Abstract
Plants change size by deforming reversibly (elastically) whenever turgor pressure changes, and by growing. The elastic deformation is independent of growth because it occurs in nongrowing cells. Its occurrence with growth has prevented growth from being observed alone. We investigated whether the two processes could be separated in internode cells of Chara corallina Klien ex Willd., em R.D.W. by injecting or removing cell solution with a pressure probe to change turgor while the cell length was continuously measured. Cell size changed immediately when turgor changed, and growth rates appeared to be altered. Low temperature eliminated growth but did not alter the elastic effects. This allowed elastic deformation measured at low temperature to be subtracted from elongation at warm temperature in the same cell. After the subtraction, growth alone could be observed for the first time. Alterations in turgor caused growth to change rapidly to a new, steady rate with no evidence of rapid adjustments in wall properties. This turgor response, together with the marked sensitivity of growth to temperature, suggested that the growth rate was not controlled by inert polymer extension but rather by biochemical reactions that include a turgor-sensitive step.
This study was undertaken to determine whether growth can be distinguished from elastic deformation when plants enlarge. Both processes are present in plants, but they occur together and are superimposed on each other when a plant becomes larger. Nevertheless, they are fundamentally different because growth results from irreversible enlargement, whereas elastic enlargement is not permanent and reverses when the deforming force is removed. At the cell level growth extends the wall permanently, whereas elastic wall deformation is reversible. Both involve water uptake because growth is associated mostly with increased cell water content, whereas elastic deformation is caused mostly by changes in P that result from changes in water content. These similar origins make the two phenomena hard to separate but, without separation, it is not possible to accurately study the growth process.
Some efforts to separate growth from elastic deformation involved plasmolyzing or freezing and thawing excised tissues to remove elastic effects of P (Ursprung and Blum, 1924; Thimann and Schneider, 1938; Ordin et al., 1956; Cleland, 1958, 1959; Brouwer, 1963; Ray and Ruesink, 1963; Burström et al., 1967; Hohl and Schopfer, 1992). Other efforts involved stretching isolated cell walls or dead or live tissues in an external apparatus (Probine and Preston, 1962; Cleland, 1967; Lockhart, 1967; Haughton and Sellen, 1969; Yamamoto et al., 1970; Fujihara et al., 1978; Kutschera and Briggs, 1988; Nonami and Boyer, 1990a). Typically, the residual enlargement after subtracting the elastic component was considered to be the growth of the plant material. As it became increasingly possible to monitor rapid changes in the dimensions of cells (Green et al., 1971; Ortega et al., 1989; Zhu and Boyer, 1992) and tissues (Hsiao et al., 1970; Vanderhoef and Stahl, 1975; Boyer and Wu, 1978, Kuzmanoff and Evans, 1981), it became necessary to rapidly distinguish growth from elastic effects. However, the usual plasmolytic and stretching techniques were either too slow or could not be adapted to intact plants, and rapid changes in enlargement increasingly were interpreted solely as growth.
Most plant enlargement results from cell enlargement in localized growing regions. Because of the complexities of these regions, Lockhart (1965a, 1965b) modeled the growth of single cells surrounded by free water. He assumed that the cell walls behaved as inert polymers stretched by P, and that wall biosynthesis was independent of growth. He considered elastic effects to be rapid and ignored them by applying the model several minutes after a new rate was achieved. Ortega (1985, 1990) extended the Lockhart treatment to account explicitly for the elastic properties of the wall. This allowed rapid effects on enlargement to be modeled, but the model remains untested because methods were unavailable to rapidly separate growth from elastic effects in experiments. The present work provides a rapid method to make this separation in live cells.
The model of Ortega (1985, 1990) was based on the superposition principle from polymer physics and showed that, for a single cell whose water uptake was not limiting, growth and elastic effects could be added according to:
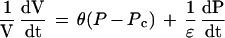 |
1 |
where (dV/dt)/V is the relative volumetric rate of enlargement (m3 s−1 m−3, or s−1), Pc is the critical turgor pressure below which growth does not occur (MPa), θ is the relative irreversible extensibility of the cell wall (s−1 MPa−1), and ε is the volumetric elastic modulus (MPa). On the right side of the equation, the first term represents growth, which is the irreversible enlargement at a steady “effective” turgor (P − Pc). The second term is the reversible elastic enlargement, which is important when P changes. Note that when P is constant, the second term becomes zero and the equation takes on the form of the Lockhart equation (dV/dt)/V = θ (P − Pc). Conversely, when a cell matures, θ becomes zero and the irreversible enlargement disappears so that only elastic effects are seen. In this equation it is important to point out that θ is a coefficient representing all of the biological and physical factors contributing to growth. It is not restricted to inert polymer effects, as originally proposed by Lockhart (1965a, 1965b).
Many studies of cell enlargement use external osmotica to vary the P. Osmotica change both the P and the solute environment in the wall, rendering it difficult to determine which factor controls enlargement. A better approach would be to alter only P without changing the environment of the wall. Ortega et al. (1988, 1989) were the first to do this kind of experiment, and they varied P by injecting silicone oil into the vacuole of cells of Phycomyces. Zhu and Boyer (1992) used a pressure probe to inject or remove cell solution to raise or lower P in the internode cells of Chara corallina. The wall environment was unaltered. Enlargement was continuously monitored. The cells were surrounded by water, causing the growth-induced water potentials associated with water uptake to be negligible (Zhu and Boyer, 1992). This latter system is the one used in the present work because it allowed P effects to be studied without considering water uptake, thus simplifying the analysis.
C. corallina grows primarily in length, at a rate essentially independent of the total length of the cell, and Equation 1 can be revised to:
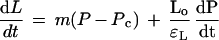 |
2 |
where dL/dt is the elongation rate (m s−1), m is the irreversible longitudinal extensibility of the cell wall (m s−1 MPa−1), Lo is the original cell length (m), and εL is the longitudinal component of the elastic modulus (MPa). Using this system to vary and control P, we were able to separate growth and elastic effects, and explore the mechanism of wall elongation with the model of Ortega (1985, 1990).
