Abstract
Leishmaniases, which are important causes of morbidity and mortality in humans and dogs, are extremely difficult to treat. Antimicrobial peptides are rarely used as alternative treatments for naturally acquired parasitic diseases. Here we report that the acylated synthetic antimicrobial peptide Oct-CA(1-7)M(2-9) is safe and effective for treating naturally acquired canine leishmaniasis.
Leishmaniases, present in 88 countries on four continents, caused 2.4 million disability-adjusted life years (DALYs) and 59,000 deaths in 2001 (34). Etiologic agents are Leishmania protozoans transmitted by sand flies. Leishmaniasis caused by Leishmania infantum is a zoonosis in the Mediterranean basin where the dog, with an extremely high prevalence of infection (25), is the main peridomestic reservoir. Leishmaniases are extremely difficult to treat in both humans and dogs, requiring long courses of organic pentavalent antimony, which is still the drug of choice despite frequent and severe side effects and increased resistance (4, 7, 27).
Antimicrobial peptides (AP), which are part of an organism's innate immunity, are ancient evolutionary weapons that have been isolated from virtually every kingdom and phylum (3). They act by permeabilizing the plasma membrane. Induction of resistance is difficult, requiring dramatic changes in phospholipid composition with pleiotropic effects on transport and enzymatic systems. AP are rarely used as alternative treatment for parasitic diseases, and there is still a paucity of information about AP activity against parasites, particularly in vivo and in clinical settings (31). For leishmaniases, positive results have been obtained in vitro using dermaseptins (10, 11, 14, 16), cecropins (1, 9), melittin (1), skin polypeptide YY (SPYY) (32), and gomesin (22). Nevertheless, assays in animal models are absent, except a brief mention of dermaseptins in treating leishmaniasis caused by Leishmania major in mice (17). To the best of our knowledge, there are no studies addressing the use of AP in naturally acquired leishmaniasis.
The acylated synthetic cecropin A-melitin hybrid AP Oct-CA(1-7)M(2-9) has shown in vitro leishmanicidal activity against Leishmania donovani and Leishmania pifanoi (8). Since this is the first study of Oct-CA(1-7)M(2-9) in a real setting, it was designed as a small clinical trial to obtain preliminary data about safety and efficacy.
Eight dogs with a recent diagnosis of canine leishmaniasis (CL) (23) were enrolled in the study. None of the dogs had been treated previously for CL. Each dog received three 5-mg doses of Oct-CA(1-7)M(2-9), except for one dog who received 10 mg, administered by slow intravenous injection. Because leishmaniasis is a life-threatening disease, and there was no previous knowledge about AP efficacy, one week after the last dose, the dogs started antimonial therapy (28).
Peripheral blood samples were collected before each dose, and one week after the last dose. Hematologic and biochemistry parameters were obtained, and titers of anti-L. infantum immunoglobulin G (IgG), IgA, and IgM were determined by an enzyme-linked immunosorbent assay (25; O. Francino et al., submitted for publication). Parasitemia was determined by real-time PCR (X. Roura, A. Sánchez, L. Ferrer, and O. Francino, submitted for publication). Briefly, DNA was prepared from 0.5 ml of dog blood-EDTA. The forward primer (5′-AACTTTCTGGTCCTCCGGGTAG-3′), reverse primer (5′-ACCCCCAGTTTCCCGCC-3′), and TaqMan probe (carboxyfluoresceine 5′-AAAAATGGGTGCAGAAAT-3′) were designed to target conserved DNA regions of the kinetoplast minicircle DNA of L. infantum. The eukaryotic 18S RNA Pre-Developed TaqMan assay reagents (Applied Biosystems) were used as an internal reference of canine genomic DNA amplification to normalize L. infantum amplification for differences in DNA content or the presence of inhibitors. Primers and probe were added at 900 and 200 nM, respectively. Each amplification was performed in triplicate in 25-μl reaction mixture (TaqMan Universal PCR Master Mix; Applied Biosystems). Thermal cycling profile was 50°C for 2 min, 95°C for 10 min, 40 cycles at 95°C for 15 s and 60°C for 60 s. Quantitative analysis of L. infantum DNA amplification was performed by the ΔΔCt method (6). Results were normalized with respect to the first determination before treatment.
One major concern about the use of AP is safety (37). Oct-CA(1-7)M(2-9) treatment did not cause any adverse events. Six months after finishing the AP course, all dogs were in good health, except for one dog that died 2 months after finishing AP therapy. This dog died from renal failure, which was probably related to leishmaniasis, as laboratory signs were present at the time of diagnosis. Thus, administration of Oct-CA(1-7)M(2-9) to dogs suffering from leishmaniasis appears to be safe. This is in contrast with the slight increase in cytotoxicity against sheep erythrocytes and murine macrophages with respect to the nonacylated parental peptide (L. Rivas, unpublished results).
