Abstract
R126638 is a new triazole agent with potent antifungal activity in vitro against various dermatophytes, Candida spp., and Malassezia spp. Its activity against Malassezia spp. in vitro was superior to that of ketoconazole, the agent currently used for the treatment of Malassezia-related infections. R126638 showed activity comparable to or lower than that of itraconazole against dermatophytes in vitro; however, in guinea pig models of dermatophyte infections, R126638 given orally consistently showed antifungal activity superior to that of itraconazole, with 50% effective doses (ED50s) three- to more than eightfold lower than those of itraconazole, depending on the time of initiation and the duration of treatment. The ED50 of R126638 in a mouse dermatophytosis model was more than fivefold lower than that of itraconazole. These data indicate that if the effects of R126638 seen when it is used to treat animals can be extrapolated to humans, the novel compound would be expected to show effects at doses lower than those of existing drugs and, hence, present a lower risk for side effects.
The possibilities for the treatment of superficial fungal infections have improved enormously during the last 30 years. Nevertheless, there remains room for new antifungals with superior efficacies and safety profiles. Oral treatment of fungal infections in dermatology has become a preferred modality for the management of these very common conditions. However, the emphasis for the discovery and development of new agents has been on systemic rather than superficial mycoses.
Several imidazoles and triazoles constitute broad-spectrum azole antifungals developed for the treatment of superficial and disseminated fungal infections (6). The target of antifungal azoles is the 14α-demethylase, a cytochrome P450 (the product of CYP51) involved in the biosynthesis of ergosterol (10). Similar P450 enzymes are present in both fungal and mammalian cells, but their precise structures are species and organ specific (10). The ideal azole antifungals are those which react strongly with fungal cytochrome P450s and that have weak or no activities against the mammalian enzyme variants.
In this paper we present evidence that the new triazole R126638 (Fig. 1) shows very high levels of antifungal activity in vitro and in models of cutaneous infections caused by Microsporum canis and Trichophyton mentagrophytes in guinea pigs and mice.
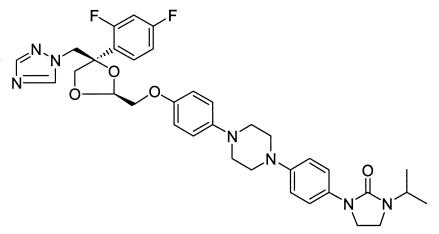
FIG. 1.
Chemical structure of R126638 (C35H39F2N7O4), or (2S-cis)-1-{4-[4-(4-{[4-(2,4-difluorophenyl)-4-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-2-yl]methoxy}phenyl)-piperazin-1-yl]phenyl}-3-(1-methylethyl)-2-imidazolidinone (molecular weight, 659.74).
MATERIALS AND METHODS
In vitro antifungal activities.
All fungi used for testing were originally isolated from humans or animals. They were stored at −80°C in the collection of the Department of Bacteriology and Mycology at the Janssen Research Foundation (now known as Johnson and Johnson Pharmaceutical Research and Development, a Division of Janssen Pharmaceutica N.V.) in dilute casein hydrolysate yeast extract-glucose medium with 10% glycerol. The species tested (and numbers of isolates of each species tested) were as follows: Candida albicans (n = 5), Candida famata (n = 1), Candida glabrata (n = 4), Candida guilliermondii (n = 1), Candida kefyr (n = 2), Candida krusei (n = 2), C. lusitaniae (n = 2), Candida parapsilosis (n = 3), Candida tropicalis (n = 4), Epidermophyton floccosum (n = 31), Microsporum audouinii (n = 9), Microsporum canis (n = 120), Microsporum cookei (n = 1), Microsporum ferrugineum (n = 2), Microsporum gallinae (n = 3), Microsporum gypseum (n = 10), Microsporum nanum (n = 1), Microsporum persicolor (n = 2), Trichophyton ajelloi (n = 10), Trichophyton concentricum (n = 5), Trichophyton mentagrophytes (n = 167), Trichophyton rubrum (n = 272), Trichophyton schoenleinii (n = 3), Trichophyton tonsurans (n = 53), Trichophyton verrucosum (n = 33), and Trichophyton violaceum (n = 13).