MATERIALS AND METHODS
Plant Materials
Several cultures of Chara corallina Klien ex Willd., em R.D.W. were grown in liquid medium as described in Zhu and Boyer (1992). Fluorescent lights and ambient sunlight above the cultures provided continuous PAR of 10 to 15 μmol photons m−2 s−1 at the surface of the water. The culture temperature was 22°C to 23°C and pH was 8.0 to 8.5. We conducted the experiments in an environmentally controlled chamber in which the temperature and light intensity were the same as those used for the cultures. We used a single internode cell, dissected by hand from a healthy thallus, for each experiment. Young, growing internode cells were from near the thallus apices, and older, nongrowing (mature) cells were from lower portions of the thallus. All of the experiments were conducted in the culture medium taken directly from the cultures.
Experimental Apparatus
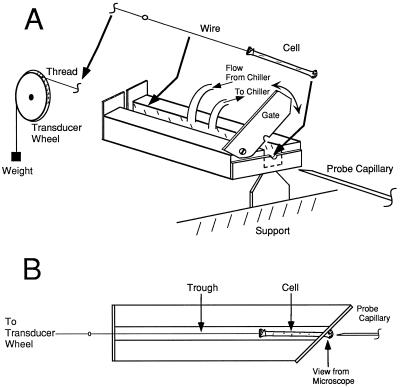
The experimental apparatus was similar to that described in Zhu and Boyer (1992), with some modifications. A trough to hold a single internode cell was made from clear acrylic. A vertical, scissors-like acrylic gate was mounted at one end of the trough (Fig. 1). A hole (800 μm in diameter) was drilled at the interface of the top and bottom jaws of the gate. Clamping the basal end in the gate hole immobilized the internode cell. In this position the node of the cell protruded from the hole in the gate and the remaining internode was suspended horizontally within the trough (Fig. 1). The hole was sealed with petroleum jelly to prevent the leakage of medium around the cell. We attached a thin steel wire to the free, apical end of the cell and passed it out the end of the trough opposite the gate. This wire was attached to a Kevlar thread that was wrapped once around a vertical plastic wheel on a position transducer (see “P and L Measurement” for details). A small weight (2.3 g) on the end of the thread ensured good contact with the transducer wheel. The trough was supported at the gate end on a vertical piece of acrylic that we attached to the top of an adjustable jack, allowing the height of the apparatus to be altered.
Figure 1.
Apparatus for measuring P and L in C. corallina. A, Perspective view. B, Top view. The cell is inserted in the trough as shown by the thick arrows. The gate is closed to immobilize the end to be probed with the capillary for measuring P. The other end is attached to a wire and thread leading to a weight. The thread is placed on a transducer wheel that turns and measures changes in L.
Temperature Control
A peristaltic pump delivered the medium to the trough (20 mL min−1) through a closed circuit of insulated tubing. Before entering the trough, the medium flowed through a temperature control unit constructed of stainless steel tubing. The tubing was bent into a flat spiral (1.5-mm i.d. of tubing bent into 6-cm flat spiral) and was clamped to the cold side of an electronic Peltier chiller (Thermoelectrics Unlimited, Wilmington, DE). The chiller could be set to give any desired medium temperature in the range of 4°C to 22°C. We used cool tap water as the heat sink for the hot side of the Peltier block. Temperatures above 23°C could be obtained by replacing this tap water with temperature-controlled warm water and switching off the Peltier block. The temperature of the medium around the cell was continuously monitored with a copper/constantan thermocouple (0.1 mm in diameter) mounted in the side of the trough. We designed (and tested) the system to minimize thermal disturbance that could be mistaken for cell elongation. Culture medium lost by evaporation from the trough was replaced by gravity-fed medium from a reserve container.
P and L Measurement
P was measured with a large pressure probe (Steudle and Zimmermann, 1974) as described in Zhu and Boyer (1992). The pressure transducer in the probe was calibrated with compressed N2 and the output was linear from 0 to 0.9 MPa. The tip of the glass microcapillary was ground at a 25o angle (relative to the long axis) until the opening had a diameter of 75 μm. This minimized plugging during P changes but had no effect on the viability of the cells. The probe was mounted on a micromanipulator to allow fine control while moving and inserting the microcapillary tip. Before beginning each experiment, the microcapillary was filled with silicone oil, and cell solution was sucked in from a growing cell (this cell was then discarded). We placed another growing cell in the trough and connected it to the position transducer (Fig. 1). We filled the trough with culture medium, inserted the microcapillary tip into the basal end of the cell immobilized in the scissors-like gate, repositioned the plunger in the probe to return the oil/cell-solution meniscus to its preinsertion position, and obtained a reading of the original cell P.
The L was continuously recorded with a position transducer (radial voltage induction transducer, RVIT, Lucas Control Systems, Hampton, VA), which was calibrated to give a linear output throughout the expected range of L.
The P Clamp
The effect of a step change in P was investigated with the P clamp method developed by Zhu and Boyer (1992). An upward P clamp involved a step increase in P followed by small further injections of cell solution to keep the new P from decreasing. After 8 to 10 min, sufficient water had moved out of the cell to concentrate the cell solution. The P was now balanced by the new osmotic potential of the cell, remaining steady at the new value, and needed no further injections. A downward P clamp involved the removal of cell solution followed by small further removals. When enough solution had been removed, the P remained at the new lower level and needed no further removals. Before each experiment, we measured the Lo and monitored L during the experiment to determine the elongation rate (dL/dt).
dL/dt at Various Temperatures
We selected growing internode cells from cultures at 23°C and exposed them to temperatures above and below the culture temperature. We measured the steady elongation rates at temperatures below 23°C by decreasing the setting in 3oC to 4oC increments with the Peltier chiller until we found a low temperature that completely inhibited elongation. The temperature was returned to 23°C and then raised incrementally until a high temperature was reached that again inhibited elongation.
