Abstract
Respiratory syncytial virus (RSV) infects all children early in life, is the most common cause of infant lower respiratory tract infections, and causes disease exacerbations in children with asthma. Episodes of lower respiratory tract infection in early life are associated with asthma development. Whether RSV infection early in life directly causes asthma or simply identifies infants who are genetically predisposed to develop subsequent wheezing is debatable. Recent studies suggest that these two explanations are not mutually exclusive, and are likely both important in asthma development. An open-label study of RSV immunoprophylaxis administered to preterm infants reduced recurrent wheezing by 50%. Clinical trials of infant RSV prevention, delay or severity reduction on the outcome of childhood asthma would confirm the causal relationship between RSV infection and asthma, and offer a primary prevention strategy.
Keywords: asthma, bronchiolitis, lower respiratory tract infection, recurrent wheezing, respiratory syncytial virus
Respiratory syncytial virus (RSV) is the most common cause of lower respiratory tract infections (LRTIs), such as bronchiolitis, among infants and young children [1–5]. Approximately 70% of infants are infected with RSV during their first year of life, and almost all children have been infected at least once by 2 years of age [6]. Bronchiolitis is an acute viral infection of the small air passages of the lungs called the bronchioles, and the infant illness is clinically defined, which may vary in different regions of the world. Infants with severe bronchiolitis, which requires hospitalization, are at increased risk of developing recurrent wheezing or childhood asthma [7–11]. A total of 31% of the children who develop asthma by school age have experienced at least one episode of bronchiolitis, which requires a healthcare visit in infancy [12]. Although the association between severe bronchiolitis and asthma is widely reported, the underlying biological mechanisms through which RSV infant infection could promote asthma development are not fully understood. Proposed mechanisms through which RSV infection could increase susceptibility to asthma include chronic epithelial and airway reactivity changes to the still developing infant lung, lung injury altering lung function and immunomodulatory changes [13–16]. An alternate explanation is that there are genetic factors that impact patterns of immune response to infectious agents, which are also linked with asthma. More likely is that both mechanisms are true and important in asthma development; genetic factors impact patterns of immune response to infectious agents such as RSV, and RSV is also a causal environmental agent in asthma development [10,17–19]. Elucidation of the relationship between RSV infection and the development of asthma, as well as a better understanding of the underlying mechanisms through which RSV causes asthma have important implications for asthma prevention and treatment. The aims of this article are to review the epidemiologic, clinical, mechanistic and genetic evidence of the relationship between RSV infection and subsequent asthma. Important areas in which further research is needed are also identified.
RSV in humans
Human RSV is a single-stranded RNA virus of the Paramyxoviridae family. It is 15,222 nucleotides in length, which yields ten genes encoding 11 proteins. Of these, eight function as structural proteins and surface glycoproteins, while the remaining two function in direct viral replication [20]. Two surface glycoproteins, the fusion protein (F) and the attachment protein (G), play an important role in infectivity and pathogenesis, and are the primary targets for the host’s protective antibodies. The G protein mediates RSV attachment to the host cell, while the F protein enables fusion between the virion and the host cell plasma membrane to permit virus entry. In in vitro experiments, the F protein further promotes the aggregation of multinucleated cells through fusion of their plasma membranes, producing the classic syncytia (hence the virus’ name) and the transmission of the virus from cell to cell.
There are two distinct subtypes of RSV, antigenic subtype A and antigenic subtype B [21,22]. Subtype A and B strains can be further divided into clusters of strains with related genotypes [23–26]. The circulation pattern of RSV strains is complex. The predominant strain changes from year to year and varies among communities at any given time [25]. In general, subtype A strains are thought to be more virulent and usually the predominant circulation strains compared with subtype B strains [26–28]. This ever changing circulation pattern of RSV may be a mechanism by which RSV evades immunity. There is evidence that strain variation influences reinfection, and the same strains can reinfect the same individual [29]. This is a very different situation from that seen with rhinovirus and influenza, where the same strain virtually never re-infects.
In the USA, RSV-induced illness results in >100,000 infant hospitalizations per year [30]. The highest morbidity risk for RSV infection occurs at approximately 2 months of age [6,31]. However, for reasons that are not fully understood, the immune response to RSV is incomplete. Reinfection with RSV occurs throughout the life [29]. RSV viral DNA was found in amniotic fluid with a sonographically normal fetus, however, the mechanism, significance and potential effects of this asymptomatic viral presence are not clear [32].
Studies of the influence of RSV infection on the development of wheezing & asthma
Retrospective studies
The occurrence of recurrent wheezing after RSV LRTI has been recognized for decades. Studies have demonstrated that children hospitalized for RSV bronchiolitis during infancy were more likely to have subsequent episodes of wheezing and asthma during the first decade of life compared with children without a history of a bronchiolitis hospitalization during infancy (Table 1) [9–11,33–40]. In a 10-year follow-up study, Pullan and Hey reported that children with RSV bronchiolitis in infancy were more likely to have further episodes of wheezing compared with controls (42 vs 19%). The difference between RSV and control groups was most pronounced during the first 4 years of life, and many children with recurrent wheezing had stopped wheezing by the time they were 6 years of age. At 10 years, wheezing was diagnosed in 6.2% of the RSV bronchiolitis group and 4.5% in the control group, which was not statistically significant [33]. We conducted a population-based birth cohort study of 90,341 children, the Tennessee Asthma Bronchiolitis Study (TABS), using data from a state-based health insurance program, and reported that infants with a healthcare visit for bronchiolitis during RSV-dominant months (December–February) were more likely to develop asthma between 4 and 5.5 years of age (RR: 1.89; 95% CI: 1.80–2.00) [41]. Similar results were presented by Mok and Simpson in a 7-year follow-up study, however, the study did not differentiate between RSV- and non-RSV-induced bronchiolitis, although most infant bronchiolitis, particularly in the first 6 months of life, is secondary to RSV [42]. Schauer et al. reported that 15.5% of children with a history of RSV bronchiolitis in infancy had had recurrent wheezing during the subsequent year, while only 3.6% of controls had ever wheezed [34]. In a retrospective case–control study, Singleton et al. reported that children who were hospitalized with RSV at less than 2 years of age were more likely to wheeze at age 3–4 years, but there was no difference at age 5–6 years from control subjects [35]. In a retrospective cohort including 150 infants with RSV bronchiolitis, Henderson et al. reported that infants with a history of a RSV bronchiolitis hospitalization were more likely to have wheezing at 30–42 months (OR: 2.3; 95% CI: 1.3–3.9), wheezing at 69–81 months (OR: 3.5; 95% CI: 1.8–6.6) and doctor-diagnosed asthma at 91 months of age (OR: 2.5; 95% CI: 1.4–4.3) as reported by parents [11]. Similarly, Fjaerli et al. found that children hospitalized with bronchiolitis, whether RSV+ or RSV−, had a higher frequency of wheezing, and were more likely to be diagnosed with asthma at 7 years of age compared with children who were not hospitalized during infancy for bronchiolitis [43]. In longer term follow-up studies, including a 20-year follow-up study, Korppi et al. reported that asthma was present in 17–22% of 18–20-year-old subjects who had RSV bronchiolitis or RSV pneumonia before age 24 months, compared with 11% in the control group [39]. Limitations exist in some of the studies cited, including small sample sizes, use of external control groups, and lack of population-based reference groups. In addition, recurrent wheeze in young children is probably not the same as asthma. Lastly, most studies focus on bronchiolitis, the most severe manifestation of respiratory viral infection, while the vast majority of infants have RSV upper respiratory infection (URI). Deficiencies exist in the literature as to whether viral infection in general poses a risk for childhood asthma.
Table 1.
Review of studies of wheezing and/or asthma in children following respiratory syncytial virus healthcare event during early childhood.
| Study (year) | Study design | Subjects with RSV infection (n) | Total (n) | Findings | Ref. |
|---|---|---|---|---|---|
| Pullan and Hey (1982) | Retrospective cohort | 130 RSV hospitalizations | 241 | No significant difference in wheezing at 10 years of age | [33] |
| Schauer et al. (2002) | Prospective cohort | 42 RSV hospitalizations | 126 | Infants with a RSV bronchiolitis hospitalization are more likely to have wheezing during the subsequent year | [34] |
| Singleton et al. (2003) | Retrospective cohort | 95 RSV hospitalizations | 208 | Infants with a RSV bronchiolitis hospitalization are more likely to wheeze at age 2–4 years, but at age 5–6 years there is no difference from control subjects | [35] |
| Sigurs et al. (1995) | Prospective cohort | 47 RSV hospitalizations | 140 | Infants with a RSV bronchiolitis hospitalization are more likely to have asthma in the subsequent 2 years | [36] |
| Sigurs et al. (2000) | Prospective cohort | 47 RSV hospitalizations | 140 | Infants with a RSV bronchiolitis hospitalization are more likely to have asthma at 7 years of age | [9] |
| Sigurs et al. (2005) | Prospective cohort | 46 RSV hospitalizations | 138 | Infants with a RSV bronchiolitis hospitalization are more likely to have allergic asthma at 13 years of age | [10] |
| Sigurs et al. (2010) | Prospective cohort | 46 RSV hospitalizations | 138 | Infants with a RSV bronchiolitis hospitalization are more likely to have allergic asthma at 18 years of age | [38] |
| Stein et al. (1999) | Prospective cohort | 207 RSV infection | 888 | Children with a RSV lower respiratory tract illness in early childhood have an increased risk of recurrent wheezing by 6 but not 13 years of age | [37] |
| Korppi et al. (2004) | Prospective cohort | 36 RSV hospitalizations | 81 | Hospitalization for RSV bronchiolitis or RSV pneumonia before age 24 months was not a significant risk factor for asthma at 18–20 years of age | [39] |
| Henderson et al. (2005) | Retrospective cohort | 150 RSV hospitalizations | 13,971 | Infants with a RSV bronchiolitis hospitalization are more likely to have wheezing at 30–43 months, wheezing at 69–81 months, and asthma at 91 months of age | [11] |
| Carroll et al. (2009)† | Retrospective cohort | 12,916 RSV healthcare encounters | 90,341 | Infants with at least one RSV healthcare encounter are more likely to have childhood asthma by 5.5 years of age | [41] |
| Escobar et al. (2010) | Retrospective cohort | 1181 RSV infections | 71,102 | Infants with a RSV infection involving hospitalization were at greater risk for recurrent wheezing at 3 years of age than those with RSV infection involving an outpatient encounter compared with infants without any healthcare visit for RSV | [40] |
The RSV healthcare encounters in this study are defined as any healthcare encounters that occured during winter months (November–April). No test has been carried out to confirm RSV.
