Abstract
Four newly synthesized ether lipid esters of cidofovir (CDV), hexadecyloxypropyl-CDV (HDP-CDV), octadecyloxyethyl-CDV (ODE-CDV), oleyloxypropyl-CDV (OLP-CDV), and oleyloxyethyl-CDV (OLE-CDV), were found to have enhanced activities against vaccinia virus (VV) and cowpox virus (CV) in vitro compared to those of CDV. The compounds were administered orally and were evaluated for their efficacies against lethal CV or VV infections in mice. HDP-CDV, ODE-CDV, and OLE-CDV were effective at preventing mortality from CV infection when treatments were initiated 24 h after viral inoculation, but only HDP-CDV and ODE-CDV maintained efficacy when treatments were initiated as late as 72 h postinfection. Oral pretreatment with HDP-CDV and ODE-CDV were also effective when they were given 5, 3, or 1 day prior to inoculation with CV, even when each compound was administered as a single dose. Both HDP-CDV and ODE-CDV were also effective against VV infections when they were administered orally 24 or 48 h after infection. In animals treated with HDP-CDV or ODE-CDV, the titers of both CV and VV in the liver, spleen, and kidney were reduced 3 to 7 log10. In contrast, virus replication in the lungs was not significantly reduced. These data indicate that HDP-CDV or ODE-CDV given orally is as effective as CDV given parenterally for the treatment of experimental CV and VV infections and suggest that these compounds may be useful for the treatment of orthopoxvirus infections in humans.
Orthopoxvirus diseases continue to pose challenges to researchers preparing for a bioterrorist release of biological weapons of mass destruction. Rapid diagnostics for smallpox and the development of effective antiviral chemotherapies are two essential components for national preparedness (16, 17). The in vitro and in vivo activities of cidofovir (CDV) against orthopoxviruses are well documented (3, 4, 8, 9, 15, 18, 20-24); however, its usefulness is limited by the requirement for intravenous administration and dose-limiting nephrotoxicity (14). Orally active compounds with prolonged therapeutic levels in blood and reduced toxicity would be desirable for mass distribution in response to an actual release of the smallpox virus.
Results from earlier studies with acyclovir and ganciclovir showed that alkoxyalkyl esters of the monophosphates of ganciclovir or acyclovir have improved oral bioavailabilities compared to those of the unmodified parent compounds and are effective against cytomegalovirus and herpes simplex virus infections (13). To improve the oral bioavailability of CDV, a novel series of analogs were synthesized by esterification with long-chain alkoxyalkanols. Hexadecyloxypropyl-CDV (HDP-CDV) and octadecyloxyethyl-CDV (ODE-CDV) were synthesized and evaluated in vitro for their efficacies against cowpox virus (CV) and vaccinia virus (VV) infections. As reported previously (15), HDP-CDV had 50% effective concentrations (EC50s) of 0.52 and 0.62 μM against CV and VV, respectively, whereas the EC50s of CDV were 42 and 31 μM, respectively. ODE-CDV also had significantly lower EC50s of 0.23 and 0.21 μM against CV and VV, respectively. Although in vitro the analogs proved to be more cytotoxic than the parent compound, CDV, their enhanced activities and selectivity indices suggested that these analogues needed to be evaluated for their oral efficacies in animal models of orthopoxvirus disease. This viewpoint is further supported by a recent report that the relative oral bioavailabilities of HDP-CDV, ODE-CDV, and oleyloxypropyl-CDV (OLP-CDV) range from 88 to 97% (6), whereas that of oral CDV is <5% (25).
The purpose of the studies reported here was to determine the comparative efficacy of parenteral CDV with those of oral HDP-CDV, ODE-CDV, OLP-CDV, and oleyloxyethyl-CDV (OLE-CDV) against lethal CV and VV infections in mice. Since we have reported previously that CDV is highly active in these models when given as single or multiple doses either prior to or after infection, similar studies were carried out with these analogs. In addition, we also determined the effect of oral treatment with HDP-CDV or ODE-CDV on the replication of CV or VV in important target organs.
MATERIALS AND METHODS
In vitro efficacy and toxicity.
The activities and toxicities of the four ether lipid esters of CDV in vitro were determined in human foreskin fibroblast cells by previously described methods (15).
Virus.
CV strain Brighton was kindly provided by John W. Huggins (U.S. Army Medical Research Institute of Infectious Disease, Frederick, Md.). VV strain WR was obtained from the American Type Culture Collection, Manassas, Va.
Mice.
Female BALB/c mice (age, 3 weeks) were obtained from Charles River Laboratories, Raleigh, N.C., and Wilmington, Va. Mice were group housed in microisolator cages and used at a quantity of 15 mice per treatment group for statistical analysis. Mice were obtained, housed, used, and euthanized according to the regulatory policies of the U.S. Department of Agriculture and the Association for Assessment and Accreditation of Laboratory Animal Care. All animal procedures were approved by Institutional Animal Care and Use Committee, University of Alabama at Birmingham, prior to initiation of the studies.
Antiviral compounds.