Elastic Behavior of Cell Walls at Various Temperatures
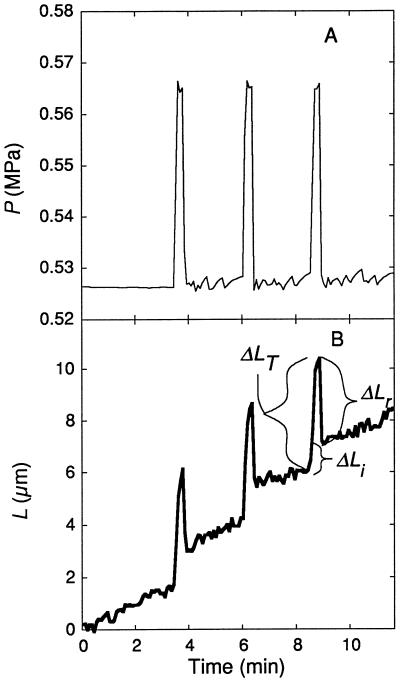
We examined the elastic behavior of the cell walls with pulses of P short enough to keep growth negligible, as described by Ortega (1994) and shown in Figure 2. Using the P clamp before each set of pulses, we adjusted the P to approximately the same value. While the cell was growing at a constant rate and constant P, cell solution was injected to increase P by 0.04 MPa for 10 s. P was then rapidly returned to the original value, producing a P pulse of 10 s. This P pulse was repeated two or three times with 2 min between each pulse. Following the set of P pulses at 23°C, additional sets were performed at various temperatures for each cell. We determined the total change in length (ΔLT) when P was increased during the P pulse. We established the reversible, elastic change in ΔLr when P was decreased at the end of the P pulse. We considered the total change in length to be the total of the reversible change and any irreversible change (ΔLi) as demonstrated in Figure 2:
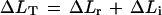 |
3 |
From the set of replicate P pulses, we calculated the mean ΔLT and ΔLr for each temperature. When comparisons were made among cells of different lengths, ΔLr, ΔLi, and ΔLT were expressed as relative length changes ΔLr/Lo, ΔLi/Lo, and ΔLT/Lo, respectively. The longitudinal component of the elastic modulus was calculated from εL = Lo(ΔP/ΔLr) for each cell.
Figure 2.
Change in L of C. corallina internode cells when P was changed rapidly with a P pulse. P pulses were given for 10 s with a pressure probe (A) and L was recorded (B). During each pulse, L increased rapidly at first and then more slowly to give the total length increase ΔLT. When the P was returned to the original level, L decreased rapidly to give the reversible component ΔLr. The ΔLi was a small, irreversible component seen whenever P increased rapidly. The variations in P (after it was returned to the original level) were generated intentionally to test for continuity between the probe contents and the cell contents. Temperature was 23°C. This cell was 13 mm long and growing at 0.014 μm s−1 for 20 min before the experiment. L is shown as the length beyond Lo at the beginning of the trace.
Data Processing
We recorded the voltage outputs from the position transducer, pressure probe, and thermocouple with a datalogger (model CR7X, Campbell Scientific, Logan, UT) and displayed them continually on a laptop computer. Voltages were recorded every 5 s. Following each experiment, we downloaded the stored data to a desktop computer for processing. The voltages were converted to L (μm), dL/dt (μm s−1), P (MPa), and temperature using the calibration factors determined during the construction of the equipment. A two-pen chart recorder gave real-time monitoring of the P and L during the experiments. Overall, the datalogger and computerized data management provided simpler and more sensitive measurements than those described by Zhu and Boyer (1992), who used only a recorder.
RESULTS
Immediately after placing the isolated internode cells into the apparatus, elongation rates were larger than in the intact plant (Zhu and Boyer, 1992). After 30 to 40 min, the elongation rate slowed to the range for intact plants and was relatively stable for the next 10 to 20 h. All of our measurements were done after the first 30 to 40 min in the apparatus.
Components of Elongation in Single Cells
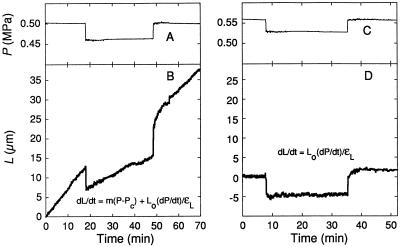
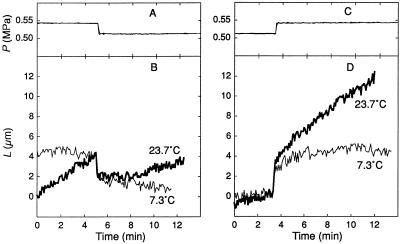
The cells changed in L when P was changed with the P clamp (Fig. 3). In a growing cell, a negative P clamp caused an instantaneous decrease in L followed by a steady elongation that was slower than before the P clamp (Fig. 3B). After a positive P clamp, an instantaneous increase in L was followed by a transition period of several minutes to a new steady elongation that was faster than before the P clamp. Similar instantaneous and transitional changes were seen in the mature cell (Fig. 3D), indicating that they were independent of the growth process. The instantaneous responses were reversible and thus elastic (ΔLr). The transitional response was not reversible and appeared to be an extended expression of ΔLi in Figure 2, which is sometimes termed a viscoelastic change. Note that the wall environment was unaltered during these measurements because cell solution was injected only into the interior of the cell. The P change was permanent, supported by a slight change in the concentration of solute normally in the cell.
Figure 3.
Changes in L during P steps in a growing (A and B) and a mature (C and D) C. corallina internode cell. The applicable equations are shown in B and D from Equation 2. The steps were generated with a pressure probe after which P was held constant by removing or injecting cell solution (P clamp). The temperature was 23°C. The cell in B was 12 mm long and growing at 0.0116 μm s−1 before P was changed. The cell in D was 21 mm long and not growing (mature). L is shown as the length beyond Lo at the beginning of the trace.
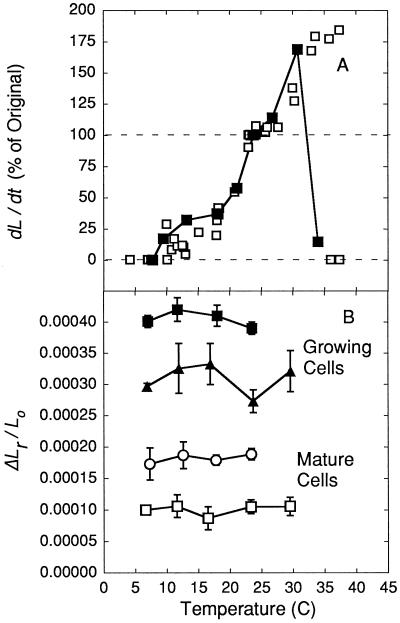
The growth rate changed with temperature but the elastic deformation did not. Figure 4A shows that growth was markedly affected between 8°C and 37°C, and was maximum in the range of 30°C to 35°C. We did not observe growth at temperatures below 5°C or above 37°C. After exposure to the low temperature, growth resumed when the cells were re-warmed. However, after exposure to the highest temperature, they were unable to grow again when cooled. In contrast, when measurements were made with P pulses as in Figure 2, the relative elastic deformation was constant at temperatures between 7oC and 30°C. This behavior was the same for growing and mature cells (Fig. 4B). The relative elastic deformation was larger for growing cells than for mature cells.