R126638, itraconazole, and ketoconazole were pure compounds synthesized at the Janssen Research Foundation, Beerse, Belgium. The test compounds were dissolved in dimethyl sulfoxide (DMSO), and a dilution series was prepared in DMSO to give concentrates that were stored at −20°C. The concentrations in the stock solutions were 100 times the final concentration of each compound. For preparation of microdilution plates, 2-μl volumes of concentrate in DMSO were added to 100-μl volumes of sterile distilled water with a computer-controlled dispenser. Addition of 100 μl of inoculated culture medium as detailed below provided the final dilution step, creating cultures with 1% (vol/vol) DMSO. Earlier tests have shown that this concentration of DMSO is not inhibitory for the species tested; all control cultures also contained 1% DMSO.
The microplate broth dilution method for Candida spp. has been described previously (7). This method has been validated against the NCCLS M27-A2 reference method for yeasts (4). Inocula were prepared overnight at 30°C in dilute casein hydrolysate-yeast extract-glucose medium with continuous rotation as described previously (5). This procedure yielded an initial concentration of approximately 107 CFU/ml, as determined by measurement of viable counts. For each isolate, 20 μl of inoculum culture was diluted in 10 ml of double-strength buffered RPMI 1640 medium with 2% glucose (7), and 100-μl volumes of the inoculated medium were added to the 100-μl volumes of solutions of the test compounds in the microdilution plates. The microdilution plates were incubated at 37°C for 48 h. Growth turbidity in the wells was read spectrophotometrically at 405 nm and corrected for the background absorbance, and MICs were recorded as the lowest concentrations of test compounds that reduced growth below 50% of the level of control growth.
For dermatophytes, cultures were prepared on Sabouraud agar (Oxoid) at 30°C for 1 to 2 weeks to bring the cultures to a full, sporulating stage of growth. Suspensions of surface material from the cultures were made in 0.05% sodium dodecyl sulfate solution and adjusted to a constant turbidity reading of an optical density at 530 nm of 1.0 ± 0.1. This procedure yielded an initial concentration of approximately 106 CFU/ml, as determined by measurement of the viable counts. For each dermatophyte isolate tested, 20 μl of inoculum suspension was diluted in 10 ml of double-strength buffered RPMI 1640 medium with 2% glucose; and 100-μl volumes were added to 100-μl volumes of serial dilutions of test compounds, as described above for the testing of the Candida yeasts. This procedure yielded an initial concentration of approximately 103 CFU/ml, as determined by measurement of the viable counts. The cultures were incubated for 5 to 7 days at 30°C until the turbidity of the control growth appeared to be adequate for spectrophotometric measurement. Growth turbidity in the wells was read spectrophotometrically at an optical density of 405 nm (corrected for background absorbance), and MICs were defined by inspection of the dose-response curves as the lowest concentrations of test compounds that reduced growth below 50% of the level of the control growth.
For tests with Malassezia spp., microdilution tests were done in a way similar to that described above for the tests with yeasts and dermatophytes, but the medium used was modified Dixon's broth and ketoconazole was used as the reference compound. Details of these tests have been described previously (11). All the isolates tested had been received or identified as M. furfur before the taxonomic revisions of the genus Malassezia into eight species (3, 9). The isolates have not been reidentified and are therefore referred to in this paper as “Malassezia spp.”
In vivo cutaneous M. canis and T. mentagrophytes infections in guinea pigs.
All experiments with animals conformed with the code of practice defined by the Animal Welfare Committee of the Janssen Research Foundation. M. canis M16/3 or T. mentagrophytes B32663 was grown on nine slants of Sabouraud agar (Oxoid) for 14 days at 25°C. The growth in all tubes was scraped off with a sterile needle and placed into 9 ml of honey diluted 1:3 in water. The suspension was briefly homogenized with an Ultra-Turrax apparatus, and 250-μl volumes were smeared on the shaved and lightly scarified dorsa of albino guinea pigs (Charles River Associates, Kisslegg, Germany). For untreated and placebo-treated animals, this procedure generated crusted, erythematous lesions of approximately 4 to 5 cm in diameter which were well developed 3 days after inoculation. Oral treatment (500 μl/guinea pig/day) with the test compounds dissolved in polyethylene glycol 200 was started either 1 h before infection (prophylaxis experiments) or 3 days after infection (therapeutic experiments) and was repeated daily for a total of 3, 6, 9, or 12 days.
Lesion severity scores were recorded by an investigator blinded to the experimental treatment on days 7, 14, and 21 after infection. A score of 4 indicated fully developed lesions with erythema and crusting and a score of 0 indicated no visible lesion. Scores of 1, 2, and 3 were used to indicate lesions with severities of approximately 25, 50, and 75% of those of fully developed lesions, respectively. The experimental groups contained a minimum of four animals each.
