Abstract
Background
There is increasing evidence that botulinum neurotoxin A may affect sensory nociceptor fibers, but the expression of its receptors in clinical pain states, and its effects in human sensory neurons, are largely unknown.
Methods
We studied synaptic vesicle protein subtype SV2A, a receptor for botulinum neurotoxin A, by immunostaining in a range of clinical tissues, including human dorsal root ganglion sensory neurons, peripheral nerves, the urinary bladder, and the colon. We also determined the effects of botulinum neurotoxins A and E on localization of the capsaicin receptor, TRPV1, and functional sensitivity to capsaicin stimuli in cultured human dorsal root ganglion neurons.
Results
Image analysis showed that SV2A immunoreactive nerve fibers were increased in injured nerves proximal to the injury (P = 0.002), and in painful neuromas (P = 0.0027); the ratio of percentage area SV2A to neurofilaments (a structural marker) was increased proximal to injury (P = 0.0022) and in neuromas (P = 0.0001), indicating increased SV2A levels in injured nerve fibers. In the urinary bladder, SV2A nerve fibers were found in detrusor muscle and associated with blood vessels, with a significant increase in idiopathic detrusor over-activity (P = 0.002) and painful bladder syndrome (P = 0.0087). Colon biopsies showed numerous SV2A-positive nerve fibers, which were increased in quiescent inflammatory bowel disease with abdominal pain (P = 0.023), but not in inflammatory bowel disease without abdominal pain (P = 0.77) or in irritable bowel syndrome (P = 0.13). In vitro studies of botulinum neurotoxin A-treated and botulinum neurotoxin E-treated cultured human sensory neurons showed accumulation of cytoplasmic vesicles, neurite loss, and reduced immunofluorescence for the heat and capsaicin receptor, TRPV1. Functional effects included dose-related inhibition of capsaicin responses on calcium imaging after acute treatment with botulinum neurotoxins A and E.
Conclusion
Differential levels of SV2A protein expression in clinical disorders may identify potential new targets for botulinum neurotoxin therapy. In vitro studies indicate that treatment with botulinum neurotoxins A and E may affect receptor expression and nociceptor function in sensory neurons.
Keywords: SV2A, human, pain, botulinum neurotoxin, neurons
Introduction
SV2A, a synaptic vesicle protein isoform, has been shown to be the high-affinity botulinum neurotoxin A receptor that mediates binding and internalization of the neurotoxin into peripheral neurons.1 Synaptic vesicle protein (SV2) is approximately 90 kDa glycoprotein component of all mammalian synaptic vesicles.2–4 In addition to the SV2A subtype protein, neuronal binding of botulinum neurotoxin also involves a ganglioside coreceptor within the presynaptic membrane.5 Cleavage of soluble NSF attachment protein receptor (SNARE) proteins within the presynaptic terminal by botulinum neurotoxin leads to prevention of the formation of a productive docking complex necessary for transmitter release, and consequent loss of function, eg, at the neuromuscular junction. Synaptosomal-associated protein (SNAP-25) is the SNARE protein that is specifically cleaved by type A toxin.1,6
Levels of SV2A, one of the SV2 isoforms to which botulinum neurotoxin A binds, are largely undetermined in clinical disorders, particularly in patients with pain and visceral dysfunction. Because botulinum neurotoxin A treatment is being considered increasingly in such conditions, we have studied SV2A protein in tissues from patients with nerve injury and pelvic visceral disorders, and the effects of botulinum neurotoxin in cultured human sensory neurons.
Recent studies in rodents have indicated the potential of botulinum neurotoxin A to affect sensory mechanisms via inhibition of neurotransmitters from sensory afferent nerves.7 A novel chimera (EA) botulinum neurotoxin A and E serotype inhibited calcitonin gene-related peptide release from trigeminal ganglionic neurons and eliminated the excitatory effects of this peptide in brain stem sensory neurons evoked by capsaicin, which activates the transient receptor potential vanilloid receptor type 1 (TRPV1).8
TRPV1 is the neuronal receptor for capsaicin (the hot ingredient of chilli peppers), low pH, heat, and inflammatory mediators, such as bradykinin, arachidonic acid and its metabolites, and mediates the perception of pain via calcium influx and membrane depolarization.9 TRPV1 expression is increased in conditions of chronic pain,10,11 and the receptor demonstrates sensitization in the presence of neurotrophic factors in models of pain.12,13 Capsaicin has been extensively used as a tool for studies of TRPV1 function.
Botulinum neurotoxin is potentially a promising treatment for chronic somatic and visceral pain,14 which by definition lasts for more than 3 months. These conditions include pelvic neurogenic hypersensitivity disorders, such as painful bladder syndrome14,15 and irritable bowel syndrome, in which pain is attributed at least in part to dysfunction or sensitization of the peripheral nervous system. The mechanisms of chronic visceral pain are complex, and have been reviewed.16 Neural plasticity may lead to chronic pain in these conditions, including upregulation of TRPV1 in peripheral nerve terminals.17–20 Botulinum neurotoxin may prevent membrane surface expression of TRPV1, thereby reducing hypersensitivity.21,22
We examined the distribution of SV2A in a range of control and clinical disease tissues, some involving pain, in order to identify patient groups that may be potential and preferential targets for botulinum neurotoxin therapy, and also determined the effects of botulinum neurotoxins A and E on sensory neuron morphology, TRPV1 expression, and responses to capsaicin in cultured human dorsal root ganglion neurons.