RSV: Respiratory syncytial virus.
Longitudinal studies
Two ongoing longitudinal studies of infants with RSV bronchiolitis suggest that RSV infection does increase the risk of wheezing and later childhood asthma [9,37]. Sigurs et al. followed a cohort of 47 Swedish infants who had RSV bronchiolitis hospitalization from December 1989 to April 1990 as well as 93 age- and gender-matched controls who were hospitalized during that same period but without RSV infection. The researchers reported increased prevalence of asthma or recurrent wheezing and allergic sensitization in children with RSV bronchiolitis at age 3, 7, 13 and 18 years [9,10,38]. At age 18 years, 39% of the children who had suffered from RSV bronchiolitis in infancy were documented to have asthma or recurrent wheezing compared with only 9% in the control group. In addition, children who had RSV bronchiolitis were more likely to have a positive skin test compared with control children (41 vs 14%) [38]. Further analysis of the longitudinal data over the entire study period suggested that children with RSV bronchiolitis during infancy were diagnosed with asthma at an earlier age compared with those of control children. Among children who had RSV LRTI as infants, reduced lung function was evident as early as the age of 7 years [38]. Somewhat different results were obtained in the Tucson Children’s Respiratory Study which prospectively enrolled 1246 infants born between 1980 and 1984 [37]. In this study, LRTI with confirmed RSV infection was associated with increased risks of infrequent and frequent wheezing at 6 years of age, but the risk was reduced as children became older, and was not statistically significant by age 13 years. Taken together, these studies demonstrate that severe RSV bronchiolitis is associated with a 30–40% increased risk of subsequent recurrent wheezing or asthma, at least within the first decade of life. The difference in asthma risk after the first decade of life between the two studies might be simply due to the differences in the populations studied, as well as the severity of infant RSV infection between the two groups. The Swedish case–control study enrolled at-risk patients, and all infants with RSV bronchiolitis were hospitalized, while the Tucson Children’s Respiratory Study was a study of infants who were not selected for asthma risk and were enrolled at birth. Furthermore, the control children in the Swedish cohort might be fundamentally different from the children who had RSV bronchiolitis, as they did not develop bronchiolitis despite enrollment during an ongoing RSV epidemic. Recently, follow-up of the children at age 22 years in the Tucson Children’s Respiratory Study has been reported. Asthma at age 22 years has been found to be associated with recurrent wheezing at age 6 years [44]. Although the relationship between RSV infection in early childhood and later asthma development was not significant at age 13 years in this cohort of children, a study of the association of asthma at age 22 years with RSV infection during early childhood will provide insights into the importance of this early life event.
Severity of RSV infection
Although numerous investigations have been conducted examining RSV infection and subsequent recurrent wheezing and asthma, most of them focus on bronchiolitis, the most severe manifestation of respiratory viral infection. Deficiencies exist in the literature as to whether viral infection, in general, poses a risk for childhood asthma. We identified a severity-dependent relationship of infant bronchiolitis severity with asthma risk using the TABS cohort, demonstrating that the more severe the infant bronchiolitis event, the greater the odds of developing childhood asthma [12]. We further demonstrated that there was a severity-dependent relationship between infant bronchiolitis with increasing severity of childhood asthma defined by hospitalization, emergency department visit for asthma or requirement for rescue corticosteroids. Although our study assessed bronchiolitis events during winter virus season, when most infant bronchiolitis is secondary to RSV, a limitation of our study is lack of identification of the viral etiology of the bronchiolitis event. Using data from a managed care organization, Escobar et al. sought to determine whether the severity of bronchiolitis affects risk of recurrent wheezing by age 3 years [40]. Compared with infants without any visit for RSV, infants with an outpatient encounter (adjusted odds ratio [AOR]: 2.07; 95% CI: 1.61–2.67), an uncomplicated hospitalization (AOR: 4.66; 95% CI: 1.61–2.67) and a prolonged hospitalization (AOR: 3.42; 95% CI: 2.01–5.82) for RSV similarly had increasing risk for recurrent wheezing at age 3 years with increased severity of infant RSV illness [40]. Both of these investigations only assessed severity levels for bronchiolitis requiring healthcare encounters; the effect of RSV infection not requiring a healthcare encounter and RSV URI has not been studied.
Evidence of causal relationship of RSV infection on asthma development
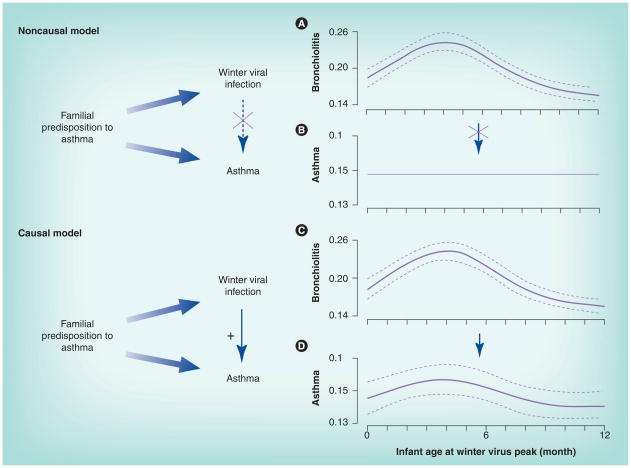
While an association between RSV infection during infancy and development of childhood asthma is well documented, causation has been long debated: does RSV infection confer a long-term change in the host, which increases the subsequent risk of asthma? Or is RSV bronchiolitis simply an early marker of an underlying predisposition for asthma? A few recent studies have aimed to address this question. In the TABS study, we specifically evaluated the causal role of winter viral respiratory epidemics in the development of asthma among nearly 100,000 infants in a retrospective birth cohort [45]. We did this by investigating the relationship between infant age at the first winter viral peak following birth and subsequent asthma risk during the fifth to sixth year of life. Infants who were 4 months of age at the peak of winter viral season were more likely to develop both clinical bronchiolitis and childhood asthma. Despite the winter viral peak shifting by nearly 2 months over the six winter viral seasons studied, the risk of asthma shifted in any given year with the shift in the peak of the winter viral peak, such that infants born approximately 4 months prior to the first winter viral peak that they encountered following birth were at the highest risk of developing childhood asthma (Figure 1). This strongly suggests a causal role of early RSV infection on asthma development. Infants may be most susceptible to RSV during this period, as infants less than 4 months of age have lost most maternal antibodies, and their IgG is at its nadir [46–48]. In addition, RSV infection during this period of infancy, when both the immune system and developing lung are immature, affects immune regulation and lung development possibly resulting in chronic effects on the airway [49]. Three additional studies have been done to assess causality between RSV infant infection and childhood asthma using a population-based study among Denmark twins [50–52]. Thomsen et al. fitted genetic variance components and direction of causation models to over 8000 twin pairs [50]. A model in which RSV hospitalization causes asthma was rejected, whereas one in which asthma causes RSV hospitalization was not. In another attempt to detect the causality between RSV and asthma, Stensballe et al. reported that the effect of RSV hospitalization on asthma was only short term (2 months after RSV hospitalization) and no longer significant 1 year later [51]. Lastly, a study of 37 monozygotic twin pairs discordant for severe RSV bronchiolitis in infancy indicated no differential effect of severity of RSV infection on the development of asthma [52]. All three twin studies utilized RSV bronchiolitis information from patient registries. However, the patient registry does not contain information on RSV outpatient visits. Twins who were discordant for RSV hospitalization were highly likely to be concordant for RSV infection given the known high infectivity. Therefore, any conclusion about causality suffers from the misclassification of twins not hospitalized with RSV infection to an uninfected group. In addition, asthma was defined at an early age in two of the three twin studies. Children were considered as having asthma as early as infancy [51] or by 3 years of age [50], which may well reflect children with ‘transient early wheezing’ but not asthma [18]. To truly understand whether RSV infection causes asthma or not, prospective trials aimed at RSV prevention, delay or severity reduction, utilizing either vaccination (not currently available), RSV immunoprophylaxis or birth timing would provide further insight into this decades old debate.
Figure 1. Noncausal and causal explanation of the relationship between winter viral infection and early childhood asthma.
In the noncausal model, a common genetic predisposition to asthma is associated with both winter viral infection and asthma, and is a confounder of the association between winter viral infection and asthma. Thus, timing of infant birth in relationship to winter virus peak has a seasonal effect on bronchiolitis (A), but not on asthma (B). In the causal model, while familial predisposition to asthma relates to both winter viral infection and asthma, winter viral infection is in the causal pathway of development of asthma. Timing of infant birth in relationship to winter virus season relates to both bronchiolitis (C) and asthma risk (D) in an identical way. The solid and dashed lines in (A), (C) and (D) are predicted probability and corresponding 95% CI of developing infant bronchiolitis and childhood asthma by infant age in months at the winter virus peak from multivariable logistic regression models. The solid line in (B) represents childhood asthma prevalence in the population.
Reprinted with permission from the American Thoracic Society from [45]. © American Thoracic Society.
