Abstract
Vitiligo is a progressive, idiopathic, pigmentation disorder of the skin, characterized by hypopigmented white lesions. PUVA therapy is the treatment of choice in the modern system of medicine. In Ayurveda, Shvitra or Kilasa is the term employed to describe hypopigmentation disorders of the skin. Shvitra is caused by various dietic and behavioral factors which aggravate the tridoshas, especially the Kapha dosha vitiating the Meda dhatu. Many Ayurvedic drugs are well known for the regeneration of melanocytes, among which Bakuchi is one. The present study was planned to study its efficacy in the regeneration of melanocytes. The outcome of treatment in 50 cases of Shvitra vis-à-vis vitiligo receiving Shvitrahara kashaya and Shvitrahara lepa was analyzed and compared. Group I (n = 25) patients were treated with Shvitrahara kashaya and Shvitrahara lepa; Group II (n = 15) patients received Shvitrahara lepa only; and the remaining 10 patients of Group III used both (Western medicine) oral psoralens and UV-A therapy. Assessment was done after 6 months with bi-monthly follow-ups. Out of 25 cases in Group I, 17 showed 80% improvement (t = 7.65; P < 0.01) in the surface area, number of lesions, pigmentation and associated symptoms like itching; out of 15 patients in Group II, 10 showed partial repigmentation, i.e. 50% (t = 5.72; P < 0.01) response was observed. In Group III, 90% response (t = 6.14; P < 0.001) in repigmentation and number of lesions as well was noted but eight patients developed adverse effects like sunburn, severe itching and gastric upset on taking oral psoralen. On the basis of results and observations, it can be concluded that Ayurvedic formulation containing Bakuchi is efficacious and has no untoward effects when compared to oral psoralens and UV-A therapy.
Keywords: Bakuchi, Haridra, haritala, psoralens, Shvitra, vitiligo
Introduction
Vitiligo (leukoderma) is a pigmentation disorder with complex causes. Depigmented patches appear on the skin, hair, mucous membranes and the retina. It can begin at any age, but in about 50% of the patients, it starts before the age of 20.[1] The emergence of white patches can be brought on by a variety of impulses. Many people report that their vitiligo first appeared following a stressful event, such as an accident, job loss, death of a family member, severe sunburn, or serious illness. It is sometimes associated with and is secondary to hypothyroidism, diabetes mellitus, and pernicious anemia.
There are mainly three theories about the underlying mechanism of vitiligo, which are as follows: (i) the first theory states that nerve endings in the skin release a chemical that is toxic to the melanocytes; (ii) the second theory states that the melanocytes simply self-destruct; and (iii) the third theory is that vitiligo is a type of autoimmune disease in which the immune system targets the body's own cells and tissues.[2]
Oral and topical psoralens have both been used with varying results in the treatment of vitiligo.[2] Psoralea corylifolia (Bakuchi) is a renowned herb and is a rich source of naturally occurring psoralen. It sensitizes human skin to the tanning effect of UV and sunlight. Autoimmune destruction is one of the major causes of destruction of melanocytes. Haratala is an arsenic compound and is widely described and used to treat immune-related disorders like rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), bronchial asthma, etc. Hence, in the present study, both were selected to evaluate their role in controlling the symptoms of vitiligo and in the regeneration of melanocytes. The trial outcome was compared with the results of PUVA therapy used in modern medicine.
Aims and objectives
To study the etiopathogenesis of Shitra vis-à-vis vitiligo in detail.
To study the role of Bakuchi and Haratala in pigment regeneration in vitiligo.
Materials and Methods
Study design
This was a randomized, controlled, clinical trial conducted in the Department of Kayachikitsa of IMS, BHU, after obtaining permission from the Institutional Ethical Review Committee of IMS.
A total of 50 uncomplicated, diagnosed cases of vitiligo at quiescent stage were registered after obtaining their informed consent. Each patient was examined in detail. Routine laboratory investigations in urine, stools, and hemoglobin and liver function tests (LFTs) were undertaken in all the cases prior to starting the treatment.
Inclusion criteria
Diagnosed cases of uncomplicated vitiligo, either segmental or generalized, involving both of sexes in the age group 16-60 years, were registered.