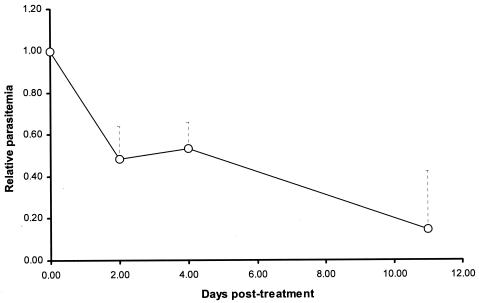
Improvement in CL is correlated with fewer symptoms, normalization of total serum protein and its electrophoresis pattern, and decrease in Ig levels. Although improvement in the general health status was reported, no changes were found in laboratory tests, probably due to a short follow-up period, since normalization may take months (26, 29). However, a progressive (P = 0.008) diminution in parasitemia (Fig. 1) was observed. Interestingly, although AP have short half-lives that rarely reach 30 min (5, 33), 1 week after the end of Oct-CA(1-7)M(2-9) therapy, parasitemia was lower (P = 0.028) than that at the time of the last dose (Fig. 1). One hypothesis is that AP may persist in serum-forming micelles that are easily phagocytosed by cells of the mononuclear phagocyte system, reaching high levels inside the parasitophorus vacuole and promoting nitric oxide synthesis through expression of the cytokine-induced form of inducible nitric oxide synthase, which kills the amastigotes (19, 30). Nonetheless, the observation of a persistent decline in parasitemia 1 week after the last dose suggests a postantibiotic-like effect interaction. This type of delayed effect has not been previously reported for AP or for L. infantum. Postantibiotic effect is a useful feature for therapies addressed to people or animals living in zones with difficult medical logistics, as tends to be the case for leishmaniases.
FIG. 1.
Relative L. infantum parasitemia after three doses of the acylated cecropin-melittin hybrid AP Oct-CA(1-7)M(2-9). The doses were administered on days 0, 2, and 4. Geometric mean and standard error of the mean (broken line) are shown.
There is a desperate need for new antileishmanial agents. Recently, important developments have been achieved: orally administered miltefosine appears to be effective for treating Indian visceral leishmaniasis caused by L. donovani (12), and liposome-encapsulated N-methylglucamine antimoniate, the “gold standard” for leishmaniasis therapy for more than 50 years, greatly enhances effectiveness in dogs and reduces toxicity (29). However, no other antileishmanial agent can compare to AP. First, curing leishmaniasis requires not only antileishmanial action but also interactions between the parasite and immune system (2, 20, 24). Since AP are also signaling molecules with multiple roles in regulating innate and adaptive immunity (15, 21, 36), they may serve as immune modulators to stimulate the nonresistant immune system or the disease-suppressed immune system (18). Second, development of resistance to AP appears to be rare (13), which is not the case for currently used antileishmanial agents.
Although more precise trials are needed, our findings show that the acylated synthetic cecropin A-melitin hybrid AP Oct-CA(1-7)M(2-9) appears to be safe and effective for treating CL. Furthermore, it is a potential powerful new drug for treating human leishmaniasis and coinfection with Leishmania and human immunodeficiency virus, an emerging extremely serious new disease with rapidly evolving resistance (35).
Acknowledgments
We thank A. Prats and M. García for help in enrolling dogs.
This work was supported in part by Laboratorios Calier S.A. L.R. was supported by grants from CAM (08.2/0054/2001.02), Plan Estratégico de Grupos en Biotecnología, and EU(QLK2-CT-2001-01404) Fondo de Investigaciones Sanitarias (C03/14). Centro de Investigaciones Biológicas belongs to the Red Española de Investigación en Patología Infecciosa.
