Abstract
Objectives
This study aims to study how Bio-Oss® can induce osteoblast differentiation in mesenchymal stem cells, the expression levels of bone related genes and mesenchymal stem cells markers using real time Reverse Transcription-Polymerase Chain Reaction.
Methods
PB-hMSCs stem preparations were obtained for gradient centrifugation from peripheral blood of healthy anonymous volunteers, using the Acuspin System-Histopaque 1077. The samples were then cultured for 7 days for RNA processing, and the expression was quantified using real time PCR.
Results
Bio-Oss® caused an induction of osteoblast transcriptional factor like RUNX2 and of bone related genes; SPP1 and FOSL1. In contrast, the expression of ENG was significantly decreased in stem cells treated with Bio-Oss® with respect to untreated cells, indicating the differentiation effect of this biomaterial on stem cells.
Conclusion
The results obtained can be relevant to enhance the understanding of the molecular mechanism of bone regeneration and can act as a model for comparing other materials with similar clinical effects.
Introduction
Bio-Oss® (Geistlich, Wolhusen, Switzerland) is a deproteinized sterilized bovine bone constituted by a calcium-deficient carbonate apatite, and is identical to human bone from a chemical and physical point of view.1-8 Bio-Oss® has a compressive strength of 35 Mpa and its porous nature (75-80% of the total volume) serves to greatly increase the surface area of the material. This increased surface area provides a substratum for an increased angiogenesis and represents a scaffold for bone formation.1-8 It has been reported that Bio-Oss® promotes osteogenesis and has a very low resorption rate. Bio-Oss® has often been used for maxillary sinus floor elevation.9-16 In some cases, it can be very advantageous to use a material that shows very little degradation such as Bio-Oss®. When Bio-Oss® is used, bone grows upward from the pre-existing bone at the sinus floor into the grafted area, maintaining the space, helping to prevent the unwanted early resorption and not showing inflammatory reaction. The success of Bio-Oss® for maxillary sinus augmentation has been confirmed also in a long-term study.17
In previous studies, by using DNA microarray containing 19,200 genes, it was identified in osteoblast-like cell lines (i.e. MG-63) cultured on Bio-Oss®, several genes where expression was differentially regulated. The differentially expressed genes cover a broad range of functional activities: 1) signal transduction, 2) transcription, 3) cell cycle regulation, 4) vesicular transport, 5) apoptosis and 6) immunity.18 Therefore, the effects of using microRNA microarray techniques, the translation regulation in osteoblasts exposed to Bio-Oss® were investigated. It was identified that miRNA regulates the transduction of genes related to skeletal development (SUFU, IGF2, IGFB4, EN1, PAPSS1) and cartilage remodeling (NOG).19
Since Bio-Oss® is always fixed onto bone and the mechanism by which Bio-Oss® acts on osteoblasts is incompletely known, it is important therefore, to attempt to acquire more insight by using human stem cells isolated from peripheral blood (PB-hMSCs).
This study aims to investigate the osteogenic differentiation of PB-hMSCs, the quantitative expression of the mRNA of specific genes, like transcriptional factor (RUNX2 and SP7), bone related genes (SPP1, COL1A1, COL3A1, BGLAP, ALPL, and FOSL1) and mesenchymal stem cells marker (ENG) which were examined by means of real time Reverse Transcription-Polymerase Chain Reaction (real time RT-PCR).
Methods
PB-hMSCs stem preparations were obtained for gradient centrifugation from peripheral blood of healthy anonymous volunteers, using the Acuspin System-Histopaque 1077 (Sigma Aldrich, Inc., St Louis, Mo, USA). Firstly, 30 ml of heparinizated peripheral blood was added to the Acuspin System-Histopaque 1077 tube and centrifugated at 1000 x g for 10 minutes. After centrifugation, the interface containing mononuclear cells was transferred in another tube, washed with PBS and centrifugated at 250 x g per 10 minutes.
The enriched mononuclear pellets was resuspended in 10 ml of Alphamem medium (Sigma Aldrich, Inc., St Louis, Mo, USA) supplemented with antibiotics (Penicillin 100 U/ml and Streptomycin 100 micrograms/ml-Sigma, Chemical Co., St Louis, Mo, USA) and amminoacids (L-Glutamine-Sigma, Chemical Co., St Louis, Mo, USA). The cells were maintained at 37°C in a fully humidified atmosphere at 5% CO2 in air. Medium was changed after 24 hours. PB-hMSC were selected for adesivity and characterized by immunoflorescence.