Materials and methods
Tissue collection
A range of tissues were used in this study, for which fully informed consent was obtained with approval of the relevant research ethics committees. Specimens were snapfrozen in liquid nitrogen and stored at −70°C until use or immersed in Zamboni’s fixative (2% w/v formalin, 0.1 M phosphate, and 15% v/v saturated picric acid) for 2 hours and stored in phosphate-buffered saline.
Specimens of nerve proximal to the site of injury (n = 6, mean age [± standard error of the mean] 29.0 ± 4.8 years, two females, range of injury delay 1.5 days to 12 months), neuroma (n = 21, mean age 23.3 ± 2.8 years, four females, range of injury delay 1.5–13 months), and dorsal root ganglia (n = 8, mean age 31.5 ± 6.0 years, one female, range of injury delay 3 days to 5 months) were obtained from patients undergoing surgery for painful neuroma relocation, brachial plexus repair, or peripheral nerve repair. Uninjured nerves (n = 7, mean age 41.6 ± 10.6 years, two females) used as nerve repair grafts during surgery served as controls.
Urinary bladder tissue specimens were obtained from control subjects under investigation for asymptomatic microscopic hematuria (control group, n = 8, mean age 51.1 [range 31–79] years, five females), idiopathic detrusor overactivity (n = 6, mean age 52.1 [range 32–73] years, four females) and painful bladder syndrome (n = 8, mean age 49.6 [range 29–71] years, six females) who met the National Institute of Diabetes and Digestive and Kidney Diseases research criteria for interstitial cystitis, as described by our group previously.23 The patients with idiopathic detrusor overactivity presented with overactive bladder symptoms, ie, urgency, with or without urge incontinence, frequency, and nocturia, and showed involuntary detrusor contractions during the filling phase of urodynamics.
Colonoscopic rectosigmoid biopsies were collected from patients with either quiescent or asymptomatic quiescent inflammatory bowel disease (n = 25, 14 ulcerative colitis, 11 Crohn’s disease, eight females, mean age 54.3 ± 3.0 [range 30–80] years), as previously described.19 Irritable bowel syndrome (n = 14, mean age 46.8 ± 8.0 [range 25–77] years) was diagnosed according to Rome II criteria, and the subjects were further classified according to Rome II criteria, as described eleswhere.10,19 Controls (n = 13, mean age 60.8 ± 4.1 [range 39–85] years, 10 females) were selected from patients who were undergoing colonoscopy for other indications (such as polyp and cancer surveillance) and had a normal colon. For inflammatory bowel disease, abdominal pain scores were recorded using a validated questionnaire and samples were subdivided into those with (n = 10, mean age 56 [range 40–66] years) or without (n = 15, mean age 57 [range 47–63] years) pain.
Immunohistochemistry
Frozen tissue sections (15 μm thickness) were postfixed in 4% w/v paraformaldehyde in 0.15 M phosphate-buffered saline for 30 minutes. Endogenous peroxidase was blocked by incubation in industrial methylated spirits containing 0.3% w/v hydrogen peroxide for 30 minutes. After rehydration with phosphate-buffered saline, the sections were incubated overnight with a primary antibody using a range of dilutions. Rabbit SV2A polyclonal antibody was obtained from Sigma-Aldrich (Dorset, UK) and used at the optimal dilution 1:750. The SV2A antibody used in this study has not been reported before, although immunohistochemistry expression profiles provided by the manufacturer indicate strong staining of neuropils in the central nervous system. Localization of SV2A immunoreactivity was similar in both prefixed and postfixed tissues. SV2A antibodies were evaluated by titration on tissue sections of dorsal root ganglia and injured nerve tissue. Immunoreaction with sensory neurons and nerve fibers diminished with increasing antibody dilution, with very weak residual immunoreactivity at 1:1000. A cocktail of monoclonal antibodies to the phosphorylated and nonphosphorylated neurofilaments of size 200 kDa (Clone N52, Sigma-Aldrich, Poole, UK) and the 57 kDa type III filament, peripherin (Novocastra Laboratories, Newcastle, UK) were used at final titers of 1:20,000 and 1:500 respectively, and acted as structural neuronal markers. Sites of primary antibody attachment were revealed using nickel-enhanced avidin-biotin peroxidase (ABC, Vector Laboratories, Peterborough, UK).
Image analysis
SV2A immunoreactivity was assessed quantitatively by computerized image analysis whereby images were captured using an Olympus DP70 camera mounted onto an Olympus BX50 microscope and analyzed using Olympus AnalySIS® (version 5.0) software. Positive immunostaining was high-lighted by setting the gray-level detection limits to threshold, and the area of highlighted immunoreactivity was obtained as percent area of the field scanned. Five random fields per tissue section were scanned at the same magnification (40×). Results were expressed as percent area. The Mann–Whitney test was used for statistical analysis (P values < 0.05 were considered statistically significant).