In vivo cutaneous T. mentagrophytes infection in mice.
Mice are generally difficult to infect reproducibly with dermatophytes commonly pathogenic for humans. For many years T. mentagrophytes var. quinckeanum strain B63868 has been used in the Janssen laboratories as a dermatophyte that gives consistent and reproducible cutaneous murine infections. The fungus was grown for 10 days on Sabouraud agar at 30°C, and the material scraped aseptically from 40 slants was pooled in 30 ml of sterile water and briefly homogenized. Volumes of 100 μl were then smeared onto the shaved and lightly scarified skin of female NMRI mice that had been immunosuppressed 4 days before challenge by subcutaneous injection of 500 μg of estradiol valerate. Estradiol pretreatment is known to inhibit innate and acquired immune defenses (1, 8). For untreated and placebo-treated animals, this procedure generated lesions of approximately 2 cm in diameter that were severely crusted and erythematous. Oral treatment (250 μl/mouse/day) with the test compounds dissolved in polyethylene glycol 200 was begun on the day of infection and was continued for 5 days. On day 7 the cutaneous lesions were scored on a scale from 0 (no visible lesion) to 3 (significant crusting and erythema). (The smaller lesions in the mice made it unrealistic to attempt a finer scale of assessment.) The experimental groups contained a minimum of four animals each.
For calculation of the 50% effective doses (ED50s) of the test compounds, a lesion score of 1 was considered a response to treatment. When feasible, ED50s were estimated from the resulting binary dose-response data by probit analysis (2). Skin lesion scores after infection with cutaneous T. mentagrophytes were evaluated as median values and 95% distribution-free confidence intervals based on the sign test. Computations for statistical analysis were carried out with the SAS (version 8.1) system.
RESULTS
In vitro antifungal activities.
As shown in Table 1, R126638 inhibited the growth of most isolates of dermatophytes, pathogenic Candida species, and Malassezia sp. isolates at concentrations below 1 μg/ml. R126638 had in vitro activity similar to or lower than that of itraconazole against dermatophytes. In vitro R126638 was twice as active as itraconazole against the panel of Candida spp. tested in these experiments, and R126638 was 10 times more active than ketoconazole against Malassezia spp. (Table 1).
TABLE 1.
MICs of test substances for panels of different fungal species or groups of species
| Fungus (no. of isolates tested) | Itraconazole or ketoconazolea MIC (μg/ml)
|
R126638 MIC (μg/ml)
|
||||
|---|---|---|---|---|---|---|
| Range | 50% | 90% | Range | 50% | 90% | |
| Candida spp.b (24) | 0.016->8 | 0.32 | >8 | 0.008->8 | 0.063 | >8 |
| Epidermophyton floccosum (31) | ≤0.004-0.5 | ≤0.004 | 0.031 | ≤0.004->2 | 0.016 | 0.13 |
| Microsporum audouinii (9) | ≤0.004-0.25 | 0.01 | 0.13 | ≤0.004-0.50 | 0.032 | 0.25 |
| Microsporum canis (120) | ≤0.004-1.0 | ≤0.004 | 0.031 | ≤0.004->2 | 0.031 | 2.0 |
| Other Microsporum spp.b (19) | ≤0.004-2.0 | 0.25 | 1.0 | ≤0.004->2 | 0.25 | >2 |
| Trichophyton ajelloi (10) | 0.008-0.5 | 0.25 | 0.5 | 0.063->2 | 0.13 | 1.0 |
| Trichophyton mentagrophytes (167) | ≤0.004-1.0 | 0.016 | 0.25 | ≤0.004->2 | 0.063 | 0.50 |
| Trichophyton rubrum (272) | ≤0.004-2.0 | 0.063 | 0.50 | ≤0.004->2 | 0.25 | 0.50 |
| Trichophyton tonsurans (53) | ≤0.004->2 | 0.063 | 0.50 | ≤0.004->2 | 0.13 | 0.50 |
| Trichophyton verrucosum (33) | ≤0.004-1.0 | ≤0.004 | 0.13 | ≤0.004->2 | 0.25 | 2.0 |
| Trichophyton violaceum (13) | ≤0.004-0.32 | 0.016 | 0.13 | ≤0.004-0.32 | 0.032 | 0.32 |
| Other Trichophyton spp.b (8) | ≤0.004->2 | ≤0.004 | ≤0.004 | ≤0.004->2 | 0.032 | >2 |
| Malassezia spp. (29) | ≤0.001-1.0 | 0.10 | 0.32 | ≤0.001-0.1 | 0.010 | 0.032 |
For Malassezia spp., ketoconazole instead of itraconazole was used as the reference compound. 50% and 90%, MICs at which 50 and 90% of the isolates in the test panel, respectively, are inhibited.