Cells were washed with PBS three times and fixed with cold methanol for 5 min at room temperature. After washing with PBS, cells were blocked with bovine albumin 3% (Sigma Aldrich, Inc., St Louis, Mo, USA) for 30 min at room temperature. The cells were incubated overnight sequentially at 4°C with primary antibodies raised against CD105 1:200, mouse (BD Biosciences, San Jose, CA, USA), CD73 1:200, mouse (Santa Cruz Biotecnology, Inc., Santa Cruz, CA, USA), CD90 1:200, mouse (Santa Cruz Biotecnology, Inc., Santa Cruz, CA, USA), CD34 1:200, mouse (Santa Cruz Biotecnology, Inc., Santa Cruz, CA, USA). They were washed with PBS and incubated for 1 hr at room temperature with secondary antibody conjugated-Rodamine goat anti-mouse 1:200 (Santa Cruz Biotecnology, Inc., Santa Cruz, CA, USA). Subsequently, cells were mounted with the Vectashield Mounting Medium with DAPI (Vector Laboratories, Inc., Burlingame, CA, USA) and observed under a fluorescence microscope (Eclipse TE 2000-E, Nikon Instruments S.p.a., Florence, Italy).
For cell culture preparations, PB-hMSCs at the second passage were grown in Alphamem medium (Sigma Aldrich, Inc., St Louis, Mo, USA) supplemented with 10% fetal calf serum, antibiotics (Penicillin 100 U/ml and Streptomycin 100 micrograms/ml- Sigma Aldrich, Inc., St Louis, Mo, USA) and amminoacids (L-Glutamine-Sigma Aldrich, Inc., St Louis, Mo, USA). The cells were maintained at 37°C in a fully humidified atmosphere at 5% CO2 in air.
For the assay, cells were collected and seeded at a density of 1x105 cells/ml into 9 cm2 (3 ml) wells by using 0.1% trypsin, 0.02% EDTA in Ca++ - and Mg–free Eagle’s buffer for cell release.
One set of wells were added with Bio-Oss® (Geistlich, Wolhusen, Switzerland) at a concentration of 10 mg/ ml. Another set of wells containing untreated cells were used as control. The medium was changed every 3 days. After seven days, when cultures were sub-confluent, cells were processed for RNA extraction.
For RNA processing, reverse transcription to cDNA was performed directly from cultured cell lysate using the TaqMAn Gene Expressio Cells-to-Ct Kit (Ambion Inc., Austin, TX, USA), following manufacturer’s instructions. Briefly, cultured cells were lysed with lysis buffer and RNA released in this solution. Cell lysate were reverse transcribed to cDNA using the RT Enzyme Mix and appropriate RT buffer (Ambion Inc., Austin, TX, USA).
Finally, the cDNA was amplified by real-time PCR using the included TaqMan Gene Expression Master Mix and the specific assay designed for the investigated genes.
Using real time PCR, expression was quantified. The gene expression levels were normalized to the expression of the housekeeping gene RPL13A and were expressed as fold changes relative to the expression of the untreated PB-hMSCs. Quantification was done with the delta/ delta calculation method.20 Forward and reverse primers and probes for the selected genes were designed using primer express software (Applied Biosystems, Foster City, CA, USA) and are listed in Table 1.
Table 1. Primer and Probes used in Real Time PCR.