Neuronal culture
Avulsed human cervical ganglia (n = 5) were obtained by ganglionectomy at the Royal National Orthopaedic Hospital, Stanmore, as a necessary part of the surgical repair procedure, with informed patient consent and approval of the research ethics committee. The ganglia were minced, enzyme-digested in 0.5% dispase (8 U/mg) and 0.2% collagenase (168 U/mg), and penicillin + streptomycin (100 μg/mL each) in Ham’s F12 nutrient medium for 3 hours, and mechanically dissociated to obtain a cell suspension. Cells were plated onto Mattek dishes coated with collagen 20 μg/mL and laminin 20 μg/mL for 20 minutes. Ham’s F12 nutrient medium containing 10% heat-inactivated fetal calf serum and recombinant human neurotrophic factors, ie, rhNGF 100 ng/mL and rhGDNF 50 ng/mL, were added, and the cells were incubated in a humid environment with 8% CO2. The medium was changed every 3–4 days. Neuronal enrichment by removal of non-neuronal cells was not carried out, so as to maximize the neuronal yield. Studies were conducted 48 hours after plating.
TRPV1 immunostaining
Established neuron cultures were treated with or without botulinum neurotoxin A 0.1 nM and 1 nM and botulinum neurotoxin E 0.1 nM for one hour, fixed in 4% paraformaldehyde for 15 minutes, and double-immunostained for TRPV1 (rabbit polyclonal anti-TRPV1 1:1000), and Gap43 (growth-associated protein mouse monoclonal 1:200), visualized with goat antirabbit Alexa 546 and goat antimouse Alexa 488 (secondary antibodies, 1:200 Molecular Probes®), and mounted in glycerol containing DABCO antifade agent, as previously described.24 TIFF images were acquired using Smartcapture 3.0 software (Digital Scientific, Cambridge, UK), on an upright Olympus microscope, at a fixed exposure of 0.5 seconds for measuring fluorescence intensity using Metafluor software. Values for fluorescence intensity were compared between the groups treated and not treated with botulinum neurotoxin. The Student’s t-test was used for statistical analysis, and P < 0.05 was considered statistically significant.
Calcium imaging
Established cultures of human dorsal root ganglion neurons were washed in phenol red free 4-(2-hydroxyethyl) piperazine-1-ethanesulfonic acid, N-(2-hydroxyethyl) piperazine-N′-(2-ethanesulfonic acid) (HEPES)-buffered Hanks-balanced salt solution, containing 0.1% bovine serum albumin, and loaded with 2 μM Fura2 AM (acetoxymethyl ester) at 37°C for 40 minutes in the dark, with further incubation in HEPES-buffered Hanks-balanced salt solution containing 0.5% bovine serum albumin for 20 minutes in the dark to allow for de-esterification of the cytosolic Fura2 AM. Experiments were conducted at 37°C in a humidified environment, as previously described.24 Live recordings of intracellular changes in bound and unbound Ca2+ ratios (340/380) were obtained before, during, and after addition of 200 nM capsaicin for baseline intracellular Ca2+, as well as changes in response to added capsaicin, in 2 mL of HEPES-buffered Hanks-balanced salt solution. A test stimulus of 200 nM capsaicin was applied for 15–30 seconds to test for capsaicin sensitivity, followed by washout and a change of medium for 30 minutes, after which a second stimulus of capsaicin 1 μM was applied. The difference in ratio between baseline to peak response was measured to give the magnitude of the response. Expressing the second response as a percentage of the first response gave the control response in the absence of botulinum neurotoxin. The effects of botulinum neurotoxins A or E were determined by applying the required concentration before the second capsaicin stimulus. Mean (± standard error of the mean) values for percent inhibition in the presence of botulinum neurotoxin were compared with those of controls without botulinum neurotoxin.
Results and discussion
In this study, we describe localization of the botulinum neurotoxin receptor protein, SV2A, in a range of human tissues, including human dorsal root ganglion sensory neurons, peripheral nerves, urinary bladder, the colon, and the skin.