Genetic studies
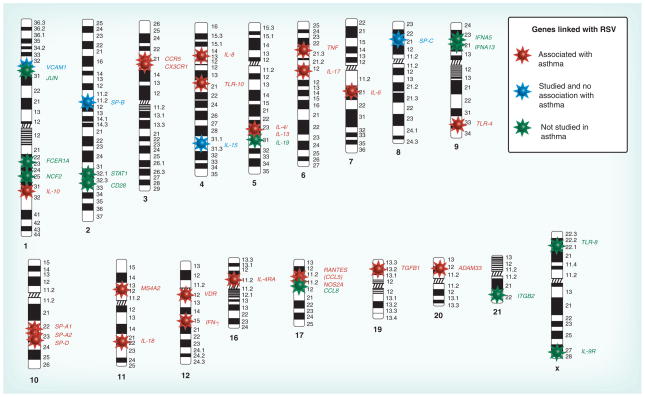
Because RSV infection is associated with asthma inception, it makes sense that genes may associate with both ailments. Figure 2 illustrates genes associated with RSV and asthma, updated from our prior review [53]. Polymorphisms in IL-10, CCR5, TLR-10, IL-4, IL-13, IL-8, IL-18, TNF, TLR-4, MS4A2, VDR, IL-4Rα, RANTES, TGFB1 and ADAM33 were associated with asthma risk, while polymorphisms in VCAM1, IL-15, SP-B and SP-C were not linked with asthma risk. The association with asthma risk of genes colored in green has not yet been studied. In the subsequent paragraphs we describe the associations with RSV and their likely importance related to asthma, as well as the functions of these genes, if known.
Figure 2. Candidate genes associated with severe RSV infection and asthma.
Genes colored in red are those associated with RSV infection with at least one significant association that overlaps with asthma studies, where the evidence of an association with asthma is strongly supported; genes colored in blue indicate the genes that are associated with RSV infection but not with asthma; genes colored in green are genes associated with RSV infection but with an unconfirmed association with asthma.
RSV: Respiratory syncytial virus.
Updated and reprinted with permission from the American Thoracic Society from [53]. © American Thoracic Society.
Multiple genes have been identified to be associated with increased risk of bronchiolitis and/or severity of RSV infection. The study of genetic associations related to RSV infection have, to date, been limited to candidate gene approaches in which only specific individual genes that are thought to be involved in the regulation of the immune response and that reflect our current concept of RSV pathogenesis are studied. Most of the genes are involved either in direct pathogen control (innate defense), immune response or modifying later immunopathology.
However, one difficulty in using a candidate gene approach is that this likely gene selection bias makes it difficult to interpret results, as genes exhibit effects that might be partially dependent on interaction with other genes or environmental factors. Avoiding some of these issues, Janssen et al. studied 384 single nucleotide polymorphisms (SNPs) in 220 candidate genes across different categories of polymorphic immune response genes in 470 infants hospitalized for RSV bronchiolitis [54]. SNPs in the innate immune genes, including vitamin D receptor gene (VDR), nitric oxide synthase (NOS2A), the Jun oncogene (JUN), and IFN-α (IFNA5), demonstrated the strongest association with RSV bronchiolitis. VDR is associated with downregulating IL-12 and IFN-γ productions, the T helper 1 (Th1) cytokines associated with pathogen clearing, VDR activation may cause a development shift of Th cells toward Th2 cytokines and thus induce asthma. Recently, Poon et al. demonstrated that SNPs of the VDR gene were associated with asthma [55]. NOS2A is well known for its antimicrobial and anti-inflammatory roles. NOS2A produces nitric oxide in response to environmental stimuli such as RSV infection, and might be associated with asthma. In the Southern California Children’s Health Study, Islam et al. reported an association of common haplotypes in the NOS2A promoter region with new-onset asthma [56]. JUN is an important transcriptional regulator in innate immune pathways; and IFNA5 is a transcriptional regulator in innate immune pathways.
RSV G protein has been identified as being the binding protein crucial for viral attachment to host cells. There is evidence that the host CX3C receptor (CX3CR) is involved in the binding of the RSV G protein to leukocytes of the host cells. CX3CR is a leukocyte receptor for fractalkine, currently the only identified member of the CX3C chemokine subfamily. Recent studies suggest that immunologic response during RSV infection is partially modified through the interaction of viral G glycoprotein with the host chemokine receptor, CX3CR1. In a study of 82 children hospitalized for RSV bronchiolitis, Amanatidou et al. demonstrated a strong association between one nonsynonymous CX3CR1 SNP, T280M and severe RSV-induced bronchiolitis [57]. Results of the association between CX3CR1 and asthma are mixed. Tremblay et al. reported a significant association of three CX3CR1 SNPs, including T280M, with asthma in a population who originated from France [58], while such an association was not found in a group of German children [59].
TLR-4 is a key regulator of both innate and adaptive immune responses that recognizes pathogen-associated molecular patterns and activates the inflammatory cells. TLR-4 contributes to immune reorganization of RSV. Recent studies in peripheral blood monocytic cells (PBMCs) suggest that RSV F protein activates the innate immune response via CD14 and TLR-4 [60,61]. TLR-4 polymorphism has been shown to be associated with increased risk of asthma in a group of Swedish children [62], while no association was seen in several other investigations [63,64].
Pulmonary surfactant consists of a lipid and protein complex, essential for normal lung function. Five surfactant proteins (SPs) are currently known (SP-A1, SP-A2, SP-B, SP-C and SP-D). In addition to its biophysical function, some surfactant components play an important role in the innate and adaptive immunity of the lung. SPs participate in host-defense pathways, such as the regulation of proinflammatory cytokine production, chemotaxis or tissue repair. Association studies have demonstrated that these SPs are associated with severe RSV infection [65–69]. Airway inflammation, a characteristic of asthma, may inhibit surfactant function. On the other hand, β2-adrenergic agonists can cause fresh surfactant to be released into alveoli which may improve asthma symptoms. SP-A and SP-D are associated with asthma, while recent study suggests that SP-B and SP-C are not associated with asthma [69,70].
RANTES is a member of the CC chemokine subfamily [71]. It is considered a major chemokine implicated in the airway inflammation of asthma. A variant in the promoter region of RANTES has been shown to be associated with high risk of asthma [72]. The impact of the RANTES pathways in RSV infection has been studied in a case–control genetic association study. The concentration of RANTES in children with RSV infection was significantly higher than those from the control children [73]. RANTES is also strongly associated with severe RSV-induced bronchiolitis [71]. By comparing 320 children with RSV bronchiolitis with 270 controls without RSV bronchiolitis, Tian et al. reported that a polymorphism in RANTES (-403 G/A) increased the transcriptional activity of the RANTES promoter, resulted in a high serum RANTES levels, and thus increased the risk of recurrent wheezing at 3 years of age after RSV bronchiolitis [74].
Interleukins are cytokines produced mainly by leukocytes. IL-4 and IL-13 are cytokines produced by type 2 T cells and are critical mediators in asthma. In a study evaluating the genetic variability of IL-4 and IL-13 in 131 children with severe RSV infection, Puthothu et al. reported a significant association between IL-13 and severe RSV infection [75]. The IL-4 590T allele was also reported to be more prevalent among children hospitalized with RSV compared with controls [76]. IL-10 is a cytokine with anti-inflammatory properties suppressing the Th1-like immune response and promoting a Th2-like response seen in asthma. IL-10 inactivates cell-mediated immune response through a combination of cytokine, chemokine and antigen presentation inhibition [77]. Genetic variability in IL-10 is associated with clinical presentation and severity of RSV infection [78–80].
IL-8 is a member of the CXC chemokine subfamily. It is a strong inductor of inflammation, mediating the activation and migration of neutrophils from peripheral blood into tissue [81]. Thus, IL-8 is important in inflammatory lung diseases such as asthma or severe infections such as RSV. Hull et al. identified a common variation in IL-8 at position -251, further demonstring that the IL-8 -251A allele was associated with increased IL-8 production, and thus increased risk of severe RSV-induced bronchiolitis in infancy [82]. The same investigators replicated these findings in a second population [83]. Puthothu et al. genotyped two IL-8 polymorphisms in the promoter region of 322 children with asthma, 131 infants with severe RSV-associated diseases and 270 controls. Although the author failed to reproduce the association between IL-8 -251 variation with RSV-induced disease, they did find that one IL-8 polymorphism and the IL-8 haplotype are associated with asthma [84]. In a family-based association study of 134 children who had bronchiolitis, Goetghebuer et al. reported that a variant in the IL-8 gene was transmitted significantly more often than expected in the children with recurrent wheezing [85].
IL-18 is a member of the IL-1 cytokine family and is expressed by a wide range of cells in humans [86]. Similar to IL-1, IL-18 participates in both innate and acquired immunity. Puthothu et al. tested six polymorphisms within the IL-18 gene to explore the association between the genetic diversity of IL-18 and severe RSV-associated respiratory disease [87]. The investigators reported the intron 133 G/C polymorphism associated with increased risk of severe RSV bronchiolitis. The association was further enhanced by haplotype analyses including all six polymorphisms. The association of IL-18 with asthma has also been studied. A functional polymorphism in IL-18 is reported to be associated with the severity of bronchial asthma in a Japanese population [88]. In a case–control study comparing patients with allergic bronchial asthma, patients with atopic dermatitis, and controls without any allergic disease, El-Mezzein et al. reported a significant increased IL-18 secretion by PBMC from patients with asthma and atopic dermatitis. The amount of IL-18 did not differ between patients with asthma and atopic dermatitis [89].
Most genetic studies of RSV infection and asthma currently focus on single genes. Gene–gene and gene–environment interactions may be more important determinants for complex situations, such as the relationship between infant RSV infection and asthma development, than have been previously recognized. For example, a recent study in asthma has demonstrated a significant gene–gene interaction between IL-13 and IL-4Rα. Polymorphisms in both genes resulted in a fivefold increased risk in asthma compared with nonrisk genotypes [90]. A significant gene–environment interaction between CD14 and endotoxin on both risk and severity of asthma has recently been reported [91–94]. Among subjects exposed to low levels of house dust endotoxin, the TT genotype for CD14/-260 appeared protective for asthma (OR: 0.09; 95% CI: 0.03–0.27). However, TT individuals with high exposure to endotoxin were more than 11 times (95% CI: 1.03–131.7) more likely to have asthma than individuals with the CC genotype [94]. As infancy is a period of rapid growth for immune and pulmonary systems, gene–environment interactions are likely to be extremely important in determining the risk that infant RSV infection confers on childhood asthma. RSV infection as an environmental ‘hit’ may be most influential among specific host genotypes and at certain critical periods of development during infancy. Understanding the mechanisms through which RSV promotes an asthma phenotype, identifying the susceptible genotype(s) as well as the critical time window of infection may help to indentify novel biological targets for therapeutics and intervention for asthma primary prevention. Another problem with current genetic research trying to establish a link between RSV and/or asthma susceptible genes is that there are insufficient replication studies. This is especially true for genes with no association reported. Even if a positive association is replicated, often the positive association observed in one report is not reproduced in subsequent studies. As a result, we are a long way from applying of information learned from genetic studies to disease prevention and intervention.