| Gene symbol | Gene name | Primer sequence (5’>3’) | Probe sequence (5’>3’) |
|---|---|---|---|
| SPP1 | osteopontin | F-GCCAGTTGCAGCCTTCTCA | CCAAACGCCGACCAAGGAAAACTCAC |
| R-AAAAGCAAATCACTGCAATTCTCA | |||
| COL1A1 | collagen type I alpha1 |
F-TAGGGTCTAGACATGTTCAGCTTTGT | CCTCTTAGCGGCCACCGCCCT |
| R-GTGATTGGTGGGATGTCTTCGT | |||
| RUNX2 | runt-related transcription factor 2 |
F-TCTACCACCCCGCTGTCTTC | ACTGGGCTTCCTGCCATCACCGA |
| R-TGGCAGTGTCATCATCTGAAATG | |||
| ALPL | alkaline phospatasi | F-CCGTGGCAACTCTATCTTTGG | CCATGCTGAGTGACACAGACAAGAAGCC |
| R-CAGGCCCATTGCCATACAG | |||
| COL3A1 | collagen, type III, alpha 1 |
F-CCCACTATTATTTTGGCACAACAG | ATGTTCCCATCTTGGTCAGTCCTATGCG |
| R-AACGGATCCTGAGTCACAGACA | |||
| BGLAP | osteocalcin | F-CCCTCCTGCTTGGACACAAA | CCTTTGCTGGACTCTGCACCGCTG |
| R-CACACTCCTCGCCCTATTGG | |||
| CD105 | endoglin | F-TCATCACCACAGCGGAAAAA | TGCACTGCCTCAACATGGACAGCCT |
| R-GGTAGAGGCCCAGCTGGAA | |||
| FOSL1 | FOS-like antigen 1 | F-CGCGAGCGGAACAAGCT | ACTTCCTGCAGGCGGAGACTGACAAAC |
| R-GCAGCCCAGATTTCTCATCTTC | |||
| SP7 | osterix | F-ACTCACACCCGGGAGAAGAA | TCACCTGCCTGCTCTGCTCCAAGC |
| R-GGTGGTCGCTTCGGGTAAA | |||
| RPL13A | ribosomal protein L13 |
F-AAAGCGGATGGTGGTTCCT | CTGCCCTCAAGGTCGTGCGTCTG |
| R-GCCCCAGATAGGCAAACTTTC |
All PCR reactions were performed in a 20 µl volume using the ABI PRISM 7500 (Applied Biosystems, Foster City, CA, USA). Each reaction contained 10 µl 2X TaqMan universal PCR master mix (Applied Biosystems, Foster City, CA, USA), 400 nM concentration of each primer and 200 nM of the probe, and cDNA. The amplification profile was initiated by 10-minute incubation at 95°C, followed by two-step amplification of 15 seconds at 95°C and 60 seconds at 60°C for 40 cycles. All experiments were performed including non-template controls to exclude reagents contamination. PCRs were performed with two biological replicates.
Results
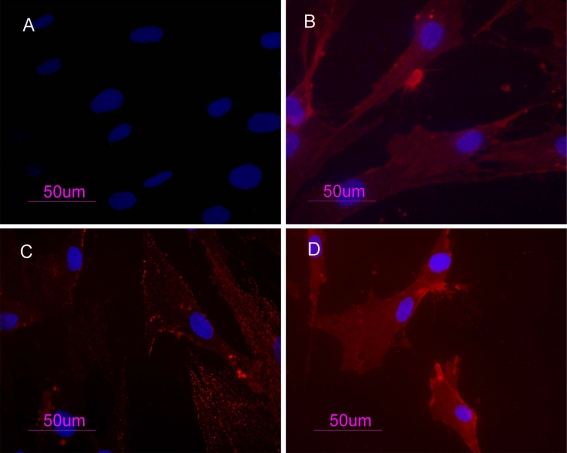
PB-hMSCs were characterized by immunofluorescence. The cell surfaces were positive for mesenchymal stem cell markers, CD105, CD90 and CD73 and negative for markers of hematopoietic origin, CD34. (Fig. 1)
Figure 1.
PB-hMSCs by indirect immunofluorescence (Rodamine). Cultured cells were positive for the mesenchymal stem cell marker CD73 (b), CD90 (c), CD105 (d) and negative for the hematopoietic markers CD34 (a). Nucleuses were stained with DAPI. Original magnification x40
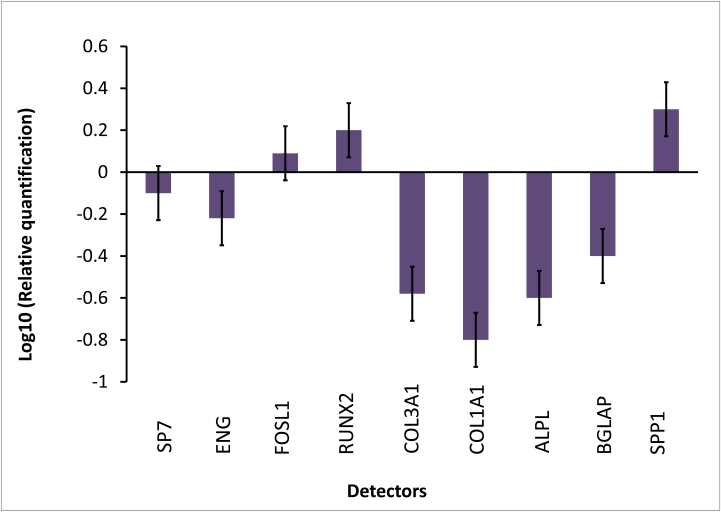
Transcriptional expressions of several osteoblast-related genes (RUNX2, SP7, SPP1, COLIA1, COL3A1, BGLAP, ALPL and FOSL1) and mesenchymal stem cells marker (ENG) were examined after 7 days of treatment with Bio-Oss®.