Dorsal root ganglia
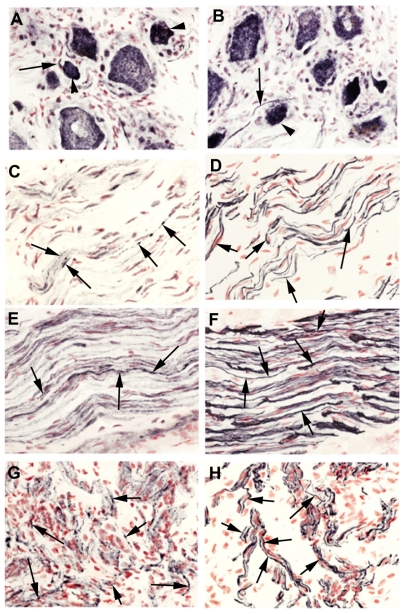
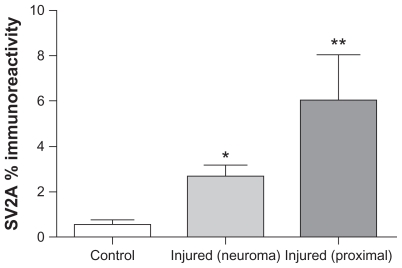
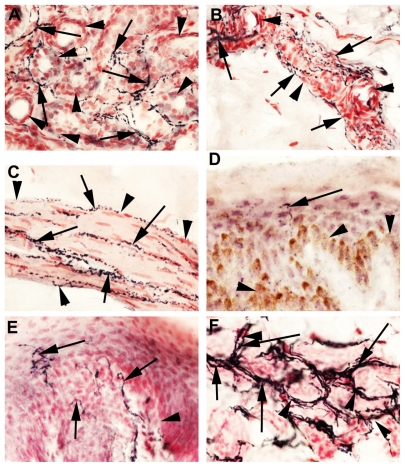
SV2A immunoreactivity was present in neurons of all sizes, although those with a smaller diameter were more intense (Figure 1A and B), suggesting that botulinum neurotoxin treatment may preferentially target nociceptor fibers. Normal uninjured peripheral nerves showed positive SV2A-immunoreactive fibers within the nerve fascicles (Figure 1C). In nerves proximal to injury and painful neuromas, SV2A immunoreactivity appeared to be increased (Figure 1E and G), and nerve immunostaining was similar to that seen with the structural nerve marker (neurofilaments, Figure 1D, F, and H). Image analysis (percent area of immunostaining) of these samples showed that SV2A was significantly increased in injured nerves compared with control uninjured nerves (P = 0.002, proximal injured nerves; P = 0.0027, neuromas), and significantly greater in proximal injured nerves compared with neuromas (P = 0.0038, Figure 2). There was no change in the percent area of neurofilaments in these specimens; when results were expressed as the ratio of SV2A to neurofilaments, there was also a significant increase proximal to injury (P = 0.0022) and in neuromas (P = 0.0001), indicating an overall increase in SV2A expression in injured nerve fibers. Analysis of the delay between injury and surgery (percent area) suggested a peak of immunoreactivity at 2–16 weeks after injury.
Figure 1.
SV2A in neuronal tissues. SV2A immunoreactivity in avulsion injured dorsal root ganglion (A and B). SV2A immunoreactivity in normal uninjured nerve (C), nerve proximal to injury (E), and in a neuroma (G); corresponding serial sections (D, F, and H) immunostained with the nerve structural marker, neurofilaments, arrows indicate neuronal fibers, magnification 40×.
Figure 2.
Image analysis (percent area) of SV2A immunoreactivity in injured nerve. Mean ± standard error of the mean of the percent area is shown.
Notes: *P = 0.0027; **P = 0.002.
Urinary bladder
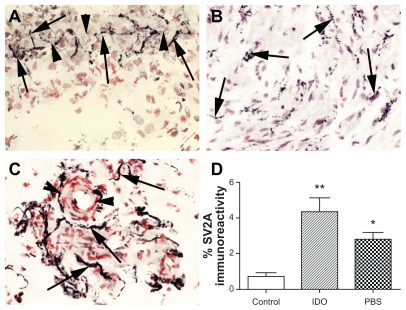
SV2A-immunoreactive nerve fibers were detected throughout the bladder, including in detrusor muscle, and were associated with blood vessels, particularly in the urothelium/suburothelial junction region (Figure 3A–C). Image analysis of detrusor muscle showed a significant increase in the idiopathic detrusor overactivity group (P = 0.002) and also in the painful bladder syndrome group (P = 0.0087, Figure 3D) compared with controls. Image analysis of neurofilaments also showed a statistically significant difference in the idiopathic detrusor overactivity group (P = 0.008) compared with the controls. Expressing the results as a ratio of SV2A to neurofilaments showed no significant difference between any of the groups, suggesting that the observed increase in SV2A may be due to increased nerve fibers which express SV2A.
Figure 3.
SV2A in the urinary bladder. SV2A-immunoreactive nerves (arrowed) in urothelium/suburothelial junction (arrowheads) (A), within the detrusor (B) and associated with blood vessel (arrowheads) within the suburothelium (C), magnification 40×, and image analysis of SV2A-immunoreactive fibers in the detrusor. Mean ± standard error of the mean of the percent area is shown (D).
Notes: *P = 0.008; **P = 0.0022.
Abbreviations: IDO, idiopathic detrusor overactivity; PBS, painful bladdersyndrome.