Exclusion criteria
Vitiligo associated with other diseases like hypothyroidism, anemia, diabetes, hypertension, tuberculosis, RA, SLE, etc.
Vitiligo patches that rapidly spread and become associated with redness, blister formation, itching during therapy or as such.
Assessment criteria
Surface area
Mild: <3.14 cm2, i.e. circular area <1 cm radius
Moderate: 3.14-12.57 cm2, i.e. circular area with a radius of 1-2 cm
Severe: >12.57 cm2, i.e. circular area with a radius of >2 cm
Surface area of white patches was measured by marking the margins of the patches one by one on a transparent paper. This sketched transparency paper was put on a graph paper and surface area noted in square centimeters.
No. of patches
Mild: <5 patches; moderate: 5-10 patches; severe: >10 patches
Itching
No itching; mild itching but no need of medication; moderate itching requiring treatment with antihistamines; and severe, i.e. not relieved by antihistamines
Color of patches
Normal skin color, reddish/erythematous, and whitish in color
Color of hair
Normal skin color, reddish/erythematous, and whitish in color
Grouping
Group I (n = 25): Patients were treated with Shvitrahara kashaya 50 ml twice a day and Shvitrahara lepa as topical application and exposed to sunlight for 10-15 minutes in between 9:00 a.m. and 11:00 a.m. after 45 minutes of intake of decoction for 6 months. Two patients did not report in the third follow up.
Group II (n = 15): Patients were treated with Shvitrahara lepa only as topical application and exposed to sunlight for 10-15 minutes in between 9:00 a.m. and 11:00 a.m. for 6 months. Four cases were dropped out because of irregular follow-ups.
Group III (n = 10): Patients were given oral psoralens at a dose of 0.6 mg//kg body weight, followed by exposure to sunlight for 10 minutes as two sittings a month for a total of 6 months (Skin Department, S.S. Hospital, IMS, BHU, Varanasi).
All the patients were advised to wear optical dark glasses during the sun exposure and for 4-5 hours thereafter. The patients were examined once in a month to record the degree of repigmentation.
Duration of trial
Total duration was 6 months, with bi-monthly follow-ups.
Follow-up and assessment
All the subjects were evaluated bi-monthly for 6 months. Efficacy of trial drug was assessed by improvement in signs and symptoms as follows:
Good: <50% decrease in surface area, lesions and itching and other above-mentioned assessment criteria
Better: 50-75% improvement in the above criteria
Best: >75% improvement in the above criteria
Statistical analysis
Non-parametrical one-way analysis of variance (ANOVA) was used to infer any significant difference among the groups if the variables were quantitative. If the test results had a significant difference, then “post-hoc test” was applied to find the pairwise difference. “Fisher's exact test” was used to find the pairwise significant difference. We used paired “t”-test and “Z”-test to see the effect of drug from baseline to different follow-ups in quantitative and qualitative variables, respectively.
Trial drugs
Shvitrahara kashaya (decoction): Bakuchi (P. corylifolia), Haridra (Curcuma longa), Khadira (Acacia catechu), Sariva (Hemidesmus indicus), Kakoudambar (Ficus hispida), Chakramarda (Cassia tora), and Chakshushya (Cassia absuss) were taken in equal quantity (1 kg each) and mixed thoroughly and ground to a coarse powder and stored in a dry container. The 50 g of the coarse powder was soaked in 500 ml of water and boiled to reduce it to 100 ml and administered in two divided doses of 50 ml each.[3,4]
Preparation of Shvitrahara lepa (ointment): A little modification in the method of preparation was done as per the advice of The Head of the Department of RasaSastra, IMS, BHU, Varanasi.[5]
To prepare 500 g of ointment, the following materials were used:
Shvitrahara kashaya: 100 ml; kalka of Shvitrahara kashaya: 25 g; sphatika: 25 g; Haratala: 25 g; mustard oil: 100 ml; bee wax: 100 g. Initially, mustard oil was boiled with Shvitrahara kashaya and kalka of Shvitrahara kashaya. Bee wax was melted in a pan and the prepared mustard oil and powders of shuddha Haratala and sphatika were added one by one by constant stirring, and the whole mixture was allowed to cool and ointment stored in dry containers.