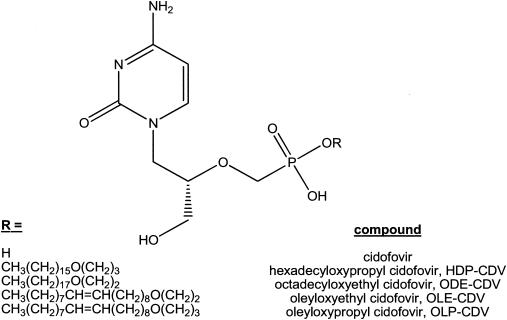
CDV (Vistide; Gilead Pharmaceuticals, Foster City, Calif.) was diluted in sterile saline to yield the desired doses in a 0.1-ml volume. It was administered intraperitoneally (i.p.) once daily for 5 days, one to two times weekly for 7 days, or as a single dose, depending on the experimental protocol. HDP-CDV and ODE-CDV were synthesized, purified, and characterized as reported previously (2, 15) and were provided as dry powders. OLP-CDV and OLE-CDV were synthesized by the same method by using oleyl bromide instead of hexadecyl bromide or octadecyl bromide (2, 15). The degrees of purity were similar to those reported for HDP-CDV and ODE-CDV. The synthesis, purification, and analytical data for these two compounds will be reported elsewhere (W. B. Wan, personal communication, 2003). The structures of the respective compounds are shown in Fig. 1.
FIG. 1.
Structures of CDV and CDV ether lipid esters.
The dry powders were weighed and dissolved in deionized water to yield the desired doses in a 0.2-ml volume for oral gavage. Each compound was administered orally once as a single dose, twice weekly, or daily for up to 5 days, depending on the experimental protocol. Uninfected mice served as toxicity controls for each compound and were treated similarly.
Experimental infections and viral pathogenesis.
Infections were initiated by intranasal inoculation of anesthetized (ketamine-xylazine) BALB/c mice (18). CV (5.3 × 105 to 9 × 105 PFU/animal) or VV (1 × 104 PFU/animal) was instilled into both nostrils with a micropipette at a total volume of 40 μl per animal. Samples of lung, liver, kidney, and spleen tissues were obtained from three mice per treatment group, as described previously (19), on days 1, 3, 5, 7, 10, 12, and 15 following CV or VV infection. Pooled organ samples were homogenized in medium (10% [wt/vol]) and frozen at −70°C until they were assayed for virus.
Virus quantitation.
Samples were thawed and assayed on Vero cells by an agarose overlay plaque assay to determine CV titers (15, 18). Briefly, samples of organ homogenates were serially diluted, a 0.2-ml volume was placed into each of 12 wells of Vero cell monolayers, and the cultures were incubated for 1 h. A solution of 0.5% agar in minimal essential medium (SeaKem, ME agarose; FMC BioProducts, Rockland, Maine) was added to each well, and the cultures were incubated for 3 days. Cultures were stained with neutral red (Gibco, Rockland, Md.) for approximately 6 h prior to enumeration of viral plaques.
Statistical evaluations.
Mortality rates were analyzed by Fisher's exact test, and the mean day of death and virus titers in tissues were analyzed by the Mann-Whitney rank sum U test. A P value ≤0.05 was considered significant.
RESULTS
In vitro activities of ether lipid esters of CDV.
It has been reported previously (15) that HDP-CDV and ODE-CDV are about 50- to 200-fold more active than CDV against CV and VV in human foreskin fibroblast cells. For comparison purposes, these results along with those for CDV and the new analogs, OLP-CDV and OLE-CDV, are included in Table 1. The EC50s of OLP-CDV were 0.4 μM for CV and 0.6 μM for VV, whereas the two viruses were inhibited by 50% by only 0.06 and 0.07 μM OLE-CDV, respectively. All four of the analogs were approximately 50- to 500-fold more active than unmodified CDV against CV and VV.
TABLE 1.
Efficacies and cytotoxicities of ether lipid esters of CDV
| Compound | VV Copenhagen
|
CV Brighton
|
||||
|---|---|---|---|---|---|---|
| EC50a (μM) | CC50a,b (μM) | Selectivity indexc | EC50 (μM) | CC50 (μM) | Selectivity index | |
| CDV | 31 ± 5.4 | >317 ± 0 | >10 | 42 ± 5.4 | >317 ± 0 | >7.5 |
| OLP-CDV | 0.4 ± 0.2 | 87 ± 15 | 218 | 0.6 ± 0.3 | 87 ± 15 | 145 |
| OLE-CDV | 0.06 ± 0.02 | 56 ± 29 | 933 | 0.07 ± 0.02 | 56 ± 29 | 800 |
| HDP-CDVd | 0.8 ± 0.4 | 31 ± 24 | 37 | 0.6 ± 0.3 | 31 ± 24 | 53 |
| ODE-CDVd | 0.2 ± 0.1 | 14 | 65 | 0.3 ± 0.3 | 14 | 49 |
Values are the means ± standard deviations of two or more assays.
CC50, 50% cytotoxic concentration.
Selectivity index = CC50 50% cytotoxic concentration/EC50.
Data are from Kern et al. (15).
Activities of HDP-CDV, ODE-CDV, OLP-CDV, and OLE-CDV against CV infections in mice.
The compounds were dissolved in deionized water at doses of 20, 6.7, or 2 mg/kg of body weight and administered to infected mice once daily for five consecutive days by oral gavage beginning 24, 48, or 72 h after viral inoculation. For comparison, CDV was administered i.p. at similar doses, although it should be noted that the molecular weight of CDV is roughly 50% of that of the ether lipid conjugates of CDV. Toxicity was associated with the 20-mg/kg doses of HDP-CDV, ODE-CDV, OLP-CDV, and OLE-CDV; and some mortality and weight loss were observed with the 10-mg/kg dose (Table 2). No toxicity was observed with the 6.7-mg/kg dose, and each compound significantly reduced the final mortality rate (P ≤ 0.01) with the initiation of therapy one or more times (Table 3). Both HDP-CDV and ODE-CDV significantly reduced the mortality rates when treatment was initiated as late as 72 h after viral inoculation. Treatment with the 2-mg/kg doses of all four analogs was ineffective. Treatment with CDV resulted in significant protection from mortality at all doses and most times of initiation, with the exception of the lowest dose administered, 2 mg/kg, at 48 or 72 h postinoculation (data not shown).