To our knowledge, this is the first report of SV2A levels in these patient groups. Our results for the distribution of SV2A in the human bladder are in agreement with others where SV2A was shown to be present in parasympathetic, sympathetic, and sensory fibers.25 Further studies, including double-staining with selective sensory and autonomic markers, are required to elucidate the nerve fiber subpopulations which show changed levels of SV2A in idiopathic detrusor overactivity and painful bladder syndrome. We have previously demonstrated a decrease of both TRPV1 and the purinergic receptor, P2X3 in the suburothelium of the urinary bladder after highly effective botulinum neurotoxin A intradetrusor injections in patients with detrusor overactivity, while structural markers showed no loss of nerve fibers.20 Intracellular proteolytic cleavage of the synaptosomal-associated protein, SNAP-25, that regulates acetylcholine exocytosis, leads to prolonged blockade of synaptic transmission while sparing nerve endings.26 The progressive decrease of TRPV1 and P2X3, over the weeks following the injection treatment implies complex or multiple mechanisms of action for botulinum neurotoxin A in the bladder, with a progressive contribution to decreased levels of these sensory receptors by amelioration of bladder hypertrophy, or by decreased expression, uptake, or axonal transport of neurotrophic factors that regulate these receptors, eg, nerve growth factor and glial-derived neurotrophic factor. Protein kinase C activation increased TRPV1 receptor surface expression in primary rat dorsal root ganglion neurons, and botulinum neurotoxin A fully blocked 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced TRPV1 surface expression,21 which could explain the acute effect on urinary urgency in patients. In primary dorsal root ganglion neurons, TRPV1 codistributes in vesicles with synaptotagmin and the vesicular protein synaptobrevin; activity-dependent delivery of channels to the neuronal surface may contribute to the buildup and maintenance of thermal inflammatory hyperalgesia in peripheral nociceptor terminals, which may be blocked by botulinum neurotoxin A.21 In accord, botulinum neurotoxin A has been shown to have an antinociceptive effect on bladder afferent pathways in patients with painful bladder syndrome or interstitial cystitis, producing both symptomatic and functional (ie, urodynamic) improvements.15
Colon
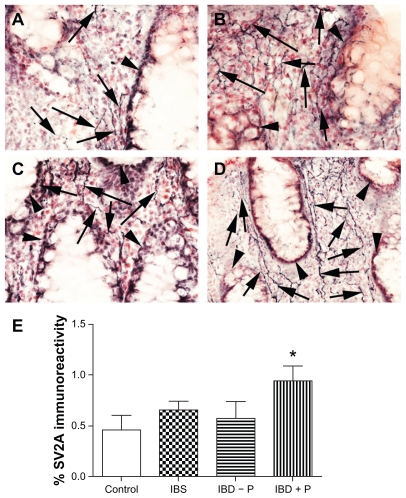
Colon biopsies from controls and from patients with irritable bowel syndrome and inflammatory bowel disease with or without painful symptoms showed SV2A-immunoreactive nerve fibers within the mucosa and muscularis mucosae (Figure 4A–D). Image analysis of SV2A mucosal fibers showed an increase only in quiescent inflammatory bowel disease with pain group (P = 0.023, Figure 4E), compared with controls, but not in those with inflammatory bowel disease without pain (P = 0.77) or in irritable bowel syndrome (P = 0.13). There was no difference between the mucosal neurofilament fibers or when results were expressed as a ratio of SV2A to neurofilaments in any of the groups studied. To our knowledge, this is the first description of SV2A in the colon mucosa of patients with irritable bowel syndrome and inflammatory bowel disease. Our finding of increased mucosal SV2A in patients with inflammatory bowel disease and abdominal pain are of interest, given that we have reported increased TRPV1 in this patient group.19 Considering the previously observed effects of botulinum neurotoxin A on TRPV1 expression in patients with detrusor overactivity, this raises interesting clinical therapeutic possibilities in inflammatory bowel disease. The regulation of SV2A expression, effect of inflammation, and efficacy of botulinum neurotoxin A in inflammatory bowel disease all deserve further investigation.
Figure 4.
SV2A in the human bowel. SV2A-immunoreactive fibers (arrowed) in control human colonic biopsies (A), irritable bowel syndrome (B), inflammatory bowel disease without pain (C) and inflammatory bowel disease with pain (D), arrowheads indicate villi, magnification 40× and image analysis of SV2A-immunoreactive fibers in control IBS and quiescent IBD with (+P) and without pain (−P). Mean ± standard error of the mean of the percent area is shown (E).
Note: *P = 0.023.
Skin
In human skin, SV2A-immunoreactive fibers were prominent around sweat glands, arrector pili, and vascular structures. Only a few intraepithelial fibers were seen in prefixed specimens (Figure 5A–D). In normal rat paw skin, SV2A strongly stained nerve fibers in the subepithelium, within the epithelium, and around sweat glands (Figure 5E–F), indicating the usefulness of this antibody for preclinical studies in rodent models. Furthermore, this provides a basis for the reduction of neurogenic inflammation in rats following transdermal botulinum neurotoxin A treatment.27 The dense innervation of SV2A-positive nerve fibers around sweat glands may explain why botulinum neurotoxin A is successful in the treatment of hyperhidrosis.28,29
Figure 5.
SV2A in human skin. SV2A-immunoreactive fibers (arrowed) in sweat glands (arrowheads) (A), around blood vessels (arrowheads) (B), arrector pili (C), and rare intraepithelial fiber, epithelial basal layer indicated by arrowhead (D) in human skin from a patient with small fiber neuropathy. Rat paw skin showing intraepithelial SV2A-immunoreactive fibers, epithelial basal layer indicated by arrowhead (E) and dense immunoreactive fibers around sweat glands (arrowheads) (F), magnification 40×.