Figure 4.
Growth rate (A) and relative elastic cell wall deformation (B) at various temperatures in C. corallina internode cells. Growth rates (A) were obtained from nine cells growing at 0.0095 to 0.024 μm s−1 at the reference temperature of 23°C (rate = 100%). Data for one individual cell are shown by ▪. Relative elastic deformation in B was obtained with P pulses as in Figure 2 from cells growing at 0.010 and 0.014 μm s−1 (closed symbols) and from mature cells that were not growing at 23°C (open symbols). Data in A are individual measurements and data in B are means ± sd of four repetitions in individual cells, as shown in Figure 2.
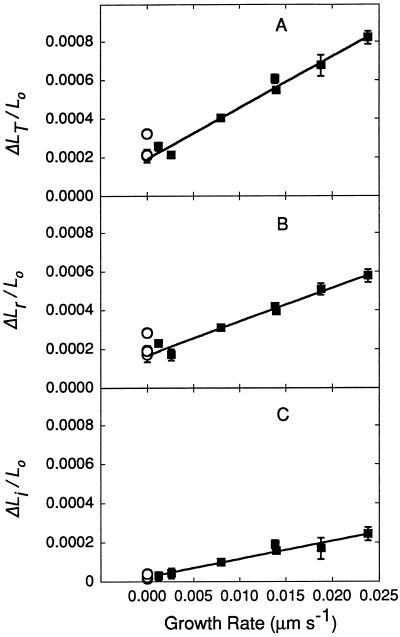
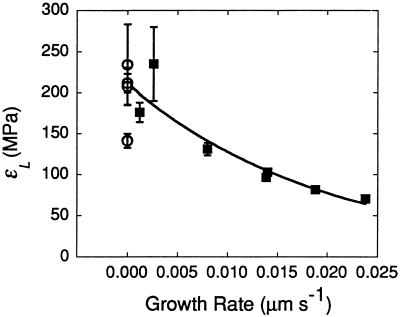
Figure 5 shows that the relative elastic deformation was linearly related to the original steady elongation rate. Cells growing rapidly at 23°C had a ΔLT/Lo of 0.00083, but others with lower growth rates had progressively smaller ΔLT/Lo (Fig. 5A). Mature cells had a ΔLT/Lo of only 0.0002 or 0.0003. Most of the variation came from differences in the elastic component ΔLr/Lo (Fig. 5B). The irreversible component also varied (ΔLi/Lo, Fig. 5C) and was always smaller in the mature cells. Figure 6 summarizes the elastic responses of the cells in terms of the longitudinal elastic modulus and shows that mature cells had a larger modulus than growing cells, i.e. mature walls were less deformable than growing walls.
Figure 5.
Relationship between growth rates at 23°C and relative length changes caused by P pulses in C. corallina internode cells. A, total; B, reversible (elastic); and C, irreversible change in length caused by P pulses as in Figure 2. Each cell was originally growing at the rate shown. Corresponding points in A, B, and C were measured in the same cell. Data are means ± sd of four repetitions in individual cells. ○, Mature cells; ▪, growing cells.
Figure 6.
Longitudinal elastic modulus (εL) at 23°C for the C. corallina internode cells in Figure 5B, calculated from the right-hand term of Equation 2. Data are means ± sd of four repetitions in individual cells, as shown in Figure 2.
Comparing Elastic Effects and Growth
When elongation was compared at high and low temperature in the same cell, only the rapid elastic and viscoelastic responses were detected at 7.3°C, and they were nearly the same as the rapid response at 23.7°C (Fig. 7). Whether P was stepped down (Fig. 7, A and B) or up (Fig. 7, C and D), these responses at cold temperatures always accounted for most of the transient response at warm temperatures. Only a slight amount of the viscoelastic response remained after the rapid response at cold temperature; this could be seen as the difference in cell length at the two temperatures between min 3.5 and 6.0 in Figure 7D. However, growth occurred at 23.7°C but was completely eliminated at 7.3°C (Fig. 7B). The time interval was kept short (20 min) between temperatures to minimize any changes in wall composition.
Figure 7.
Comparison of elongation at 23.7°C and 7.3°C when a C. corallina internode cell was subjected to a P step of 0.04 MPa downward (A and B) or upward (C and D). At 23.7°C (heavy trace), the cell was growing. At 7.3°C (light trace), the same cell was not growing. A and C show the superimposed P step for the two temperatures in the same cell. B and D show the rapid elongation responses to the P step superimposed in the same cell. L has been adjusted for the superposition in B and D.
Analyzing Growth with the Elongation Growth Equation (Eq. 2)
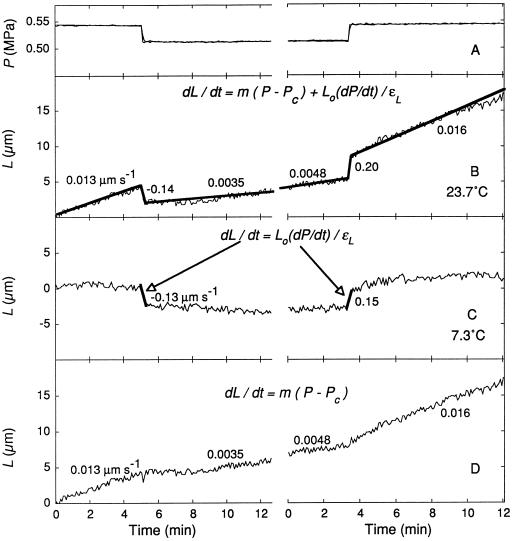
From the behavior described above it can be seen that a simple subtraction of the rapid response at 7.3°C from the total response at 23.7°C would produce a curve lacking elastic and rapid viscoelastic effects, showing growth alone. Accordingly, we subtracted them (heavy lines in Fig. 8C) from the total elongation (Fig. 8B) to obtain the growth in Figure 8D. The rate of rapid change was similar when P was stepped down (−0.13 and −0.14 μm s−1) or up (0.15 and 0.20 μm s−1) at the two temperatures. After the subtraction there was no evidence of rapid transients in the response to P. Growth changed immediately and smoothly. The immediate change indicated that P altered a step involved in growth that was not part of the elastic or rapid viscoelastic responses. The graphs indicate the applicable form of the growth equation (Eq. 2) and show the growth component, i.e. m(P − Pc), that was changed by P after the subtraction in Figure 8D.