Observations and Results
The youngest patient was a 16-year-old girl and the oldest patient was of 62 years. In 35 patients, the disease had started around 20 years of age and majority of them were females. Out of 50 patients, 16 had a family history of vitiligo. All routine laboratory investigations were within normal limits except for the presence of intestinal worms in 14 cases. Exposed areas of the body were involved in 40 patients, front of legs in 26 patients, hands in 24, and face and neck in 19 cases. Genitals were the least affected area; only two male patients had registered. Segmental vitiligo was found in 72% of cases. Twenty-four patients had <5 lesions, 18 (23.3%) had <10 lesions and 8 patients had >10 lesions. Pitta Kaphaj prakriti was predominant in majority of the cases. Inter-group comparison shows that F value is 16.69 (P < 0.001HS) and 2.51 (P > 0.05NS) before and after treatment, respectively. The effect of therapy in groups is shown in Tables 1–3 below.
Table 1.
Effect of Shvitrahara decoction and ointment on total surface area of mild, moderate and severe patches
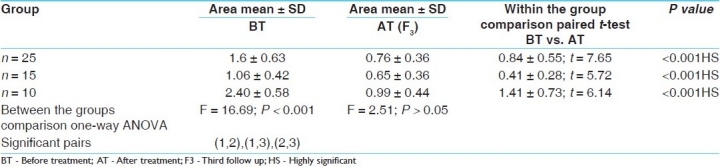
Table 3.
Effect of Shvitrahara kashaya and lepa on % of eosinophils and liver function tests
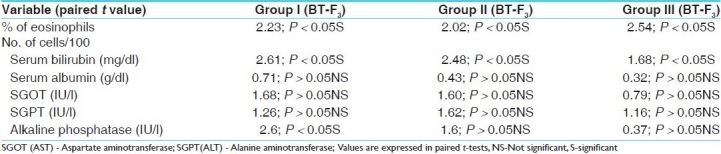
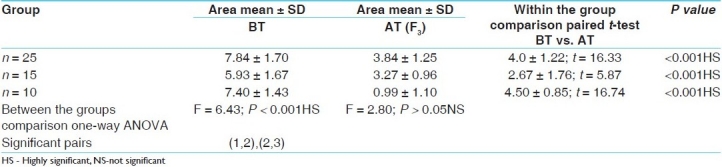
Table 2.
Effect of trial drug on number of lesions
Discussion
In the Ayurvedic system of medicine, Bakuchi (P. corylifolia) seeds are used for the treatment of vitiligo. Psoralens have been used as topical and systemic applications for vitiligo since decades.[6] In our clinical trial, in the first 2 months of therapy, the initial response to treatment was erythema of the lesion in 22, 8 and 6 cases of Group I, II and III, respectively, while itching and blister formation was observed in the remaining cases. Blister formation was more frequently observed in Group III cases using methoxypsoralen and UV-A; but it was observed in three patients of Group I and one patient of Group II. For the blisters, application of a soothing lotion or cream was prescribed. Later, the psoralen lotion was used in a more diluted form. After 8-10 weeks of therapy, erythema was followed by repigmentation. In 35 cases, the repigmentation was fast with t = 16.33, P < 0.01 and t = 5.37, P < 0.01, respectively, in mild patches group, but was delayed in patients who had >10 lesions. Best results were obtained in Group I and Group II, where Shvitrahara decoction and ointment were administered orally and topically as well, followed by exposure to sun rays. But Group III patients responded with tanning, blister formation, severe irritation, sunburn and itching upon taking the modern therapy. However, no significant alterations were seen in the LFTs in all the three groups (P > 0.05).