Biological evidence of how RSV causes asthma
Immune response
Infant immune modulation is one proposed mechanism through which RSV may cause subsequent asthma. It has been shown that RSV viral infection in infancy may alter the subsequent Th1/Th2 immune response, enhance Th2 sensitization to aeroallergens, and thus induce the development of a chronic asthma phenotype [95,96]. Alteration of Th1 and Th2 cytokine levels have been linked to severe RSV bronchiolitis. Severe RSV disease is associated with Th2 polarization of the lung immune response and may then ‘sensitize’ the host’s response towards allergic responses against other molecules [97]. In animal studies, RSV primary infection during the neonatal period predisposes BALB/c mice to a more severe disease upon reinfection in adulthood compared to mice with delayed RSV primary infection, which is linked to T cells, IL-13 and IgE [98–101]. Upon infection, innate cytokines, including type I interferons, as well as IL-12 and IL-18 are generated [102]. In response to RSV infection, airway epithelial cells produce a spectrum of cytokines and chemokines, including IL-10, IL-8, RANTES, macrophage inflammatory protein (MIP)-1β, CCL2 and eotaxin. Levels of these molecules are correlated with the severity of the disease [103]. IFN-γ expression in PBMCs is reduced among infants with more severe RSV bronchiolitis compared with those with mild disease [104]. In addition, hospitalized infants were reported to have diminished IFN-γ production from PBMCs during and in the months after RSV bronchiolitis, but only in those children who later developed asthma [105]. However, it is unclear if the PBMCs reflect the local response that occurs in the lung following acute RSV infection.
RSV F protein is a strong agonist of the pattern-recognition receptor TLR-4. Activation of TLR-4 in the respiratory epithelium mediates inception of asthma caused by house dust mites (HDMs), the most frequent cause of childhood allergic asthma [106]. As TLR-4 is a pattern recognition receptor that is not antigen-specific, a high RSV pulmonary load would activate TLR-4 to initiate an allergic response against environmental allergens, of which HDM is the most common. In this case, more severe RSV should correlate with higher viral loads and consequent potent TLR-4 activation followed by allergic asthma [107,108].
In understanding the possible link between acute viral infection and pathogenesis of a chronic inflammatory disease such as asthma, Kim et al. developed a mouse model of chronic lung disease that resembled asthma in humans [109]. The authors reported that the chronic inflammatory disease arose independently of an adaptive immune response and is driven by IL-13 produced by macrophages that are stimulated by CD1d-dependent TCR-invariant natural killer (NK)T cells. The authors concluded that persistent activation of a novel NKT cell–macrophage innate immune axis was required for the transition from viral infection to chronic lung disease [109].
Neuro-immune interactions
Recent studies have reported that RSV infection affects neurogenic inflammation in the airways. RSV infection in early life promotes an overexpression of nerve growth factor (NGF) and its receptors in the developing lungs of both animal models and humans [110,111]. NGF is a key regulatory element of neuronal development and responsiveness [112]. It controls the structural development of peripheral afferent and efferent neurons, and exerts ‘neuronal plasticity’, an ability of the nervous system to change their functional activity in response to developmental and environmental changes [113]. Thus, RSV-induced overexpression of NGF can cause airway hyperreactivity during and after the infection by driving both short- and long-term changes in the distribution and reactivity of sensory and motor nerves across the pulmonary systems. In animal studies, Auais et al. reported that NGF overexpression is critical for neurogenic-mediated mucosal edema and for innate lymphocytic and monocytic responses in RSV-infected airways, suggesting neuro-immune interactions in the pathophysiology of local and systemic inflammation [114]. Lastly, patients with bronchial asthma and allergic rhinoconjunctivitis have high serum levels of NGF, suggesting an important pathogenic role of neurotrophins in allergic disorders [115].
Lung development
Lung development is a process beginning by 4 weeks of gestation and continuing for years after birth [116]. At birth, the lung starts to differentiate alveoli (alveolarization), which continues for 2–3 years postnatally. The temporal sequence of alveolarization corresponds with the age at which children are most likely to have a RSV LRTI. RSV LRTI is known to cause lung injury resulting in chronically diminished lung function throughout childhood, and may cause persistent airway hyperreactivity and chronic epithelial changes [117–120]. In rat models, virally infected outbred rats during early life exhibited abnormal alveolar development and bronchiolar hypoplasia which were associated with abnormalities in pulmonary function; subsequent continued postnatal lung growth could not compensate for early virus-induced abnormalities in alveolar and bronchiolar growth [121]. Whether or not there are long-term consequences on lung function resulting from viral-induced acute inflammatory responses, along with efforts to repair viral-induced damage to lung tissue, has been studied. Weanling rats infected with parainfluenza virus develop a chronic asthma phenotype characterized by episodic and reversible airway obstruction. Furthermore, this asthma phenotype was genotype specific (only occurred in a genetically susceptible brown Norway strain, as opposed to the resistant Th1-skewed F344 strain), and were induced at a critical time point during development [122,123].
Critical RSV-susceptibility period during infancy
Relevant to both the immune and pulmonary responses, the stage of development may affect the severity of infection, and in turn the long-term consequences of infection. In other words, the impact of RSV infection may have the greatest impact during a particular ‘susceptibility’ period during infancy. We have shown that infants born in the fall in the Northern hemisphere and hence approximately 4 months of age at the peak of the winter viral season have the highest risk of developing both bronchiolitis and childhood asthma [45]. Animal studies have also found that viral infection had a much more marked effect on neonatal rats in the proliferative stage of lung growth than on weanlings in the equilibrated stage of lung growth [121]. Using a murine model, Culley et al. demonstrated that age at first RSV infection determined the pattern of T-cell-mediated disease during reinfection in adulthood [98]. Neonatal priming by RSV infection increased inflammatory cell recruitment (including Th2 cells and eosinophils) during reinfection, whereas delayed priming led to enhanced IFN-γ production and less severe disease during reinfection. Thus, LRTIs during an active period of immune and lung development could adversely affect these processes and result in airway remodeling or interfere with the generation of new alveoli [49]. Uhl et al. compared viral-induced structural abnormalities between virus-susceptible brown Norway rats with virus-resistant F344 rats infected with parainfluenza virus as neonates [122]. Following infection they reported abnormal pulmonary function in brown Norway rats and no physiologic abnormalities in F344 rats. Subsequent studies in rats infected as weanlings, with more fully developed alveoli, confirmed that the postviral asthma-like phenotype was independent of alveolar dysplasia [123]. These findings strongly suggest that the impact of infant viral infection on the developing immune and lung systems and subsequently resulting in an asthma phenotype may have to occur during a critical susceptibility period and in genetically susceptible hosts.
RSV prevention, delay or severity reduction & asthma
Ultimately, to address the question of whether RSV infection during infancy causes asthma, showing that prevention or delay in infant infection prevents asthma will be necessary. In randomized clinical trials, RSV immunoprophylaxis has been proven to have efficacy in reducing RSV-related morbidity in high-risk infants [124,125]. Currently, means to prevent infant RSV infection are limited to include birth timing, avoidance and use of RSV immunoprophylaxis.
RSV immunoprophylaxis is one of the most costly pediatric medications. Two products, RSV-IVIG (RespiGam® [MedImmune, LLC, Gaithersburg, MD, USA], no longer available) and palivizumab (Synagis® [MedImmune, LLC, Gaithersburg, MD, USA]), have been licensed by the US FDA in the last decade for use in preventing severe RSV infections in high-risk infants, including children younger than 24 months with chronic lung disease (bronchopulmonary dysplasia), other high-risk cardiopulmonary conditions and certain preterm infants. Both products are administered monthly to high-risk infants throughout the RSV season. Ribavirin (1-β-D-ribofuranosyl-12-triazole-3-carboximide) is a synthetic nucleoside analog with a broad-spectrum and in vivo activity against RNA and DNA viruses. It can be used to treat RSV infection through preventing RSV reproduction, therefore minimizing potential RSV induced complications such as pneumonia or bronchiolitis.
There is very limited data, in the form of two small studies, which suggests that RSV immunoprophylaxis may lower the likelihood for the development of asthma. The first is a small investigation of 13 children identified retrospectively as having received RSV immunoprophylaxis and a matched retrospective comparison group of 26 children who did not receive immunoprophylaxis studied at a mean age of 8 years, showing that receipt of RSV immunoprophylaxis during infancy may have long-term effects on respiratory and immunologic parameters relevant to the development of asthma [126]. The second study is an industry-sponsored investigation of open-label compassionate-use RSV immunoprophylaxis among a European cohort of preterm infants. In total, 191 infants received palivizumab and were not hospitalized for RSV, and 230 did not receive palivizumab. There are substantial limitations to the design and selection of subjects for both groups, including use of RSV immunoprophylaxis on a compassionate use basis with no delineated indications or standard recommendations for use in each child [127,128]. In addition, the age of the children in this investigation at follow-up was only 19–43 months, and ‘recurrent wheezing’ during early childhood, not asthma, was the outcome of interest. We are involved in a large-scale study of the impact of reducing RSV morbidity on asthma inception, which is the next step in answering the question of whether RSV causes asthma [126,129].