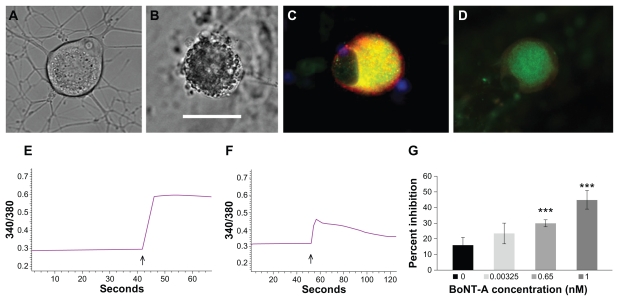
Morphological effects of botulinum neurotoxin in dorsal root ganglia
Under differential interference contrast optics, normal untreated human dorsal root ganglion neurons showed few cytoplasmic vesicles and densely branched neurites ( Figure 6A). Neurons treated with botulinum neurotoxin E 1 nM for one hour showed dense accumulation of cytoplasmic vesicles, and loss of neurites after 24 hours. Human dorsal root ganglion neurons treated with botulinum neurotoxin E 0.1 nM for 48 hours also showed accumulation of cytoplasmic vesicles and loss of neurites up to 3 days later (Figure 6B). These effects were observed with comparable doses of both botulinum neurotoxins A and E. These changes are expected to be greater and more rapid in cultured neurons where the whole cell is exposed to the toxin, compared with the in vivo situation where only the nerve terminals may be initially exposed. The morphological effects of botulinum neurotoxin A and E treatment, ie, neurite loss and vesicle accumulation in the sensory neurons of the dorsal root ganglia observed in this study, are likely to result from disruption of the process of fusion of intracellular vesicles containing the proteins necessary for the structural and functional integrity of the axon, in a manner similar to that observed in motor neurons, whereby cleavage of SNARE proteins within the presynaptic terminal by botulinum neurotoxin leads to prevention of the formation of the productive docking complex necessary for transmitter release, and consequent loss of function.24 A similar action of a recombinant chimera of botulinum neurotoxins A and E has been described in sensory neurons of the trigeminal ganglion affecting calcitonin gene-related peptide release.13
Figure 6.
In vitro effects of botulinum neurotoxin A and E treatment in human dorsal root ganglion neurons. Differential interference contrast image of a normal untreated neuron showing few intracellular organelles and densely branched neurites (A). Similar image of a neuron treated with botulinum neurotoxin E for 48 hours, showing dense accumulation of cytoplasmic vesicles and loss of neurites (B). Immunofluorescent image of an untreated neuron with membrane-bound intense TRPV1 localization (red) surrounding cytoplasmic Gap43 (green) appearing yellow in merged areas (C). Similar image showing diminished fluorescence intensity after one hour of botulinum neurotoxin A treatment (D). Sample trace of baseline ratio of bound and unbound Ca2+ and increase in ratio after capsaicin application (arrow, E). Trace showing diminished response to capsaicin (arrow) following pretreatment with 0.65 nM botulinum neurotoxin A (F). Graph showing dose-related percent inhibition of capsaicin responses following acute botulinum neurotoxin A treatment (G). Bar = 45 μm in B, applies to A, C, and D.
Note: ***P = 0.0036 for 0.65 nM botulinum neurotoxin A and P = 0.005 for 1 nM botulinum neurotoxin A.
TRPV1 immunofluorescence in dorsal root ganglia
The effect of treatment with botulinum neurotoxins A and E on TRPV1 localization in cultured human dorsal root ganglion neurons was determined by treating the neurons with botulinum neurotoxin A 0.1 nM and 1 nM and botulinum neurotoxin E at 1 nM, for one hour, before fixing with paraformaldehyde. Neurons not treated with botulinum neurotoxin showed cytoplasmic Gap43 immunostaining (green, Figure 6C), surrounded by membrane-bound red TRPV1 immunofluorescence. This pattern and intensity of staining were greatly diminished in botulinum neurotoxin-treated neurons (Figure 6D). Quantification of TRPV1 immunostained images showed approximately 60% reduction in signal intensity compared with untreated controls, at the concentrations used here, indicating the potency of the toxin in exerting its effects at low concentrations applied acutely for one hour. The average fluorescence intensity value (arbitrary units) for untreated controls was 39.3 ± 2.7 (n = 23 neurons), reduced to 14.17 ± 2.27 (n = 8 neurons, P < 0.0001) after botulinum neurotoxin A 0.1 nM treatment, 17.15 ± 3.8 (n = 5 neurons, P < 0.0001) after botulinum neurotoxin A 1 nM, and 16.8 ± 1.76 (n = 27 neurons, P < 0.0001) after botulinum neurotoxin E 1 nM treatment for one hour.
Calcium imaging
With single-cell studies using calcium imaging, we observed diminished calcium influx in response to the TRPV1 ligand, capsaicin, following application of botulinum neurotoxins A or E (Figure 6). Control neurons not treated with botulinum neurotoxin showed, on average, 16.6% ± 5% inhibition (n = 6 neurons) caused by the known desensitization due to repeat capsaicin stimulation. The first stimulus was used to identify capsaicin-sensitive neurons and the second stimulus was used to test the effect of botulinum neurotoxin after washout and change of medium, as described by our group previously. 24 Ten minutes of incubation with 0.00325 nM botulinum neurotoxin A resulted in 23.4% ± 6.7% inhibition (n = 11 neurons, P = 0.27); 0.65 nM botulinum neurotoxin A resulted in 29.98% ± 2.3% inhibition (n = 6 neurons, P = 0.0036); and 1 nM botulinum neurotoxin A resulted in 45.1% ± 6% inhibition (n = 5 neurons, P = 0.005). Botulinum neurotoxin E-treated neurons showed 58.6% ± 8.4% inhibition (n = 4 neurons, P = 0.008) after one hour of treatment with 0.1 nM botulinum neurotoxin E, and 97.8% ± 1.5% inhibition (n = 6 neurons, P < 0.01) after 48 hours of treatment with 0.1 nM botulinum neurotoxin E. Botulinum neurotoxin E 1 nM treatment for 10 minutes resulted in 25.04% ± 5.1% inhibition of capsaicin responses (n = 6 neurons, P = 0.19).