Figure 8.
Growth behavior in response to P steps for the cell in Figure 7. A, P steps down and up; B, total elongation at 23.7°C; C, total elongation at 7.3°C in the same cell; and D, growth obtained by subtracting heavy lines in C from corresponding locations in B. The relevant form of Equation 2 is shown in each graph. Data for thin lines are running averages of 20 s for measurements every 5 s, and data for heavy lines are regressions for the data in the thin lines underneath. Numbers beside heavy lines are rates obtained from slopes of the regressions. L is shown as the length beyond Lo at the beginning of the trace.
DISCUSSION
Separating Elastic Effects from Growth
The cell walls of C. corallina internodes displayed elastic deformation whenever P changed during growth. The deformation was an inevitable consequence of the change in force on the walls. It combined with growth to create a complex response mixing early rapid elongation, viscoelastic deformation, and, later, steady elongation that blurred interpretation. Because elastic and viscoelastic deformation occurred when the cells were not growing, they could be separated from the growth process. It is important that the deformation of mature cells could not be used to correct for elastic and viscoelastic behavior in growing cells. Mature cells always displayed elastic responses that were smaller than in growing cells, probably because of differences in wall composition. Instead, we measured the elastic responses alone when growth did not occur at cold temperatures, and subtracted it from the total elongation in the same cell when growth did occur at warm temperatures. The residue gave the actual elongation due to growth. With this method, the growth that emerged responded to changes in P. If P decreased, the growth rate immediately decreased, and if P increased, the growth rate immediately increased. There was no evidence for rapid transients of growth rate or rapid changes in m or Pc as P changed, and growth smoothly came to a new steady rate.
The key to the method was the invariable nature of the elastic component as temperature changed. Inert polymers often display stable elastic behavior over a considerable range of temperatures above the glass-transition temperature (Sperling, 1992). Temperatures in the range around room temperature that we used with C. corallina are above the glass-transition temperature. Cross-linking and semicrystalline components likely to be present in cell walls tend to increase the thermal stability of elastic behavior (Sperling, 1992). Tomos et al. (1981) similarly observed stable elastic effects at temperatures around room temperature in Tradescantia virginiana cells. The thermal stability suggests that elastic behavior is purely physical and can be expected to be present in all plant cells.
The viscoelastic behavior also appeared to be largely physical because it was present in mature cells and thus was independent of growth. In contrast to the reversible elastic responses, it was largely irreversible and probably can be attributed to a displacement of wall polymers that was not reversed when P returned to its original level. It was particularly apparent during a step-up in P, but with our method the rapid portion of this viscoelastic component was subtracted from elongation, removing it from observation. There remained only a small residual portion expressed slowly, and we could observe little if any effect of it on the steady growth of the cells.
Green et al. (1971) did not report elastic responses in single cells of growing Nitella, although Kamiya et al. (1963), Probine and Preston (1962), and Metraux et al. (1980) observed such responses in nongrowing cell walls of Nitella. Probine and Preston (1962) found that the magnitude of the elastic response of the isolated cell walls was correlated with the previous growth rate of the intact cells, as we also observed. Green et al. (1971) interpreted cell elongation entirely as growth, and rapid changes in growth rate were attributed to alterations in Pc. However, P was changed with osmotica during the experiments, and the need to change bathing solutions around the cells may have obscured elastic effects in the first seconds after the change (Cleland, 1971). Ortega et al. (1989, 1991) attempted to quantify the elastic response in single cells of Phycomyces to step-up and pulse-up in P produced with a pressure probe and by using the equation reported in Ortega (1985). However, they encountered technical difficulties that complicated the interpretation of the results (Ortega et al., 1991). Zhu and Boyer (1992) reported elastic changes in growing C. corallina cells but lacked a method for quantitatively separating them from growth.
In our work elastic effects were clearly seen, but to evaluate them it was essential to change P rapidly without other complicating factors. We used a single cell from an alga surrounded with water that did not have large, growth-induced water potentials (Zhu and Boyer, 1992). These potentials are prevalent in growing multicellular tissues and change when P changes, making interpretation difficult (Nonami and Boyer, 1990b; Boyer, 1993; Maruyama and Boyer, 1994; Nonami et al., 1997). Our method of injecting solution from other C. corallina cells altered P alone in a natural fashion, avoiding the complications of these potentials and changing growth without altering the chemical environment of the wall. There were no large solute concentrations from external osmotica that can change wall behavior, as demonstrated by Zhu and Boyer (1992). The cells were alive and displayed protoplasmic streaming during the experiments (Zhu and Boyer, 1992). Thus, we observed normal growth and elastic effects. By accurately and continuously measuring cell dimensions, we could separate elastic effects experimentally and analytically.
In multicellular plants it is more difficult to separate elastic effects from growth. In some studies osmotica and freeze/thawing were used to eliminate P after a period of growth, and the remaining irreversible deformation was determined (Cleland, 1958, 1959; Hohl and Schopfer, 1992). This treatment prevented the tissue from being used further and was not suitable for intact plants. As a practical matter, it is worth noting that the method of estimating elastic deformation in C. corallina using P steps has promise for estimating elastic deformation in multicellular tissues.
Significance of the Temperature Response
There was a remarkable difference in the thermal response of growth and elastic behavior. Growth rates varied from zero to maximum, then returned to zero as the temperature rose from 5oC to 35oC to 37°C. The elastic behavior was unaffected by the same range of temperatures. This difference implies different mechanisms for the two processes.