Lastly, an open-label study assessing the impact of treating (not preventing) active RSV bronchiolitis with the antiviral agent ribavirin showed a reduction in the risk of asthma and allergic sensitization at 6 years of age among children 2 years of age and less who did (n = 40) and did not (n = 44) receive ribavirin for RSV bronchiolitis [130]. This study also has limitations, in that those who did and did not receive ribavirin differed significantly in age of RSV bronchiolitis, prematurity and disease severity [130].
Expert commentary & five-year view
RSV viral infection is a leading worldwide cause of serious LRTI and resultant morbidity and mortality in infancy and early childhood. RSV LRTIs are strongly associated and likely causal in the development of asthma, the most common chronic disease in childhood, causing significant morbidity and mortality throughout life. To date, most studies of asthma after RSV infection have focused on the most severe RSV episode during infancy, which only comprises a very small percentage of RSV-related respiratory illness. Studies on severity of RSV bronchiolitis measured by the type of healthcare encounter indicated a severity-dependent relationship between RSV bronchiolitis and risk of asthma. The more severe the bronchiolitis healthcare encounter is during infancy, the higher the risk of developing asthma later. While a common genetic predisposition for severe infant RSV viral infection and childhood asthma is likely to result in an increased risk of both early viral infection and subsequent recurrent wheezing, evidence also suggests that RSV viral infection has the capacity to directly cause subsequent recurrent wheezing and asthma. Although RSV infection in early life is significantly associated with subsequent recurrent wheezing and asthma, and recent data supports a causal role, ultimately, whether prevention or modification of the host response to RSV infection during infancy can prevent or decrease the risk of developing asthma must be answered in a randomized clinical trial. The following studies/steps will aid in identifying novel biological targets for primary disease prevention and treatment:
To evaluate whether modification of infant RSV infection risk or severity can prevent asthma using strategies such as RSV immunoprophylaxis and birth timing;
To understand the mechanisms through which infant RSV infection impacts the development of asthma, and to understand and identify the critical time periods during which RSV infection confers the greatest impact on asthma risk;
While RSV infection is ubiquitous in young children, not all infants who develop bronchiolitis later develop asthma, thus, understanding how RSV infection interacts with genetic and other environmental risk factors will be important in asthma prevention, as well as selecting high-risk populations for primary prevention interventions.
Key issues.
Respiratory syncytial virus (RSV) is the leading cause of hospitalization for lower respiratory tract infection in infants.
RSV bronchiolitis is associated with increased risk of recurrent wheezing and asthma until early adulthood.
A common genetic predisposition for both RSV infection and asthma leads to increased risk of both diseases.
RSV infection during infancy might increase the susceptibility to asthma by impairing the developing immune and pulmonary systems of infants.
An RSV prevention trial would provide proof of the causal relationship between RSV infection and development of asthma.
Knowledge of the causal relationship between RSV infection and asthma offers the potential of preventing or altering the phenotypic expression of childhood asthma in susceptible hosts.
Acknowledgments
Financial & competing interests disclosure
Pingsheng Wu has received funding from the NIH (grant no. K12 HD 043483). Tina V Hartert has received funding from the NIH (grant no. AI77930). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Glezen WP, Loda FA, Clyde WA, Jr, et al. Epidemiologic patterns of acute lower respiratory disease of children in a pediatric group practice. J Pediatr. 1971;78(3):397–406. doi: 10.1016/s0022-3476(71)80218-4. [DOI] [PubMed] [Google Scholar]
- 2.Carlsen KH, Orstavik I, Halvorsen K. Viral infections of the respiratory tract in hospitalized children. A study from Oslo during a 90 months’ period. Acta Paediatr Scand. 1983;72(1):53–58. doi: 10.1111/j.1651-2227.1983.tb09663.x. [DOI] [PubMed] [Google Scholar]
- 3.Wright AL, Taussig LM, Ray CG, Harrison HR, Holberg CJ. The Tucson Children’s Respiratory Study. II Lower respiratory tract illness in the first year of life. Am J Epidemiol. 1989;129(6):1232–1246. doi: 10.1093/oxfordjournals.aje.a115243. [DOI] [PubMed] [Google Scholar]
- 4.Jartti T, Lehtinen P, Vuorinen T, et al. Respiratory picornaviruses and respiratory syncytial virus as causative agents of acute expiratory wheezing in children. Emerg Infect Dis. 2004;10(6):1095–1101. doi: 10.3201/eid1006.030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calvo C, Garcia-Garcia ML, Blanco C, Pozo F, Flecha IC, Perez-Brena P. Role of rhinovirus in hospitalized infants with respiratory tract infections in Spain. Pediatr Infect Dis J. 2007;26(10):904–908. doi: 10.1097/INF.0b013e31812e52e6. [DOI] [PubMed] [Google Scholar]
- 6.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140(6):543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 7.Noble V, Murray M, Webb MS, Alexander J, Swarbrick AS, Milner AD. Respiratory status and allergy nine to 10 years after acute bronchiolitis. Arch Dis Child. 1997;76(4):315–319. doi: 10.1136/adc.76.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray M, Webb MS, O’Callaghan C, Swarbrick AS, Milner AD. Respiratory status and allergy after bronchiolitis. Arch Dis Child. 1992;67(4):482–487. doi: 10.1136/adc.67.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000;161(5):1501–1507. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 10.Sigurs N, Gustafsson PM, Bjarnason R, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171(2):137–141. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 11.Henderson J, Hilliard TN, Sherriff A, Stalker D, Al Shammari N, Thomas HM. Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: a longitudinal birth cohort study. Pediatr Allergy Immunol. 2005;16(5):386–392. doi: 10.1111/j.1399-3038.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 12••.Carroll KN, Wu P, Gebretsadik T, et al. The severity-dependent relationship of infant bronchiolitis on the risk and morbidity of early childhood asthma. J Allergy Clin Immunol. 2009;123(5):1055–1061. doi: 10.1016/j.jaci.2009.02.021. First study to demonstrate that the risk and severity of asthma is associated with the risk and severity of infant bronchiolitis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartz H, Buning-Pfaue F, Turkel O, Schauer U. Respiratory syncytial virus induces prostaglandin E2, IL-10 and IL-11 generation in antigen presenting cells. Clin Exp Immunol. 2002;129(3):438–445. doi: 10.1046/j.1365-2249.2002.01927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsen GL, Colasurdo GN. Neural control mechanisms within airways: disruption by respiratory syncytial virus. J Pediatr. 1999;135(2 Pt 2):21–27. [PubMed] [Google Scholar]
- 15.Hall GL, Hantos Z, Sly PD. Altered respiratory tissue mechanics in asymptomatic wheezy infants. Am J Respir Crit Care Med. 2001;164(8 Pt 1):1387–1391. doi: 10.1164/ajrccm.164.8.2012148. [DOI] [PubMed] [Google Scholar]
- 16.Hibbert ME, Hudson IL, Lanigan A, Landau LI, Phelan PD. Tracking of lung function in healthy children and adolescents. Pediatr Pulmonol. 1990;8(3):172–177. doi: 10.1002/ppul.1950080308. [DOI] [PubMed] [Google Scholar]
- 17.Lemanske RF., Jr Issues in understanding pediatric asthma: epidemiology and genetics. J Allergy Clin Immunol. 2002;109(Suppl 6):S521–S524. doi: 10.1067/mai.2002.124564. [DOI] [PubMed] [Google Scholar]
- 18.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332(3):133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 19.Openshaw PJ, Yamaguchi Y, Tregoning JS. Childhood infections, the developing immune system, and the origins of asthma. J Allergy Clin Immunol. 2004;114(6):1275–1277. doi: 10.1016/j.jaci.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 20.Collins PL, Crowe JE. Respiratory syncytial virus and metapneumovirus. In: Knipe DM, Howley PM, editors. Field Virology. Lippincott-Williams & Wilkins; Philadelphia, PA, USA: 2007. pp. 1601–1646. [Google Scholar]
- 21.Anderson LJ, Hierholzer JC, Tsou C, et al. Antigenic characterization of respiratory syncytial virus strains with monoclonal antibodies. J Infect Dis. 1985;151(4):626–633. doi: 10.1093/infdis/151.4.626. [DOI] [PubMed] [Google Scholar]
- 22.Mufson MA, Orvell C, Rafnar B, Norrby E. Two distinct subtypes of human respiratory syncytial virus. J Gen Virol. 1985;66(Pt 10):2111–2124. doi: 10.1099/0022-1317-66-10-2111. [DOI] [PubMed] [Google Scholar]
- 23.Cane PA, Pringle CR. Respiratory syncytial virus heterogeneity during an epidemic: analysis by limited nucleotide sequencing (SH gene) and restriction mapping (N gene) J Gen Virol. 1991;72(Pt 2):349–357. doi: 10.1099/0022-1317-72-2-349. [DOI] [PubMed] [Google Scholar]
- 24.Storch GA, Anderson LJ, Park CS, Tsou C, Dohner DE. Antigenic and genomic diversity within group A respiratory syncytial virus. J Infect Dis. 1991;163(4):858–861. doi: 10.1093/infdis/163.4.858. [DOI] [PubMed] [Google Scholar]