The TRPV1 receptor is actively transported in vesicles associated with the proteins synaptotagmin IX and snapin, and inserted in the plasma membrane by protein kinase C-regulated exocytosis, via a botulinum neurotoxin A-sensitive mechanism. 26 In agreement with this phenomenon, our results show the accumulation of vesicles within the cytoplasm of botulinum neurotoxin A-treated and botulinum neurotoxin E-treated neurons, correlating with diminished TRPV1 signal intensity and capsaicin responses. Botulinum neurotoxin effects on sensory neurons may thus be mediated by decreasing levels of sensory receptors, and blockade of their translocation to the plasma membrane. Further work using larger cohorts of different patient groups, together with further characterization of SV2A immunoreactivity, are required to confirm the current findings. In addition, localization and identification of SNARE proteins in cultured dorsal root ganglia neurons, with and without botulinum neurotoxin treatment, would support our findings, and will form the focus of future research. The functional effects observed in this study, along with the distribution and levels of the receptor SV2A in normal and diseased human tissues, offer the potential of targeting a range of clinical conditions.
Conclusion
Differential levels of SV2A protein and changes in clinical disorders may provide potential and preferential targets for botulinum neurotoxin therapy, including painful neuromas, urinary bladder disorders, and inflammatory bowel disease.30,31 Novel designed agents based on neurotoxins and directed to molecular mechanisms underlying clinical disorders thus hold great promise for addressing unmet clinical needs. Selective targeted effects may advance treatment, eg, on sensory mechanisms in urinary bladder disorders, which may provide efficacy without motor dysfunction, and thereby avoid the need for bladder catheterization. Botulinum neurotoxin A and E treatment in cultured human sensory neurons produced morphological, molecular, and functional effects, supporting their use in clinical pain and hypersensitivity disorders, and the strategy of designing novel neurotoxins to target subsets of sensory nerve fibers.
Acknowledgment
We thank Syntaxin Ltd and Cancer Research UK, London Research Institute, for their support of this research.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Dong M, Yeh F, Tepp WH, et al. SV2 is the protein receptor for botulinum neurotoxin A. Science. 2006;312(5773):592–596. doi: 10.1126/science.1123654. [DOI] [PubMed] [Google Scholar]
- 2.Simpson LL. The action of botulinal toxin. Rev Infect Dis. 1979;1(4):656–662. doi: 10.1093/clinids/1.4.656. [DOI] [PubMed] [Google Scholar]
- 3.Buckley K, Kelly RB. Identification of a transmembrane glycoprotein specific for secretory vesicles of neural and endocrine cells. J Cell Biol. 1985;100(4):1284–1294. doi: 10.1083/jcb.100.4.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feany MB, Lee S, Edwards RH, Buckley KM. The synaptic vesicle protein SV2 is a novel type of transmembrane transporter. Cell. 1992;70(5):861–867. doi: 10.1016/0092-8674(92)90319-8. [DOI] [PubMed] [Google Scholar]
- 5.Bajjalieh SM, Peterson K, Shinghal R, Scheller RH. SV2, a brain synaptic vesicle protein homologous to bacterial transporters. Science. 1992;257(5074):1271–1273. doi: 10.1126/science.1519064. [DOI] [PubMed] [Google Scholar]
- 6.Dong M, Tepp WH, Liu H, Johnson EA, Chapman ER. Mechanism of botulinum neurotoxin B and G entry into hippocampal neurons. J Cell Biol. 2007;179(7):1511–1522. doi: 10.1083/jcb.200707184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duggan MJ, Quinn CP, Chaddock JA, et al. Inhibition of release of neurotransmitters from rat dorsal root ganglia by a novel conjugate of a Clostridium botulinum toxin A endopeptidase fragment and Erythrina cristagalli lectin. J Biol Chem. 2002;277(38):34846–34852. doi: 10.1074/jbc.M202902200. [DOI] [PubMed] [Google Scholar]
- 8.Meng J, Ovsepian SV, Wang J, et al. Activation of TRPV1 mediates calcitonin gene-related peptide release, which excites trigeminal sensory neurons and is attenuated by a retargeted botulinum toxin with antinociceptive potential. J Neurosci. 2009;29(15):4981–4992. doi: 10.1523/JNEUROSCI.5490-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benham CD, Davis JB, Randall AD. Vanilloid and TRP channels: a family of lipid-gated cation channels. Neuropharmacology. 2002;42(7):873–888. doi: 10.1016/s0028-3908(02)00047-3. [DOI] [PubMed] [Google Scholar]