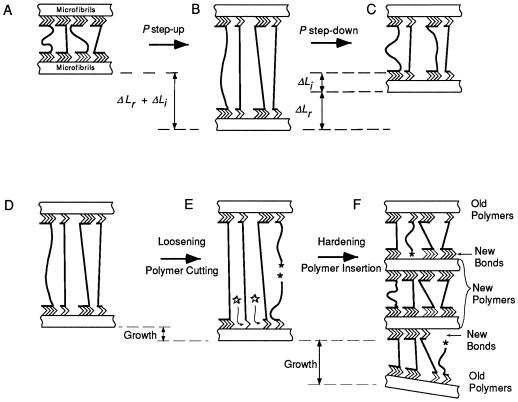
Several models have been suggested to account for cell enlargement (Passioura and Fry, 1992; Carpita and Gibeaut, 1993; Passioura, 1994; Roberts, 1994; Carpita et al., 1996), and Figure 9 shows their central molecular features. According to the models, during a P step-up, more tethers come under tension in the matrix polysaccharides linking cellulose microfibrils (ΔLr, Fig. 9, A and B) and some tethers undergo displacement (ΔLi, Fig. 9, A and B). The increased tension decreases the range of kinetic motion of the tethers. When the process is reversed, molecular motion is regained (ΔLr, Fig. 9, B and C) but the displacement is not reversed (ΔLi, Fig. 9C). These physical phenomena are present whether or not growth occurs. Their presence during growth does not imply that they control or contribute to growth, but rather that they are expressed as separate events while growth is ongoing.
Figure 9.
Diagrammatic representation of elastic changes and growth in a molecular unit of the primary wall. A, Cellulose microfibrils connected through hydrogen bonds (>>>>) to tethering matrix polysaccharides, two of which are load-bearing. B, More tethers become load-bearing (ΔLr) and some tethers are displaced (ΔLi) when P increases. C, Some tethers are released from load-bearing but displacements are not fully reversed when P decreases. D, Wall at high P (as in B) is loosened in E by breaking covalent bonds (★) and hydrogen bonds (⋆) probably through enzymatic action. F, Inserting new wall polymers and forming new hydrogen bonds hardens the wall in E. Not shown are wall proteins or layering of various polysaccharides in the wall.
By comparison, growth appears to involve many enzymatic events, including the cutting of tethers (Wong and Maclachlan 1979; Huber and Nevins, 1981; Yamamoto and Nevins, 1981; Hayashi and Maclachlan, 1984; Fry, 1989; Fry et al., 1992; Wu et al., 1996) and possible enzymatic breaking of hydrogen bonds (Cosgrove, 1993; McQueen-Mason and Cosgrove, 1995). The wall loosens as a result (Fig. 9E). The loosening is probably countered by reconnecting cut tethers (Smith and Fry, 1991; Nishitani and Tominaga, 1992; Hetherington and Fry, 1993) and synthesizing and inserting new wall polymers (Roberts, 1994), hardening the matrix (Fig. 9F). Hardening prevents rupture of the wall, which is the disastrous end result of continued loosening. Temperature probably has large effects on growth because the enzyme reactions involve chemical bonds having high activation energies.
Elastic behavior has been observed in many plants (Kamiya et al., 1963; Tomos et al., 1981; Steudle and Jeschke, 1983; Steudle et al., 1983; Nonami and Boyer, 1990a), and its molecular nature suggests that it will be present whenever the wall is placed under tension by P. As a result, growth models incorporate it by adding elastic effects to other dimensional effects (Ortega, 1985, 1990; Nonami and Boyer, 1990a). When the elastic effects are subtracted, the remaining ones are mostly the result of biochemical loosening and hardening of the wall (Fig. 9, D–F). This suggests that the term m(P − Pc) of Equation 2 is mostly biochemically controlled.
The Role of P
Lockhart (1965a, 1965b) noted the similarity between the extension of inert polymers and cell growth when a force is applied. He proposed that P is the force causing growth by acting on the wall as an inert polymer. Accordingly, with an increase in P, growth rate would increase; when P decreased, growth rate would decrease. Our work showed a similar P response during growth. However, the deformation of inert polymers generally showed few thermal effects in the narrow range of temperatures that we used (Sperling, 1992). For example ΔLr clearly is a property of inert materials and showed little thermal response in the C. corallina wall. If the inert polymer model is correct, growth similarly should have displayed little temperature response. The large growth response actually observed argues against the inert polymer model and suggests a biochemical mechanism.
Increased P undoubtedly stretched the wall more, causing inert elastic deformation as in Figure 9, A and B. We eliminated most of it by subtraction, but a growth response remained and was apparent within 1 min, suggesting that the magnitude of P may have rapidly altered a growth factor involving biochemical events. The exact way the magnitude of P might participate is unclear, but it should be noted that Robinson and Cummins (1976) reported little insertion of cellulose and matrix polymers into the wall of pea stem cells when P was low. The delivery of matrix polymers normally involved vesicles visible in the cytoplasm that fused with the plasmalemma. Upon fusion, the vesicles opened to the wall and immediately expelled their contents to the wall because of the force of P. At low P the vesicle contents were inserted more slowly into the wall, and without P no wall insertion occurred. Thus, the magnitude of P might play a role in wall assembly (Fig. 9F) that could be highly temperature responsive.
From different evidence, others concluded that growth rates were controlled more by biochemical factors than by the deformation of inert wall polymers. Haughton and Sellen (1969) used temperature to vary the deformation of isolated cell walls but found the effects to be too small to account for the sensitivity of growth to temperature. Ray and Ruesink (1962) suggested that there was a biochemical reaction close to the terminal steps in wall enlargement because of the rapidity of the growth response to temperature in living oat coleoptiles. Roberts (1994) noted that nearly all wall polymers were newly synthesized in primary walls of the outer, growth-limiting epidermal cells of multicellular organisms, and concluded that this synthetic activity must be central to wall growth. Zhu and Boyer (1992) used chemical inhibitors to decrease energy metabolism in C. corallina, and found growth to be inhibited despite high P. They suggested that metabolism controlled growth.
Zhu and Boyer (1992) found that growth was eliminated below a threshold P and only responded to P well above normal levels. This behavior suggests that the growth process could be entirely metabolic, with little involvement of P other than as an initial triggering event. Although the present work confirms the involvement of metabolism, the growth rate changed when P changed, suggesting a pressure-sensitive step in metabolism. This discrepancy needs further investigation.