The results of real-time PCR showed that after 1 week of treatment, compared to the control cells, the osteogenic transcription factors RUNX2 and two bone related genes FOSL1 and SPP1 were up-regulated.
COL1A1 and COL3A, BGLAP, SP7 and ALPL were decreased in the presence of Bio-Oss® at day 7, like the stem cell marker ENG. (Fig. 2)
Figure 2.
Gene expression analysis of BM-hMSCs after 7 days of treatment with BioOss®
Discussion
Histologic reports in the literature shows that most of the Bio-Oss® particles were surrounded by newly formed mature, compact bone with well-organized osteons.2,5,7,8 In some fields, osteoblasts were observed in the process of apposing bone directly on the particle surface. No gaps were present at the bone-particles interface, and the bone was always in close contact with the particles.9-13 No inflammatory cell infiltrate was present around the particles or at the interface of the bone.
In order to highlight the exact mechanism of how Bio-Oss® acts on PB-hMSCs, changes in expression of bone related marker genes (RUNX2, SPP1, SP7, COLIA1, COL3A1, BGLAP, ALPL and FOSL1) and mesenchymal stem cells marker (ENG) were investigated using real-time RT-PCR.
In this study, mesenchymal stem cells from peripheral blood were isolated and characterized by morphology and immunophenotype. Isolated PB-hMSCs showed fibroblast-like morphology and were positive for MSC surface molecules (CD90, CD105, CD73) and negative for markers of haematopoietic progenitors (CD34).
After 7 days of treatment with Bio-Oss® the expression levels of osteodifferentiation genes were measured by relative quantification methods using real-time RT-PCR.
Two transcriptional factors had an opposite expression. RUNX2 was up-regulated in treated PB-hMSCs with respect to controls, while SP7 was down-expressed.
RUNX2 is a key transcriptional modulator of osteoblast differentiation which plays a fundamental role in osteoblast maturation and homeostasis. RUNX2-null mice despite normal skeletal patterning have no osteoblasts and consequently bone tissue. RUNX2 at the early stage of embryogenesis determines the osteoblast lineage from multipotent mesenchymal stem cells.21
SP7 is a zinc finger transcriptional factor which regulates bone formation and osteoblast differentiation in vitro and in vivo and is downstream of RUNX2; however, in this experimental model, the SP7 expression was down-regulated during osteogenic induction, probably because this gene regulates the later stages of osteoblast differentiation and bone development.22
Also, BGLAP, a bone specific protein involved in mineralization and bone resorption was expressed early in osteogenic progression of PB-hMSCs trated with Bio-Oss®.
Another investigated gene, up-regulated in treated stem cells, was FOSL-1 which encodes for Fra-1, a component of the dimeric transcription factor activator protein-1 (Ap-1), which is composed mainly of Fos (c-Fos, FosB, Fra-1 and Fra-2) and Jun proteins (c-Jun, JunB and JunD). AP-1 sites are present in the promoters of many developmentally regulated osteoblast genes, including alkaline phosphatase, collagen I, osteocalcin. McCabe et al. demonstrated that differential expression of Fos and Jun family members could play a role in the developmental regulation of bone-specific gene expression and, as a result, may be functionally significant for osteoblast differentiation.23
ENG (CD105), a surface marker used to define a bone marrow stromal cell population capable of multilineage differentiation, was down expressed in treated PB-hMSCs with respect to control during the 7 days, indicating the differentiation effect of this biomaterial on stem cells.24 The disappearance of the CD105 antigen during osteogenesis suggests that this protein, like others in the TFG-ك superfamily, is involved in the regulation of osteogenesis.25
Bio-Oss® also modulates the expression of genes encoding for collagenic extracellular matrix proteins like collagen type 1-alpha-1 (COL1A1) and collagen type 3-alpha-1 (COL3A1). COL1A1, and COL3A1 were significantly down expressed compared to the control when exposed to Bio-Oss®, probably because this gene is activated in the late stage of differentiation and is related to extracellular matrix synthesis.