Among the main ingredients of Shvitrahara kashaya and lepa, Bakuchi is a renowned herb with many therapeutic properties.[7] It has been extensively used by all the Ayurvedic scholars in hypopigmention with great success. It contains psoralens, which on exposure to the sun bring out melanin in the depigmented lesions. Haridra, whose synonyms are named after its beneficial effects on the skin, is a potent drug with adaptogenic, hypoglycemic, antimicrobial, antiallergic, hepatoprotective and antioxidant properties.[8] Here, its role is to protect the skin from the irritating effects of Bakuchi and as an emollient. Chakramarda and Chakshusya are bestowed with similar properties and are popular for skin disorders and have potent antimicrobial and antiallergic properties. Purified Haratala, an arsenic compound, was used in the ointment. This was selected on the basis of its reference in Rasa Ratna Samucchaya. Purified Haratala is bestowed with immune modulating properties. It is one of the ingredients of the Mallasindoor, Talakewar Ras, which is widely used for some autoimmune disorders like psoriasis, allergic bronchial asthma, etc. in which the etiopathogenesis is deranged immunity.[9] Arsenic is absorbed through skin in addition to other routes. In Shvitra, the deranged immune system destroys the pigment synthesizing melanocytes. Haratala breaks this pathogenesis and prevents the self-destruction of melanocytes. The impact of decoction and methoxypsoralen was studied on liver and was assessed by the eosinophils % and LFTs. There was no alteration in the values of SGOT, SGPT, and alkaline phosphatase in Group I and Group II after 6 months of clinical trial. Bakuchi and Haratala have no toxic effects on the body. It was also noticed that modern PUVA therapy was associated with considerable side effects like GI upset, itching and painful blisters, thereby limiting its use in patients.
Conclusion
Shvitra or Kilasa is a Krichra Sadhya, Tridoshaj pigmentation disorder of the skin. It is caused by various dietic and behavioral factors which aggravate tridoshas, especially the Kapha dosha vitiating the Medo dhatu. It can be correlated with vitiligo, a complex pigmented disorder of the skin.
A clinical trial with Shvitrahara kashaya containing Bakuchi, Haridra (powerful antioxidant), etc. in the decoction form, and the same drugs along with Haratala (immunomodulating) on topical use in vitiligo showed that the formulation is a safe remedy with significant pigment regenerating capacity. It did not show any untoward effects as encountered with the PUVA therapy of modern medicine.
This is a preliminary study and more research is needed to prove the immune modulating effect of Haratala.
References
- 1.Braunwald, Kasper, et al. Part-7, Section 54. 17th ed. Vol. 1. New York: McGraw Hill; 2008. Harrison's - Principles of Internal Medicine; pp. 324–6. [Google Scholar]
- 2.Njoo md, Westerhof W. Vitiligo, Pathogenesis and treatment. Am J Clin Dermatol. 2001;2:167–81. doi: 10.2165/00128071-200102030-00006. [DOI] [PubMed] [Google Scholar]
- 3.Chikitsa Sthana, 7/161-178. Vol. 2. Varanasi: Chowkhamba Sanskrit Series; 1998. Agnivesha, Charaka Samhita, Kashinath Shastri, Y. Upadhyay; pp. 273–8. [Google Scholar]
- 4.Sushruta, Sushruta Samhita. In: Nidanasthana. 14th ed. 32-33. Shastri Ambika Datta., editor. Vol. 5. Varanasi: Chowkhamba Sanskrit Series; 2004. p. 25. [Google Scholar]
- 5.Visarpadi Chikitsanam. 2nd ed. 200-209. Vol. 20. Varanasi: 1962. Rasa Ratna Sammucchaya with commentary of Atri Dev Gupta by Pandit. Dharmananda Sharma; pp. 358–9. Reprint 1999. [Google Scholar]
- 6.Sushruta, Sushruta Samhita, Shastri Ambika Datta. Chikitsasthana. 14th ed. 17-24. Vol. 9. Varanasi: Chowkhamba Sanskrit Series; 2004. pp. 52–3. [Google Scholar]
- 7.Part 1. 11th ed. Varanasi: Chowkamba Sanskrit Series; 2002. Bhava Prakasha of Sri Bhavamishra by Brahmasankara Mishra and Rupalalji Vaisya; pp. 123–4. Bakuchi, Haritakyadi Varga. [Google Scholar]
- 8.Ibid Bhava Prakasha Nighantu Haridra Haritakyadi Varga. pp. 114–5.
- 9.Michel L, Dupuy A, Jean-Louis F, Sors A, Poupon J, Viguier M, et al. Arsenic trioxide induces apoptosis of cutaneous T cell lymphoma cells: Evidence for a partially caspase-independent pathway and potentiation by ascorbic acid (vitamin C) J Invest Dermatol. 2003;121:881–93. doi: 10.1046/j.1523-1747.2003.12479.x. [DOI] [PubMed] [Google Scholar]