- 25.Peret TC, Hall CB, Schnabel KC, Golub JA, Anderson LJ. Circulation patterns of genetically distinct group A and B strains of human respiratory syncytial virus in a community. J Gen Virol. 1998;79(Pt 9):2221–2229. doi: 10.1099/0022-1317-79-9-2221. [DOI] [PubMed] [Google Scholar]
- 26.Peret TC, Hall CB, Hammond GW, et al. Circulation patterns of group A and B human respiratory syncytial virus genotypes in 5 communities in North America. J Infect Dis. 2000;181(6):1891–1896. doi: 10.1086/315508. [DOI] [PubMed] [Google Scholar]
- 27.Walsh EE, McConnochie KM, Long CE, Hall CB. Severity of respiratory syncytial virus infection is related to virus strain. J Infect Dis. 1997;175(4):814–820. doi: 10.1086/513976. [DOI] [PubMed] [Google Scholar]
- 28.Hall CB, Walsh EE, Schnabel KC, et al. Occurrence of groups A and B of respiratory syncytial virus over 15 years: associated epidemiologic and clinical characteristics in hospitalized and ambulatory children. J Infect Dis. 1990;162(6):1283–1290. doi: 10.1093/infdis/162.6.1283. [DOI] [PubMed] [Google Scholar]
- 29.Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163(4):693–698. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- 30.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA. 1999;282(15):1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 31.Kim HW, Arrobio JO, Brandt CD, et al. Epidemiology of respiratory syncytial virus infection in Washington, D.C. I. Importance of the virus in different respiratory tract disease syndromes and temporal distribution of infection. Am J Epidemiol. 1973;98(3):216–225. doi: 10.1093/oxfordjournals.aje.a121550. [DOI] [PubMed] [Google Scholar]
- 32.Baschat AA, Towbin J, Bowles NE, Harman CR, Weiner CP. Prevalence of viral DNA in amniotic fluid of low-risk pregnancies in the second trimester. J Matern Fetal Neonatal Med. 2003;13(6):381–384. doi: 10.1080/jmf.13.6.381.384. [DOI] [PubMed] [Google Scholar]
- 33.Pullan CR, Hey EN. Wheezing, asthma, and pulmonary dysfunction 10 years after infection with respiratory syncytial virus in infancy. Br Med J (Clin Res Ed) 1982;284(6330):1665–1669. doi: 10.1136/bmj.284.6330.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schauer U, Hoffjan S, Bittscheidt J, et al. RSV bronchiolitis and risk of wheeze and allergic sensitisation in the first year of life. Eur Respir J. 2002;20(5):1277–1283. doi: 10.1183/09031936.02.00019902. [DOI] [PubMed] [Google Scholar]
- 35.Singleton RJ, Redding GJ, Lewis TC, et al. Sequelae of severe respiratory syncytial virus infection in infancy and early childhood among Alaska native children. Pediatrics. 2003;112(2):285–290. doi: 10.1542/peds.112.2.285. [DOI] [PubMed] [Google Scholar]
- 36.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B, Bjorksten B. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics. 1995;95(4):500–505. [PubMed] [Google Scholar]
- 37••.Stein RT, Sherrill D, Morgan WJ, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354(9178):541–545. doi: 10.1016/S0140-6736(98)10321-5. Important study reporting that children with a respiratory syncytial virus (RSV) lower respiratory tract illness in early childhood have an increased risk of recurrent wheezing by the age of 6 years but not by the age of 13 years in a birth cohort study. [DOI] [PubMed] [Google Scholar]
- 38••.Sigurs N, Aljassim F, Kjellman B, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65(12):1045–1052. doi: 10.1136/thx.2009.121582. Important study reporting that children with RSV hosptialization in infancy were more likely to have asthma by age 18 years compared with age- and gender-matched controls. [DOI] [PubMed] [Google Scholar]
- 39.Korppi M, Piippo-Savolainen E, Korhonen K, Remes S. Respiratory morbidity 20 years after RSV infection in infancy. Pediatr Pulmonol. 2004;38(2):155–160. doi: 10.1002/ppul.20058. [DOI] [PubMed] [Google Scholar]
- 40.Escobar GJ, Ragins A, Li SX, Prager L, Masaquel AS, Kipnis P. Recurrent wheezing in the third year of life among children born at 32 weeks’ gestation or later: relationship to laboratory-confirmed, medically attended infection with respiratory syncytial virus during the first year of life. Arch Pediatr Adolesc Med. 2010;164(10):915–922. doi: 10.1001/archpediatrics.2010.177. [DOI] [PubMed] [Google Scholar]
- 41.Carroll KN, Wu P, Gebretsadik T, et al. Season of infant bronchiolitis and estimates of subsequent risk and burden of early childhood asthma. J Allergy Clin Immunol. 2009;123(4):964–966. doi: 10.1016/j.jaci.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mok JY, Simpson H. Outcome of acute lower respiratory tract infection in infants: preliminary report of seven-year follow-up study. Br Med J (Clin Res Ed) 1982;285(6338):333–337. doi: 10.1136/bmj.285.6338.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fjaerli HO, Farstad T, Rod G, Ufert GK, Gulbrandsen P, Nakstad B. Acute bronchiolitis in infancy as risk factor for wheezing and reduced pulmonary function by seven years in Akershus County, Norway. BMC Pediatr. 2005;5:31. doi: 10.1186/1471-2431-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Stern DA, Morgan WJ, Halonen M, Wright AL, Martinez FD. Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet. 2008;372(9643):1058–1064. doi: 10.1016/S0140-6736(08)61447-6. Important study reporting that asthma at age 22 years is associated with recurrent wheezing at age 6 years in a birth cohort study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45•.Wu P, Dupont WD, Griffin MR, et al. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med. 2008;178(11):1123–1129. doi: 10.1164/rccm.200804-579OC. Important study providing strong evidence that winter virus is in the causal pathway of development of childhood asthma by school age. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hartter HK, Oyedele OI, Dietz K, Kreis S, Hoffman JP, Muller CP. Placental transfer and decay of maternally acquired antimeasles antibodies in Nigerian children. Pediatr Infect Dis J. 2000;19(7):635–641. doi: 10.1097/00006454-200007000-00010. [DOI] [PubMed] [Google Scholar]
- 47.de FA, Hall AJ, Unicomb L, Chakraborty J, Yunus M, Sack RB. Maternal measles antibody decay in rural Bangladeshi infants – implications for vaccination schedules. Vaccine. 1998;16(6):564–568. doi: 10.1016/s0264-410x(97)00245-4. [DOI] [PubMed] [Google Scholar]
- 48.Buckley RHT-B. NK-cell systems. In: Behrman RE, Kliegman RM, Arvin AM, Nelson WE, editors. Nelson Textbook of Pediatrics. W.B. Saunders Company; Philadelphia, PA, USA: 1996. pp. 561–567. [Google Scholar]
- 49.Gern JE, Rosenthal LA, Sorkness RL, Lemanske RF., Jr Effects of viral respiratory infections on lung development and childhood asthma. J Allergy Clin Immunol. 2005;115(4):668–674. doi: 10.1016/j.jaci.2005.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomsen SF, van der SS, Stensballe LG, et al. Exploring the association between severe respiratory syncytial virus infection and asthma: a registry-based twin study. Am J Respir Crit Care Med. 2009;179(12):1091–1097. doi: 10.1164/rccm.200809-1471OC. [DOI] [PubMed] [Google Scholar]
- 51.Stensballe LG, Simonsen JB, Thomsen SF, et al. The causal direction in the association between respiratory syncytial virus hospitalization and asthma. J Allergy Clin Immunol. 2009;123(1):131–137. doi: 10.1016/j.jaci.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 52.Poorisrisak P, Halkjaer LB, Thomsen SF, et al. Causal direction between respiratory syncytial virus bronchiolitis and asthma studied in monozygotic twins. Chest. 2010;138(2):338–344. doi: 10.1378/chest.10-0365. [DOI] [PubMed] [Google Scholar]
- 53.Singh AM, Moore PE, Gern JE, Lemanske RF, Jr, Hartert TV. Bronchiolitis to asthma: a review and call for studies of gene–virus interactions in asthma causation. Am J Respir Crit Care Med. 2007;175(2):108–119. doi: 10.1164/rccm.200603-435PP. [DOI] [PubMed] [Google Scholar]
- 54••.Janssen R, Bont L, Siezen CL, et al. Genetic susceptibility to respiratory syncytial virus bronchiolitis is predominantly associated with innate immune genes. J Infect Dis. 2007;196(6):826–834. doi: 10.1086/520886. Important genetic study identifying genes associated with RSV bronchiolitis. Candidate genes were identified across different categories of polymorphic immune responses. [DOI] [PubMed] [Google Scholar]
- 55.Poon AH, Laprise C, Lemire M, et al. Association of vitamin D receptor genetic variants with susceptibility to asthma and atopy. Am J Respir Crit Care Med. 2004;170(9):967–973. doi: 10.1164/rccm.200403-412OC. [DOI] [PubMed] [Google Scholar]
- 56.Islam T, Breton C, Salam MT, et al. Role of inducible nitric oxide synthase in asthma risk and lung function growth during adolescence. Thorax. 2010;65(2):139–145. doi: 10.1136/thx.2009.114355. [DOI] [PubMed] [Google Scholar]
- 57.Amanatidou V, Sourvinos G, Apostolakis S, Tsilimigaki A, Spandidos DA. T280M variation of the CX3C receptor gene is associated with increased risk for severe respiratory syncytial virus bronchiolitis. Pediatr Infect Dis J. 2006;25(5):410–414. doi: 10.1097/01.inf.0000214998.16248.b7. [DOI] [PubMed] [Google Scholar]
- 58.Tremblay K, Lemire M, Provost V, et al. Association study between the CX3CR1 gene and asthma. Genes Immun. 2006;7(8):632–639. doi: 10.1038/sj.gene.6364340. [DOI] [PubMed] [Google Scholar]
- 59.Depner M, Kormann MS, Klopp N, et al. CX3CR1 polymorphisms are associated with atopy but not asthma in German children. Int Arch Allergy Immunol. 2007;144(1):91–94. doi: 10.1159/000102620. [DOI] [PubMed] [Google Scholar]