TABLE 2.
Toxicities of oral ether lipid esters in BALB/c mice
| Treatment and dose (mg/kg)a | Mortality
|
MDDb | |
|---|---|---|---|
| No. of mice that died/total no. tested | % | ||
| HDP-CDVc | |||
| 20 | 4/10 | 40 | 9.5 ± 2.5 |
| 10 | 1/10 | 10 | 3.0 ± 0 |
| 7.5 | 1/10 | 10 | 7.0 ± 0 |
| 5.0 | 5/10 | 50 | 2.0 ± 0 |
| 2.5 | 1/8 | 13 | 10.0 ± 0 |
| ODE-CDV | |||
| 20 | 10/10 | 100 | 8.2 ± 1.8 |
| 10 | 4/10 | 40 | 10.8 ± 0.5 |
| 5 | 1/10 | 10 | 6.0 ± 0 |
| 2.5 | 1/10 | 10 | 3.0 ± 0 |
| 1.25 | 1/9 | 11 | 6.0 ± 0 |
| OLP-CDV | |||
| 20 | 10/10 | 100 | 9.7 ± 2.5 |
| 10 | 0/10 | 0 | |
| OLE-CDV | |||
| 20 | 6/10 | 60 | 9.0 ± 0 |
| 10 | 2/10 | 20 | 7.0 ± 5.7 |
The test compounds were prepared daily in water and delivered orally in 0.2-ml doses. Animals were treated once daily for 5 days.
MDD, mean ± standard deviation day of death.
Data represent the results from three separate experiments.
TABLE 3.
Effects of oral treatment with HDP-CDV, ODE-CDV, OLP-CDV, and OLE-CDV on mortality of BALB/c mice inoculated intranasally with CV
| Treatment and time (h) of administrationa | Mortality
|
P value for mortality | MDDb | P value for MDD | |
|---|---|---|---|---|---|
| No. of mice that died/total no. tested | % | ||||
| Placebo (saline at 24 h) | 15/15 | 100 | 9.7 ± 0.6 | ||
| CDV | |||||
| 24 | 0/15 | 0 | <0.001 | ||
| 48 | 0/15 | 0 | <0.001 | ||
| 72 | 5/15 | 33 | <0.001 | 13.2 ± 3.0 | <0.01 |
| Placebo (water at 24 h) | 15/15 | 100 | 9.3 ± 0.6 | ||
| HDP-CDV | |||||
| 24 | 6/15 | 40 | 0.001 | 9.5 ± 4.8 | NSc |
| 48 | 12/14 | 86 | NS | 10.5 ± 3.7 | NS |
| 72 | 7/15 | 47 | <0.01 | 12.7 ± 3.3 | <0.001 |
| ODE-CDV | |||||
| 24 | 3/13 | 23 | <0.001 | 9.3 ± 6.1 | NS |
| 48 | 6/14 | 43 | <0.01 | 12.7 ± 4.9 | 0.01 |
| 72 | 7/13 | 54 | 0.02 | 11.6 ± 4.1 | 0.07 |
| OLP-CDV | |||||
| 24 | 12/14 | 86 | NS | 11.4 ± 2.5 | <0.01 |
| 48 | 4/14 | 29 | <0.001 | 12.5 ± 3.7 | 0.09 |
| 72 | 12/14 | 86 | NS | 10.3 ± 2.1 | 0.02 |
| OLE-CDV | |||||
| 24 | 8/15 | 53 | <0.01 | 13.0 ± 6.2 | NS |
| 48 | 5/15 | 33 | <0.001 | 12.0 ± 3.4 | <0.001 |
| 72 | 11/14 | 79 | NS | 11.5 ± 4.5 | 0.02 |
All test compounds except CDV were prepared daily in water and delivered orally at 6.7 mg/kg in 0.2-ml doses; CDV was prepared in sterile saline and delivered i.p. at 6.7 mg/kg in 0.1-ml doses. The animals were treated once daily for 5 days beginning 24, 48, or 72 h after viral inoculation.
MDD, mean ± standard deviation day of death.
NS, not significant compared to the results for the placebo-treated controls.
Activities of HDP-CDV, ODE-CDV, OLP-CDV, and OLE-CDV against VV infections in mice.
The compounds were dissolved in deionized water at doses of 10, 5, or 2.5 mg/kg and administered to infected mice once daily for five consecutive days by oral gavage beginning at 24, 48, or 72 h after viral inoculation. For comparison, CDV was administered i.p. at similar doses. At 5 mg/kg, HDP-CDV, ODE-CDV, and OLE-CDV significantly reduced the final mortality rates (P ≤ 0.01) at 24 or 48 h postinoculation (Table 4). None of the compounds exhibited activity when treatments were initiated at 72 h postinoculation. The positive control, CDV, was highly effective (P ≤ 0.001) at all dosages and all times, with the exception of the lowest dose administered, 2.5 mg/kg, at 72 h postinoculation (data not shown).
TABLE 4.
