, the fact that (−D)hBD2 has the same charge (+7) as mfaBD2 indicates that these effects do not derive merely from an increase in charge with respect to hBD2.
Bacterial inactivation kinetics were determined under low-salt conditions (SPB) for the same E. coli ML-35 and S. aureus 710A strains, as well as Enterococcus faecalis A16, and the three peptides showed substantial differences in their time-killing traces (Fig. 2E and F). Again, hBD2 was more active towards E. coli, while mfaBD2 was almost two logs more efficient in inactivating S. aureus 710A at 120 min. All three peptides efficiently killed Enterococcus faecalis within 10 min under the same conditions (≥5 log decrease), but mfaBD2 was the most efficient at lower peptide concentrations (Fig. 2F), confirming a superior activity towards the gram-positive microorganisms. These results are in line with the permeabilization kinetics. Both kinetics experiments indicate a more rapid inactivation of E. coli by hBD2 than (−D)hBD2, while the MICs are similar. On the other hand, both peptides have similar MICs and killing/permeabilization kinetics towards S. aureus and Enterococcus faecalis. This may indicate that the peptides act via different mechanisms on the gram-negative bacterium, with that used by the more completely structured hBD2 being more effective, while both peptides may use a similar, less efficient mechanism on the gram-positive microorganisms.
In conclusion, our data indicate that the amino acid sequence variations observed in the human and monkey β-defensins 2, which have been found to result from positive selection, do in fact reflect on the antimicrobial activity of these peptides, and that the macaque peptide seems more efficient in inactivating S. aureus, Enterococcus faecalis, and C. albicans. It is thus conceivable that the evolution of these peptides may have occurred, at least in part, in response to different environmental pressures, so as to modulate the direct antimicrobial activity. However, how important a factor this may have been with respect to others will require a more extensive analysis of the peptides' activity on a wider panel of microorganisms and should also take into account conditions likely to be present in vivo.
We have also found evidence that the N-terminal stretch present in the human (and great ape) peptide, a stable helix, may be a unique feature of these peptides that depends on the presence of Asp in position 4. It appears to have a significant effect on antimicrobial activity and may also have an effect on the chemotactic activity of these peptides, another potential cause of positive selection, as an Asp residue occurs in an analogous position in MIP-3α, which shares the same CCR6 receptor on dendritic cells with hBD2, in a stretch that seems to be required for receptor activation (9, 12).
Acknowledgments
This research was supported in part by grants from the Italian Ministry of Universities and Scientific Research (PRIN 2002) and a region Friuli-VG grant. N. Antcheva is supported and I. Zelezetsky is partially supported by a grant from the V Framework EU PANAD project QLK2-CT-2000-00411. M. Boniotto is the recipient of a long-term fellowship from the University of Trieste.
REFERENCES
- 1.Boniotto, M., N. Antcheva, I. Zelezetsky, A. Tossi, V. Palumbo, M. V. Verga Falzacappa, S. Sgubin, L. Braida, A. Amoroso, and S. Crovella. 2003. A study of host defence peptide β-defensin 3 in primates. Biochem. J. 374:707-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boniotto, M., A. Tossi, M. Del Pero, S. Sgubin, N. Antcheva, D. Santon, J. Masters, and S. Crovella. 2003. Evolution of the β-defensin 2 gene in primates. Genes Immun. 4:251-257. [DOI] [PubMed] [Google Scholar]
- 3.Del Pero, M., M. Boniotto, D. Zuccon, P. Cervella, A Spano, A. Amoroso, and S. Crovella. 2002. β-Defensin 1 gene variability among non-human primates. Immunogenetics 53:907-913. [DOI] [PubMed] [Google Scholar]
- 4.Giangaspero, A., L. Sandri, and A. Tossi. 2001. Amphipathic alpha helical antimicrobial peptides. Eur. J. Biochem. 268:5589-5600. [DOI] [PubMed] [Google Scholar]
- 5.Hao, H. N., J. Zhao, G. Lotoczky, W. E. Grever, and W. D. Lyman. 2001. Induction of human beta-defensin-2 expression in human astrocytes by lipopolysaccharide and cytokines. J. Neurochem. 77:1027-1035. [DOI] [PubMed] [Google Scholar]
- 6.Harder, J., J. Bartels, E. Christophers, and J. M. Schroder. 1997. A peptide antibiotic from human skin. Nature 387:861. [DOI] [PubMed] [Google Scholar]
- 7.Harder, J., U. Meyer-Hoffert, L. M. Teran, L. Schwichtenberg, J. Bartels, S. Maune, and J. M. Schroder. 2000. Mucoid Pseudomonas aeruginosa, TNF-alpha, and IL-1beta, but not IL-6, induce human beta-defensin-2 in respiratory epithelia. Am. J. Respir. Cell Mol. Biol. 22:714-721. [DOI] [PubMed] [Google Scholar]
- 8.Hengstenberg, W., and M. L. Morse. 1969. An improved method of synthesis of o-nitrophenyl β-d-galactopyranoside 6-phosphate. Carbohydr. Res. 10:463-465. [Google Scholar]
- 9.Hoover, D. M., C. Boulegue, D. Yang, J. J. Oppenheim, K. Tucker, W. Lu, and J. Lubkowski. 2002. The structure of human macrophage inflammatory protein-3alpha /CCL20. Linking antimicrobial and CC chemokine receptor-6-binding activities with human beta-defensins. J. Biol. Chem. 277:37647-37654. [DOI] [PubMed] [Google Scholar]
- 10.Hoover, D. M., K. R. Rajashankar, R. Blumenthal, A. Puri, J. J. Oppenheim, O. Chertov, and J. Lubkowski. 2000. The structure of human beta-defensin-2 shows evidence of higher order oligomerization. J. Biol. Chem. 275:2911-32918. [DOI] [PubMed] [Google Scholar]
- 11.Liu, A. Y., D. Destoumieux, A. V. Wong, C. H. Park, E. V. Valore, L. Liu, and T. Ganz. 2002. Human beta-defensin-2 production in keratinocytes is regulated by interleukin-1, bacteria, and the state of differentiation. J. Investig. Dermatol. 118:275-281. [DOI] [PubMed] [Google Scholar]
- 12.Perez-Canadillas, J. M., A. Zaballos, J. Gutierrez, R. Varona, F. Roncal, J. P. Albar, G. Marquez, and M. Bruix. 2001. NMR solution structure of murine CCL20/MIP-3alpha, a chemokine that specifically chemoattracts immature dendritic cells and lymphocytes through its highly specific interaction with the beta-chemokine receptor CCR6. J. Biol. Chem. 276:28372-28379. [DOI] [PubMed] [Google Scholar]
- 13.Singh, P. K., H. P. Jia, K. Wiles, J. Hesselberth, L. Liu, B. A. Conway, E. P. Greenberg, E. V. Valore, M. J. Welsh, T. Ganz, B. F. Tack, and P. B. McCray, Jr. 1998. Production of beta-defensins by human airway epithelia. Proc. Natl. Acad. Sci. USA 95:14961-14966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tossi, A., C. Tarantino, and D. Romeo. 1997. Design of synthetic antimicrobial peptides based on sequence analogy and amphipathicity. Eur. J. Biochem. 250:549-558. [DOI] [PubMed] [Google Scholar]
- 15.Yang, D., A. Biragyn, L. W. Kwak, and J. J. Oppenheim. 2002. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 23:291-296. [DOI] [PubMed] [Google Scholar]