See Materials and Methods.
In vivo activity of R126638 against cutaneous dermatophyte infections caused by M. canis or T. mentagrophytes in guinea pigs.
Tables 2 and 3 show the results of various prophylactic oral treatment experiments (in which treatment was started at day 0) and therapeutic oral treatment experiments (in which treatment was started at day 3) with R126638 and itraconazole in models of M. canis and T. mentagrophytes cutaneous infections in guinea pigs, respectively. In both models, R126638 consistently outperformed itraconazole at equivalent test doses. Comparison of the results obtained on days 7 and 14 shows that R126638 reduced the lesion severity more rapidly than itraconazole did.
TABLE 2.
ED50 of itraconazole and R126638 for guinea pigs infected with M. canisa
| Treatment day
|
Dosages tested (mg/kg)b | Drug | ED50 (mg/kg) assessed on day:
|
|||
|---|---|---|---|---|---|---|
| Start | End | 7 | 14 | 21 | ||
| 0 | 3 | 0, 1.25, 2.5, 5, 10 | Itraconazole | >10 | >10 | >10 |
| 0 | 3 | 0, 1.25, 2.5, 5, 10 | R126638 | <1.25 | 9.31 | >10 |
| 0 | 6 | 0, 0.16, 0.32 (n = 3), 0.63, 1.25 | Itraconazole | >1.25 | >1.25 | >1.25 |
| 0 | 6 | 0, 0.16, 0.32, 0.63, 1.25 | R126638 | 0.45 | 0.59 | 0.77 |
| 0 | 12 | 0 (n = 21), 0.31, 0.63, 1.25 (n = 8), 2.5 (n = 12), 5 | Itraconazole | 0.89 | 1.16 | 1.18 |
| 0 | 12 | 0 (n = 21), 0.31, 0.63, 1.25 (n = 8), 2.5 (n = 12), 5 | R126638 | <0.31 | <0.31 | <0.31 |
| 3 | 3 | 0, 1.25, 2.5, 5, 10 | Itraconazole | >10 | >10 | >10 |
| 3 | 3 | 0, 1.25, 2.5, 5, 10 | R126638 | 2.35 | <1.25 | <1.25 |
| 3 | 6 | 0, 0.16, 0.32, 0.63, 1.25 | Itraconazole | >1.25 | >1.25 | >1.25 |
| 3 | 6 | 0, 0.16, 0.32, 0.63, 1.25 | R126638 | 0.63 | 0.67 | 0.45 |
A response was defined as a lesion score of less than 2.
The test groups comprised four animals each unless indicated otherwise in parentheses.
TABLE 3.
ED50 of itraconazole and R126638 for guinea pigs infected with T. mentagrophytesa
| Treatment day
|
Dosage tested (mg/kg)b | Drug | ED50 (mg/kg) assessed on day:
|
|||
|---|---|---|---|---|---|---|
| Start | End | 7 | 14 | 21 | ||
| 0 | 3 | 0, 1.25, 2.5, 5, 10 | Itraconazole | >10 | 9.31 | 7.26 |
| 0 | 3 | 0, 1.25, 2.5, 5, 10 | R126638 | 2.66 | 2.50 | 1.77 |
| 0 | 6 | 0, 0.16, 0.32, 0.63, 1.25 | Itraconazole | 0.67 | 0.89 | >1.25 |
| 0 | 6 | 0, 0.16, 0.32, 0.63, 1.25 | R126638 | 0.23 | <0.16 | <0.16 |
| 0 | 12c | 0, 1.25, 2.5, 5 | Itraconazole | <1.25 | <1.25 | <1.25 |
| 0 | 12c | 0, 0.31, 0.63, 1.25, 2.5 | R126638 | <0.32 | <0.32 | <0.32 |
| 3 | 3 | 0, 1.25, 2.5, 5, 10 | Itraconazole | >10 | 3.54 | 3.54 |
| 3 | 3 | 0, 1.25, 2.5, 5, 10 | R126638 | 1.77 | 1.33 | <1.25 |
| 3 | 6 | 0, 0.16, 0.32, 0.63, 1.25 | Itraconazole | 1.18 | >1.25 | >1.25 |
| 3 | 6 | 0, 0.16, 0.32, 0.63, 1.25 | R126638 | <0.16 | 0.23 | 0.23 |
A response was defined as a lesion score of less than 2.