- 60.Kurt-Jones EA, Popova L, Kwinn L, et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1(5):398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 61.Tulic MK, Hurrelbrink RJ, Prele CM, et al. TLR4 polymorphisms mediate impaired responses to respiratory syncytial virus and lipopolysaccharide. J Immunol. 2007;179(1):132–140. doi: 10.4049/jimmunol.179.1.132. [DOI] [PubMed] [Google Scholar]
- 62.Fageras BM, Hmani-Aifa M, Lindstrom A, et al. A TLR4 polymorphism is associated with asthma and reduced lipopolysaccharide-induced interleukin-12(p70) responses in Swedish children. J Allergy Clin Immunol. 2004;114(3):561–567. doi: 10.1016/j.jaci.2004.04.050. [DOI] [PubMed] [Google Scholar]
- 63.Raby BA, Klimecki WT, Laprise C, et al. Polymorphisms in Toll-like receptor 4 are not associated with asthma or atopy-related phenotypes. Am J Respir Crit Care Med. 2002;166(11):1449–1456. doi: 10.1164/rccm.200207-634OC. [DOI] [PubMed] [Google Scholar]
- 64.Yang IA, Barton SJ, Rorke S, et al. Toll-like receptor 4 polymorphism and severity of atopy in asthmatics. Genes Immun. 2004;5(1):41–45. doi: 10.1038/sj.gene.6364037. [DOI] [PubMed] [Google Scholar]
- 65.Ghildyal R, Hartley C, Varrasso A, et al. Surfactant protein A binds to the fusion glycoprotein of respiratory syncytial virus and neutralizes virion infectivity. J Infect Dis. 1999;180(6):2009–2013. doi: 10.1086/315134. [DOI] [PubMed] [Google Scholar]
- 66.Griese M. Respiratory syncytial virus and pulmonary surfactant. Viral Immunol. 2002;15(2):357–363. doi: 10.1089/08828240260066279. [DOI] [PubMed] [Google Scholar]
- 67.Lahti M, Lofgren J, Marttila R, et al. Surfactant protein D gene polymorphism associated with severe respiratory syncytial virus infection. Pediatr Res. 2002;51(6):696–699. doi: 10.1203/00006450-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 68.Lofgren J, Ramet M, Renko M, Marttila R, Hallman M. Association between surfactant protein A gene locus and severe respiratory syncytial virus infection in infants. J Infect Dis. 2002;185(3):283–289. doi: 10.1086/338473. [DOI] [PubMed] [Google Scholar]
- 69.Puthothu B, Forster J, Heinze J, Heinzmann A, Krueger M. Surfactant protein B polymorphisms are associated with severe respiratory syncytial virus infection, but not with asthma. BMC Pulm Med. 2007;7:6. doi: 10.1186/1471-2466-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Puthothu B, Krueger M, Heinze J, Forster J, Heinzmann A. Haplotypes of surfactant protein C are associated with common paediatric lung diseases. Pediatr Allergy Immunol. 2006;17(8):572–577. doi: 10.1111/j.1399-3038.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- 71.Amanatidou V, Sourvinos G, Apostolakis S, et al. RANTES promoter gene polymorphisms and susceptibility to severe respiratory syncytial virus-induced bronchiolitis. Pediatr Infect Dis J. 2008;27(1):38–42. doi: 10.1097/INF.0b013e31814d4e42. [DOI] [PubMed] [Google Scholar]
- 72.Lachheb J, Chelbi H, Hamzaoui K, Hamzaoui A. Association between RANTES polymorphisms and asthma severity among Tunisian children. Hum Immunol. 2007;68(8):675–680. doi: 10.1016/j.humimm.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 73.Sheeran P, Jafri H, Carubelli C, et al. Elevated cytokine concentrations in the nasopharyngeal and tracheal secretions of children with respiratory syncytial virus disease. Pediatr Infect Dis J. 1999;18(2):115–122. doi: 10.1097/00006454-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 74.Tian M, Liu F, Wen GY, Shi SY, Chen RH, Zhao DY. Effect of variation in RANTES promoter on serum RANTES levels and risk of recurrent wheezing after RSV bronchiolitis in children from Han, Southern China. Eur J Pediatr. 2009;168(8):963–967. doi: 10.1007/s00431-008-0870-3. [DOI] [PubMed] [Google Scholar]
- 75.Puthothu B, Krueger M, Forster J, Heinzmann A. Association between severe respiratory syncytial virus infection and IL13/IL4 haplotypes. J Infect Dis. 2006;193(3):438–441. doi: 10.1086/499316. [DOI] [PubMed] [Google Scholar]
- 76.Hoebee B, Rietveld E, Bont L, et al. Association of severe respiratory syncytial virus bronchiolitis with interleukin-4 and interleukin-4 receptor α polymorphisms. J Infect Dis. 2003;187(1):2–11. doi: 10.1086/345859. [DOI] [PubMed] [Google Scholar]
- 77.Moore KW, de Waal MR, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Ann Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 78.Hoebee B, Bont L, Rietveld E, et al. Influence of promoter variants of interleukin-10, interleukin-9, and tumor necrosis factor-α genes on respiratory syncytial virus bronchiolitis. J Infect Dis. 2004;189(2):239–247. doi: 10.1086/380908. [DOI] [PubMed] [Google Scholar]
- 79.Wilson J, Rowlands K, Rockett K, et al. Genetic variation at the IL10 gene locus is associated with severity of respiratory syncytial virus bronchiolitis. J Infect Dis. 2005;191(10):1705–1709. doi: 10.1086/429636. [DOI] [PubMed] [Google Scholar]
- 80.Gentile DA, Doyle WJ, Zeevi A, et al. Cytokine gene polymorphisms moderate illness severity in infants with respiratory syncytial virus infection. Hum Immunol. 2003;64(3):338–344. doi: 10.1016/s0198-8859(02)00827-3. [DOI] [PubMed] [Google Scholar]
- 81.Kunkel SL, Standiford T, Kasahara K, Strieter RM. Interleukin-8 (IL-8): the major neutrophil chemotactic factor in the lung. Exp Lung Res. 1991;17(1):17–23. doi: 10.3109/01902149109063278. [DOI] [PubMed] [Google Scholar]
- 82.Hull J, Thomson A, Kwiatkowski D. Association of respiratory syncytial virus bronchiolitis with the interleukin 8 gene region in UK families. Thorax. 2000;55(12):1023–1027. doi: 10.1136/thorax.55.12.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hull J, Ackerman H, Isles K, et al. Unusual haplotypic structure of IL8, a susceptibility locus for a common respiratory virus. Am J Hum Genet. 2001;69(2):413–419. doi: 10.1086/321291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Puthothu B, Krueger M, Heinze J, Forster J, Heinzmann A. Impact of IL8 and IL8-receptor α polymorphisms on the genetics of bronchial asthma and severe RSV infections. Clin Mol Allergy. 2006;4:2. doi: 10.1186/1476-7961-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goetghebuer T, Isles K, Moore C, Thomson A, Kwiatkowski D, Hull J. Genetic predisposition to wheeze following respiratory syncytial virus bronchiolitis. Clin Exp Allergy. 2004;34(5):801–803. doi: 10.1111/j.1365-2222.2004.1947.x. [DOI] [PubMed] [Google Scholar]
- 86.Dinarello CA. IL-18: a Th1-inducing, proinflammatory cytokine and new member of the IL-1 family. J Allergy Clin Immunol. 1999;103(1 Pt 1):11–24. doi: 10.1016/s0091-6749(99)70518-x. [DOI] [PubMed] [Google Scholar]
- 87.Puthothu B, Krueger M, Forster J, Heinze J, Weckmann M, Heinzmann A. Interleukin (IL)-18 polymorphism 133C/G is associated with severe respiratory syncytial virus infection. Pediatr Infect Dis J. 2007;26(12):1094–1098. doi: 10.1097/INF.0b013e3181453579. [DOI] [PubMed] [Google Scholar]
- 88.Harada M, Obara K, Hirota T, et al. A functional polymorphism in IL-18 is associated with severity of bronchial asthma. Am J Respir Crit Care Med. 2009;180(11):1048–1055. doi: 10.1164/rccm.200905-0652OC. [DOI] [PubMed] [Google Scholar]
- 89.El-Mezzein RE, Matsumoto T, Nomiyama H, Miike T. Increased secretion of IL-18 in vitro by peripheral blood mononuclear cells of patients with bronchial asthma and atopic dermatitis. Clin Exp Immunol. 2001;126(2):193–198. doi: 10.1046/j.1365-2249.2001.01664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Howard TD, Koppelman GH, Xu J, et al. Gene–gene interaction in asthma: IL4RA and IL13 in a Dutch population with asthma. Am J Hum Genet. 2002;70(1):230–236. doi: 10.1086/338242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eder W, Klimecki W, Yu L, et al. Opposite effects of CD 14/-260 on serum IgE levels in children raised in different environments. J Allergy Clin Immunol. 2005;116(3):601–607. doi: 10.1016/j.jaci.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 92.Simpson A, John SL, Jury F, et al. Endotoxin exposure, CD14, and allergic disease: an interaction between genes and the environment. Am J Respir Crit Care Med. 2006;174(4):386–392. doi: 10.1164/rccm.200509-1380OC. [DOI] [PubMed] [Google Scholar]
- 93.Williams LK, McPhee RA, Ownby DR, et al. Gene–environment interactions with CD14 C-260T and their relationship to total serum IgE levels in adults. J Allergy Clin Immunol. 2006;118(4):851–857. doi: 10.1016/j.jaci.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 94.Zambelli-Weiner A, Ehrlich E, Stockton ML, et al. Evaluation of the CD14/-260 polymorphism and house dust endotoxin exposure in the Barbados Asthma Genetics Study. J Allergy Clin Immunol. 2005;115(6):1203–1209. doi: 10.1016/j.jaci.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 95.Martinez FD, Stern DA, Wright AL, Taussig LM, Halonen M. Differential immune responses to acute lower respiratory illness in early life and subsequent development of persistent wheezing and asthma. J Allergy Clin Immunol. 1998;102(6 Pt 1):915–920. doi: 10.1016/s0091-6749(98)70328-8. [DOI] [PubMed] [Google Scholar]
- 96.Macaubas C, de Klerk NH, Holt BJ, et al. Association between antenatal cytokine production and the development of atopy and asthma at age 6 years. Lancet. 2003;362(9391):1192–1197. doi: 10.1016/s0140-6736(03)14542-4. [DOI] [PubMed] [Google Scholar]
- 97.Stephens R, Randolph DA, Huang G, Holtzman MJ, Chaplin DD. Antigen-nonspecific recruitment of Th2 cells to the lung as a mechanism for viral infection-induced allergic asthma. J Immunol. 2002;169(10):5458–5467. doi: 10.4049/jimmunol.169.10.5458. [DOI] [PubMed] [Google Scholar]