- 10.Akbar A, Yiangou Y, Facer P, Walters JR, Anand P, Ghosh S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut. 2008;57(7):923–929. doi: 10.1136/gut.2007.138982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Facer P, Casula MA, Smith GD, et al. Differential expression of the capsaicin receptor TRPV1 and related novel receptors TRPV3, TRPV4 and TRPM82 in normal human tissues and changes in traumatic and diabetic neuropathy. BMC Neurol. 2007;7:11. doi: 10.1186/1471-2377-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shu X, Mendell LM. Acute sensitization by NGF of the response of small-diameter sensory neurons to capsaicin. J Neurophysiol. 2001;86(6):2931–2938. doi: 10.1152/jn.2001.86.6.2931. [DOI] [PubMed] [Google Scholar]
- 13.Anand U, Otto WR, Casula MA, et al. The effect of neurotrophic factors on morphology, TRPV1 expression and capsaicin responses of cultured human DRG sensory neurons. Neurosci Lett. 2006;399(1–2):51–56. doi: 10.1016/j.neulet.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 14.Ney JP, Joseph KR. Neurologic uses of botulinum neurotoxin type A. Neuropsychiatr Dis Treat. 2007;3(6):785–798. doi: 10.2147/ndt.s1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith CP, Radziszewski P, Borkowski A, Somogyi GT, Boone TB, Chancellor MB. Botulinum toxin a has antinociceptive effects in treating interstitial cystitis. Urology. 2004;64(5):871–875. doi: 10.1016/j.urology.2004.06.073. [DOI] [PubMed] [Google Scholar]
- 16.Cervero F, Laird JM. Visceral pain. Lancet. 1999;353(9170):2145–2148. doi: 10.1016/S0140-6736(99)01306-9. [DOI] [PubMed] [Google Scholar]
- 17.Mukerji G, Yiangou Y, Agarwal SK, Anand P. Transient receptor potential vanilloid receptor subtype 1 in painful bladder syndrome and its correlation with pain. J Urol. 2006;176(2):797–801. doi: 10.1016/j.juro.2006.03.074. [DOI] [PubMed] [Google Scholar]
- 18.Apostolidis A, Brady C, Yiangou Y, et al. Parallel changes in suburothelial vanilloid receptor TRPV1 (VR1) and pan-neuronal marker PGP9.5 immunoreactivity in patients with neurogenic detrusor overactivity (NDO) following intravesical resiniferatoxin treatment. Eur Urol. 2003;(Suppl 2(1)):391. doi: 10.1111/j.1464-410X.2003.04722.x. [DOI] [PubMed] [Google Scholar]
- 19.Akbar A, Yiangou Y, Facer P, et al. Expression of the TRPV1 receptor differs in quiescent inflammatory bowel disease with or without abdominal pain. Gut. 2010;59(6):767–774. doi: 10.1136/gut.2009.194449. [DOI] [PubMed] [Google Scholar]
- 20.Apostolidis A, Popat R, Yiangou Y, et al. Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J Urol. 2005;174(3):977–982. doi: 10.1097/01.ju.0000169481.42259.54. [DOI] [PubMed] [Google Scholar]
- 21.Morenilla-Palao C, Planells-Cases R, Garcia-Sanz N, Ferrer-Montiel A. Regulated exocytosis contributes to protein kinase C potentiation of vanilloid receptor activity. J Biol Chem. 2004;279(24):25665–25672. doi: 10.1074/jbc.M311515200. [DOI] [PubMed] [Google Scholar]
- 22.Kuner R. Central mechanisms of pathological pain. Nat Med. 2010;16(11):1258–1266. doi: 10.1038/nm.2231. [DOI] [PubMed] [Google Scholar]
- 23.Mukerji G, Yiangou Y, Agarwal SK, Anand P. Increased cannabinoid receptor 1-immunoreactive nerve fibers in overactive and painful bladder disorders and their correlation with symptoms. Urology. 2010;75(6):1514, e1515–e1520. doi: 10.1016/j.urology.2009.12.051. [DOI] [PubMed] [Google Scholar]
- 24.Anand U, Otto WR, Sanchez-Herrera D, et al. Cannabinoid receptor CB2 localisation and agonist-mediated inhibition of capsaicin responses in human sensory neurons. Pain. 2008;138(3):667–680. doi: 10.1016/j.pain.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Coelho A, Dinis P, Pinto R, et al. Distribution of the high-affinity binding site and intracellular target of botulinum toxin type A in the human bladder. Eur Urol. 2010;57(5):884–890. doi: 10.1016/j.eururo.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 26.Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol Rev. 2000;80(2):717–766. doi: 10.1152/physrev.2000.80.2.717. [DOI] [PubMed] [Google Scholar]
- 27.Carmichael NM, Dostrovsky JO, Charlton MP. Peptide-mediated transdermal delivery of botulinum neurotoxin type A reduces neurogenic inflammation in the skin. Pain. 2010;149(2):316–324. doi: 10.1016/j.pain.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 28.Glaser DA. Treatment of axillary hyperhidrosis by chemodenervation of sweat glands using botulinum toxin type A. J Drugs Dermatol. 2004;3(6):627–631. [PubMed] [Google Scholar]
- 29.Laing TA, Laing ME, O’Sullivan ST. Botulinum toxin for treatment of glandular hypersecretory disorders. J Plast Reconstr Aesthet Surg. 2008;61(9):1024–1028. doi: 10.1016/j.bjps.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 30.Ranoux D, Attal N, Morain F, Bouhassira D. Botulinum toxin type A induces direct analgesic effects in chronic neuropathic pain. Ann Neurol. 2008;64(3):274–283. doi: 10.1002/ana.21427. [DOI] [PubMed] [Google Scholar]
- 31.Smith HS. Botulinum toxins for analgesia. Pain Physician. 2009;12(3):479–481. [PubMed] [Google Scholar]