REFERENCES
- 1.Akuffo, H., D. Hultmark, A. Engstom, D. Frohlich, and D. Kimbrell. 1998. Drosophila antibacterial protein, cecropin A, differentially affects non-bacterial organisms such as Leishmania in a manner different from other amphipathic peptides. Int. J. Mol. Med. 1:77-82. [DOI] [PubMed] [Google Scholar]
- 2.Altet, L., O. Francino, L. Solano-Gallego, C. Renier, and A. Sanchez. 2002. Mapping and sequencing of the canine NRAMP1 gene and identification of mutations in leishmaniasis-susceptible dogs. Infect. Immun. 70:2763-2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreu, D., and L. Rivas. 1998. Animal antimicrobial peptides: an overview. Biopolymers 47:415-433. [DOI] [PubMed] [Google Scholar]
- 4.Baneth, G., and S. E. Shaw. 2002. Chemotherapy of canine leishmaniosis. Vet. Parasitol. 106:315-324. [DOI] [PubMed] [Google Scholar]
- 5.Bauer, R. J., M. L. White, N. Wedel, B. J. Nelson, N. Friedmann, A. Cohen, W. N. Hustinx, and A. H. Kung. 1996. A phase I safety and pharmacokinetic study of a recombinant amino terminal fragment of bactericidal/permeability-increasing protein in healthy male volunteers. Shock 5:91-96. [DOI] [PubMed] [Google Scholar]
- 6.Bieche, I., B. Parfait, V. Le Doussal, M. Olivi, M. C. Rio, R. Lidereau, and M. Vidaud. 2001. Identification of CGA as a novel estrogen receptor-responsive gene in breast cancer: an outstanding candidate marker to predict the response to endocrine therapy. Cancer Res. 61:1652-1658. [PubMed] [Google Scholar]
- 7.Carrió, J., and M. Portús. 2 May 2002, posting date. In vitro susceptibility to pentavalent antimony in Leishmania infantum strains is not modified during in vitro or in vivo passages but is modified after host treatment with meglumine antimoniate. BMC Pharmacol. 2:11. [Online.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chicharro, C., C. Granata, R. Lozano, D. Andreu, and L. Rivas. 2001. N-terminal fatty acid substitution increases the leishmanicidal activity of CA(1-7)M(2-9), a cecropin-melittin hybrid peptide. Antimicrob. Agents Chemother. 45:2441-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz Achirica, P., J. Ubach, A. Guinea, D. Andreu, and L. Rivas. 1998. The plasma membrane of Leishmania donovani promastigotes is the main target for CA(1-8)M(1-18), a synthetic cecropin A-melittin hybrid peptide. Biochem. J. 330:453-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feder, R., A. Dagan, and A. Mor. 2000. Structure-activity relationship study of antimicrobial dermaseptin S4 showing the consequences of peptide oligomerization on selective cytotoxicity. J. Biol. Chem. 275:4230-4238. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez, C., A. Mor, F. Dagger, P. Nicolas, A. Hernandez, E. L. Benedetti, and I. Dunia. 1992. Functional and structural damage in Leishmania mexicana exposed to the cationic peptide dermaseptin. Eur. J. Cell Biol. 59:414-424. [PubMed] [Google Scholar]
- 12.Jha, T. K., S. Sundar, C. P. Thakur, P. Bachmann, J. Karbwang, C. Fischer, A. Voss, and J. Berman. 1999. Miltefosine, an oral agent, for the treatment of Indian visceral leishmaniasis. N. Engl. J. Med. 341:1795-1800. [DOI] [PubMed] [Google Scholar]
- 13.Koczulla, A. R., and R. Bals. 2003. Antimicrobial peptides: current status and therapeutic potential. Drugs 63:389-406. [DOI] [PubMed] [Google Scholar]
- 14.Kustanovich, I., D. E. Shalev, M. Mikhlin, L. Gaidukov, and A. Mor. 2002. Structural requirements for potent versus selective cytotoxicity for antimicrobial dermaseptin S4 derivatives. J. Biol. Chem. 277:16941-16951. [DOI] [PubMed] [Google Scholar]
- 15.Lillard, J. W., P. N. Boyaka, O. Chertov, J. J. Oppenheim, and J. R. McGhee. 1999. Mechanisms for induction of acquired host immunity by neutrophil peptide defensins. Proc. Natl. Acad. Sci. USA 96:651-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mor, A., and P. Nicolas. 1994. The NH2-terminal alpha-helical domain 1-18 of dermaseptin is responsible for antimicrobial activity. J. Biol. Chem. 269:1934-1939. [PubMed] [Google Scholar]
- 17.Nicolas, P., and A. Mor. 1995. Peptides as weapons against microorganisms in the chemical defense system of vertebrates. Annu. Rev. Microbiol. 49:277-304. [DOI] [PubMed] [Google Scholar]
- 18.Raj, P. A., and A. R. Dentino. 2002. Current status of defensins and their role in innate and adaptive immunity. FEMS Microbiol. Lett. 206:9-18. [DOI] [PubMed] [Google Scholar]
- 19.Rivas, L., M. Velasco, J. M. Díaz-Guerra, P. Díaz-Achirica, D. Andreu, and L. Boscá. 1996. CA(1-8)M(1-18), a cecropin A-melittin hybrid peptide, induces nitric oxide, apoptosis and leishmanicidal activity in macrophages, p. 755-756. In R. Ramage and R. Epton (ed.), Peptides. Mayflowers Scientific Ltd., Kingswinford, United Kingdom.