The test groups comprised four animals each unless indicated otherwise.
In these experiments, eight animals were included in each test group.
In the M. canis model, treatment with R126638 at doses of 1.25 mg/kg of body weight or more begun on day 0 and continued daily for 12 days eliminated visible cutaneous lesions from all animals from day 7 onward, resulting in an ED50 lower than the lowest dose tested (0.32 mg/kg; Table 2). Similar prophylactic treatment regimens (begun on day 0) continued for just 3 or 6 days also markedly reduced lesion scores from day 7 onward, although with these shorter durations of treatment complete eradication of the lesions was not achieved until day 21. Clear reductions in lesion severity were achieved with itraconazole at doses of 1.25 mg/kg and higher under all test conditions when treatment began on day 0 (Table 2), but the ED50s showed that the efficacy of itraconazole was usually less than that of R126638 under all conditions tested. Even when initiation of treatment was delayed to day 3 postinfection and continued for only 3 days, all R126638-treated animals had lesion scores of only 0 or 1 by day 14, even with the lowest dose tested (1.25 mg/kg), whereas under the same treatment conditions, itraconazole-treated animals showed no reduction in lesion severity scores below a score of 2 at any of the three sampling times, even when 10-mg/kg dosages were used (Table 2).
The results for the animals with T. mentagrophytes infections (Table 3) showed that R126638 had even more potent activity in this model than it did in the model of M. canis infections. For 12-day treatments started on day 0, both itraconazole and R126638 reduced lesion severity scores below 2 for all animals from day 7 onward at the lowest doses tested (1.25 mg/kg for itraconazole, 0.32 mg/kg for R126638). Six days of treatment with R126638 starting on day 0 led to reductions in cutaneous lesion severity to a mean score of only 1 from day 7 onward at doses of 0.32 mg/kg and higher, with complete eradication of the lesions achieved by day 21 with doses of 0.63 and 1.25 mg/kg (ED50, <0.16 mg/kg; Table 3). Even 3-day treatments with R126638 begun on day 0 led to the complete eradication of lesions by day 21 for doses of 5 and 10 mg/kg (ED50, 1.8 mg/kg; Table 3). Under the same test conditions itraconazole never reduced the mean T. mentagrophytes lesion scores to 0, but a reduction in the severity of the lesions was recorded when a dose of 1.25 mg/kg was given prophylactically for 6 days. When treatment was started on day 3 postinfection and continued for only 3 or 6 days, R126638 showed efficacy and the ED50s of R126638 were lower than those of itraconazole (Table 3).
In vivo activity against murine dermatophytosis caused by T. mentagrophytes.
Detailed results for the experiments with T. mentagrophytes are presented in Table 4. At all three dosage regimens tested (1.25, 2.5, and 5 mg/kg daily for 5 days), R126638 reduced the lesion severity scores to 0 or 1 by day 7 postinfection; the ED50 based on a lesion severity score of 0 or 1, indicating a response, was calculated to be <0.63 mg/kg. At the same test doses, only the 5-mg/kg itraconazole treatment produced responses comparable to the produced by R126638. The ED50 of itraconazole was calculated to be 3.02 mg/kg (95% confidence interval, 2.39 to 3.97 mg/kg).
TABLE 4.
Results of treatment of murine cutaneous T. mentagrophytes infections with itraconazole and R126638
| Treatment | Dose (mg/kg) | No. of animals | Median skin lesion score (95% confidence interval)a |
|---|---|---|---|
| Placebo | 0 | 24 | 3 (3-3) |
| Itraconazole | 0.63 | 6 | 3 (3-3) |
| R126638 | 0.63 | 6 | 1 (1-3) |
| Itraconazole | 1.25 | 6 | 3 (3-3) |
| R126638 | 1.25 | 12 | 1 (0-2) |
| Itraconazole | 2.5 | 18 | 2.5 (0-3) |
| R126638 | 2.5 | 18 | 0 (0-1) |
| Itraconazole | 5 | 12 | 0.5 (0-1) |
| R126638 | 5 | 10 | 0 (0-1) |
Skin lesion scores were assessed on day 7.