Compared to levels in control cells, after 7 days of treatment, ALPL was down-regulated. Alkaline phosphatase regulates mineralization of bone matrix. Several studies have demonstrated that the potency of individual substances to induce alkaline phosphatase varies in a species-dependent manner. Glucocorticoids such as dexamethasone are potent inducers in human and rat stromal cells, but they have no effect on alkaline phosphatase activity in mouse stromal cells.26,27 On the contrary, bone morphogenetic proteins (BMPs) are potent inducers of osteogenesis in both mouse and rat bone marrow stromal cells but Diefenderfer et al. showed that BMP-2 alone is a poor osteoblast inducer in human marrow derived from stromal cells.28,29
SSP1 encodes osteopontin, which is a phosphoglycoprotein of bone matrix and it is the most representative non collagenic component of extracellular bone matrix.30 Osteopontin is actively involved in bone resorbitive processes directly by ostoclasts.31 Osteopontin produced by osteoblasts, shows high affinity to the molecules of hydroxylapatite in extracellular matrix and it is a chemo-attractant to osteoclasts.32 In this study, osteopontin was significantly down expressed when exposed to Bio-Oss®. Therefore Bio-Oss® seemed to act by reducing bone resorption processes.
Conclusion
The present study showed the effect of Bio-Oss® on PB-hMSCs in the early differentiation stages, as indicated by the activation of bone related markers RUNX1, FOSL1 and SPP1. The down regulation of genes such as SP7, ALPL and collagens demonstrated that 1 week of treatment was not enough for osteoblast differentiation. Moreover, it was decided to perform the experiment after 7 days in order to get information on the early stages of stimulation. The general understanding, therefore, is that more investigations with different time points are needed in order to get a global comprehension of the molecular events related to Bio-Oss®. The reported model is useful to investigate the effects of different substances on stem cells.
Acknowledgements
This work was supported by FAR from the University of Ferrara (FC), Ferrara, Italy, and from Regione Emilia Romagna, Programma di Ricerca Regione Università, 2007–2009, Area 1B: Patologia osteoarticolare: ricerca pre-clinica e applicazioni cliniche della medicina rigenerativa, Unità Operativa n. 14.
References
- 1.Berglundh T, Lindhe J. Healing around implants placed in bone defects treated with Bio-Oss. An experimental study in the dog. Clin Oral Implants Res 1997. Apr;8(2):117-124 10.1034/j.1600-0501.1997.080206.x [DOI] [PubMed] [Google Scholar]
- 2.Piattelli M, Favero GA, Scarano A, Orsini G, Piattelli A. Bone reactions to anorganic bovine bone (Bio-Oss) used in sinus augmentation procedures: a histologic long-term report of 20 cases in humans. Int J Oral Maxillofac Implants 1999. Nov-Dec;14(6):835-840 [PubMed] [Google Scholar]
- 3.Valentini P, Abensur D. Maxillary sinus floor elevation for implant placement with demineralized freeze-dried bone and bovine bone (Bio-Oss): a clinical study of 20 patients. Int J Periodontics Restorative Dent 1997. Jun;17(3):232-241 [PubMed] [Google Scholar]
- 4.Rodriguez A, Anastassov GE, Lee H, Buchbinder D, Wettan H. Maxillary sinus augmentation with deproteinated bovine bone and platelet rich plasma with simultaneous insertion of endosseous implants. J Oral Maxillofac Surg 2003. Feb;61(2):157-163 10.1053/joms.2003.50041 [DOI] [PubMed] [Google Scholar]