Abbreviations:
- P
turgor pressure
- L
length
Footnotes
This study was supported by the National Science Foundation (grant no. IBN-9603956 to J.K.E.O.) and the Department of Energy (grant no. DE-FG02-87ER13776 to J.S.B.).
LITERATURE CITED
- Boyer JS. Temperature and growth-induced water potential. Plant Cell Environ. 1993;16:1099–1106. [Google Scholar]
- Boyer JS, Wu G. Auxin increases the hydraulic conductivity of auxin-sensitive hypocotyl tissue. Planta. 1978;139:227–237. doi: 10.1007/BF00388634. [DOI] [PubMed] [Google Scholar]
- Brouwer R. The influence of the suction tension of the nutrient solutions on growth, transpiration and diffusion pressure deficit of bean leaves. Acta Bot Neerl. 1963;12:248–261. [Google Scholar]
- Burström H, Uhrström GI, Wurscher R. Growth, turgor, water potential and Young's modulus in pea internodes. Physiol Plant. 1967;20:213–231. [Google Scholar]
- Carpita NC, Gibeaut DM. Structural models of primary cell walls in the flowering plants: consistency of molecular structure with the physical properties of walls during growth. Plant J. 1993;3:1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Carpita NC, McCann M, Griffing LR. The plant extracellular matrix: news from the cell's frontier. Plant Cell. 1996;8:1451–1463. doi: 10.1105/tpc.8.9.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R. A separation of auxin-induced cell wall loosening into its plastic and elastic components. Physiol Plant. 1958;11:599–609. [Google Scholar]
- Cleland R. Effect of osmotic concentration on auxin-action and on irreversible and reversible expansion of the Avena coleoptile. Physiol Plant. 1959;12:809–825. [Google Scholar]
- Cleland R. Extensibility of isolated cell walls: measurement and changes during cell elongation. Planta. 1967;74:182–191. doi: 10.1007/BF00384842. [DOI] [PubMed] [Google Scholar]
- Cleland R. Cell wall extension. Annu Rev Plant Physiol. 1971;22:197–222. [Google Scholar]
- Cosgrove DJ. How do plant cell walls extend? Plant Physiol. 1993;102:1–6. doi: 10.1104/pp.102.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry SC. Cellulases, hemicellulases and auxin-stimulated growth: a possible relationship. Physiol Plant. 1989;75:532–536. [Google Scholar]
- Fry SC, Smith RC, Renwick KF, Martin DJ, Hodge SK, Matthews KJ. Xyloglucan endotransglycosylase, a new wall-loosening enzyme activity from plants. Biochem J. 1992;282:821–828. doi: 10.1042/bj2820821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihara S, Yamamoto R, Masuda Y. Viscoelastic properties of plant cell walls. I. Mathematical formulation for stress relaxation with consideration for pre-extension rate. Biorheology. 1978;15:63–75. doi: 10.3233/bir-1978-15201. [DOI] [PubMed] [Google Scholar]
- Green PB, Erickson RO, Buggy J. Metabolic and physical control of cell elongation rate: in vivo studies in Nitella. Plant Physiol. 1971;47:423–430. doi: 10.1104/pp.47.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughton PM, Sellen DB. Dynamic mechanical properties of the cell wall of some green algae. J Exp Bot. 1969;20:516–535. [Google Scholar]
- Hayashi T, Maclachlan GA. Pea xyloglucan and cellulase. III. Metabolism during lateral expansion of pea epicotyl cells. Plant Physiol. 1984;76:739–742. doi: 10.1104/pp.76.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington PR, Fry SC. Xyloglucan endotransglycosylase activity in carrot cell suspensions during cell elongation and somatic embryogenesis. Plant Physiol. 1993;103:987–992. doi: 10.1104/pp.103.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl M, Schopfer P. Physical extensibility of maize coleoptile cell walls: apparent plastic extensibility is due to elastic hysteresis. Planta. 1992;187:498–504. doi: 10.1007/BF00199968. [DOI] [PubMed] [Google Scholar]
- Hsiao TC, Acevedo E, Henderson DW. Maize leaf elongation: continuous measurements and close dependence on plant water status. Science. 1970;168:590–591. doi: 10.1126/science.168.3931.590. [DOI] [PubMed] [Google Scholar]
- Huber DJ, Nevins DJ. Partial purification of endo- and exo-β-d-glucanase enzymes from Zea mays seedlings and their involvement in cell-wall autohydrolysis. Planta. 1981;151:206–214. doi: 10.1007/BF00395171. [DOI] [PubMed] [Google Scholar]
- Kamiya N, Tazawa M, Takata T. The relation of turgor pressure to cell volume in Nitella with special reference to mechanical properties of the cell wall. Protoplasma. 1963;57:501–521. [Google Scholar]
- Kutschera U, Briggs WR. Growth, in vivo extensibility, and tissue tension in developing pea internodes. Plant Physiol. 1988;86:306–311. doi: 10.1104/pp.86.1.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmanoff KM, Evans ML. Kinetics of adaptation to osmotic stress in lentil (Lens culinaris Med.) roots. Plant Physiol. 1981;68:244–247. doi: 10.1104/pp.68.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart JA. An analysis of irreversible plant cell elongation. J Theor Biol. 1965a;8:264–275. doi: 10.1016/0022-5193(65)90077-9. [DOI] [PubMed] [Google Scholar]
- Lockhart JA (1965b) Cell extension. In J Bonner, JE Varner, eds, Plant Biochemistry. Academic Press, New York, pp 826–849
- Lockhart JA. Physical nature of irreversible deformation of plant cells. Plant Physiol. 1967;42:1545–1552. doi: 10.1104/pp.42.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama S, Boyer JS. Auxin action on growth in intact plants: threshold turgor is regulated. Planta. 1994;193:44–50. [Google Scholar]
- McQueen-Mason SJ, Cosgrove DJ. Expansin mode of action on cell walls. Plant Physiol. 1995;107:87–100. doi: 10.1104/pp.107.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metraux J-P, Richmond PA, Taiz L. Control of cell elongation in Nitella by endogenous cell wall pH gradients. Plant Physiol. 1980;65:204–210. doi: 10.1104/pp.65.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani K, Tominaga R. Endo-xyloglucan transferase, a novel class of glycosyltransferase that catalyzes transfer of a segment of xyloglucan molecule to another xyloglucan molecule. J Biol Chem. 1992;267:21058–21064. [PubMed] [Google Scholar]