DISCUSSION
The results of this preclinical study show that R126638 is a potent triazole antifungal with efficacy in vitro and in vivo against pathogens causing common superficial mycoses. Susceptibility tests with R126638 indicate that it has in vitro activity against dermatophytes comparable to or somewhat lower than that of itraconazole. However, in vivo, R126638 greatly outperformed itraconazole in cutaneous models of M. canis and T. mentagrophytes infections in guinea pigs, often reducing lesion scores to 0 when it was used at remarkably low dosages in prophylactic and therapeutic treatment regimens. R126638 also outperformed itraconazole in a cutaneous model of T. mentagrophytes dermatophytosis in the mouse. The reasons for the differential comparative activity of R126638 versus that of itraconazole in vitro and in vivo are not yet fully explained, but a lower potential for protein binding of R126638 may be a significant factor contributing to its therapeutic success in the animal models.
R126638 matched itraconazole in terms of its potency against Candida spp. in vitro. Its efficacy in vivo in models of vaginal or cutaneous Candida infections remains to be confirmed, but by comparison with the findings of the results of the in vitro and in vivo studies with dermatophytes, a similarly impressive level of activity is anticipated.
R126638 was 10 times more potent than ketoconazole as an inhibitor of growth of Malassezia spp. in vitro. Ketoconazole is the favored therapy for Malassezia-related infections in the clinic, so the potential of development of R126638 for the treatment of pityriasis versicolor, seborrhoeic dermatitis, and Malassezia folliculitis merits further investigation.
Our findings reveal that R126638 is a drug of promise for development as a new triazole for dermatological use. It may offer efficacy and safety benefits because of the lower dosages and shorter durations of therapy that can be used compared with those required for the other azoles.
Acknowledgments
This study was supported by a grant from the IWT-Vlaanderen (grant 030023 to J.A.).
REFERENCES
- 1.Carlsten, H., R. Holmdahl, and A. Tarkowski. 1991. Analysis of the genetic encoding of oestradiol suppression of delayed-type hypersensitivity in (NZB × NZW) F1 mice. Immunology 73:186-190. [PMC free article] [PubMed] [Google Scholar]
- 2.Finney, D. J. 1971. Statistical methods in biological assay, 3rd ed. Charles Griffen & Company, Cambridge, United Kingdom.
- 3.Guého, E., G. Midgley, and J. Guillot. 1996. The genus Malassezia with description of four new species. Antonie Leeuwenhoek 69:337-355. [DOI] [PubMed] [Google Scholar]
- 4.NCCLS. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, 2nd ed., vol. 17, no. 9. NCCLS, Wayne, Pa.
- 5.Odds, F. C. 1992. Antifungal susceptibility testing of Candida spp. by relative growth measurement at single concentrations of antifungal agents. Antimicrob. Agents Chemother. 36:1727-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Odds, F. C., A. J. P. Brown, and N. A. R. Gow. 2003. Antifungal agents: mechanisms of action. Trends Microbiol. 11:272-279. [DOI] [PubMed] [Google Scholar]
- 7.Odds, F. C., L. Vranckx, and F. Woestenborghs. 1995. Antifungal susceptibility testing of yeasts: evaluation of technical variables for test automation. Antimicrob. Agents Chemother. 39:2051-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Styrt, B., and B. Sugarman. 1991. Estrogens and infection. Rev. Infect. Dis. 13:1139-1150. [DOI] [PubMed] [Google Scholar]
- 9.Sugita, T., M. Takashima, T. Shinoda, H. Suto, T. Unno, R. Tsuboi, H. Ogawa, and A. Nishikawa. 2002. New yeast species, Malassezia dermatis, isolated from patients with atopic dermatitis. J. Clin. Microbiol. 40:1363-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanden Bossche, H., L. Koymans, and H. Moereels. 1995. P450 inhibitors of use in medical treatment: focus on mechanisms of action. Pharmacol. Ther. 67:79-100. [DOI] [PubMed] [Google Scholar]
- 11.Van Gerven, F., and F. C. Odds. 1995. The anti-Malassezia furfur activity in vitro and in experimental dermatitis of six imidazole antifungal agents—bifonazole, clotrimazole, flutrimazole, ketoconazole, miconazole and sertaconazole. Mycoses 38:389-393. [DOI] [PubMed] [Google Scholar]