Effects of oral treatment with HDP-CDV, ODE-CDV, OLP-CDV, and OLE-CDV on mortality of BALB/c mice inoculated intranasally with VV
| Treatment and time (h) of administrationa | Mortality
|
P value for mortality | MDDb | P value for MDD | |
|---|---|---|---|---|---|
| No. of mice that died/total no. tested | % | ||||
| Placebo (saline at 24 h) | 15/15 | 100 | 6.8 ± 0.4 | ||
| CDV | |||||
| 24 | 0/15 | 0 | <0.001 | ||
| 48 | 4/15 | 27 | <0.001 | 7.8 ± 0.5 | 0.01 |
| 72 | 0/15 | 0 | <0.001 | ||
| Placebo (water at 24 h) | 15/15 | 100 | 6.8 ± 0.7 | ||
| HDP-CDV | |||||
| 24 | 2/14 | 13 | <0.001 | 11.0 ± 4.2 | <0.05 |
| 48 | 10/15 | 67 | <0.05 | 8.0 ± 1.2 | <0.01 |
| 72 | 14/15 | 93 | NSc | 7.4 ± 0.9 | 0.07 |
| ODE-CDV | |||||
| 24 | 0/15 | 0 | <0.001 | ||
| 48 | 6/15 | 40 | 0.001 | 8.0 ± 3.0 | 0.06 |
| 72 | 15/15 | 100 | NS | 7.3 ± 0.8 | <0.01 |
| OLP-CDV | |||||
| 24 | 11/15 | 73 | NS | 9.6 ± 1.3 | <0.001 |
| 48 | 12/15 | 80 | NS | 7.3 ± 1.7 | <0.01 |
| 72 | 15/15 | 100 | NS | 7.0 ± 0.8 | <0.05 |
| OLE-CDV | |||||
| 24 | 4/15 | 27 | <0.001 | 7.5 ± 3.3 | NS |
| 48 | 9/15 | 60 | <0.05 | 7.4 ± 0.7 | <0.01 |
| 72 | 14/14 | 100 | NS | 6.5 ± 0.5 | NS |
The test compounds were prepared in water and delivered orally at 5 mg/kg in 0.2-ml doses. CDV was prepared in sterile saline and was delivered i.p. at 5 mg/kg in 0.1-ml doses. The animals were treated once daily for 5 days beginning 24, 48, or 72 h after viral inoculation.
MDD, mean ± standard deviation day of death.
NS, not significant compared to the results for the the placebo-treated controls.
Effect of pretreatment with HDP-CDV or ODE-CDV on CV infections in mice.
We have reported previously that CDV can protect mice infected with CV or VV when it is given as early as 5 days prior to infection (18). HDP-CDV and ODE-CDV were also evaluated for their prophylactic activities by treating mice by oral gavage with 10 or 5 mg/kg and 5 or 2.5 mg/kg, respectively, beginning 5, 3, or 1 day prior to viral inoculation. For comparison, CDV was given i.p. at 10 or 5 mg/kg. Groups treated beginning 5 days prior to infection were dosed once daily for five consecutive days up to day 0, which was the day of viral inoculation. The groups that began treatment 3 days prior to infection received a total of three daily doses prior to the day of infection, and the groups that began treatment 1 day prior to infection received only a single dose 24 h prior to viral inoculation. The results in Table 5 indicate that HDP-CDV and ODE-CDV, as well as CDV, were highly protective against mortality due to CV infection when they were given 1 to 5 days prior to infection.
TABLE 5.
Effect of preinfection oral treatment with HDP-CDV or ODE-CDV on mortality of BALB/c mice inoculated intranasally with CV
| Treatment day and treatment (dose [mg/kg])a | Mortality
|
P value for mortality | MDDb | P value for MDD | |
|---|---|---|---|---|---|
| No. of mice that died/total no. tested | % | ||||
| Day −5 (water placebo) | 15/15 | 100 | 10.1 ± 0.8 | ||
| Day −5 | |||||
| CDV (10) | 1/15 | 7 | <0.001 | 12.0 ± 0 | 0.09 |
| CDV (5) | 1/15 | 7 | <0.001 | 17.0 ± 0 | 0.07 |
| HDP-CDV (10) | 0/15 | 0 | <0.001 | ||
| HDP-CDV (5) | 0/15 | 0 | <0.001 | ||
| ODE-CDV (5) | 3/15 | 20 | <0.001 | 5.7 ± 5.5 | NSc |
| ODE-CDV (2.5) | 5/13 | 38 | <0.001 | 8.6 ± 4.2 | NS |
| Day −3 | |||||
| CDV (10) | 2/15 | 13 | <0.001 | 9.0 ± 5.7 | NS |
| CDV (5) | 6/15 | 40 | <0.01 | 11.0 ± 4.9 | 0.05 |
| HDP-CDV (10) | 5/14 | 36 | <0.001 | 9.6 ± 5.2 | NS |
| HDP-CDV (5) | 3/13 | 23 | <0.001 | 15.0 ± 4.0 | <0.01 |
| ODE-CDV (5) | 2/15 | 13 | <0.001 | 11.0 ± 0 | 0.07 |
| ODE-CDV (2.5) | 1/15 | 7 | <0.001 | 13.0 ± 0 | 0.07 |
| Day −1 | |||||
| CDV (10) | 1/15 | 7 | <0.001 | 12.0 ± 0 | 0.09 |
| CDV (5) | 5/15 | 33 | <0.001 | 11.4 ± 1.7 | 0.06 |
| HDP-CDV (10) | 4/14 | 29 | <0.001 | 9.3 ± 4.9 | NS |
| HDP-CDV (5) | 6/15 | 40 | <0.01 | 10.7 ± 0.5 | 0.06 |
| ODE-CDV (5) | 0/14 | 0 | <0.001 | ||
| ODE-CDV (2.5) | 4/15 | 27 | <0.001 | 11.5 ± 1.7 | <0.05 |
CDV was prepared in sterile saline and delivered i.p. in 0.1-ml doses. HDP-CDV and ODE-CDV were prepared in deionized water and delivered orally in 0.2-ml doses. The animals were treated once daily beginning on day −5, −3, or −1 through day 0 (the viral inoculation day).
MDD, mean ± standard deviation day of death.
NS, not significant compared to the results for the placebo-treated controls.
Effect of single-dose treatment with HDP-CDV or ODE-CDV on CV infections in mice.