- 98•.Culley FJ, Pollott J, Openshaw PJ. Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood. J Exp Med. 2002;196(10):1381–1386. doi: 10.1084/jem.20020943. Important study demonstrating that the timing of infection during infancy has an impact on subsequent asthma development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tregoning JS, Yamaguchi Y, Harker J, Wang B, Openshaw PJ. The role of T cells in the enhancement of respiratory syncytial virus infection severity during adult reinfection of neonatally sensitized mice. J Virol. 2008;82(8):4115–4124. doi: 10.1128/JVI.02313-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dakhama A, Park JW, Taube C, et al. The enhancement or prevention of airway hyperresponsiveness during reinfection with respiratory syncytial virus is critically dependent on the age at first infection and IL-13 production. J Immunol. 2005;175(3):1876–1883. doi: 10.4049/jimmunol.175.3.1876. [DOI] [PubMed] [Google Scholar]
- 101.Dakhama A, Lee YM, Ohnishi H, et al. Virus-specific IgE enhances airway responsiveness on reinfection with respiratory syncytial virus in newborn mice. J Allergy Clin Immunol. 2009;123(1):138–145. doi: 10.1016/j.jaci.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 102.Peebles RS, Jr, Graham BS. Pathogenesis of respiratory syncytial virus infection in the murine model. Proc Am Thorac Soc. 2005;2(2):110–115. doi: 10.1513/pats.200501-002AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Welliver RC, Garofalo RP, Ogra PL. β-chemokines, but neither T helper type 1 nor T helper type 2 cytokines, correlate with severity of illness during respiratory syncytial virus infection. Pediatr Infect Dis J. 2002;21(5):457–461. doi: 10.1097/00006454-200205000-00033. [DOI] [PubMed] [Google Scholar]
- 104.Aberle JH, Aberle SW, Dworzak MN, et al. Reduced interferon-γ expression in peripheral blood mononuclear cells of infants with severe respiratory syncytial virus disease. Am J Respir Crit Care Med. 1999;160(4):1263–1268. doi: 10.1164/ajrccm.160.4.9812025. [DOI] [PubMed] [Google Scholar]
- 105.Renzi PM, Turgeon JP, Marcotte JE, et al. Reduced interferon-γ production in infants with bronchiolitis and asthma. Am J Respir Crit Care Med. 1999;159(5 Pt 1):1417–1422. doi: 10.1164/ajrccm.159.5.9805080. [DOI] [PubMed] [Google Scholar]
- 106.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15(4):410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Devincenzo JP, Wilkinson T, Vaishnaw A, et al. Viral load drives disease in humans experimentally infected with respiratory syncytial virus. Am J Respir Crit Care Med. 2010;182(10):1305–1314. doi: 10.1164/rccm.201002-0221OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang Y, Bai C, Li K, Adler KB, Wang X. Role of airway epithelial cells in development of asthma and allergic rhinitis. Respir Med. 2008;102(7):949–955. doi: 10.1016/j.rmed.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 109••.Kim EY, Battaile JT, Patel AC, et al. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat Med. 2008;14(6):633–640. doi: 10.1038/nm1770. Important study demonstrating that transition from respiratory viral infection into chronic lung disease requires persistent activation of a novel natural killer T cell marcophage innate immune axis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hu C, Wedde-Beer K, Auais A, Rodriguez MM, Piedimonte G. Nerve growth factor and nerve growth factor receptors in respiratory syncytial virus-infected lungs. Am J Physiol Lung Cell Mol Physiol. 2002;283(2):L494–L502. doi: 10.1152/ajplung.00414.2001. [DOI] [PubMed] [Google Scholar]
- 111•.Tortorolo L, Langer A, Polidori G, et al. Neurotrophin overexpression in lower airways of infants with respiratory syncytial virus infection. Am J Respir Crit Care Med. 2005;172(2):233–237. doi: 10.1164/rccm.200412-1693OC. Important study reporting that RSV infection in early life promotes an overexpression of nerve growth factor and its receptors in the developing airways. [DOI] [PubMed] [Google Scholar]
- 112.Kernie SG, Parada LF. The molecular basis for understanding neurotrophins and their relevance to neurologic disease. Arch Neurol. 2000;57(5):654–657. doi: 10.1001/archneur.57.5.654. [DOI] [PubMed] [Google Scholar]
- 113.Wright M, Piedimonte G. Respiratory syncytial virus prevention and therapy: past, present, and future. Pediatr Pulmonol. 2011;46(4):324–347. doi: 10.1002/ppul.21377. [DOI] [PubMed] [Google Scholar]
- 114••.Auais A, Adkins B, Napchan G, Piedimonte G. Immunomodulatory effects of sensory nerves during respiratory syncytial virus infection in rats. Am J Physiol Lung Cell Mol Physiol. 2003;285(1):L105–L113. doi: 10.1152/ajplung.00004.2003. Important study demonstrating that nerve growth factor overexpression is critical for neurogenic-mediated immune responses in RSV-infected airways, suggesting an important role of neuro-immune interactions in the pathophysiology of local and systemic inflamation against respiratory virus. [DOI] [PubMed] [Google Scholar]
- 115••.Braun A, Lommatzsch M, Lewin GR, Virchow JC, Renz H. Neurotrophins: a link between airway inflammation and airway smooth muscle contractility in asthma? Int Arch Allergy Immunol. 1999;118(2–4):163–165. doi: 10.1159/000024056. Important study suggesting that neurotrophins are important in the pathogenetics of allergic disorders. [DOI] [PubMed] [Google Scholar]
- 116.American Thoracic Society ad hoc Statement Committee. Mechanisms and limits of induced postnatal lung growth. Am J Respir Crit Care Med. 2004;170(3):319–343. doi: 10.1164/rccm.200209-1062ST. [DOI] [PubMed] [Google Scholar]
- 117.Hall WJ, Hall CB. Clinical significance of pulmonary function tests. Alterations in pulmonary function following respiratory viral infection. Chest. 1979;76(4):458–465. doi: 10.1378/chest.76.4.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lukacs NW, Tekkanat KK, Berlin A, et al. Respiratory syncytial virus predisposes mice to augmented allergic airway responses via IL-13-mediated mechanisms. J Immunol. 2001;167(2):1060–1065. doi: 10.4049/jimmunol.167.2.1060. [DOI] [PubMed] [Google Scholar]
- 119.Tekkanat KK, Maassab HF, Cho DS, et al. IL-13-induced airway hyperreactivity during respiratory syncytial virus infection is STAT6 dependent. J Immunol. 2001;166(5):3542–3548. doi: 10.4049/jimmunol.166.5.3542. [DOI] [PubMed] [Google Scholar]
- 120.Peebles RS, Jr, Sheller JR, Collins RD, et al. Respiratory syncytial virus infection does not increase allergen-induced type 2 cytokine production, yet increases airway hyperresponsiveness in mice. J Med Virol. 2001;63(2):178–188. [PubMed] [Google Scholar]
- 121.Castleman WL, Sorkness RL, Lemanske RF, Grasee G, Suyemoto MM. Neonatal viral bronchiolitis and pneumonia induces bronchiolar hypoplasia and alveolar dysplasia in rats. Lab Invest. 1988;59(3):387–396. [PubMed] [Google Scholar]
- 122.Uhl EW, Castleman WL, Sorkness RL, Busse WW, Lemanske RF, Jr, McAllister PK. Parainfluenza virus-induced persistence of airway inflammation, fibrosis, and dysfunction associated with TGF-β 1 expression in brown Norway rats. Am J Respir Crit Care Med. 1996;154(6 Pt 1):1834–1842. doi: 10.1164/ajrccm.154.6.8970378. [DOI] [PubMed] [Google Scholar]
- 123.Kumar A, Sorkness RL, Kaplan MR, Lemanske RF., Jr Chronic, episodic, reversible airway obstruction after viral bronchiolitis in rats. Am J Respir Crit Care Med. 1997;155(1):130–134. doi: 10.1164/ajrccm.155.1.9001301. [DOI] [PubMed] [Google Scholar]
- 124.Groothuis JR, Simoes EA, Levin MJ, et al. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. The Respiratory Syncytial Virus Immune Globulin Study Group. N Engl J Med. 1993;329(21):1524–1530. doi: 10.1056/NEJM199311183292102. [DOI] [PubMed] [Google Scholar]
- 125.The Impact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102(3 Pt 1):531–537. [PubMed] [Google Scholar]
- 126••.Wenzel SE, Gibbs RL, Lehr MV, Simoes EA. Respiratory outcomes in high-risk children 7 to 10 years after prophylaxis with respiratory syncytial virus immune globulin. Am J Med. 2002;112(8):627–633. doi: 10.1016/s0002-9343(02)01095-1. Important study suggesting that RSV immunoprophylaxis administration in infancy may lower the risk of subsequent development of childhood asthma. [DOI] [PubMed] [Google Scholar]
- 127.Simoes EA, Groothuis JR, Carbonell-Estrany X, et al. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr. 2007;151(1):34–42. 42.e1. doi: 10.1016/j.jpeds.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 128••.Meissner HC, Long SS. Respiratory syncytial virus infection and recurrent wheezing: a complex relationship. J Pediatr. 2007;151(1):6–7. doi: 10.1016/j.jpeds.2007.02.034. Important study suggesting that anti-RSV theapy during early childhood may reduce the risk of subsequent development of asthma and allergic sensization. [DOI] [PubMed] [Google Scholar]
- 129.Simoes EA. Treatment and prevention of respiratory syncytial virus lower respiratory tract infection. Long-term effects on respiratory outcomes. Am J Respir Crit Care Med. 2001;163(3 Pt 2):S14–S17. doi: 10.1164/ajrccm.163.supplement_1.2011112. [DOI] [PubMed] [Google Scholar]
- 130.Chen CH, Lin YT, Yang YH, et al. Ribavirin for respiratory syncytial virus bronchiolitis reduced the risk of asthma and allergen sensitization. Pediatr Allergy Immunol. 2008;19(2):166–172. doi: 10.1111/j.1399-3038.2007.00610.x. [DOI] [PubMed] [Google Scholar]