- 5.Tadjoedin ES, de Lange GL, Bronckers AL, Lyaruu DM, Burger EH. Deproteinized cancellous bovine bone (Bio-Oss) as bone substitute for sinus floor elevation. A retrospective, histomorphometrical study of five cases. J Clin Periodontol 2003. Mar;30(3):261-270 10.1034/j.1600-051X.2003.01099.x [DOI] [PubMed] [Google Scholar]
- 6.Merkx MA, Maltha JC, Stoelinga PJ. Assessment of the value of anorganic bone additives in sinus floor augmentation: a review of clinical reports. Int J Oral Maxillofac Surg 2003. Feb;32(1):1-6 10.1054/ijom.2002.0346 [DOI] [PubMed] [Google Scholar]
- 7.Maiorana C, Sommariva L, Brivio P, Sigurtà D, Santoro F. Maxillary sinus augmentation with anorganic bovine bone (Bio-Oss) and autologous platelet-rich plasma: preliminary clinical and histologic evaluations. Int J Periodontics Restorative Dent 2003. Jun;23(3):227-235 [PubMed] [Google Scholar]
- 8.Hallman M, Cederlund A, Lindskog S, Lundgren S, Sennerby L. A clinical histologic study of bovine hydroxyapatite in combination with autogenous bone and fibrin glue for maxillary sinus floor augmentation. Results after 6 to 8 months of healing. Clin Oral Implants Res 2001. Apr;12(2):135-143 10.1034/j.1600-0501.2001.012002135.x [DOI] [PubMed] [Google Scholar]
- 9.Haas R, Baron M, Donath K, Zechner W, Watzek G. Porous hydroxyapatite for grafting the maxillary sinus: a comparative histomorphometric study in sheep. Int J Oral Maxillofac Implants 2002. May-Jun;17(3):337-346 [PubMed] [Google Scholar]
- 10.Artzi Z, Nemcovsky CE, Dayan D. Bovine-HA spongiosa blocks and immediate implant placement in sinus augmentation procedures. Histopathological and histomorphometric observations on different histological stainings in 10 consecutive patients. Clin Oral Implants Res 2002. Aug;13(4):420-427 10.1034/j.1600-0501.2002.130411.x [DOI] [PubMed] [Google Scholar]
- 11.Sennerby L, Lundgren S. Histologic aspects of simultaneous implant and graft placement. In: Jensen OT, editor. The sinus bone graft. Chicago: Quintessence Publishing Co; 1999; 95-105. [Google Scholar]
- 12.Landi L, Pretel RW, Jr, Hakimi NM, Setayesh R. Maxillary sinus floor elevation using a combination of DFDBA and bovine-derived porous hydroxyapatite: a preliminary histologic and histomorphometric report. Int J Periodontics Restorative Dent 2000. Dec;20(6):574-583 [PubMed] [Google Scholar]
- 13.Froum SJ, Tarnow DP, Wallace SS, Rohrer MD, Cho SC. Sinus floor elevation using anorganic bovine bone matrix (OsteoGraf/N) with and without autogenous bone: a clinical, histologic, radiographic, and histomorphometric analysis–Part 2 of an ongoing prospective study. Int J Periodontics Restorative Dent 1998. Dec;18(6):528-543 [PubMed] [Google Scholar]
- 14.Wheeler SL. Sinus augmentation for dental implants: the use of alloplastic materials. J Oral Maxillofac Surg 1997. Nov;55(11):1287-1293 10.1016/S0278-2391(97)90186-5 [DOI] [PubMed] [Google Scholar]
- 15.Wallace SS, Froum SJ, Tarnow DP. Histologic evaluation of a sinus elevation procedure: a clinical report. Int J Periodontics Restorative Dent 1996. Feb;16(1):46-51 [PubMed] [Google Scholar]
- 16.Wallace SS, Froum SJ. Effect of maxillary sinus augmentation on the survival of endosseous dental implants. A systematic review. Ann Periodontol 2003. Dec;8(1):328-343 10.1902/annals.2003.8.1.328 [DOI] [PubMed] [Google Scholar]
- 17.Sartori S, Silvestri M, Forni F, Icaro Cornaglia A, Tesei P, Cattaneo V. Ten-year follow-up in a maxillary sinus augmentation using anorganic bovine bone (Bio-Oss). A case report with histomorphometric evaluation. Clin Oral Implants Res 2003. Jun;14(3):369-372 10.1034/j.1600-0501.2003.140316.x [DOI] [PubMed] [Google Scholar]