It has also been reported that treatment with only one or two doses of CDV significantly reduces mortality rates among CV- or VV-infected mice due to the long intracellular half-life of this drug (3, 7, 18, 20). In a similar study, we gave HDP-CDV at a single dose of 25 or 12.5 mg/kg or ODE-CDV at 20 or 10 mg/kg on days −5, −3, −1, +1, or + 3 prior to or after intranasal CV inoculation. All regimens with both compounds and at all times of initiation provided significant protection from mortality (Table 6). In addition, CDV given at 30 mg/kg as a single dose i.p. was also protective.
TABLE 6.
Effect of oral pre- or postinfection single-dose treatment with HDP-CDV or ODE- CDV on the mortality of BALB/c mice inoculated intranasally with CV
| Treatment day and treatment (dose [mg/kg])a | Mortality
|
P value for mortality | MDDb | P value for MDD | |
|---|---|---|---|---|---|
| No. of mice that died/total no. tested | % | ||||
| +24 h, placebo | |||||
| Saline | 9/15 | 60 | 12.2 ± 2.8 | ||
| Deionized H2O | 7/15 | 47 | 11.9 ± 2.9 | ||
| Day −5 | |||||
| CDV (30) | 4/15 | 27 | <0.05 | 11.5 ± 2.5 | NSc |
| HDP-CDV (12.5) | 3/15 | 20 | <0.05 | 10.3 ± 2.1 | NS |
| ODE-CDV (10) | 1/15 | 7 | <0.05 | 11.0 ± 0 | NS |
| Day −3 | |||||
| CDV (30) | 6/15 | 40 | NS | 11.7 ± 3.9 | NS |
| HDP-CDV (12.5) | 0/15 | 0 | <0.001 | ||
| ODE-CDV (10) | 0/15 | 0 | <0.001 | ||
| Day −1 | |||||
| CDV (30) mg/kg | 0/15 | 0 | <0.001 | ||
| HDP-CDV (12.5) mg/kg | 0/15 | 0 | <0.001 | ||
| ODE-CDV (10) | 2/15 | 14 | <0.05 | 10.0 ± 1.4 | NS |
| +24 h | |||||
| CDV (30) | 0/15 | 0 | <0.001 | ||
| HDP-CDV (12.5) | 0/15 | 0 | <0.001 | ||
| ODE-CDV (10) | 1/15 | 7 | <0.05 | 21.0 ± 0 | NS |
| Day 3 | |||||
| CDV (30) | 1/15 | 7 | <0.05 | 15.0 ± 0 | NS |
| HDP-CDV (12.5) | 2/15 | 13 | <0.05 | 11.5 ± 2.1 | NS |
| ODE-CDV (10) | 1/15 | 7 | <0.05 | 16.0 ± 0 | NS |
CDV was prepared in sterile saline and delivered i.p. in 0.1-ml doses. HDP-CDV was prepared in deionized water and delivered orally in 0.2-ml doses. The animals were treated one time with a single dose on day −5, −3, or −1 before viral inoculation or at 24 h or day 3 after viral inoculation.
MDD, mean ± standard deviation day of death.
NS, not significant compared to the results for the placebo-treated controls combined.
Effect of HDP-CDV or ODE-CDV on the pathogenesis of CV and VV infections in mice.
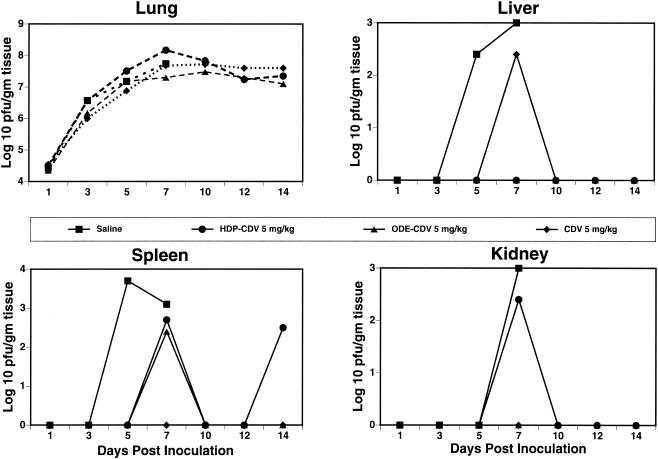
To determine the effect of treatment with HDP-CDV or ODE-CDV on the replication of CV in the target organs of mice, animals were inoculated with CV and treated orally with 5 mg of HDP-CDV or ODE-CDV per kg once daily for 5 days beginning 24 h after infection. On various days postinfection the animals were euthanized, and their tissues were removed and assayed for CV. All of the treatment regimens resulted in significant reductions in mortality rates and 3 to 5 log10 decreases in virus titers in liver, spleen, and kidney tissues. There were no alterations in virus titers in lung tissues; however, all treated control mice survived. Similar results were observed with CDV given i.p. (Fig. 2).
FIG. 2.
Effect of daily oral treatment with HDP-CDV or ODE-CDV on the pathogenesis of CV infection in mice. Treatment was initiated 24 h after viral inoculation and was continued once daily for five consecutive days. Points represent the mean log10 PFU per gram of tissue.
In order to determine the effects of these compounds on the replication of VV in tissues, HDP-CDV or ODE-CDV was given at 5 mg/kg once daily for 5 days beginning 24 h after viral inoculation. Both HDP-CDV and ODE-CDV reduced the levels of viral replication by ≥2 logs in the liver, spleen, and kidney tissues of VV-infected mice (Fig. 3). As was the case for CV, the titers in lung tissue remained unaffected, although all treated mice survived. Again, similar results were obtained with CDV given i.p.
FIG. 3.