- Nonami H, Boyer JS. Wall extensibility and cell hydraulic conductivity decrease in enlarging stem tissues at low water potentials. Plant Physiol. 1990a;93:1610–1619. doi: 10.1104/pp.93.4.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonami H, Boyer JS. Primary events regulating stem growth at low water potentials. Plant Physiol. 1990b;93:1601–1609. doi: 10.1104/pp.93.4.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonami H, Wu Y, Boyer JS. Decreased growth-induced water potential: a primary cause of growth inhibition at low water potentials. Plant Physiol. 1997;114:501–509. doi: 10.1104/pp.114.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordin L, Applewhite TH, Bonner J. Auxin-induced water uptake by Avena coleoptile sections. Plant Physiol. 1956;31:44–53. doi: 10.1104/pp.31.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega JKE. Augmented growth equation for cell wall expansion. Plant Physiol. 1985;79:318–320. doi: 10.1104/pp.79.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega JKE. Governing equations for plant cell growth. Physiol Plant. 1990;79:116–121. [Google Scholar]
- Ortega JKE. Plant and fungal cell growth: governing equations for cell wall extension and water transport. Biomimetics. 1994;2:215–227. [Google Scholar]
- Ortega JKE, Manica KJ, Keanini FG. Phycomyces: turgor pressure behavior during the light and avoidance growth responses. Photochem Photobiol. 1988;48:697–703. [Google Scholar]
- Ortega JKE, Smith ME, Erazo AJ, Bell SA, Espinosa MA. Volumetric elastic moduli of growing plant cells (abstract no. 470) Plant Physiol. 1991;96:S-73. [Google Scholar]
- Ortega JKE, Zehr EG, Keanini RG. In vivo creep and stress relaxation experiments to determine the wall extensibility and yield threshold for the sporangiophores of Phycomyces. Biophys J. 1989;56:465–475. doi: 10.1016/S0006-3495(89)82694-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passioura JB. The physical chemistry of the primary cell wall: implications for the control of expansion rate. J Exp Bot. 1994;45:1675–1682. [Google Scholar]
- Passioura JB, Fry SC. Turgor and cell expansion: beyond the Lockhart equation. Aust J Plant Physiol. 1992;19:565–576. [Google Scholar]
- Probine MC, Preston RD. Cell growth and the structure and mechanical properties of the wall in internode cells of Nitella opaca. II. Mechanical properties of the walls. J Exp Bot. 1962;13:111–127. [Google Scholar]
- Ray PM, Ruesink AW. Kinetic experiments on the nature of the growth mechanism in oat coleoptile cells. Dev Biol. 1962;4:377–397. [Google Scholar]
- Ray PM, Ruesink AW. Osmotic behavior of oat coleoptile tissue in relation to growth. J Gen Physiol. 1963;47:83–101. doi: 10.1085/jgp.47.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts K. The plant extracellular matrix: in a new expansive mood. Curr Opin Cell Biol. 1994;6:688–694. doi: 10.1016/0955-0674(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Robinson DG, Cummins WR. Golgi apparatus secretion in plasmolysed Pisum sativum L. Protoplasma. 1976;90:369–379. [Google Scholar]
- Smith RC, Fry SC. Endotransglycosylation of xyloglucans in plant cell suspension cultures. Biochem J. 1991;279:529–535. doi: 10.1042/bj2790529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling LH. Introduction to Polymer Physics. New York: John Wiley & Sons; 1992. [Google Scholar]
- Steudle E, Jeschke WD. Water transport in barley roots. Planta. 1983;158:237–248. doi: 10.1007/BF01075260. [DOI] [PubMed] [Google Scholar]
- Steudle E, Ziegler H, Zimmermann U. Water relations of the epidermal bladder cells of Oxalis carnosa Molina. Planta. 1983;159:38–45. doi: 10.1007/BF00998812. [DOI] [PubMed] [Google Scholar]
- Steudle E, Zimmermann U. Determination of the hydraulic conductivity and of reflection coefficients in Nitella flexilis by means of direct cell-turgor pressure measurements. Biochim Biophys Acta. 1974;332:399–412. [Google Scholar]
- Thimann KV, Schneider CL. The role of salts, hydrogen-ion concentration and agar in the response of the Avena coleoptile to auxins. Am J Bot. 1938;25:270–280. [Google Scholar]
- Tomos AD, Steudle E, Zimmermann U, Schulze E-D. Water relations of leaf epidermal cells of Tradescantia virginiana. Plant Physiol. 1981;68:1135–1143. doi: 10.1104/pp.68.5.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursprung A, Blum G. Eine Methode zur Messung des Wand- und Turgordruckes der Zelle usw. Jahrb Wiss Bot. 1924;63:1–110. [Google Scholar]
- Vanderhoef LN, Stahl CA. Separation of the two responses to auxin by means of cytokinin inhibition. Proc Natl Acad Sci USA. 1975;72:1822–1825. doi: 10.1073/pnas.72.5.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Y, Maclachlan GA. 1,3-β-Glucanases from Pisum sativum seedlings. II. Substrate specificities and enzyme action patterns. Biochim Biophys Acta. 1979;571:256–269. doi: 10.1016/0005-2744(79)90096-2. [DOI] [PubMed] [Google Scholar]
- Wu SC, Blumer JM, Darvill AG, Albersheim P. Characterization of an endo-β-1,4-glucanase gene induced by auxin in elongating pea epicotyls. Plant Physiol. 1996;110:163–170. doi: 10.1104/pp.110.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto R, Nevins DJ. Coleoptile growth-inducing capacities of exo-β-(1,3)-glucanases from fungi. Physiol Plant. 1981;51:118–122. [Google Scholar]
- Yamamoto R, Shinozaki K, Masuda Y. Stress relaxation properties of plant cell walls with special reference to auxin action. Plant Cell Physiol. 1970;11:947–956. [Google Scholar]
- Zhu GL, Boyer JS. Enlargement in Chara studied with a turgor clamp: growth rate is not determined by turgor. Plant Physiol. 1992;100:2071–2080. doi: 10.1104/pp.100.4.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]