- 18.Carinci F, Piattelli A, Degidi M, Palmieri A, Perrotti V, Scapoli L, et al. Genetic effects of anorganic bovine bone (Bio-Oss) on osteoblast-like MG63 cells. Arch Oral Biol 2006. Feb;51(2):154-163 10.1016/j.archoralbio.2005.06.006 [DOI] [PubMed] [Google Scholar]
- 19.Annalisa P, Furio P, Ilaria Z, Anna A, Luca S, Marcella M, et al. Anorganic bovine bone and a silicate-based synthetic bone activate different microRNAs. J Oral Sci 2008. Sep;50(3):301-307 10.2334/josnusd.50.301 [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001. Dec;25(4):402-408 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 21.Ducy P, Starbuck M, Priemel M, Shen J, Pinero G, Geoffroy V, et al. A Cbfa1-dependent genetic pathway controls bone formation beyond embryonic development. Genes Dev 1999. Apr;13(8):1025-1036 10.1101/gad.13.8.1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002. Jan;108(1):17-29 10.1016/S0092-8674(01)00622-5 [DOI] [PubMed] [Google Scholar]
- 23.McCabe LR, Banerjee C, Kundu R, Harrison RJ, Dobner PR, Stein JL, et al. Developmental expression and activities of specific fos and jun proteins are functionally related to osteoblast maturation: role of Fra-2 and Jun D during differentiation. Endocrinology 1996. Oct;137(10):4398-4408 10.1210/en.137.10.4398 [DOI] [PubMed] [Google Scholar]
- 24.Jin HJ, Park SK, Oh W, Yang YS, Kim SW, Choi SJ. Down-regulation of CD105 is associated with multi-lineage differentiation in human umbilical cord blood-derived mesenchymal stem cells. Biochem Biophys Res Commun 2009. Apr;381(4):676-681 10.1016/j.bbrc.2009.02.118 [DOI] [PubMed] [Google Scholar]
- 25.Haynesworth SE, Baber MA, Caplan AI. Cell surface antigens on human marrow-derived mesenchymal cells are detected by monoclonal antibodies. Bone 1992;13(1):69-80 10.1016/8756-3282(92)90363-2 [DOI] [PubMed] [Google Scholar]
- 26.Leboy PS, Beresford JN, Devlin C, Owen ME. Dexamethasone induction of osteoblast mRNAs in rat marrow stromal cell cultures. J Cell Physiol 1991. Mar;146(3):370-378 10.1002/jcp.1041460306 [DOI] [PubMed] [Google Scholar]
- 27.Beresford JN, Joyner CJ, Devlin C, Triffitt JT. The effects of dexamethasone and 1,25-dihydroxyvitamin D3 on osteogenic differentiation of human marrow stromal cells in vitro. Arch Oral Biol 1994. Nov;39(11):941-947 10.1016/0003-9969(94)90077-9 [DOI] [PubMed] [Google Scholar]
- 28.Balk ML, Bray J, Day C, Epperly M, Greenberger J, Evans CH, et al. Effect of rhBMP-2 on the osteogenic potential of bone marrow stromal cells from an osteogenesis imperfecta mouse (oim). Bone 1997. Jul;21(1):7-15 10.1016/S8756-3282(97)00075-6 [DOI] [PubMed] [Google Scholar]
- 29.Diefenderfer DL, Osyczka AM, Garino JP, Leboy PS. Regulation of BMP-induced transcription in cultured human bone marrow stromal cells. J Bone Joint Surg Am 2003;85-A(3)(Suppl 3):19-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKee MD, Farach-Carson MC, Butler WT, Hauschka PV, Nanci A. Ultrastructural immunolocalization of noncollagenous (osteopontin and osteocalcin) and plasma (albumin and alpha 2HS-glycoprotein) proteins in rat bone. J Bone Miner Res 1993. Apr;8(4):485-496 10.1002/jbmr.5650080413 [DOI] [PubMed] [Google Scholar]
- 31.Dodds RA, Connor JR, James IE, Rykaczewski EL, Appelbaum E, Dul E, et al. Human osteoclasts, not osteoblasts, deposit osteopontin onto resorption surfaces: an in vitro and ex vivo study of remodeling bone. J Bone Miner Res 1995. Nov;10(11):1666-1680 10.1002/jbmr.5650101109 [DOI] [PubMed] [Google Scholar]
- 32.Ohtsuki C, Kamitakahara M, Miyazaki T. Bioactive ceramic-based materials with designed reactivity for bone tissue regeneration. J R Soc Interface 2009. Jun;6(Suppl 3):S349-S360 [DOI] [PMC free article] [PubMed] [Google Scholar]