Effect of daily oral treatment with HDP-CDV or ODE-CDV on the pathogenesis of VV infection in mice. Treatment was initiated 24 h after viral inoculation and was continued once daily for five consecutive days. Points represent the mean log10 PFU per gram of tissue.
Since CV is only moderately sensitive to the action of CDV, we postulated that higher doses of the drugs might be necessary to reduce viral titers in the lungs. The levels of HDP-CDV and ODE-CDV in lung tissue were previously reported to be 43 and 28 times lower than those found in liver tissue, respectively, and 5.8 and 4.8 times lower than those found in kidney tissue, respectively (6); and we observed good antiviral activity in liver and kidney tissues. To test this hypothesis, we used higher doses of HDP-CDV or ODE-CDV given twice weekly and determined their effects on viral titers in the same target organs. The results of this experiment are shown in Fig. 4. When HDP-CDV or ODE-CDV was given at 30 mg/kg on days 1 and 5 after viral inoculation, treatment reduced the level of viral replication by ≥2.5 log10 in the liver, spleen, and kidney tissues of CV-infected mice. There were 1 to 2 log10 reductions in CV titers in lung tissue on days 3 and 5 after treatment with HDP-CDV or ODE-CDV at 30 mg/kg, which was suggestive of a small dose-response and which indicated a correlation between drug levels in lung tissue and the effect on viral replication.
FIG. 4.
Effect of oral treatment with HDP-CDV or ODE-CDV twice weekly on the pathogenesis of CV infection in mice. Treatment was started 24 and 96 h after viral inoculation. Points represent the mean log10 PFU per gram of tissue.
DISCUSSION
CDV has been reported to be highly effective against orthopoxvirus infection in vitro and in murine models of CV and VV infections (3, 4, 8, 9, 15, 18, 20-24) and is the drug of choice for the treatment of potential outbreaks of smallpox or monkeypox and vaccination complications. Although it has good activity in experimental systems, its usefulness in humans is limited by nephrotoxicity and a lack of activity when it is administered orally. A number of approaches have been taken to enhance the activities of orally administered nucleosides and nucleotides, including the synthesis of prodrugs, as was done for acyclovir (Valtrex), ganciclovir (Valcyte), penciclovir (Famvir), tenofovir (Viread), and adefovir (Preveon); conjugation to various lipid molecules to facilitate oral absorption (11-13); or the synthesis of ether lipid esters of CDV, as in the present study. It was previously reported (11-13) that alkylglycerol phosphate or alkoxypropyl phosphate esters of acyclovir and ganciclovir have greater oral bioavailabilities in rodents and are active orally in animal models of herpes simplex virus, murine cytomegalovirus, and woodchuck hepatitis virus infections.
To obtain better oral bioavailability with CDV, a similar methodology was used to produce HDP-CDV and HDP-cyclic CDV (HDP-cCDV) (2, 15). A comparison of the activities of the parent nucleotides CDV and cCDV and the alkoxyalkyl ester analogs against VV and CV indicated that while 25 to 50 μM CDV or cCDV was required to inhibit the replication of the orthopoxviruses tested, HDP-CDV and HDP-cCDV analogs were active at levels 50- to 200-fold less than the levels required for the activities of the parent molecules (15). Although the cytotoxicities of the analogs were increased over those of the parent compounds, the selectivity indices of HDP-CDV and HDP-cCDV were considerably increased over those of the parent compounds (15).
HDP-CDV, ODE-CDV, and OLP-CDV are efficiently absorbed when they are given orally to mice, resulting in levels in plasma which would be well above the levels required for the in vitro inhibition of replication of orthopoxviruses (6, 15). It was further demonstrated that the cellular uptake of the analog HDP-[2-14C]CDV is 11- to 23-fold greater than that of [2-14C]CDV and that the intracellular levels of CDV-diphosphate, the active antiviral agent, are 100 times greater than those of CDV (1). This enhanced uptake and conversion to the antiviral diphosphate form appear to be responsible for the significant enhancement in activity seen against both orthopoxvirus (15) and herpesvirus (2) infections in vitro.
As part of the present studies, the activities of two additional ether lipid conjugates, OLP-CDV and OLE-CDV, were compared with that of CDV in vitro; and both compounds were more active than either HDP-CDV or ODE-CDV. The oral bioavailabilities and antiviral efficacies of all four analogs were demonstrated in studies with animals. HDP-CDV, ODE-CDV, and OLE-CDV were active against CV and VV at nontoxic dosages; and treatment could be delayed until 48 to 72 h postinfection and still decrease the mortality rates. On the basis of the results of initial experiments evaluating the effects of the four compounds on mortality due to CV or VV infection and the pharmacokinetic profile of HDP-CDV, the analogs HDP-CDV and ODE-CDV were selected for further evaluations. In all experiments, both of the active analogs administered orally were as effective at reducing mortality and viral replication in the target organs as CDV administered parenterally. In addition, both were active either prophylactically or therapeutically, as described previously for CDV (18).
It is interesting that even though orally administered HDP-CDV and ODE-CDV were significantly more active than CDV against both viruses in vitro, they were equally effective in reducing viral replication in the liver, spleen, and kidney but no more effective than CDV in reducing viral replication in the lungs. In a study by Bray et al. (4), in which CDV was given subcutaneously at 100 mg/kg, virus titers in the lungs were significantly reduced. Similarly, when Smee et al. (22) administered CDV directly to the lung via aerosol or intranasal instillation, the virus titers in the lungs were also significantly reduced, further suggesting that oral administration of 5 to 10 mg of HDP-CDV or ODE-CDV per kg in our studies did not achieve sufficient levels of drug in the lung to reduce viral replication, whereas higher concentrations were more effective. In contrast to the levels of compound observed after oral HDP-CDV administration, the levels of the drug in the lungs are much lower after parenteral administration of CDV; and the majority of the drug is found in the kidneys, with significant amounts found in the liver (6). Conversely, when HDP-CDV and ODE-CDV were given orally, they were present at much higher levels in the liver than in the kidney and should maintain therapeutic levels in tissue with less nephrotoxicity. Finally, although the maximum concentrations of total drug were higher in the lung with oral HDP-CDV and ODE-CDV administration than with parenteral CDV administration, 1.2 versus 0.4 nmol/g (6), the levels of the key metabolite, CDV-diphosphate, in lung tissue have not been measured.