- 20.Sacks, D., and A. Sher. 2002. Evasion of innate immunity by parasitic protozoa. Nat. Immunol. 3:1041-1047. [DOI] [PubMed] [Google Scholar]
- 21.Salzet, M. 2002. Antimicrobial peptides are signaling molecules. Trends Immunol. 23:283-284. [DOI] [PubMed] [Google Scholar]
- 22.Silva, P. I., S. Daffre, and P. Bulet. 2000. Isolation and characterization of gomesin, an 18-residue cysteine-rich defense peptide from the spider Acanthoscurria gomesiana hemocytes with sequence similarities to horseshoe crab antimicrobial peptides of the tachyplesin family. J. Biol. Chem. 275:33464-33470. [DOI] [PubMed] [Google Scholar]
- 23.Slappendel, R. J., and L. Ferrer. 1998. Leishmaniasis, p. 450-458. In C. E. Green (ed.), Infectious diseases of the dog and cat, 2nd ed. W. B. Saunders Company, Philadelphia, Pa.
- 24.Solano-Gallego, L., J. Llull, G. Ramos, C. Riera, M. Arboix, J. Alberola, and L. Ferrer. 2000. The Ibizian hound presents a predominantly cellular immune response against natural Leishmania infection. Vet. Parasitol. 90:37-45. [DOI] [PubMed] [Google Scholar]
- 25.Solano-Gallego, L., P. Morell, M. Arboix, J. Alberola, and L. Ferrer. 2001. Prevalence of Leishmania infantum infection in dogs living in an area of canine leishmaniasis endemicity using PCR on several tissues and serology. J. Clin. Microbiol. 39:560-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solano-Gallego, L., C. Riera, X. Roura, L. Iniesta, M. Gallego, J. E. Valladares, R. Fisa, S. Castillejo, J. Alberola, L. Ferrer, M. Arboix, and M. Portus. 2001. Leishmania infantum-specific IgG, IgG1 and IgG2 antibody responses in healthy and ill dogs from endemic areas. Evolution in the course of infection and after treatment. Vet. Parasitol. 96:265-276. [DOI] [PubMed] [Google Scholar]
- 27.Sundar, S., D. K. More, M. K. Singh, V. P. Singh, S. Sharma, A. Makharia, P. C. Kumar, and H. W. Murray. 2000. Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the Indian epidemic. Clin. Infect. Dis. 31:1104-1107. [DOI] [PubMed] [Google Scholar]
- 28.Valladares, J. E., C. Riera, J. Alberola, M. Gallego, M. Portus, C. Cristofol, C. Franquelo, and M. Arboix. 1998. Pharmacokinetics of meglumine antimoniate after administration of a multiple dose in dogs experimentally infected with Leishmania infantum. Vet. Parasitol. 75:33-40. [DOI] [PubMed] [Google Scholar]
- 29.Valladares, J. E., C. Riera, P. Gonzalez Ensenyat, A. Diez-Cascon, G. Ramos, L. Solano-Gallego, M. Gallego, M. Portus, M. Arboix, and J. Alberola. 2001. Long term improvement in the treatment of canine leishmaniosis using an antimony liposomal formulation. Vet. Parasitol. 97:15-21. [DOI] [PubMed] [Google Scholar]
- 30.Velasco, M., M. J. Diaz Guerra, P. Diaz Achirica, D. Andreu, L. Rivas, and L. Bosca. 1997. Macrophage triggering with cecropin A and melittin-derived peptides induces type II nitric oxide synthase expression. J. Immunol. 158:4437-4443. [PubMed] [Google Scholar]
- 31.Vizioli, J., and M. Salzet. 2002. Antimicrobial peptides versus parasitic infections? Trends Parasitol. 18:475-476. [DOI] [PubMed] [Google Scholar]
- 32.Vouldoukis, I., Y. Shai, P. Nicolas, and A. Mor. 1996. Broad spectrum antibiotic activity of the skin-PYY. FEBS Lett. 380:237-240. [DOI] [PubMed] [Google Scholar]
- 33.Wade, D., A. Boman, B. Wahlin, C. M. Drain, D. Andreu, H. G. Boman, and R. B. Merrifield. 1990. All-D amino acid-containing channel-forming antibiotic peptides. Proc. Natl. Acad. Sci. USA 87:4761-4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization. 2002. Annex 3. Burden of disease in DALYs by cause, sex and mortality stratum in WHO regions, estimates for 2001. In The World Health Report 2002, p. 192-197. World Health Organization, Geneva, Switzerland. [Online.] http://www.who.int/whr/2002/en/whr2002_annex3.pdf.
- 35.World Health Organization. May 2000, posting date. The leishmaniases and Leishmania/HIV co-infections. Fact sheet no. 116. World Health Organization, Geneva, Switzerland. [Online.] http://www.who.int/inf-fs/en/fact116.html.
- 36.Yang, D., A. Biragyn, L. W. Kwak, and J. J. Oppenheim. 2002. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 23:291-296. [DOI] [PubMed] [Google Scholar]
- 37.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389-395. [DOI] [PubMed] [Google Scholar]