A correlation between in vitro and in vivo efficacy is further demonstrated by the observation that murine cytomegalovirus is greater than 100-fold more sensitive to CDV and 40,000-fold more sensitive to the CDV analogs than CV or VV (2, 15). Treatment of murine cytomegalovirus infections with CDV, HDP-CDV, or ODE-CDV is highly effective in reducing viral replication in all tissues, including the lungs, of treated animals (E. R. Kern, D. J. Bidanset, D. C. Quenelle, C. B. Hartline, W. B. Wan, J. R. Beadle, and K. Y. Hostetler, Abstr. 34th Intersci. Conf. Antimicrob. Agents Chemother., abstr. V-1289, 2003). Therefore, it appears that in vitro sensitivity and blood and tissue drug levels are in fact important determinants of antiviral efficacy and that the levels of drug in different tissues may vary, depending on the uptake and conversion of the drug to the active metabolite.
HDP-CDV and ODE-CDV are both orally bioavailable, have high degrees of antiviral efficacy, and persist in tissues for relatively long periods of time (6, 15). The present results comparing CDV administered i.p. with HDP-CDV and ODE-CDV administered orally show that when these compounds are given orally their activities are at least equivalent to that of CDV given parenterally. Our results are consistent with pharmacokinetic data indicating the oral bioavailabilities of HDP-CDV, ODE-CDV, and OLP-CDV; the persistence of these compounds in tissues up to 72 h after oral administration; and the persistence of therapeutic drug levels in critical organs (lung, liver, and kidney) after administration of a single oral dose of 10 mg/kg. This translates into oral dosing once or twice weekly instead of daily. Since the level of drug exposure in the kidneys is reported to be low with orally administered HDP-CDV or ODE-CDV (6), one would anticipate reduced nephrotoxic adverse events. Oral HDP-CDV and ODE-CDV were at least equivalent to i.p. CDV in these studies and should be effective when used for prophylaxis, postexposure prophylaxis, or treatment for smallpox and other orthopoxvirus infections, including monkeypox infections, which have become a problem due to unexpected outbreaks and increasing incidences of natural transmission (5, 10).
Acknowledgments
This work was supported by Public Health Service contract NO1-AI-15439, from NIAID, NIH, Bethesda, Md.; NIH grants EY11832 and AI29164; and the Department of the Army (grant 17-01-2-0071). The U.S. Army Medical Research Acquisition Activity, Ft. Detrick, Md., is the awarding acquisition office.
The content of this article does not necessarily reflect the position or policy of the U.S. government, and no official endorsement should be inferred.
Excellent technical assistance for in vitro analyses was kindly provided by Kathy A. Keith, and excellent technical assistance for in vivo analyses was kindly provided by Bridgett P. Herrod.
REFERENCES
- 1.Aldern, K. A., S. Ciesla, K. L. Winegarden, and K. Y. Hostetler. 2003. Increased antiviral activity of 1-O-hexacecyloxypropyl-[2-14C] cidofovir in MRC-5 human lung fibroblasts is explained by unique cellular uptake and metabolism. Mol. Pharmacol. 63:678-681. [DOI] [PubMed] [Google Scholar]
- 2.Beadle, J. R., C. Hartline, K. A. Aldern, N. Rodriguez, E. Harden, E. R. Kern, and K. Y. Hostetler. 2002. Alkoxyalkyl esters of cidofovir exhibit multiple-log enhancement of antiviral activity against cytomegalovirus and herpesvirus replication in vitro. Antimicrob. Agents Chemother. 46:2381-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray, M., M. Martinez, D. F. Smee, D. Kefauver, E. Thompson, and J. W. Huggins. 2000. Cidofovir protects mice against lethal aerosol or intranasal cowpox virus challenge. J. Infect. Dis. 181:10-19. [DOI] [PubMed] [Google Scholar]
- 4.Bray, M., M. Martinez, D. Kefauver, M. West, and C. Roy. 2002. Treatment of aerosolized cowpox virus infection in mice with aerosolized cidofovir. Antivir. Res. 54:129-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2003. Multistate outbreak of monkeypox—Illinois, Indiana, and Wisconsin, 2003. Morb. Mortal. Wkly. Rep. 52:537-540. [PubMed] [Google Scholar]
- 6.Ciesla, S. L., J. Trahan, W. B. Wan, J. R. Beadle, K. A. Aldern, G. R. Painter, and K. Y. Hostetler. 2003. Esterification of cidofovir with alkoxyalkanols increases oral bioavailability and diminishes drug accumulation in kidney. Antivir. Res. 59:163-171. [DOI] [PubMed] [Google Scholar]
- 7.Cundy, K. C., A. M. Bidgood, G. Lynch, J. P. Shaw, L. Griffin, and W. A. Lee. 1996. Pharmacokinetics, bioavailability, metabolism, and tissue distribution of cidofovir (HPMPC) and cyclic HPMPC in rats. Drug Metab. Dispos. 24:745-752. [PubMed] [Google Scholar]
- 8.De Clercq, E., T. Sakuma, M. Baba, R. Pauwels, J. Balzarini, I. Rosenberg, and A. Holy. 1987. Antiviral activity of phosphonylmethoxyalkyl derivatives of purine and pyrimidines. Antivir. Res. 8:261-272. [DOI] [PubMed] [Google Scholar]
- 9.De Clercq, E., and J. Neyts. 1993. Efficacy of (S)-1-(3-hydroxy-2-phosphonylmethoxy-propyl) cytosine for the treatment of lethal vaccinia virus infections in severe combined immune deficiency (scid) mice. J. Med. Virol. 41:242-246. [DOI] [PubMed] [Google Scholar]
- 10.Heymann, D. L., M. Szczeniowski, and K. Esteves. 1988. Re-emergence of monkeypox in Africa: a review of the past six years. Br. Med. Bull. 54:693-702. [DOI] [PubMed] [Google Scholar]
- 11.Hostetler, K. Y., J. R. Beadle, G. D. Kini, M. F. Gardner, K. N. Wright, T.-H. Wu, and B. E. Korba. 1997. Enhanced oral absorption and antiviral activity of 1-O-octadecyl-sn-glycero-3-phospho-acyclovir and related compounds in hepatitis B virus infection, in vitro. Biochem. Pharmacol. 53:1815-1822. [DOI] [PubMed] [Google Scholar]
- 12.Hostetler, K. Y., J. R. Beadle, W. E. Hornbuckle, C. A. Bellezza, I. A. Tochkov, P. J. Cote, J. L. Gerin, B. E. Korba, and B. C. Tennant. 2000. Antiviral activities of oral 1-O-hexadecylpropanediol-3-phospho-ganciclovir and acyclovir in woodchucks with chronic woodchuck hepatitis virus infection. Antimicrob. Agents Chemother. 44:1964-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hostetler, K. Y., R. J. Rybak, J. R. Beadle, M. F. Gardner, K. A. Aldern, K. N. Wright, and E. R. Kern. 2001. In vitro and in vivo activity of 1-O-hexadecylpropanediol-3-phospho-ganciclovir and 1-O-hexadecylpropanediol-3-phospho-penciclovir in cytomegalovirus and herpes simplex virus infections. Antivir. Chem. Chemother. 12:61-70. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson, M. A., S. Wilson, H. Stanley, C. Holtzer, J. Cherrington, and S. Safrin. 1999. Phase I study of combination therapy with intravenous cidofovir and oral gangciclovir for cytomegalovirus retinitis in patients with AIDS. Clin. Infect. Dis. 28:528-533. [DOI] [PubMed] [Google Scholar]
- 15.Kern, E. R., C. Hartline, E. Harden, K. Keith, N. Rodriguez, J. R. Beadle, and K. Y. Hostetler. 2002. Enhanced inhibition of orthopoxvirus replication in vitro by alkoxyalkyl esters of cidofovir and cyclic cidofovir. Antimicrob. Agents Chemother. 46:991-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LeDuc, J. W., I. Damon, D. A. Relman, J. Huggins, and P. B. Jahrling. 2002. Smallpox research activities: U. S. interagency collaboration, 2001. Emerg. Infect. Dis. 8:743-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LeDuc, J. W., and P. B. Jahrling. 2001. Strengthening national preparedness for smallpox: an update. Emerg. Infect. Dis. 7:155-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quenelle, D. C., D. J. Collins, and E. R. Kern. 2003. Efficacy of multiple and single dose cidofovir in vaccinia and cowpox virus infections of mice. Antimicrob. Agents Chemother. 47:3275-3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rybak, R. J., J. Zemlika, Y. Qiu, C. B. Hartline, and E. R. Kern. 1999. Effective treatment of murine cytomegalovirus infections with methlenecyclopropane analogues of nucleosides. Antivir. Res. 43:175-188. [DOI] [PubMed] [Google Scholar]
- 20.Smee, D. F., K. W. Bailey, and R. W. Sidwell. 2001. Treatment of lethal vaccinia virus respiratory infections in mice with cidofovir. Antivir. Chem. Chemother. 12:71-76. [DOI] [PubMed] [Google Scholar]
- 21.Smee, D. F., K. W. Bailey, and R. W. Sidwell. 2000. Treatment of cowpox virus respiratory infections in mice with ribavirin as a single agent or followed sequentially by cidofovir. Antivir. Chem. Chemother. 11:303-309. [DOI] [PubMed] [Google Scholar]
- 22.Smee, D. F., K. W. Bailey, M. H. Wong, and R. W. Sidwell. 2000. Intranasal treatment of cowpox virus respiratory infections in mice with cidofovir. Antivir. Res. 47:171-177. [DOI] [PubMed] [Google Scholar]
- 23.Smee, D. F., K. W. Bailey, M. H. Wong, and R. W. Sidwell. 2001. Effects of cidofovir on the pathogenesis of a lethal vaccinia virus respiratory infection in mice. Antivir. Res. 52:55-62. [DOI] [PubMed] [Google Scholar]
- 24.Snoeck, R., A. Holý, C. Dewolf-Peeters, J. Van Den Oord, E. DeClercq, and G. Andrei. 2002. Antivaccinia activities of acyclic nucleoside phosphonate derivatives in epithelial cells and organotypic cultures. Antimicrob. Agents Chemother. 46:3356-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wachsman, M., B. G. Petty, K. C. Cundy, H. S. Jaffe, P. E. Fisher, A. Pastelak, and P. S. Lietman. 1996. Pharmacokinetics, safety and bioavailability of HPMPC (cidofovir) in human immunodeficiency virus-infected subjects. Antivir. Res. 29:153-161. [DOI] [PubMed] [Google Scholar]